Abstract
Phytoremediation is becoming more prevalent globally. Literature on phytoremediation strategies in western China is relatively scarce. The present research sought to fill this gap by examining the effects of trace elements such as Cd, Cr, Pb, and Zn on growth, physiological traits, tolerances, and accumulation characteristics in 2-year-old saplings of Ligustrum obtusifolium. The gradient of trace element concentration was determined by adding exogenous trace elements to the soil in a pot experiment: CK (no exogenous trace element), T1, T2, and T3 (Cd 0, 2, 5, and 10 mg/kg; Cr 0, 300, 500, and 700 mg/kg; Pb 0, 400, 800, and 1200 mg/kg; and Zn 0, 300, 500, and 1000 mg/kg, respectively). The results indicated that Chla, Chlb, and total Chls significantly decreased (p < 0.05) in the leaves of L. obtusifolium, with the lowest value obtained during soil treatment with T3. Along with the increase in trace element concentration, the net increase in height, root biomass, aerial biomass, and total biomass was reduced significantly. The net growth of L. obtusifolium under Cr stress did not differ significantly from that of CK at T1; however, the net growth of L. obtusifolium under Cr stress was considerably reduced at T2 and T3. The antioxidant enzyme activity of L. obtusifolium increased under different trace element stresses and first increased and then decreased as trace element levels increased. It was found that the SOD, POD, CAT, and APX activity of L. obtusifolium peaks at T2 under Cd and Zn stress in contrast to a peak at T1 under Cr and Pb stress. The contents of trace elements in L. obtusifolium roots, stems, and leaves increased along with the increase in soil trace element levels. Cr, Pb, and Zn threshold values can be set at 300 mg/kg, 400 mg/kg, and 300 mg/kg, respectively. This is carried out by using a reference index of the biomass of L. obtusifolium decreased by 10%, while the Cd threshold value needs to be further studied. L. obtusifolium would be an appropriate plant for phytoremediation of Cr-polluted soil, compared to Cd, Pb, and Zn absorption and accumulation. It showed optimal antioxidant enzyme activity and transfer ability under soil Cr contents of 300 mg/kg, and the growth of L. obtusifolium was not restricted. Therefore, L. obtusifolium was particularly suitable for phytoremediation of Cr pollution in areas in western China.
1. Introduction
Population growth is increasing worldwide, which impacts the forest landscape [1,2,3]. Similarly, industrialization poses considerable challenges to the natural environment [4,5], which are exacerbated by human intensification [6,7,8]. Coal mining threatens the natural landscape. The creation of green patches among the coal mining industry is vital for industrial development since they are a vital component of greening and environmental protection [8,9]. Humans and plants suffer from trace elements’ long-term effects [10,11,12,13]. The toxicity, abundance, and persistence of these substances have contributed to a global problem [14,15,16]. It has been established that trace element exposure can have a significant impact on various health conditions in practice [10,17,18,19]. Asthma, allergies, bone ailments, and lifetime cancer risk are some of these challenges. Ingestion of foods and beverages, skin contact, and inhaling air are some of the most common ways trace elements enter the body [18,20,21,22]. Industrialized areas have been the focus of many studies regarding trace elements [3,16,23,24,25,26]. While many studies have been conducted recently concerning urban woody trees regarding phytoremediation, very few studies have been conducted on deciduous shrubs. These shrubs deserve immediate attention.
The coal mining industry and natural landscape have coexisted for a long time in Ningxia, one of the largest coal provinces in China [16]. Trace elements are present in the mining area and surrounding soil in higher concentrations than the background value [16]. This is potentially harmful to urban development and ecosystem services [27,28,29,30]. Trace elements may be considered abiotic stress factors that affect plant growth and development. In addition to inhibiting plant growth, excessive trace element absorption reduces biomass accumulation, reduces chlorophyll synthesis, and suppresses photosynthesis [30,31]. This stress impacts SOD, POD, CAT, and APX activities, resulting in physiological disorders and plant death. The trace elements cadmium (Cd), chromium (Cr), lead (Pb), and zinc (Zn) are easy for plants to absorb, and they migrate rapidly through the soil [32]. Since trace elements are difficult to degrade in the environment, they may also affect ecosystem provisions by impacting plant growth. Soil ecology in the Huinong district of Shizuishan in Ningxia must be protected from trace element pollution by remediating mining area soil in China.
It has been found that several solutions have been relatively successful in addressing soil pollution caused by trace elements, including chemical treatments. However, they are expensive and environmentally unfriendly. Soil cannot be cleaned of toxic trace elements using these techniques. Consequently, an environmentally friendly, effective, and affordable solution is necessary to solve this problem. Phytoremediation is a promising method of removing or reducing metal contaminants from the environment. It is possible to use phytoremediation techniques based on hyperaccumulators, which are plants that absorb metals and chemicals from the soil and metabolize them [33]. The research on hyperaccumulators under trace element stress has been extensive. It has been demonstrated by Hossain that plants absorb substances from sediments during phytoextraction and store them in their cells during phytodegradation, during which they convert them into nontoxic ones [34]. Ligustrum lucidum has been found to contain trace elements, such as Pb, Cu, and Zn, which are mainly deposited in the roots and are associated with root-hoarding plants [35]. It has been demonstrated that the treatment of L. lucidum leaves with heavy metal lead inhibited both photosynthesis and coenzyme Q10 responses. The inhibition was stronger when the concentration of heavy metal lead increased. However, L. lucidum still has high net photosynthetic capacity and carbon uptake capacity under mild to moderate pollution conditions. Thus, even though L. lucidum grows slowly in Pb-contaminated areas, it may still be useful as a remediation species [36]. L. lucidum leaves contain higher Cr content than the other five species of arboreal and bush plants. Their ability to enrich Sc and V is stronger [37]. Ligustrum obtusifolium has low mortality rates in unsanitary landfills rich in Ca, Mg, Cr, Cd, so it is recommended as one of the possible species suitable for landfill remediation in South Korea [38].
Currently, local species are preferred for the remediation of trace-element-contaminated soils since they are easier to screen than other species. The L. obtusifolium is a common shrub in the northern parts of China, and it has many advantages, including its tolerance of cold, drought, and salinity [39]. Since it is a shrub species, it has a greater biomass and faster growth rate than general enriched herbaceous plants. Therefore, the average mass concentration of Cd, Cr, Zn, and Pb in the soil of Shizuishan is reported slightly higher than the background value in the soil of the Yinchuan Plain in western China [40]. This study aims to determine the critical growth value of L. obtusifolium, a dominant greening tree species in the region. It is imperative to determine how L. obtusifolium responds to heavy metal stress, such as Cd, Cr, and Pb, and how these elements affect its physiological response. In light of the above aspects, this study also aims to provide a basis for phytoremediation of heavy metal and trace element pollution in green spaces in western China.
2. Materials and Methods
2.1. Materials and Experimental Design
The Senmiao Botanical Garden in Yinchuan city in Ningxia, China provided us with 2-year-old young L. obtusifolium trees. We simulated the local soil type (sandy loam) to ensure the survival of these plants in pots. In order to accomplish this, we also collected soil from the Senmiao Botanical Garden. This soil was configured to be sandy loam soil containing 50% to 60% sand. The basic physico-chemical characteristics of soil are presented in Table 1, in which the mass of the different trace elements is calculated per unit of air-dried soil mass. Cd content in the test soil was 0.31 mg/kg, Cr content was 71.41 mg/kg, Pb content was 31.61 mg/kg, and Zn content was 125 mg/kg. In May 2015, the sandy loam soil was passed through a 4 mm sieve and evenly sprayed with pure CdCl2·2.5H2O, CrCl3·6H2O, Pb (CH3COO)2·3H2O, and Zn (CH3COO)2·2H2O. Once the soil had been thoroughly mixed in, the soil was loaded into plastic barrels (height 30 cm × width 30 cm), containing 7.5 kg of soil per barrel. In accordance with the “Soil Environmental Quality Standard” (GB 15618-2018), we produced soils with trace elements such as Cd, Cr, Pb, and Zn under different gradient concentrations (consider adding pure metal and air-dried soil) (Table 2). We randomly transplanted L. obtusifolium saplings with consistent growth into soil containing one trace element on 10 June 2015. This was after one month of aging the soil (5 pots/treatment, 1 sapling/pot), watering thoroughly, and placing them in a cool, ventilated environment to grow saplings. All potted experimental saplings were cultivated at the experimental base of the State Key Laboratory of Seedling Bioengineering at Senmiao Botanical Garden in Yinchuan city. This was performed after domesticating the plants for one month. A greenhouse with a transparent roof, open all around, was constructed at the experimental base. The experimentation period began at this point. The trial was conducted with regular field management and timely watering. Testing was completed after 90 days, and destructive sampling was performed after the indicators were measured.

Table 1.
The basic physico-chemical characteristics of soils (mean ± standard error).

Table 2.
Treatment elements and levels in the pot cultivation (mg/kg).
2.2. Measurement of Indicators
2.2.1. Determination of Chlorophyll Content
Healthy and mature functional leaves of L. obtusifolium that were fully expanded at the tip of the sprouting strip were used to determine the levels of photosynthetic pigments. The chlorophyll in the plants was extracted using acetone. L. obtusifolium leaves were ground and extracted completely before the absorbance of the leaves was determined at 645 and 663 nm using a general analysis TU-1901 dual-beam UV/VIS spectrophotometer to determine the chlorophyll a (Ch1a), chlorophyll b (Ch1b), and total chlorophyll (Chls) content.
2.2.2. Determination of Growth and Biomass
L. obtusifolium was measured using a ruler before sampling. The plant was thoroughly washed with ultrapure water after it had been harvested. Afterwards, we rinsed the roots with ultrapure water after removing the trace element ions that had been adsorbing to the root surface using sodium EDTA (20 mmol/L). Following this, the parts were baked at 105 °C for 30 min and dried at 80 °C until a constant weight was achieved. Lastly, the dry mass of the aboveground and belowground components was determined.
2.2.3. Determination of Physiological and Biochemical Indicators
Nitroblue tetrazolium (NBT) was used to determine superoxide dismutase (SOD) activity. It was denoted by the enzyme activity unit (U) as a photochemical reduction of 50% of NBT per unit time. The enzyme activity was expressed in units of U/g. In order to measure catalase (CAT) activity, the ultraviolet absorption method was used [4]. A decrease of 0.1 absorbance unit per minute is considered to be one enzyme activity unit. Ascorbate peroxidase (APX) activity was measured using ultraviolet spectrophotometry, while peroxidase (POD) activity was measured using the guaiacol colorimetric method [41,42].
2.2.4. Determination of Trace Element Content
The dried leaves of each plant were crushed using a ball mill, and an analytical balance was used to accurately measure 0.050 g of dry powder (1/10,000th). Microwave digesters (Leeman SW-4, German) were used to digest and fix the volume. Trace element concentrations in digestion solution were determined using inductively coupled plasma-emission spectrometry (ICP-OES, Thermo Fisher iCAP 6300, Cambridge, UK).
2.3. Parameter Calculation
The translocation factor was calculated using the following formula to determine the extent to which the plant was capable of transferring trace elements aboveground:
Transfer coefficient = trace element content in the aboveground part/trace element content in the root.
2.4. Data Analysis
We analyzed and processed experimental data using SPSS 22.0. The data were expressed as mean ± standard error. One-way analysis of variance was performed to examine the effects of trace element stress at various mass concentrations on the growth, physiology, biomass, and absorption and accumulation of trace elements in the L. obtusifolium. Each index was tested for significance using Duncan’s test (p < 0.05) in different treatment groups. OriginPro 2023 was used to create the illustrations.
3. Results
3.1. Effects of Different Trace Elements on Leaf Chlorophyll Content of L. obtusifolium
As shown in Figure 1, Cha, Chb, Chls, and Chlorophyll a/b of L. obtusifolium showed a downward trend with an increase in soil Cd, Cr, Pb, and Zn content, respectively (p < 0.05). The difference between different treatment groups is significant at different Pb and Zn concentrations. Chlorophyll is not significant after treatment with a Cr concentration of 700 mg/kg. At 10 mg/kg Cd treatment concentration, Chls and Chla are significantly correlated with the control group (Figure 2).
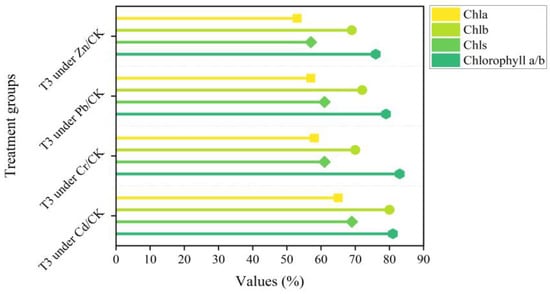
Figure 1.
Comparison of chlorophyll content in T3 treatment group.
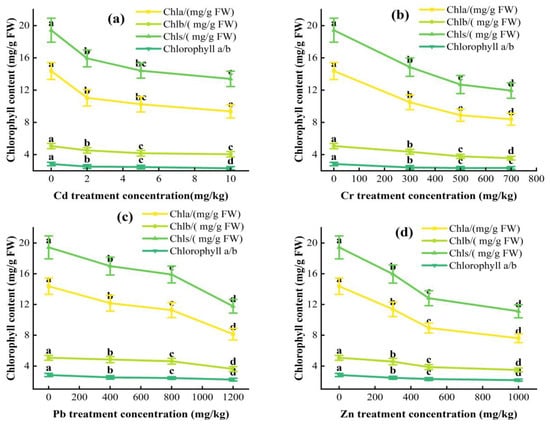
Figure 2.
Trace element stress on leaf chlorophyll contents of L. obtusifolium under four trace elements (a–d) under the same treatment time; different lowercase letters indicate significant differences between different stress concentrations (p < 0.05).
3.2. Effects of Different Trace Elements on the Growth Indices of L. obtusifolium
As shown in Table 3, different trace elements significantly affected the net increase in height and biomass of L. obtusifolium plants. With an increase in Cd, Pb, and Zn content in the soil, the net growth in plant height, root, and aerial and total biomass significantly declined, respectively (p < 0.05). However, under soil Cr stress, no significant difference was observed between plant height and biomass in the T1 treatment group and CK ratio. Moreover, when the soil Cr content increased in the T2 and T3 groups, plant height and biomass decreased considerably (p < 0.05).

Table 3.
The effects of different trace element stress on the plant height and the total biomass of L. obtusifolium (mean ± SE).
3.3. Antioxidant Enzyme Response under Cd Stress
Figure 3 shows that Cd stress increases antioxidant enzyme activity in L. obtusifolium. With an increase in Cd content in the soil, the activity of antioxidant enzymes (SOD, POD, CAT, and APX) in L. obtusifolium leaves increased initially and then decreased (p < 0.05). The maximum values were observed in the T2 group, which were 291%, 179%, 141%, and 422% of CK, respectively.
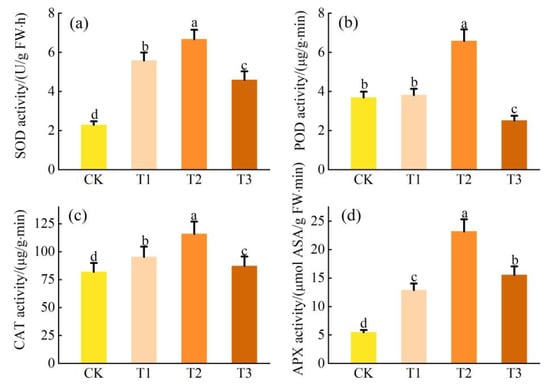
Figure 3.
Activities of antioxidant enzyme SOD activity (a), POD activity (b), CAT activity (c), and APX activity (d) in the leaves of L. obtusifolium exposed during 90 days to 0, 2, 5, or 10 mg Cd kg−1 soil. Different letters on the top of bars indicate significant differences at p < 0.05 level as determined by Duncan’s multiple range test.
3.4. Antioxidant Enzyme Response under Cr Stress
The antioxidant enzyme activity in L. obtusifolium plants first increased and then decreased with the increase in soil Cr content (p < 0.05; Figure 4). The activities of SOD, POD, CAT, and APX in L. obtusifolium leaves reached the maximum value under soil Cr content in the T1 group, which were 288%, 202%, 160%, and 364% of CK, respectively.
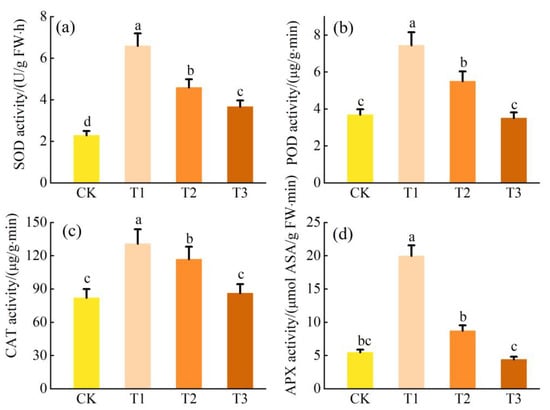
Figure 4.
Activities of antioxidant enzyme SOD activity (a), POD activity (b), CAT activity (c), and APX activity (d) in the leaves of L. obtusifolium exposed during 90 days to 0, 300, 500, or 700 mg Cr kg−1 soil. Different letters on the top of bars indicate significant differences at p < 0.05 level as determined by Duncan’s multiple range test.
3.5. Antioxidant Enzyme Response under Pb Stress
Under Pb stress, the activities of SOD, POD, CAT, and APX in L. obtusifolium leaves showed a similar trend as they first increased and then decreased with an increase in soil Pb content (p < 0.05; Figure 5). The maximum values observed in the T1 group were 426%, 257%, 162%, and 517% of CK, respectively.
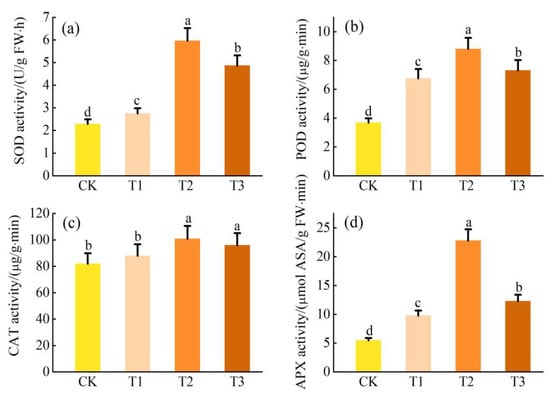
Figure 5.
Activities of antioxidant enzyme SOD activity (a), POD activity (b), CAT activity (c), and APX activity (d) in the leaves of L. obtusifolium exposed during 90 days to 0, 400, 800, or 1200 mg Pb kg−1 soil. Different letters on the top of bars indicate significant differences at p < 0.05 level as determined by Duncan’s multiple range test.
3.6. Antioxidant Enzyme Response under Zn Stress
Under Zn stress, the activities of SOD, POD, and APX in L. obtusifolium leaves tended to increase at first and then decrease (p < 0.05) with an increase in soil Zn content, reaching the maximum values in the T2 treatment group (261%, 239%, and 415% of CK, respectively; Figure 6). Moreover, the SOD, POD, and APX activities decreased significantly as the soil Zn content continued to increase in the T3 group (p < 0.05). Although CAT activity in the L. obtusifolium leaves did not change significantly with the increase in soil Zn content in the T1 and CK groups (p < 0.05), it was considerably higher than that in the T2 and T3 groups (123% and 117% of CK, respectively).
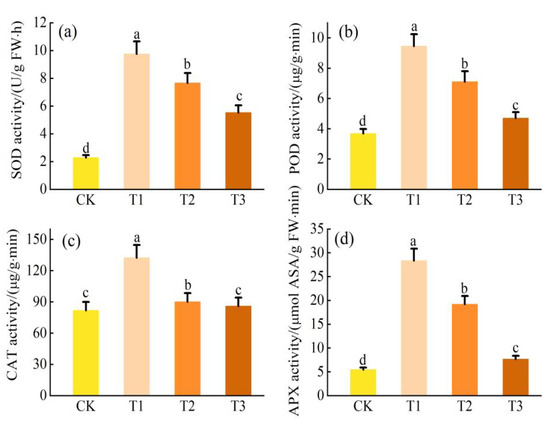
Figure 6.
Activities of antioxidant enzyme SOD activity (a), POD activity (b), CAT activity (c), and APX activity (d) in the leaves of L. obtusifolium exposed during 90 days to 0, 300, 500, or 1000 mg Zn kg−1 soil. Different letters on the top of bars indicate significant differences at p < 0.05 level as determined by Duncan’s multiple range test.
3.7. Capacity of L. obtusifolium Plants to Accumulate and Transport Trace Elements
When trace element content in the soil increased, the accumulated trace element content in each part of the L. obtusifolium plant also increased (p < 0.05; Table 4). Under Cd, Pb, and Zn stress, the content of trace elements accumulated in the L. obtusifolium plant was ranked in the following order: roots > stems > leaves. Under Cd and Pb stress, the transfer coefficient of the L. obtusifolium plant decreased with an increase in soil Pb content (p < 0.05), while under Zn stress, it tended to increase at first and then decrease with the increase in soil Zn content (p < 0.05). It reached the maximum in the T2 group, which was 111% of CK. Under soil Cr stress, the transfer coefficient of L. obtusifolium first increased and then decreased with the increase in soil Cr content. The maximum value was observed in the T1 group (1.05).

Table 4.
The ability of concentration and translation of trace elements by L. obtusifolium (mean ± SE).
4. Discussion
4.1. Effects of Different Trace Elements on the Chlorophyll Content of L. obtusifolium
Chlorophyll content is commonly used to determine the presence of trace elements in plants [42]. The degradation of chlorophyll in plants directly reduces the efficiency of their photosynthetic process, resulting in nutrient scarcity and sluggish or no growth. It was observed in this study that several individual trace elements significantly inhibited the chlorophyll content in L. obtusifolium. The chlorophyll content of L. obtusifolium leaves decreased because of Cd stress. It has been previously observed that Lonicera japonica was found to have decreased Cha and Chb contents as Cd concentration increased, which is consistent with our results [43]. Cd stress may affect pigment synthesis, resulting in a reduction in chlorophyll content. L. obtusifolium also showed similar chlorophyll content changes under Cr stress. There has been evidence that Cr stress can lead to damage to the ultrastructure of chlorophyll and thylakoids in plants, resulting in a decrease in chlorophyll content. The authors Liu, Li, and Meng also observed significantly reduced chlorophyll content in Zelkova schneideriana under Pb stress [4,43,44]. A combination of Pb and Cu was applied to Citrus aurantium L. The results showed that these metals significantly reduced photoactivity, increased lipid peroxide levels, suppressed photosynthesis and growth, and inhibited mineral nutrient absorption [45]. Glińska has demonstrated that Zn alters the ultrastructure of root meristem cells and results in slight recombination of chloroplast thylakoids, causing the thylakoids to lose their structure and decreasing the content of photosynthetic pigment [46]. The chlorophyll content of senescent leaves was found to decrease significantly over time during the aging process, with Cha reducing more than Chb. The same genus of plants, L. ovalifolium forms a special protection mechanism against Pb metal concentration stress. This is because of the interaction of photopigments and total phenolic contents [47,48]. Consequently, Cha/b can serve as a biomarker for leaf aging and a sensitive measure of leaf stress caused by trace elements. The study found that the reduction in Cha/b content was caused by single trace element treatments in L. obtusifolium leaves. This indicates that trace element stress damages the chlorophyll membrane system, resulting in chlorophyll degradation and inactivation, as well as an acceleration of leaf aging.
4.2. Effect of Different Trace Elements on the Growth of L. obtusifolium
It was found that trace element stress adversely affected the growth of L. obtusifolium. There was a general downward trend in the height and biomass of L. obtusifolium following an increase in soil Cd content, with varying degrees of suppression. A similar study by Huang, 2020, found that Calendula officinalis and Althaea rosea (L.) Cavan did not grow or produce biomass as their soil Cd content increased [49]. We found no significant differences in height, growth, or biomass accumulation between the treatment and control groups in our study when soil Cr content was up to 300 mg/kg in the T1 treatment group, indicating that L. obtusifolium could grow normally in soil with such soil Cr content. In contrast, when the Cr concentration increased, the net growth and biomass of the plants declined significantly. The growth of L. obtusifolium was inhibited under Pb stress. Plants growing in environments with high levels of Pb were observed to exhibit root growth inhibition, inhibition of root apical cell division, distortion of mitosis, damage to microtubules, and decreased cell membrane stability, resulting in abnormal plant growth [33]. We found that excessive soil Zn content limited the growth of L. obtusifolium, despite Zn being an essential micronutrient for higher plants [37]. In contrast to the control, L. obtusifolium showed significantly lower growth and biomass accumulation when soil Zn was increased. As Zn concentration increased, the plant biomass increased and then decreased in Sinapis alba L. and Sasa argenteostriata (Regel) [50,51]. The growth of citrus plants was also inhibited by excessive Zn stress. Zn stress reduces the ability of plants to divide cells and grow roots, resulting in a reduction in biomass accumulation.
The China Soil Environmental Capacity Collaboration Group developed a method for determining critical soil content in 2018. It is recommended that the concentration of harmful substances in the soil be reduced to between 5% and 10% (the upper and lower limits of the yield) of the maximum allowable concentration [52]. Compared to the control group, the subground, aboveground, and total biomass of L. obtusifolium was reduced by 10% when the soil concentration of Cr, Pb, and Zn was within 300 mg/kg, 400 mg/kg, and 300 mg/kg, respectively. These values can therefore be used to set critical cutoff values for Cr, Pb, and Zn in soil when growing L. obtusifolium. L. obtusifolium biomass was reduced by >10% when the soil Cd concentration was 2 mg/kg. Further research is needed to determine the cutoff value for Cd for planting L. obtusifolium.
4.3. Antioxidant Enzyme Response to Trace Element Stress in L. obtusifolium
Plants are more sensitive to trace element toxicity, which increases the production of ROS. ROS include superoxide radicals, hydroxyl radicals, and hydrogen peroxide radicals [49]. Plants are affected by ROS that can damage their membranes, proteins, and nucleic acids [51]. Plants are activated by their antioxidant enzyme system to resist external adverse environmental conditions and maintain their normal growth when they are under stress. SOD, POD, CAT, and APX are components of this system that are activated to enhance plant resistance to adverse environmental conditions. Four trace elements were evaluated in this study. L. obtusifolium leaves exposed to trace element stress showed increased antioxidant enzyme activity, indicating activation of stress defense mechanisms that reduce ROS-mediated oxidative damage. As a consequence of Cd and Zn stress, SOD, POD, CAT, and APX activities in L. obtusifolium peaked in the T2 group, while under Cr stress, they peaked in the T1 group. Increasing soil trace element content resulted in a decrease in antioxidant enzyme activity in the plant. Consequently, L. obtusifolium demonstrates different tolerance thresholds for different trace elements when it is individually treated with these elements. As the concentration of trace elements exceeds its tolerance level, it affects the concentration of oxygen in plant cells. In the process, biological substances within the cell, including enzymes, are damaged, resulting in a decreased level of antioxidant enzyme activity.
4.4. Trace Element Accumulation and Transport Capacity of L. obtusifolium
The presence of exogenous trace elements in the soil is associated with a significant increase in trace element accumulation in each part of the plant. Our study indicates a correlation between high accumulation capacity and low plant biomass in L. obtusifolium plants under trace element stress. Hence, plants growing in trace element-contaminated soil have a high accumulation capacity that can limit their growth and biomass production. Generally, plants that grow in an environment that contains trace elements can accumulate those elements in their roots. Roots absorb these trace elements, which inhibit SOD activity and root metabolism, reduce root vitality, and prevent the transfer of these trace elements to the aboveground parts. In this way, trace elements are less likely to have negative effects on the photosynthetic machinery and metabolism that are toxic to the organism [45]. It was found that under Cd, Pb, and Zn stress, L. obtusifolium roots accumulated trace elements to a much greater extent than the aboveground parts, possibly resulting in a reduced ability of the plant to transfer trace elements. Furthermore, plant roots are the first barrier to trace elements, so these metals are inhibited strongly by the roots of the plant. It is believed that the lignin and cellulose present in the root cell wall have the ability to bind divalent and trivalent trace element ions, thus inhibiting the transfer of trace elements upwards [53]. In the presence of soil with a Cr content of 300 mg/kg, L. obtusifolium showed an excellent transfer coefficient of 1.05, indicating its translocation factor ability. The enrichment of the same genus plant L. lucidum for Zn and Pb was also concentrated in the roots of the plant. This is consistent with the enrichment results we obtained. L. lucidum exposure to Zn and Pb can decrease ROS and MDA content and increase anti-oxidative enzyme activities [54]. Furthermore, the plants of L. obtusifolium that were treated with this concentration exhibited no growth inhibition and had optimal antioxidant enzyme activity, demonstrating that L. obtusifolium was able to grow under soils containing 300 mg/kg of Cr. It was shown that vegetation responds successfully to various factors [55,56,57,58,59].
5. Conclusions
This study demonstrated a dose-dependent inhibition of chlorophyll synthesis by a single trace element in Ligustrum obtusifolium. It was found that exposure to Cd, Pb, and Zn stress inhibited the growth and biomass accumulation of L. obtusifolium, as well as increasing its antioxidant enzyme activity. There were critical values of 400 mg/kg and 300 mg/kg for Pb and Zn, respectively. Phytoremediation of soil pollution with Cr is more suitable for L. obtusifolium than with Cd, Pb, and Zn based on its absorption and accumulation characteristics. L. obtusifolium demonstrated optimal antioxidant enzyme activity and transfer ability at 300 mg/kg soil Cr content. Therefore, L. obtusifolium is suitable for phytoremediation of trace-element-contaminated areas in Ningxia, western China.
Author Contributions
Conceptualization, W.C., M.A. and C.L.; methodology, W.C., M.A., Z.C. and C.L.; software, W.C., M.A. and Z.C.; validation, W.C., M.A. and C.L.; formal analysis, W.C., M.A. and Z.C.; investigation, W.C., M.A., Z.C. and C.L.; resources, C.L.; data curation, W.C., M.A. and C.L.; writing—original draft preparation, W.C., M.A. and Z.C.; writing—review and editing, M.A. and C.L.; visualization, W.C., M.A., Z.C. and C.L.; supervision, M.A. and C.L.; project administration, M.A. and C.L.; funding acquisition, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been supported by Foreign Young Talent Program (Program number: QN2022168001L); Chongqing Housing and Urban-rural Construction Committee (Program number: Chengkezi-2022-6-3); Chongqing Municipality Key Forestry Research Project (2021-9); Forestry Extension Project of China Central Finance (No. Yulinketui 2023-8); China Postdoctoral Science Foundation (2021M703137); and Chongqing Postdoctoral Science Foundation (cstc2021jcyj-bsh0080).
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to express their gratitude to the staff of the Senmiao Botanical Garden in Yinchuan City in China for their valuable assistance with the experimental sample.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arif, M.; Qi, Y.; Dong, Z.; Wei, H. Rapid Retrieval of Cadmium and Lead Content from Urban Greenbelt Zones Using Hyperspectral Characteristic Bands. J. Clean. Prod. 2022, 374, 133922. [Google Scholar] [CrossRef]
- Southerland, V.A.; Brauer, M.; Mohegh, A.; Hammer, M.S.; Van Donkelaar, A.; Martin, R.V.; Apte, J.S.; Anenberg, S.C. Global Urban Temporal Trends in Fine Particulate Matter (Pm2·5) and Attributable Health Burdens: Estimates from Global Datasets. Lancet Planet. Heath 2022, 6, e139–e146. [Google Scholar] [CrossRef] [PubMed]
- Zlobina, V.L.; Medovar, Y.A.; Yushmanov, I.O. Assessing the Hazard of Environmental Pollution by Landfills of Industrial and Municipal Wastes. Water Resour. 2021, 48, 420–426. [Google Scholar] [CrossRef]
- Li, M.; Verburg, P.H.; Van Vliet, J. Global Trends and Local Variations in Land Take Per Person. Landsc. Urban Plann. 2022, 218, 104308. [Google Scholar] [CrossRef]
- Zhao, J.; Xiao, Y.; Sun, S.; Sang, W.; Axmacher, J.C. Does China’s Increasing Coupling of ‘Urban Population’and ‘Urban Area’growth Indicators Reflect a Growing Social and Economic Sustainability? J. Environ. Manage. 2022, 301, 113932. [Google Scholar] [CrossRef] [PubMed]
- Ciprari, E.; Ancillotto, L.; Mori, E.; Studer, V.; Chessa, C. Rescue Data as an Alternative for Assessing Trends and Phenological Changes in Two Invasive Parakeet Species. Urban Ecosyst. 2022, 25, 1199–1206. [Google Scholar] [CrossRef]
- Cui, X.; Geng, Y.; Sun, R.; Xie, M.; Feng, X.; Li, X.; Cui, Z. Distribution, Speciation and Ecological Risk Assessment of Heavy Metals in Jinan Iron & Steel Group Soils from China. J. Clean. Prod. 2021, 295, 126504. [Google Scholar] [CrossRef]
- Ma, Y.; Egodawatta, P.; McGree, J.; Liu, A.; Goonetilleke, A. Human Health Risk Assessment of Heavy Metals in Urban Stormwater. Sci. Total Environ. 2016, 557, 764–772. [Google Scholar] [CrossRef]
- Men, C.; Liu, R.; Xu, F.; Wang, Q.; Guo, L.; Shen, Z. Pollution Characteristics, Risk Assessment, and Source Apportionment of Heavy Metals in Road Dust in Beijing, China. Sci. Total Environ. 2018, 612, 138–147. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, Z.; Huang, K.; Wang, X.; Cheng, L.; Zeng, L.; Zhou, Y.; Jing, T. Multiple Exposure Pathways and Health Risk Assessment of Heavy Metal (Loid) S for Children Living in Fourth-Tier Cities in Hubei Province. Environ. Int. 2019, 129, 517–524. [Google Scholar] [CrossRef]
- Chen, X.; Guo, M.; Feng, J.; Liang, S.; Han, D.; Cheng, J. Characterization and Risk Assessment of Heavy Metals in Road Dust from a Developing City with Good Air Quality and from Shanghai, China. Environ. Sci. Pollut. Res. 2019, 26, 11387–11398. [Google Scholar] [CrossRef]
- Hong, N.; Guan, Y.; Yang, B.; Zhong, J.; Zhu, P.; Ok, Y.S.; Hou, D.; Tsang, D.C.W.; Guan, Y.; Liu, A. Quantitative Source Tracking of Heavy Metals Contained in Urban Road Deposited Sediments. J. Hazard. Mater. 2020, 393, 122362. [Google Scholar] [CrossRef]
- Mirzaei Aminiyan, M.; Baalousha, M.; Mousavi, R.; Mirzaei Aminiyan, F.; Hosseini, H.; Heydariyan, A. The Ecological Risk, Source Identification, and Pollution Assessment of Heavy Metals in Road Dust: A Case Study in Rafsanjan, Se Iran. Environ. Sci. Pollut. Res. 2018, 25, 13382–13395. [Google Scholar] [CrossRef]
- Fei, X.; Lou, Z.; Xiao, R.; Ren, Z.; Lv, X. Source Analysis and Source-Oriented Risk Assessment of Heavy Metal Pollution in Agricultural Soils of Different Cultivated Land Qualities. J. Clean. Prod. 2022, 341, 130942. [Google Scholar] [CrossRef]
- Mamak, M.; Kicińska, A. Environmental Indicators for Evaluation of Chromium Content in Soils on the Example of an Inoperative Tanning Plant. Hum. Ecol. Risk Assess. 2019, 25, 2056–2072. [Google Scholar] [CrossRef]
- Zhang, S.; Ni, X.; Arif, M.; Zheng, J.; Stubbs, A.; Li, C. Nacl Improved Cd Tolerance of the Euhalophyte Suaeda Glauca but Not the Recretohalophyte Limonium Aureum. Plant Soil 2020, 449, 303–318. [Google Scholar] [CrossRef]
- Arif, M.; Jiajia, L.; Tahir, M.; Jie, Z.; Li, C. Environmental Literacy Scenarios Lead to Land Degradation and Changes in Riparian Zones: Implications for Policy in China. Land Degrad. Dev. 2023, 34, 156–172. [Google Scholar] [CrossRef]
- Goudarzi, G.; Alavi, N.; Geravandi, S.; Idani, E.; Behrooz, H.R.A.; Babaei, A.A.; Alamdari, F.A.; Dobaradaran, S.; Farhadi, M.; Mohammadi, M.J. Health Risk Assessment on Human Exposed to Heavy Metals in the Ambient Air Pm 10 in Ahvaz, Southwest Iran. Int. J. Biometeorol. 2018, 62, 1075–1083. [Google Scholar] [CrossRef]
- Lisiak-Zielińska, M.; Borowiak, K.; Budka, A.; Kanclerz, J.; Janicka, E.; Kaczor, A.; Żyromski, A.; Biniak-Pieróg, M.; Podawca, K.; Mleczek, M. How Polluted Are Cities in Central Europe?-Heavy Metal Contamination in Taraxacum Officinale and Soils Collected from Different Land Use Areas of Three Representative Cities. Chemosphere 2021, 266, 129113. [Google Scholar] [CrossRef]
- Jabbo, J.N.; Isa, N.M.; Aris, A.Z.; Ramli, M.F.; Abubakar, M.B. Geochemometric Approach to Groundwater Quality and Health Risk Assessment of Heavy Metals of Yankari Game Reserve and Its Environs, Northeast Nigeria. J. Clean. Prod. 2022, 330, 129916. [Google Scholar] [CrossRef]
- Nechita, C.; Iordache, A.M.; Lemr, K.; Levanič, T.; Pluhacek, T. Evidence of Declining Trees Resilience under Long Term Heavy Metal Stress Combined with Climate Change Heating. J. Clean. Prod. 2021, 317, 128428. [Google Scholar] [CrossRef]
- Arif, M.; Changxiao, L. Impacts of Environmental Literacy on Ecological Networks in the Three Gorges Reservoir, China. Ecol. Indic. 2022, 145, 109571. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Liu, Y.; Hai, X.; Shanguan, Z.; Deng, L. Driving Factors of Ecosystem Services and Their Spatiotemporal Change Assessment Based on Land Use Types in the Loess Plateau. J. Environ. Manage. 2022, 311, 114835. [Google Scholar] [CrossRef]
- Wu, D.; Yu, X.; Lai, M.; Feng, J.; Dong, X.; Peng, W.; Su, S.; Zhang, X.; Wan, L.; Jacobs, D.F.; et al. Diversified Effects of Co-Planting Landscape Plants on Heavy Metals Pollution Remediation in Urban Soil Amended with Sewage Sludge. J. Hazard. Mater. 2021, 403, 123855. [Google Scholar] [CrossRef]
- Gunarathne, V.; Ashiq, A.; Ramanayaka, S.; Wijekoon, P.; Vithanage, M. Biochar from Municipal Solid Waste for Resource Recovery and Pollution Remediation. Environ. Chem. Lett. 2019, 17, 1225–1235. [Google Scholar] [CrossRef]
- Xie, S.W.; Guo, X.S.; Yang, F.; Huang, Q.; Chen, M.J.; Wei, X.; Liu, C.S. Accumulation Characteristics, Geochemical Fractions Distribution and Ecological Risk of Heavy Metals in Soils of Urban Parks in Guangzhou, China. Ecol. Environ. Sci. 2022, 31, 2206–2215. [Google Scholar] [CrossRef]
- Liu, X.; Arif, M.; Wan, Z.; Zhu, Z. Dynamic Evaluation of Coupling and Coordinating Development of Environments and Economic Development in Key State-Owned Forests in Heilongjiang Province, China. Forests 2022, 13, 2069. [Google Scholar] [CrossRef]
- Xie, W.J.; Wang, S.X. Research on the Coupling Coordination between Economic Development and Ecological Environment—A Case Study of Ecological Civilization Construction of Qinghai Province. Plateau Sci. Res. 2020, 4, 36–45. [Google Scholar] [CrossRef]
- Xiao, R.; Guo, D.; Ali, A.; Mi, S.; Liu, T.; Ren, C.; Li, R.; Zhang, Z. Accumulation, Ecological-Health Risks Assessment, and Source Apportionment of Heavy Metals in Paddy Soils: A Case Study in Hanzhong, Shaanxi, China. Environ. Pollut. 2019, 248, 349–357. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Yang, Y.; Ni, X.; Arif, M.; Charles, W.; Li, C. Trace Elements in Soils of a Typical Industrial District in Ningxia, Northwest China: Pollution, Source, and Risk Evaluation. Sustainability 2020, 12, 1868. [Google Scholar] [CrossRef]
- Zhu, L.A.; Yin, A.H.; Lin, L.W.; Liu, Y.; Zhang, H.H. Accumulation Characteristics, Influencing Factors and Evaluation of Heavy Metals in Surface Soil in Urban Forest Park of Foshan. Ecol. Environ. 2021, 30, 849–856. [Google Scholar] [CrossRef]
- Yang, G.-L.; Zheng, M.-M.; Tan, A.-J.; Liu, Y.-T.; Feng, D.; Lv, S.-M. Research on the Mechanisms of Plant Enrichment and Detoxification of Cadmium. Biology 2021, 10, 544. [Google Scholar] [CrossRef]
- Kumar, R.; Ivy, N.; Bhattacharya, S.; Dey, A.; Sharma, P. Coupled Effects of Microplastics and Heavy Metals on Plants: Uptake, Bioaccumulation, and Environmental Health Perspectives. Sci. Total Environ. 2022, 836, 155619. [Google Scholar] [CrossRef]
- Hossain, M.B.; Masum, Z.; Rahman, M.S.; Yu, J.; Noman, M.A.; Jolly, Y.N.; Begum, B.A.; Paray, B.A.; Arai, T. Heavy Metal Accumulation and Phytoremediation Potentiality of Some Selected Mangrove Species from the World’s Largest Mangrove Forest. Biology 2022, 11, 1144. [Google Scholar] [CrossRef]
- Zhou, P.-F.; Zhang, S.-W.; Luo, M.; Wei, H.-B.; Song, Q.; Fang, B.; Zhuang, H.-J.; Chen, H.-Y. Characteristics of Plant Diversity and Heavy Metal Enrichment and Migration under Different Ecological Restoration Modes in Abandoned Mining Areas. Environ. Sci. 2022, 43, 985–994. [Google Scholar] [CrossRef]
- Liu, M.S. Effect of Different Concentration of Lead on Photosynthesis of Ligustrum Lucidum. Master’s Thesis, Central South University of Forestry & Technology, Changsha, China, 2021. [Google Scholar]
- Rucandio, M.I.; Petit-Domínguez, M.D.; Fidalgo-Hijano, C.; García-Giménez, R. Biomonitoring of Chemical Elements in an Urban Environment Using Arboreal and Bush Plant Species. Environ. Sci. Pollut. Res. 2011, 18, 51–63. [Google Scholar] [CrossRef]
- Kim, K.D.; Lee, E.J. Potential Tree Species for Use in the Restoration of Unsanitary Landfills. Environ. Manage. 2005, 36, 1–14. [Google Scholar] [CrossRef]
- Yu, Z.; Feng, X.Y.; Ji, L.R.; Ren, X.H.; Zhang, S.W. Study on Cold Tolerance of 12 Foreign Tree Species in Wuwei. For. Sci. Technol. 2012, 37, 6–8. [Google Scholar] [CrossRef]
- Shi, T.C.; Wang, Z.Q.; Cao, Y.Y.; Yang, J.F.; Ma, G.L.; Yang, B.G. Evalution on Heavy Metal Levels and Potential Ecological Risks in Agricultural Soils. J. Ningxia Univ. 2021, 42, 180–183. [Google Scholar] [CrossRef]
- Chai, C.R.; Mu, L.Q.; Liang, M.; Wang, R.S. Physiological Responses of Six Northern Greening Shrubs to Water Stress. J. Northeast For. Univ. 2012, 40, 12–15. [Google Scholar] [CrossRef]
- Elavarthi, S.; Martin, B. Spectrophotometric Assays for Antioxidant Enzymes in Plants. In Plant Stress Tolerance: Methods and Protocols; Sunkar, R., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 273–280. [Google Scholar]
- Liu, Z.; He, X.; Chen, W. Effects of Cadmium Stress on the Growth and Physiological Characteristics of Lonicera Japonica. Chin. J. Appl. Ecol. 2009, 20, 40–44. [Google Scholar]
- Meng, F.; Gao, Y.; Feng, Q. Discovery and Mechanism Study of a Novel Chromium-Accumulating Plant, Lonicera Japonica Thunb. Environ. Sci. Pollut. Res. 2019, 26, 13812–13817. [Google Scholar] [CrossRef] [PubMed]
- Giannakoula, A.; Therios, I.; Chatzissavvidis, C. Effect of Lead and Copper on Photosynthetic Apparatus in Citrus (Citrus aurantium L.) Plants. The Role of Antioxidants in Oxidative Damage as a Response to Heavy Metal Stress. Plants 2021, 10, 155. [Google Scholar] [CrossRef]
- Glińska, S.; Gapińska, M.; Michlewska, S.; Skiba, E.; Kubicki, J. Analysis of Triticum Aestivum Seedling Response to the Excess of Zinc. Protoplasma 2016, 253, 367–377. [Google Scholar] [CrossRef]
- Ito, H.; Saito, H.; Fukui, M.; Tanaka, A.; Arakawa, K. Poplar Leaf Abscission through Induced Chlorophyll Breakdown by Mg-Dechelatase. Plant Sci. 2022, 324, 111444. [Google Scholar] [CrossRef]
- Gajic, G.; Mitrovic, M.; Pavlovic, P.; Stevanovic, B.; Djurdjevic, L.; Kostic, O. An assessment of the tolerance of ligustrum ovalifolium hassk. to traffic-generated Pb using physiological and biochemical markers. Ecotoxicol. Environ. Saf. 2009, 72, 1090–1101. [Google Scholar] [CrossRef]
- Huang, Y.; Zu, L.; Zhang, M.; Yang, T.; Zhou, M.; Shi, C.; Shi, F.; Zhang, W. Tolerance and Distribution of Cadmium in an Ornamental Species Althaea Rosea Cavan. Int. J. Phytorem. 2020, 22, 713–724. [Google Scholar] [CrossRef]
- Liao, J.; Li, N.; Yang, Y.; Yang, J.; Tian, Y.; Luo, Z.; Jiang, M. Tolerance and Heavy Metal Accumulation Characteristics of Sasa Argenteostriata (Regel) Eg Camus under Zinc Single Stress and Combined Lead–Zinc Stress. Toxics 2022, 10, 450. [Google Scholar] [CrossRef]
- Shahbazi, M.; Tohidfar, M.; Aliniaeifard, S.; Yazdanpanah, F.; Bosacchi, M. Transgenic Tobacco Co-Expressing Flavodoxin and Betaine Aldehyde Dehydrogenase Confers Cadmium Tolerance through Boosting Antioxidant Capacity. Protoplasma 2021, 259, 965–979. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Q.; Liu, P.; Zhang, Y. Effects of Polyethylene and Heavy Metal Cadmium on the Growth and Development of Brassica Chinensis Var. Chinensis. Water Air Soil Pollut. 2022, 233, 426. [Google Scholar] [CrossRef]
- Krzesłowska, M. The Cell Wall in Plant Cell Response to Trace Metals: Polysaccharide Remodeling and Its Role in Defense Strategy. Acta Physiol. Plant. 2011, 33, 35–51. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, F.; He, Z.; Chen, X.; Mwamba, T.M. Photosynthesis performance and antioxidative enzymes response of Melia azedarach and Ligustrum lucidum plants under Pb–Zn mine tailing conditions. Front. Plant Sci. 2020, 11, 571157. [Google Scholar] [CrossRef]
- Jiajia, L.; Arif, M.; Dongdong, D.; Xin, H.; Qianwen, G.; Fan, Y.; Changxiao, L. The diversity of plant communities in different habitats can lead to distinct methanotrophic communities. Rhizosphere 2023, 26, 100690. [Google Scholar] [CrossRef]
- Yuancai, Q.; Muhammad, A.; Dong, Z.; Ting, W.; Qin, Y.; Bo, P.; Peng, W.; Wei, H. The effect of hydrological regimes on the concentrations of nonstructural carbohydrates and organic acids in the roots of Salix matsudana in the Three Gorges Reservoir, China. Ecol. Indic. 2022, 142, 109176. [Google Scholar] [CrossRef]
- Qianwen, G.; Arif, M.; Zhongxun, Y.; Jie, Z.; Xinrui, H.; Dongdong, D.; Fan, Y.; Changxiao, L. Plant species composition and diversity along successional gradients in arid and semi-arid regions of China. For. Ecol. Manag. 2022, 524, 120542. [Google Scholar] [CrossRef]
- Arif, M.; Jiajia, L.; Dongdong, D.; Xinrui, H.; Qianwen, G.; Fan, Y.; Songlin, Z.; Changxiao, L. Effect of topographical features on hydrologically connected riparian landscapes across different land-use patterns in colossal dams and reservoirs. Sci. Total Environ. 2022, 851, 158131. [Google Scholar] [CrossRef]
- Jiajia, L.; Lijuan, L.; Muhammad, A.; Dongdong, D.; Xin, H.; Changxiao, L. Newly formed riparian microhabitats simplify bacterial community structure and diversity. J. Soils Sediments 2023, 23, 1927–1943. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).