Effect of High-Temperature Paraffin Impregnation on the Properties of the Amorphous Cellulose Region Based on Molecular Dynamics Simulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Model Establishment
2.2. Dynamic Simulation
3. Results and Discussion
3.1. Energy
3.1.1. Balance of the System
3.1.2. Total Potential Energy and Non-Bond Energy
3.2. Mean Square Displacement of Paraffin
3.3. Lattice Parameters and Density
3.4. Hydrogen Bonding
3.5. Mechanical Properties
4. Conclusions
- The total potential energy was minimized when the system temperature was 100 °C. The difference between the non-bonding and total potential energy was the minimum, and the inter-atomic repulsive force within the molecule was the weakest. This confirmed that the number of hydrogen bonds in the system was the highest, and the structure of the cellulose was the most stable.
- The paraffin molecular diffusion coefficient peaked at a system temperature of 210 °C. The temperature rise increased the activity of the paraffin molecules, increasing the intensity of movement and consequently leading to the largest cell volume at this temperature, resulting in the lowest density and weakest water absorption.
- The mechanical parameters were calculated with Lamé’s constant in the temperature range of 100–210 °C. The results show that Young’s modulus (E) and shear modulus (G) were the largest at the system temperature of 100 °C, and the wood had the best rigidity; the Poisson’s ratio (γ) and K/G values were the largest at the system temperature of 210 °C, and the cellulose ductility and plasticity were the best. Therefore, according to the needs of the actual application process, the wood can be heat treated at the appropriate temperature selected during production and processing in order to improve the utilization of wood.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pelaez-Samaniego, M.R.; Yadama, V.; Lowell, E.; Espinoza-Herrera, R. A review of wood thermal pretreatments to improve wood composite properties. Wood Sci. Technol. 2013, 47, 1285–1319. [Google Scholar] [CrossRef]
- Kamperidou, V.; Barboutis, I.; Vasileiou, V. Influence of thermal treatment on mechanical strength of scots pine (Pinus sylvestris L.) wood. Wood Res. 2014, 59, 373–378. [Google Scholar]
- Guo, Y.; Wang, W.; Jiang, X. Molecular Dynamics Study on Mechanical Properties of Cellulose with Water Molecules Diffusion Behavior at Different Oxygen Concentrations. Forests 2023, 14, 371. [Google Scholar] [CrossRef]
- Esteves, B.; Ferreira, H.; Viana, H.; Ferreira, J.; Domingos, I.; Cruz-Lopes, L.; Nunes, L. Termite Resistance, Chemical and Mechanical Characterization of Paulownia tomentosa Wood before and after Heat Treatment. Forests 2021, 12, 1114. [Google Scholar] [CrossRef]
- Durmaz, E.; Ucuncu, T.; Karamanoglu, M.; Kaymakci, A. Effects of heat treatment on some characteristics of scots pine (Pinus sylvestris L.) wood. BioResources 2019, 14, 9531–9543. [Google Scholar] [CrossRef]
- Fang, C.H.; Cloutier, A.; Blanchet, P.; Koubaa, A. Densification of wood veneers combined with oil-heat treatment. Part I: Dimensional stability. BioResources 2011, 6, 373–385. [Google Scholar] [CrossRef]
- Wang, J.Y.; Cooper, P.A. Effect of oil type, temperature and time on moisture properties of hot oil-treated wood. Holz Als Roh-Und Werkst. 2005, 63, 417–422. [Google Scholar] [CrossRef]
- Amthor, J. Paraffin dispersions for waterproofing of particle board. Holz Als Roh-Und Werkst. 1972, 30, 422–429. [Google Scholar] [CrossRef]
- Esteves, B.; Nunes, L.; Domingos, I.; Pereira, H. Improvement of termite resistance, dimensional stability and mechanical properties of pine wood by paraffin impregnation. Eur. J. Wood Wood Prod. 2014, 72, 609–615. [Google Scholar] [CrossRef]
- Reinprecht, L.; Repák, M. The impact of paraffin-thermal modification of beech wood on its biological, physical and mechanical properties. Forests 2019, 10, 1102. [Google Scholar] [CrossRef]
- Humar, M.; Kržišnik, D.; Lesar, B.; Thaler, N.; Ugovšek, A.; Zupanic, K.; Žlahtic, M. Thermal modification of wax-impregnated wood to enhance its physical, mechanical, and biological properties. Holzforschung 2017, 71, 57–64. [Google Scholar] [CrossRef]
- Karplus, M.; Petsko, G.A. Molecular dynamics simulations in biology. Nature 1990, 347, 631–639. [Google Scholar] [CrossRef]
- González, M.A. Force fields and molecular dynamics simulations. École Thématique Société Française Neutron 2011, 12, 169–200. [Google Scholar] [CrossRef]
- Lee, S.H.; Rossky, P.J. A comparison of the structure and dynamics of liquid water at hydrophobic and hydrophilic surfaces—A molecular dynamics simulation study. J. Chem. Phys. 1994, 100, 3334–3345. [Google Scholar] [CrossRef]
- Meier, R.J.; Maple, J.R.; Hwang, M.J.; Hagler, A.T. Molecular Modeling Urea- and Melamine-Formaldehyde Resins. 1. A Force Field for Urea and Melamine. J. Phys. Chem. 1995, 99, 5445–5456. [Google Scholar] [CrossRef]
- Hou, T.; Zhang, W.; Xu, X. Binding Affinities for a Series of Selective Inhibitors of Gelatinase-A Using Molecular Dynamics with a Linear Interaction Energy Approach. J. Phys. Chem. B 2001, 105, 5304–5315. [Google Scholar] [CrossRef]
- Theodorou, D.N.; Suter, U.W. Detailed molecular structure of a vinyl polymer glass. Am. Chem. Soc. 1984, 187, 1467–1478. [Google Scholar] [CrossRef]
- Wang, X.; Tang, C.; Wang, Q.; Li, X.; Hao, J. Selection of Optimal Polymerization Degree and Force Field in the Molecular Dynamics Simulation of Insulating Paper Cellulose. Energies 2017, 10, 1377. [Google Scholar] [CrossRef]
- Onyon, P.F. Polymer Handbook. Nature 1972, 238, 56. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Li, X. Molecular dynamics study on mechanical properties of cellulose with air/nitrogen diffusion behavior. BioResources 2018, 13, 7900–7910. [Google Scholar] [CrossRef]
- Maple, J.R.; Hwang, M.J.; Stockfisch, T.P.; Dinur, U.; Waldman, M.; Ewig, C.S.; Hagler, A.T. Derivation of class II force fields. I. Methodology and quantum force field for the alkyl functional group and alkane molecules. J. Comput. Chem. 1994, 15, 162–182. [Google Scholar] [CrossRef]
- Andersen, H.C. The role of long ranged forces in determining the structure and properties of liquid water. J. Chem. Phys. 1983, 79, 4576–4584. [Google Scholar] [CrossRef]
- Berendsen, H.J.; Postma, J.V.; Van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Ewald, P. Evaluation of optical and electrostatic lattice potentials. Ann. Der Phys. 1921, 64, 253–287. [Google Scholar] [CrossRef]
- Ouyang, F.; Wang, W. Effect of Thermo-Hydro-Mechanical Treatment on Mechanical Properties of Wood Cellulose: A Molecular Dynamics Simulation. Forests 2022, 13, 903. [Google Scholar] [CrossRef]
- Michael, P.; Tildesley, D.J. Computer Simulation of Liquids; Oxford University Press: Oxford, UK, 2017. [Google Scholar] [CrossRef]
- Maple, J.R.; Hwang, M.J.; Stockfisch, T.P.; Hagler, A.T. Derivation of class II force fields. III. Characterization of a quantum force field for alkanes. Isr. J. Chem. 1994, 34, 195–231. [Google Scholar] [CrossRef]
- Wang, W.; Cao, Y.; Sun, L.; Wu, M. Effect of Temperature on Formaldehyde Diffusion in Cellulose Amorphous Region: A Simulation Study. BioResources 2021, 16, 3200–3213. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, W.; Guo, Y.; Dai, M. Effect of Pressurized Hydrothermal Treatment on the Properties of Cellulose Amorphous Region Based on Molecular Dynamics Simulation. Forests 2023, 14, 314. [Google Scholar] [CrossRef]


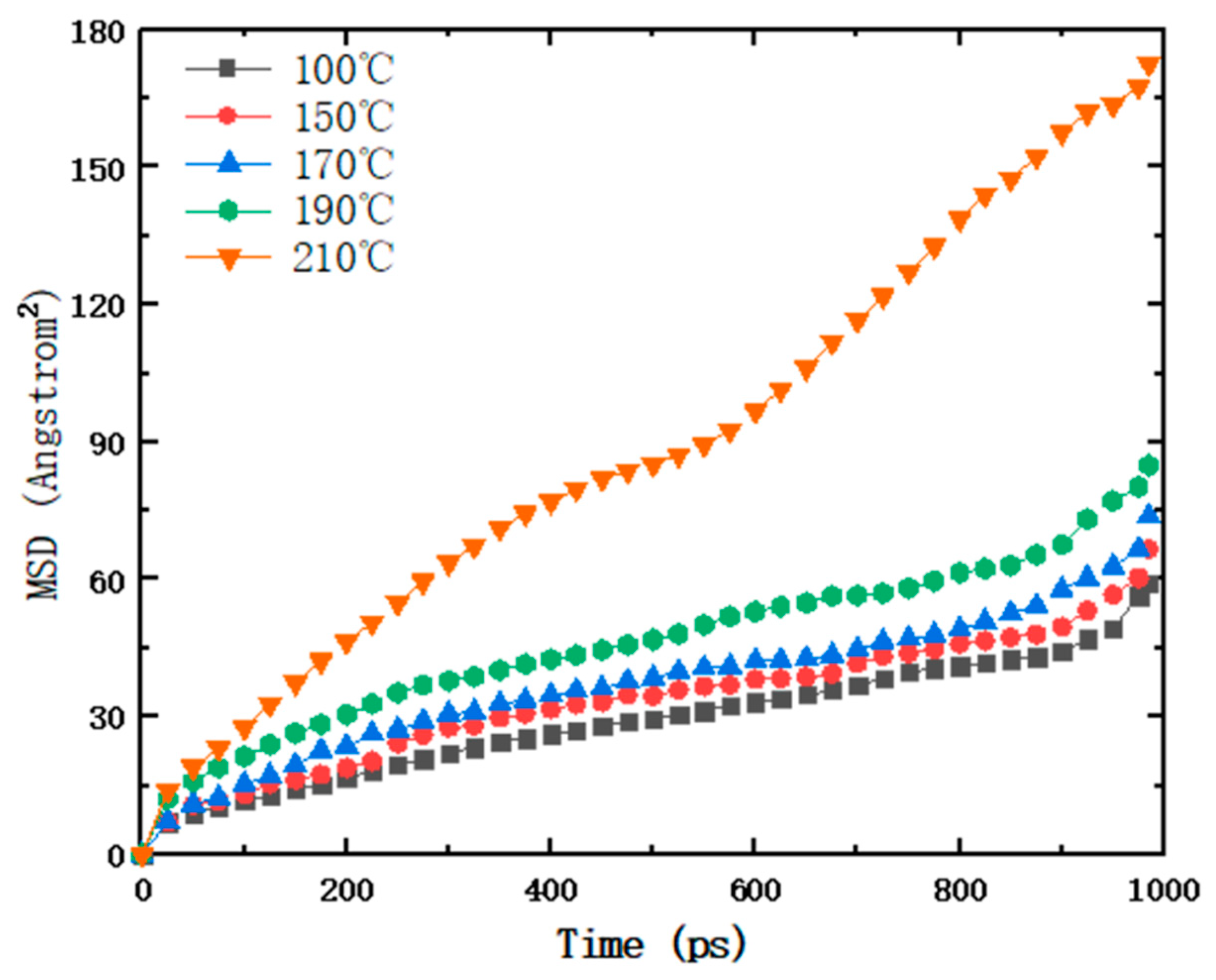
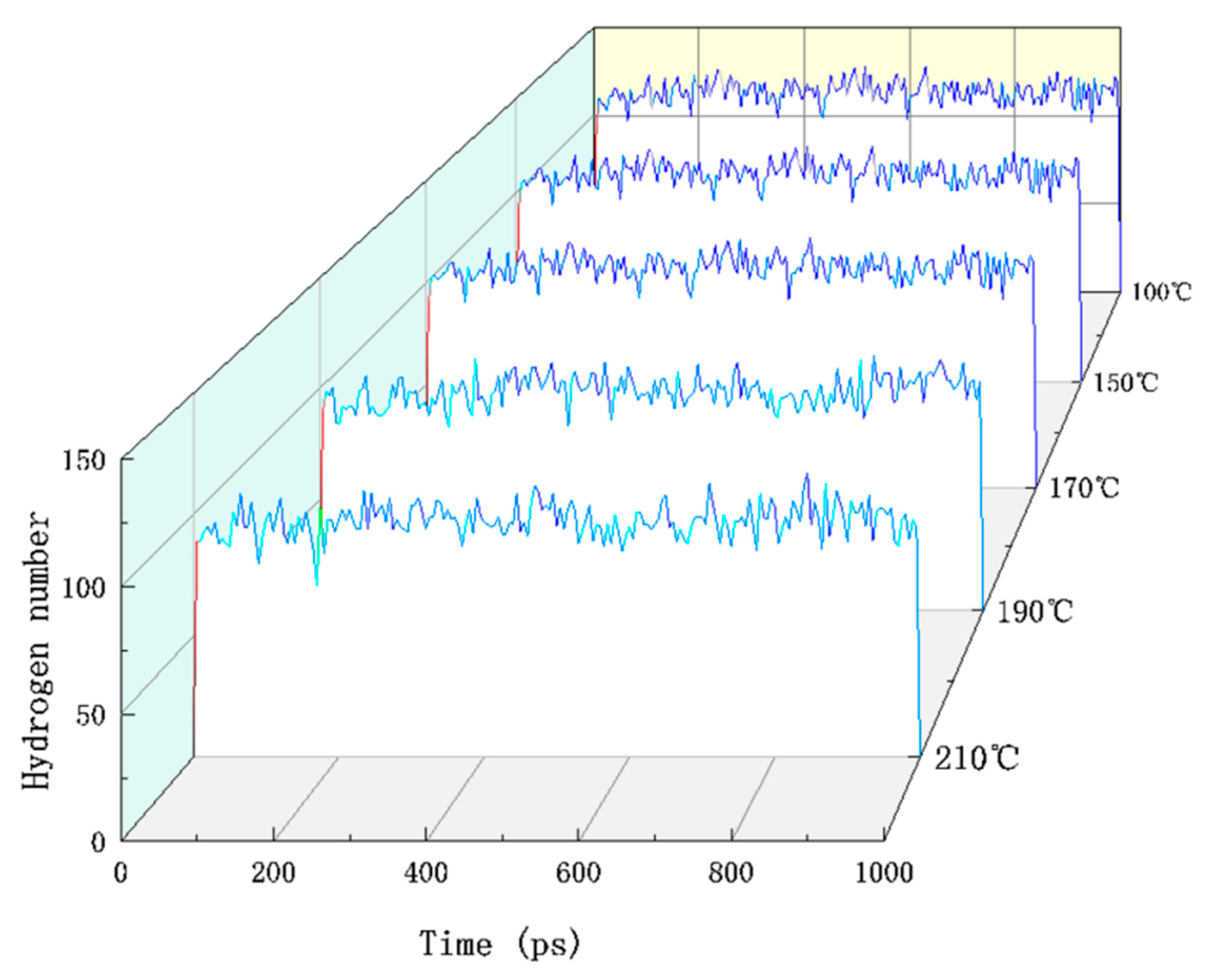
| Temperature (°C) | 100 | 150 | 170 | 190 | 210 |
|---|---|---|---|---|---|
| ETotal | 2769.277 | 3280.921 | 3426.100 | 3739.676 | 3938.125 |
| Ebond | 1788.714 | 2268.072 | 2393.878 | 2701.807 | 2871.446 |
| Enonbond | 980.563 | 1012.849 | 1032.222 | 1037.869 | 1066.679 |
| Temperature (°C) | 100 | 150 | 170 | 190 | 210 |
|---|---|---|---|---|---|
| k | 0.03724014 | 0.05294676 | 0.05457696 | 0.06585672 | 0.19829112 |
| D (A2/ps) | 0.00620669 | 0.00882446 | 0.00909616 | 0.01097612 | 0.03304852 |
| R2 | 0.99329621 | 0.98541197 | 0.87490145 | 0.86396499 | 0.97642928 |
| Temperature (°C) | Cell Parameters (Å) | Volume (A3) | Density (g/cm3) | ||
|---|---|---|---|---|---|
| The Length | The Width | The Height | |||
| 100 | 24.69 | 24.69 | 24.69 | 15,044.329 | 1.100 |
| 150 | 24.79 | 24.79 | 24.79 | 15,241.504 | 1.086 |
| 170 | 24.82 | 24.82 | 24.82 | 15,296.172 | 1.082 |
| 190 | 24.92 | 24.92 | 24.92 | 15,470.951 | 1.070 |
| 210 | 24.94 | 24.94 | 24.94 | 15,517.501 | 1.066 |
| Temperature (°C) | λ | μ | E | G | γ | K/G |
|---|---|---|---|---|---|---|
| 100 | 17.1525 | 2.3641 | 6.8059 | 2.3641 | 0.4394 | 7.9220 |
| 150 | 18.0467 | 2.3246 | 6.7085 | 2.3246 | 0.4429 | 8.430 |
| 170 | 19.8403 | 1.4500 | 4.2512 | 1.4500 | 0.4659 | 14.3469 |
| 190 | 20.5715 | 1.3973 | 4.1030 | 1.3973 | 0.4681 | 15.3889 |
| 210 | 20.2425 | 1.3308 | 3.9103 | 1.3308 | 0.4691 | 15.8774 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, Z.; Wang, W.; Hua, Y.; Cang, S. Effect of High-Temperature Paraffin Impregnation on the Properties of the Amorphous Cellulose Region Based on Molecular Dynamics Simulation. Forests 2023, 14, 1068. https://doi.org/10.3390/f14061068
Qu Z, Wang W, Hua Y, Cang S. Effect of High-Temperature Paraffin Impregnation on the Properties of the Amorphous Cellulose Region Based on Molecular Dynamics Simulation. Forests. 2023; 14(6):1068. https://doi.org/10.3390/f14061068
Chicago/Turabian StyleQu, Zening, Wei Wang, Youna Hua, and Shilong Cang. 2023. "Effect of High-Temperature Paraffin Impregnation on the Properties of the Amorphous Cellulose Region Based on Molecular Dynamics Simulation" Forests 14, no. 6: 1068. https://doi.org/10.3390/f14061068
APA StyleQu, Z., Wang, W., Hua, Y., & Cang, S. (2023). Effect of High-Temperature Paraffin Impregnation on the Properties of the Amorphous Cellulose Region Based on Molecular Dynamics Simulation. Forests, 14(6), 1068. https://doi.org/10.3390/f14061068






