Abstract
Due to the rapid development of China’s economy, the demand for wood is steadily increasing. Eucalyptus species have been introduced in large quantities because of their fast growth, strong adaptability, and wide utility. To understand the phenological changes in introduced Eucalyptus in its new range, we carried out a field investigation to examine leaf functional and chemical defense traits of three introduced species (E. saligna, E. grandis and E. robusta) over latitudinal and altitudinal gradients in southern China. We sampled multiple stands of each species, and measured the leaf physical characteristics (e.g., leaf width, leaf thickness, and specific leaf area [SLA]), leaf nitrogen (N) and phosphorus (P) content, and phenolic compounds. We found that many functional traits (e.g., leaf size and thickness) decreased at lower latitudes, especially in E. grandis, possibly to reduce heat and water loss under higher temperatures. In E. grandis, we found that leaf P was lower at higher latitudes and altitude, and phenolics increased with elevation, while in E. robusta, both leaf N and P decreased with altitude. These findings suggested that both species were more conservative in resource allocation, with E. grandis possessing enhanced chemical defenses in response to the conditions experienced at higher elevations. In addition, we found the tree populations at the northern range limit of E. robusta had lower SLA, suggesting a more conservative growth strategy, In contrast, small populations in the northern part of the ranges of E. grandis had higher SLA, indicating range expansion at the edge of the species’ geographic distribution. Overall, it is particularly important to consider intraspecific trait differences across wide geographic areas when studying the spread of invasive species in the new range.
1. Introduction
Over the last few centuries, thousands of woody species have been transported from their native forests and other areas for multiple purposes, and have become naturalized and invasive in other parts of the world [1]. Although many of these trees have highly detrimental effects on invaded habitats [2], some of these species have only become naturalized or invasive relatively recently, and as such, little is known regarding their invasion ecology, specifically how they have adapted and responded to environmental conditions in their new range [3]. Examining the variation in leaf traits within and among species across contrasting sites can provide vital information on plant performance and adaptation to local environments [4,5]. Environmental factors act as selective filters on plant genotypes, particularly along abiotic gradients [6]. For example, with changes in latitude come variations in climate, soil, and biotic interactions that shape differences in leaf functional and chemical traits [7,8]. Similarly, environmental changes across elevations underlie morphological and physiological trait differences between plants at high and low altitudes [9]. Different populations along gradients have variation in tolerance to stressors, with many plant species displaying similar traits under the same conditions [10]. Therefore, environmental gradients are natural laboratories for investigating the response of species to climate change and novel habitats [11].
The success of an introduced species in the new range is dependent on many factors [12]. Following introduction, populations may be exposed to the selection of individuals with traits that are better matched with the physiological requirements of the new environment [13]. Phenotypic plasticity is another important factor in the spread of invasive species across large geographic areas [14], while biotic interactions with native species (e.g., herbivores and pathogens) may play a major role in the success of introduced species [12]. Previous research has shown that successful invaders often display high phenotypic plasticity, strong chemical defenses, and can persist in a wide range of environments [15,16]. While a lot of research focused on trait variation among native and non-native species across environmental gradients [17,18], fewer studies focused on population-level differences, particularly within introduced tree species. Understanding intraspecific variation of introduced trees across latitudinal and altitudinal gradients may provide useful ecological insights, and also help identify the most productive and useful genotypes for future applications.
Eucalyptus is an important genus in forestry with many species displaying fast growth, high phenotypic plasticity, strong adaptability, short rotation cycle, and great economic value [19]. Due to these characteristics, Eucalyptus has been introduced into many countries around the world [19,20,21]. Eucalyptus was first introduced to China in the late 1800s [22], with E. globulus, E. tereticornis, E. camaldulensis, and E. robusta planted mainly as ornamental trees [23,24]. By the end of the 20th century, due to increasing population growth and rapid economic development, the demand for wood in China greatly increased [25]. In the 1990s, there was a program that introduced many new Eucalyptus provenances into China as part of the Chinese-Australian collaborative Research and Development project on the introduction, domestication, and silviculture of cold tolerant eucalypts (coordinated by CERC and CSIRO) [25,26]. The plantation area in China increased each year, reaching 5.46 million ha in 2018 [27]. Currently, China has the second largest area of Eucalyptus plantations in the world, mostly in the southern parts of the country [28]. Eucalyptus can spread naturally from plantations via seed onto nearby hillsides and coastal areas [29]. The introduction and spread of Eucalyptus led to multiple ecological and agricultural problems in southern China, such as soil degradation and loss of native biodiversity [30,31,32]. For example, Eucalyptus can inhibit the survival of adjacent seedlings and the germination of native plants and crop species through allelopathy [33].
A large body of evidence strongly supports the notion that leaf morphology and characteristics have climate-related adaptive significance [34]. The environmental filtering hypothesis predicts that with increases in environmental stress, plant species will display higher stress tolerance in their traits [35]. Many studies have shown that variations in climate can lead to significant changes in the function of plant leaves [8], especially changes in temperature [36], rainfall [37], and other environmental conditions [38,39]. Specifically, annual average temperature and annual average precipitation are highly correlated with latitude, and are thought to be important drivers underlying the relationship between leaf traits and latitude [40]. As early as the 19th century, biogeographers noticed that leaves in low latitudes were usually larger in size [41], with leaf size decreasing with increases in altitude [42]. However, the relationship between leaf size and environmental factors is not fixed, and may vary in different regions or different species [41]. Leaf area and specific leaf area (SLA) are related to plant photosynthesis and primary productivity, which is very important for understanding plant responses to climate change [43]; while leaf nutrient content affects nutrient cycling in forests [37]. Previously, it was found that leaf width was negatively correlated with latitude, and leaf area was negatively correlated with altitude [44]. Gong et al. [8] studied the characteristics of leaves in northeastern China and found that SLA decreased with increases in latitude. SLA may be an adaptive strategy of plants to environmental changes to maximize photosynthetic rate [43], with higher SLA values indicating a larger light capture area of leaves [37,45]. The growth rate hypothesis (GRH) predicts that leaf nitrogen and phosphorus will decrease with increases in latitude, because growth rate is higher in warm environments, resulting in a high demand for phosphorus for RNA, ATP production, and protein synthesis [46]. Previous studies revealed that leaf nitrogen and phosphrous decreased with increasing latitude and altitude [47,48].
Many plants possess leaf defense mechanisms that protect them against herbivores and pathogens that may affect plant growth and productivity [49]. For example, phenolic compounds (such as tannins) are generally considered to increase the resistance of trees to herbivorous insects [50]. Biological interactions are thought to be higher at lower latitudes [51]. As such, plant species at low latitudes are expected to display higher degrees of defense (i.e., the low latitude high defense hypothesis, LLHD) [52,53]. Using a meta-analysis approach, it was found that the chemical defense ability of plants at high latitudes is significantly greater [54], which did not support the LLHD. In another study, evergreen broad-leaved species had higher chemical defenses in low latitude areas and deciduous broad-leaved species had higher physical defenses at low latitudes [4]. Plants at high altitudes have been shown to exhibit elevated concentrations of phenolic compounds [55]. For example, in three species from the tribe Lactuceae (Asteraceae), it was found that flavonoid and phenolic acid contents were positively correlated with altitude [56]. Increased concentration of phenolic compounds in plant leaves at greater elevations is likely a response to extreme abiotic conditions, such as higher irradiation, wind exposure, and snow cover [57].
In the current study, we focus on three eucalypt species that have been widely used in forestry in southern China, namely E. saligna Sm., E. grandis W.Hill, and E. robusta Sm. (Figure 1; Supplementary Table S1). All three species are native to eastern Australia, where E. saligna and E. grandis are distributed from the coast to elevations of around 800 m, and E. robusta occurs predominantly in coastal areas, where it is usually found in swamps and along estuaries [58,59]. Both E. saligna and E. grandis grow rapidly, with the former used mainly in the paper industry throughout the world [60,61,62], and the latter widely used for pulp and wood [63]. Oil extracted from the leaves of E. grandis is also used for cosmetics and medicinal purposes [64]. Eucalyptus saligna was introduced to China in the 1970s [65], where it was planted in Hunan, Guangdong, Guangxi, Fujian, Jiangxi, and Zhejiang provinces [66], while E. grandis was mostly planted in Guangdong and Guangxi [64]. Eucalyptus robusta is a fast-growing and highly productive timber species, that has been extensively planted to produce high yield forests in southern China [67]. The species is utilized in the paper making and biomass energy industries [68]. Leaves and bark of E. robusta are also used in traditional medicine for the treatment of influenza, dysentery, malaria, and scalds [69].
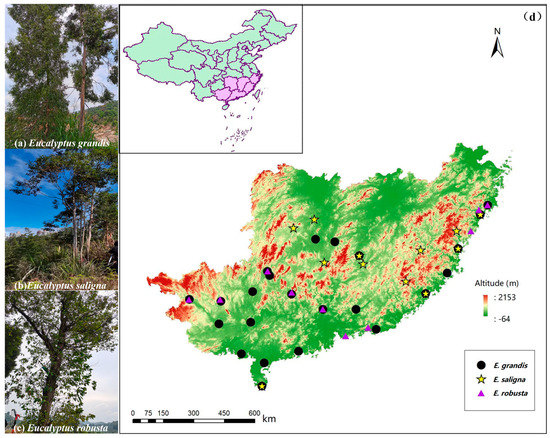
Figure 1.
The study species, including (a) Eucalyptus grandis, (b) E. saligna, and (c) E. robusta. Locations of tree stands of the three introduced species sampled for leaf functional and chemical defense traits in southern China are shown in (d). The full details of each sampling location are presented in Table 1.
The purpose of this study is to investigate the changes in leaf functional, chemical, and defense traits of the three Eucalyptus species across latitudinal and altitudinal gradients in southeastern China. Due to the high morphological variation observed in their native range, and since they have been successfully introduced and naturalized in many countries around the world, we expect leaf functional and chemical defense traits of the three focal Eucalyptus species to significantly vary along latitudinal and altitudinal gradients and across climates. Therefore, we test the following hypotheses: (1) leaf functional traits (e.g., size, thickness, and SLA) will decrease with the increase in latitude and elevation due to lower temperatures and precipitation, and (2) leaf nitrogen and phosphorus concentrations will reduce with increases in latitude and altitude, while phenolic compounds will be higher at lower latitudes (associated with warmer and humid environments), as well as at higher elevations (due to increased abiotic stressors). In addition, we asked what is the relative influence (%) of altitude, latitude, temperature, and precipitation to changes in leaf functional and plant defense traits in the introduced Eucalyptus species?
2. Materials and Methods
2.1. Study Area and Sample Collection
Naturalized and planted stands of E. saligna, E. grandis, and E. robusta were selected from across the range of each species in southern China (this included both forest and urban areas) (Figure 1). In our study, we define ‘naturalized’ tree stands as those where there was evidence of them being localized and reproducing regularly [70]. Initially, we used the Chinese Virtual Herbarium (https://www.cvh.ac.cn, accessed on 20 June 2021) to identify potential areas with reproducing trees. However, although general details were given regarding the collections of herbarium specimens (e.g., at the city- or province-level), often, precise locations were not available for tree stands of each species in the online database. As such, we used Street View in Baidu Maps (https://map.baidu.com, accessed on 3 July 2021 to 15 May 2022) to identify and locate potential populations of the species in Zhejiang, Fujian, Jiangxi, Hunan, Guangxi, and Guangdong provinces. The sampling latitude ranged from 20°16′8″ N to 28°21′43″ N, where we targeted tree stands in a range of environments, including coastal areas, inland flats (on both sides of roads or rivers), and high mountains and forests (Figure 1).
We sampled leaf material from 23 tree stands of E. grandis, 13 tree stands of E. saligna, and 10 tree stands of E. robusta (Table 1). For larger tree stands, we collected leaves from at least 6 randomly selected individuals at least 10 m apart, while for smaller populations (i.e., 6 individuals or less), leaves were sampled from all individuals present. During our sampling, we selected mature trees that were at least several years old. An extendable pole pruner was used to cut mature leaves (without insect pests or other damage) from a high branch in the middle of the crown of the tree facing the sun. In total, 14 leaves were collected from each individual (4 leaves for leaf functional traits and 10 leaves for chemical analysis) and immediately placed in plastic bags and stored in a cooler box to keep in optimal condition. At each location, the latitude and longitude were recorded, and notes were taken regarding whether the trees at each site were planted or naturally occurring (Table 1). The location information was later used to determine the altitude of the sampling point (using https://www.advancedconverter.com, accessed on 18 June 2022). A voucher specimen of each individual sampled, including buds, flowers, and fruits where possible, were taken to verify species identification. All samples were returned to Jiangsu University, where they were placed into the refrigerator before processing.

Table 1.
Tree stands of the introduced Eucalyptus species sampled for leaf, chemical and plant defense traits in southern China, showing location details (including latitude and longitude), local climate (mean annual temperature [MAT] and mean annual precipitation [MAP]), whether trees were planted or sprouted naturally (i.e., naturalized), and other notes about the sites. Locations where both naturalized and (+) planted trees were observed are also noted, as are soil type (obtained from https://www.resdc.cn, accessed on 20 April 2023) and other details.
2.2. Leaf Functional Trait Measurements
We measured six leaf functional traits from each tree sampled in the field (from 4 leaves per individual). Leaf size can be a useful indicator of plant growth, and is strongly linked to plant ecological strategies [71]. In our study, all leaf samples were scanned and blade length, blade width (at the widest point), and leaf area were measured using Intelligent Leaf Area Measurement software (Zhejiang Topp Yunnong Technology Co., Ltd., Hangzhou, China). Leaf thickness was measured from fresh leaves within 12 h after collection. For each leaf, we measured the thickness from four random points on the blade (avoiding the veins) using a digital Vernier caliper (Ningbo Deli Tools Co., Ltd., Ningbo, China) [72]. The petiole length of each leaf was measured using a digital Vernier caliper (Ningbo Deli Tools Co., Ltd., Ningbo, China). The leaves were then oven-dried at 80 °C for 48 h to constant mass, and their dry weights were determined with an analytical balance. For each leaf, we calculated the SLA, since it is positively related to the relative growth rate [73,74] and is an indicator of plant response to resource availability [75]. The SLA was calculated as the total leaf area (in square millimeters) divided by the total dry mass of the leaf (in grams) using the formula of Wright and Westoby [76].
2.3. Chemical Analysis and Plant Defense Traits
We measured leaf nitrogen and phosphorus for chemical analysis and the phenolic compounds (i.e., tannins) for the metrics of the leaf defense traits. The leaves were oven-dried at 65 °C for 48 h, and sent to Ruiyang Biology in Nanjing, China for analysis. Total nitrogen (N) and total phosphorus (P) were determined using the Kjeldahl method and ICP-AES/molybdenum antimony resistance colorimetry methods, respectively. Leaf N and P concentrations are important indicators for the plant nutrition status [72]. Moreover, N and P levels can be strongly correlated with leaf-herbivory because they are often limiting with respect to herbivore nutritional requirements [77]. Phenolic compounds were chosen for examining the plant defense because they are known to be herbivore feeding deterrents in many plant taxa [7]. The phenolic compounds were measured from each leaf sample using phosphomolybdic acid and phosphotungstic acid colorimetry methods [78].
2.4. Climatic Variables
Climatic conditions at each sampling location were characterized using a subset of the bioclimatic variables downloaded from the WorldClim database (http://www.worldclim.org/, accessed on 8 July 2022) [79]. Initially, we used a range of bioclimatic variables (following the approach used by Moreira et al. [7]), including BIO1 (mean annual temperature, MAT), BIO5 (max temp of the warmest month), BIO6 (min temp of the coldest month), BIO12 (mean annual precipitation, MAP), BIO13 (precipitation of the wettest month), BIO14 (precipitation of the driest month), and BIO15 (precipitation seasonality). Upon producing simple linear regressions between each of the measured traits and the climatic factors, we found MAT and MAP to be the most informative variables [8,80,81]. As such, MAT and MAP were used for all data analyses.
2.5. Data Analysis
We log transformed all of the trait data to ensure that they were more normally distributed prior to the analyses [8]. Linear mixed-effects models were used to investigate the separate and interactive effects of latitude, altitude, and climate variables (MAT and MAP) on the leaf functional and plant defense traits of all species. In this analysis, altitude, latitude, MAT, and MAP were fixed effects, while species was a random factor. Mixed-effects models were performed using R version 4.2.2 (R Core Development Team). To visualize the significant interaction effects in the mixed-effects models, contour plots were generated using the R package visreg (visualization of regression models). To assess the relative influence of the environmental variables on each trait, the proportion of variance for each factor was calculated, and a variance ratio diagram was produced using the R package ggplot. A one-way analysis of variance (ANOVA) in SPSS version 26 (IBM, Chicago, IL, USA) was used to examine the differences in leaf functional and plant defense traits of each species across locations. Significant differences (at p < 0.05) between the pairwise mean values were assessed using Tukey’s honest significant difference (HSD) test. Simple linear regression plots were used to explore the relationship between all functional traits and climatic variables, latitude, and altitude, and were generated using Origin (OriginLab, Northampton, MA, USA)
3. Results
3.1. Changes in the Leaf Functional Traits across Latitudinal, Altitudinal, and Climatic Gradients
We found that latitude and climatic variables (MAT and MAP) had a significant effect on the leaf length of all species when the data were pooled (p < 0.05, Table 2). Latitude and altitude had a significant effect on leaf width, while SLA was significantly affected by altitude and MAP (p < 0.05, Table 2). All environmental factors significantly impacted leaf area, while leaf thickness was significantly affected by most factors apart from latitude (Table 2). Many interactions between environmental factors were significant for leaf width and leaf area (e.g., latitude × MAT, altitude × MAT × MAP, and altitude × latitude × MAT × MAP, p < 0.05, Table 2). For leaf length, leaf thickness, and SLA, most interactions between environmental factors were significant (except for altitude × MAT for all three traits and altitude × latitude for SLA, Table 2).

Table 2.
Significance levels shown for mixed-effects model testing for the effects of latitude (Lat), altitude (Alt), mean annual temperature (MAT), and mean annual precipitation (MAP) on leaf functional traits in all samples of Eucalyptus species introduced in southern China and all interactions. Values highlighted in bold indicate a significance at p < 0.05.
When the data for all species were pooled, we found that leaf width and leaf thickness increased with higher latitudes (Figure 2; see Supplementary Figures S1–S3 for individual species contour plots). In E. grandis, leaf width, leaf thickness, and SLA were significantly positively correlated with latitude (R2 was 0.170, 0.306 and 0.106, respectively, p < 0.05, Figure 3a, Supplementary Figure S4a). The leaf length, leaf width, leaf area, leaf thickness, and petiole length of all species decreased with increases in altitude (Figure 2). For E. grandis, there was a significant negative correlation between both leaf length and leaf area, and altitude (R2 was 0.200 and 0.170, respectively, p < 0.05, Supplementary Figure S4b). In E. robusta, there was a significant negative correlation between leaf width and elevation (R2 = 0.395, p = 0.03, Figure 3b). Differences for all leaf traits among locations were significant in each species (p < 0.05, Figure 4a, Supplementary Figure S5). In E. grandis, the population at the site with the highest elevation (Wan’an) had the smallest leaves with the highest SLA (Figure 4a). By contrast, the population of E. grandis at the coastal city of Xiamen had the largest and thickest leaves, with the more southern populations of Nanning and Xuwen having significantly lower SLA than many other locations (Figure 4a). A similar pattern was observed for E. saligna with the population at the highest altitude having the highest SLA, while the population at Xiamen had the largest leaf length, width, area, and thickness (Figure 4a, Supplementary Figure S5). In E. robusta, the most southern population at Shenzhen had the highest SLA and thinnest leaves (Figure 4a).
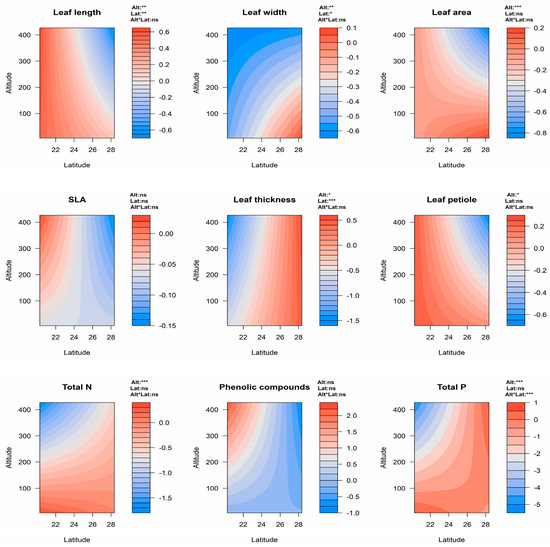
Figure 2.
Contour plots illustrating the effects of altitude (Alt) and latitude (Lat), and their interactions on all leaf functional and chemical defense traits in all of the Eucalyptus study species. For the statistical results, see Supplementary Table S2. Significance of the main effects (Alt and Lat) and the interactions (Alt*Lat) are indicated as ns (i.e., non−significant, p ≥ 0.05), * (p < 0.05), ** (p < 0.01), *** (p < 0.001) in the upper right corner of each plot. Abbreviations: SLA (specific leaf area), total P (total phosphorus), and total N (total nitrogen).
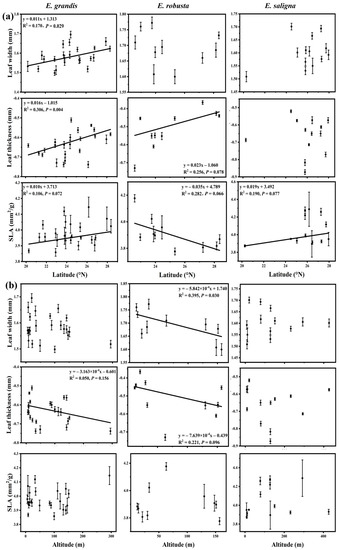
Figure 3.
Pattern of key leaf functional traits of introduced Eucalyptus grandis, E. saligna, and E. robusta across (a) latitude and (b) altitude in southern China. Traits shown are leaf width, leaf thickness, and specific leaf area (SLA). Black dots represent the mean ± standard error (SE). Relationships between other leaf functional traits and latitudinal and altitudinal gradients for each species are presented in Supplementary Figure S4.
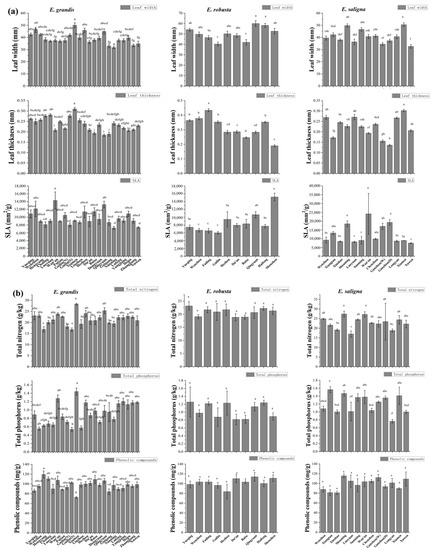
Figure 4.
Differences in (a) leaf functional and (b) plant defense traits across locations of introduced Eucalyptus grandis, E. robusta, and E. saligna in southern China. Significant differences (p < 0.05, Tukey’s HSD post-hoc comparison) among sites are denoted by different letters above the bars. Abbreviations: SLA (specific leaf area), total P (total phosphorus), and total N (total nitrogen).
We found that the leaf length of all Eucalyptus species increased with higher MAT, while leaf width, leaf thickness, and petiole length reduced with increases in MAT (Figure 5, see Supplementary Figures S6–S8 for the contour plots of each individual species). In E. grandis, the leaf width and leaf thickness were significantly negatively correlated with MAT (R2 was 0.137 and 0.233, respectively, p < 0.05, Figure 6a). For E. robusta, there was a significantly negative correlation between leaf thickness and MAT (R2 = 0.413, p = 0.03, Figure 6a). The leaf length, leaf area, leaf thickness, and petiole length in all species decreased with higher MAP (Figure 5). Specifically, in E. grandis there was a significant negative correlation between both leaf thickness and leaf area, and MAP (R2 was 0.176 and 0.235, respectively, p < 0.05, Figure 6b, Supplementary Figure S9b).
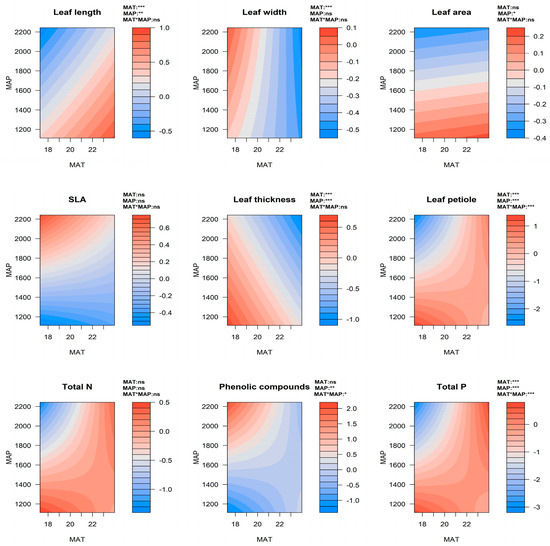
Figure 5.
Contour plots illustrating the effects of mean annual temperature (MAT) and mean annual precipitation (MAP), and their interactions on all leaf functional and chemical defense traits in all of the Eucalyptus study species. For the statistical results, see Supplementary Table S3. Significance of the main effects (MAT and MAP) and the interactions (MAT*MAP) are indicated as ns (i.e., non-significant, p ≥ 0.05), * (p < 0.05), ** (p < 0.01), *** (p < 0.001) in the upper right corner of each plot. Abbreviations: SLA (specific leaf area), total P (total phosphorus), and total N (total nitrogen).
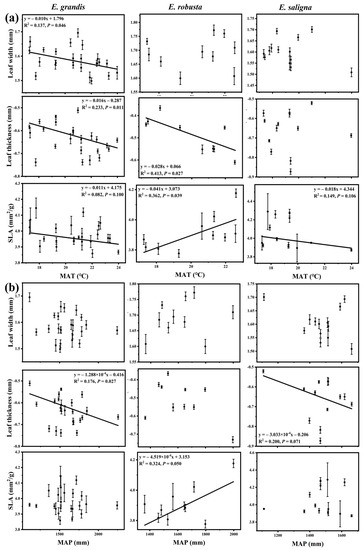
Figure 6.
Relationship between the key functional traits of introduced Eucalyptus grandis, E. saligna, and E. robusta and (a) mean annual temperature (MAT) and (b) mean annual precipitation (MAP) in southern China. Traits shown are leaf width, leaf thickness, and specific leaf area (SLA). The black dots represent the mean ± standard error (SE). Patterns of other leaf functional traits across latitudinal and altitudinal gradients for each species are presented in Supplementary Figure S9.
3.2. Variation in Plant Defense Traits along Altitudinal, Latitudinal, and Climatic Gradients
We found that changes in altitude had a significant effect on both total N and total P, while total P was also significantly affected by MAP (p < 0.05, Table 3). All plant defense traits were significantly affected by interactions between environmental factors. For example, total N and phenolic compounds were significantly impacted by the interactions between latitude, MAT, and MAP, as well as between altitude, latitude, MAT, and MAP (p < 0.05, Table 3). Total P and phenolic compounds were both significantly affected by the interaction between altitude, latitude, and MAT (p < 0.05, Table 3).

Table 3.
Significance levels shown for the mixed-effects model testing for the effects of latitude (Lat), altitude (Alt), mean annual temperature (MAT), and mean annual precipitation (MAP) on plant defense traits in all samples of Eucalyptus species introduced in southern China and all interactions. Values highlighted in bold indicate significance at p < 0.05.
Total N and total P of all species decreased with both higher latitudes and increases in altitude (Figure 2). In contrast, levels of phenolic compounds increased with higher elevations (Figure 2). For E. grandis, there was a negative correlation between total P and latitude (R2 = 0.487, p < 0.001), and a positive correlation between phenolic compounds and altitude (R2 = 0.310, p = 0.002, Supplementary Figure S10). In E. robusta and E. saligna, there was a negative correlation between total N and altitude (R2 was 0.460 and 0.341, respectively, p < 0.05), and for the former species, there was also a negative correlation between total P and altitude (R2 = 0.659, p = 0.003, Supplementary Figure S10b). Differences in all leaf chemical defense traits among locations in E. grandis were significant (p < 0.05, Figure 4). In E. grandis, the coastal population at Xiamen had the highest total leaf N and P, and lowest concentration of phenolic compounds (Figure 4). Total leaf N and P were significantly different among locations in E. saligna (Figure 4). In this species, total P was significantly lower at the highest altitude site of Longyan (Figure 4). There were no significant differences among locations in any of the measured chemical defense traits in E. robusta (Figure 4).
For all species, total P decreased with increased MAT, and reduced with higher MAP, while phenolic compounds increased with increases in MAP (Figure 5). In E. grandis, there was a positive correlation between total P and MAT and between phenolic compounds and MAP (R2 was 0.477 and 0.426, respectively, p < 0.001); while total N negatively correlated with MAP (R2 = 0.293, p = 0.005, Supplementary Figure S11). In E. saligna, total N and phenolic compounds were positively correlated with MAP (R2 was 0.384 and 0.349, p < 0.05, Supplementary Figure S11b).
3.3. Relative Influence of the Environmental Factors on Leaf Functional and Plant Defense Traits
In our analyses of the relative influence of environmental factors on leaf functional and plant defense traits in all species (Figure 7), we found that many traits were highly affected by latitude. Of all of the environmental factors, leaf width, leaf area, total P, and total N were mostly affected by latitude (54.5%, 34.3%, 34.3%, and 43.6%, respectively, Figure 7). MAT also highly affected the total P content in the study species (31.9%), while leaf area was highly influenced by both MAT and MAP (25.5% and 24.1%, respectively, Figure 7). Leaf length, leaf thickness, petiole length, SLA, and phenolic compounds were mainly affected by MAT (39.9%, 40.8%, 52.9% 35.0%, and 64.5%, respectively), with SLA also being highly influenced by MAP (30.6%, Figure 7).
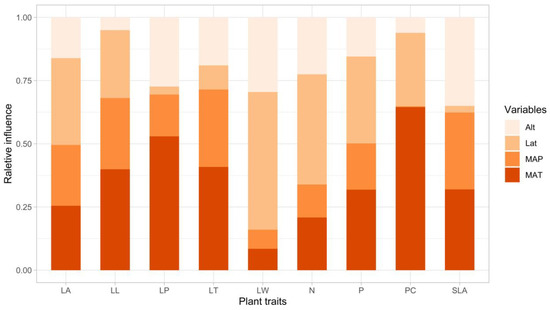
Figure 7.
Effects of altitude (Alt), latitude (Lat), mean annual temperature (MAT), and mean annual precipitation MAP) on each leaf functional and chemical defense trait, including leaf area (LA), leaf length (LL), leaf width (LW), leaf petiole length (LP), leaf thickness (LT), total nitrogen (N), total phosphorus (P), phenolic compounds (PC), and specific leaf area (SLA).
4. Discussion
Population-level variation in leaf functional and plant chemical defenses across environmental gradients can provide vital information regarding resource acquisition and utilization, competitiveness, and invasiveness of plant species [82,83]. Yet, few studies have examined the in-situ trait variations in invasive trees across latitudinal, altitudinal, and climatic gradients [84,85]. To our knowledge, the present study is the first to investigate the leaf functional and plant defense trait variations in populations of multiple Eucalyptus species that are widely used in forestry, within the introduced range in China. The findings from this study highlight how closely related and co-occurring tree species can differ markedly in their resource use strategy in the introduced range, which has important implications for their potential invasiveness.
4.1. Variation in the Leaf Functional Traits of Introduced Eucalyptus Species across Clines
Overall, our results revealed that the leaf width and leaf thickness of all species combined increased with increases in latitude, with E. grandis displaying a greater leaf width, leaf thickness, and SLA at higher latitudes (Figure 2 and Figure 3). In contrast, most leaf functional traits of all species decreased with higher elevation (Figure 2). As such, our first hypothesis that leaf functional traits would reduce with increases in latitude and altitude due to lower temperatures and precipitation was only partially supported. With higher latitudes in China, temperature and precipitation decrease and hydrothermal conditions deteriorate [86]. Consequently, greater leaf size in such environments would maximize photosynthetic rates to enhance plant growth [87,88]. Moreover, smaller and narrower leaves that enable plants to maintain their physiological equilibrium via rapid heat dissipation across a smaller area may be advantageous in lower latitudes with higher temperatures [42]. Our finding of thinner leaves in Eucalyptus populations at lower latitudes with higher temperatures (i.e., in the south of China) is consistent with patterns observed in Eriophorum vaginatum and Cassiope tetragona, where the southern populations in Alaska also had thinner leaves [89]. As the case with smaller leaves, thinner leaves can maintain optimal temperature under warmer conditions, since they facilitate rapid heat transfer across the thin boundary layer [41,86]. Our result of SLA in E. grandis decreasing at lower latitudes is consistent with some previous studies [11,88,90]. Lower SLA is associated with nutrient retention and the prevention of desiccation in plants, that could be a useful strategy in hotter environments [87].
The results from the current study are consistent with many previous studies demonstrating leaf size as negatively correlated with altitude [44,91]. With increasing altitude, temperature decreases, restricting leaf size in plants [92]. Smaller leaves reduce water loss, thereby improving the plant response to exposure and dryness at high altitudes [41,91,93]. Lower temperatures and precipitation, as well as weaker light intensities, may inhibit plant growth, and therefore more resources need to be allocated to foliage construction, reducing SLA [94].
4.2. Leaf Chemical and Defense Traits across Clines
Consistent with our second hypothesis, we found that leaf N and P concentrations of Eucalyptus species reduced with increases in latitude and altitude (Figure 2). Previous studies have demonstrated that temperature, water availability, and light can strongly influence N and P concentrations in leaves of many plant species. Of these environmental factors, temperature has been shown to be a major factor influencing leaf chemistry across latitude and altitude [95]. For example, in a study of 1280 plant species, Reich and Oleksyn [96] found that overall leaf N and P declined with increases in MAT towards the equator, which is consistent with our research results (Figure 5). Other studies, have shown declines in leaf N and P associated with lower temperatures at higher elevations in Metrosideros and evergreen woody plants [47,95,97,98,99]. Changes in precipitation across latitude and altitude have also been shown to be related to concentrations of leaf phosphorus in many species [100]. Low precipitation limits soil weathering, organic matter production, and mineralization, resulting in the slow release of soil phosphorus; therefore, reducing the phosphorus content of plant leaves [101].
Concentration of phenolics in plant leaves are strongly affected by climate and season, with tannin production influenced by photoperiod, soil pH, and water availability [102,103,104]. We found that the phenolic compounds of all Eucalyptus species increased with elevation (Figure 2), which was consistent with our second hypothesis, and agreed with the results of Monschein et al. [105], which revealed that the harsher environmental and climatic conditions at higher altitudes were associated with an increase in flavonols in the tissues of Epilobium angustifolium. Other studies have reported that phenolic compounds are higher in warm and humid environments. For instance in oak trees, it was found that leaves contained higher water content and higher concentration of tannins in warmer climates [106]. In our study, there was not a clear relationship between phenolic compounds and latitude or MAT (Supplementary Figures S10 and S11), although we did find that phenolics increased with MAP in both E. saligna and E. grandis (Supplementary Figure S11b), highlighting how changes in precipitation may influence plant defense in these species. In evergreen broadleaved trees, it was found that while the tannin content decreased with latitude, leaf thickness in these species increased (which was thought to be a substitute for phenolics as a physical defense mechanism) [4]. We also found that leaf thickness of these Eucalyptus species increased with latitude, indicating a similar mechanism operating in these species in the introduced range in southern China.
4.3. Contribution of Environmental Factors to the Potential Spread of Introduced Eucalyptus Species
Understanding the environmental factors that limit the range of species is vital for exploring the potential of introduced plants to invade new areas, and predict their response to global changes [107]. Both leaf functional and plant defense traits of invasive plants may change in the introduced range, and over time adapt to the new environmental conditions [108]. In addition, many invasive species are thought to be more responsive to changes in the environment than native species, and display a high degree of phenotypic plasticity, thereby facilitating their expansion into new areas [14]. While previous studies have demonstrated the role of phenotypic plasticity or local adaptation in the success of alien species in the introduced range [109,110], the relative importance of each strategy to different invaders has not been fully appreciated.
In our study, E. grandis and E. saligna populations at environmental range limits at the highest elevations displayed higher maximum photosynthetic capture rates (i.e., smaller leaves with higher SLA) and higher phenolic compounds (Figure 4). In more coastal populations of E. grandis (e.g., Xiamen), which were exposed to higher precipitation and more benign temperatures, leaves were larger with higher total P and N and lower phenolic compounds (Figure 4). In E. robusta, the populations at the northern range limit, which are exposed to colder winter temperatures, had a more conservative resource allocation strategy (with lower SLA), which was in contrast to E. grandis, where the southern populations in warmer and more humid environments tended to have lower SLA (Figure 4). The higher SLA at the most northern limits of the range of E. grandis may be indicative of these populations expanding their ranges into the north [111]. Our variance partitioning analysis revealed that temperature was an important factor influencing leaf size, total P, SLA, and phenolic compounds (i.e., MAT, Figure 7). During our surveys, we could not find any populations of any of the study species north of Taizhou (Zhejiang Province) and Changsha (Hunan Province). Areas to the north of these cities experience colder winters (e.g., the lowest temperatures in Changsha and Taizhou in the last decade were −6 °C and −17 °C, respectively), indicating that temperature may be a limiting factor for these species.
The trait variation we observed along clines could be due to plasticity and/or local adaptation in the introduced range. Given that these are long-lived trees, much of the variation observed is likely due to phenotypic plasticity. Many previous studies support the notion that plasticity, rather than adaptive ecotypes, is a driver of invasiveness in many invaders (e.g., Alternanthera philoxeroides [112]). Invasive species that reproduce clonally and have undergone fewer introduction events are thought to employ phenotypic plasticity as a primary strategy for exploiting new habitats (e.g., due to lower genetic variation) [113]. In contrast, multiple introductions and outcrossing (including hybridization with other closely related species) is associated with invaders that possess higher genetic variation that can enable them to adapt to many different habitats [114,115]. Many Eucalyptus species display outcrossing and have a high propensity to hybridize in the native range [116,117,118]. In China, there have been multiple introductions of the three study species over the last 130 years, as well as closely related species they could hybridize with [119]. Previously, it was found that phenotypic plasticity in combination with local adaptation led to the success of invasive Prunella vulgaris [120]. As such, adaptive processes cannot be excluded as a mechanism for some of the trait variations observed in the current study. The high degree of trait variation observed here has implications for the effects of climate change on the spread of these introduced Eucalyptus species in China. For instance, invaders with high phenotypic plasticity are likely to be able to spread further towards the poles under climate warming [121]. Future studies involving genetic studies on adaptation to environmental variables and the response of seedlings to varying resource availability (Liu et al., unpublished data) will likely shed light on how introduced tree species may change upon settling in a new range.
5. Conclusions
Intraspecific differences in leaf functional traits along environmental gradients revealed that the three introduced Eucalyptus species from this study can exploit a wide range of habitats through changes in leaf functional, chemical, and plant defense traits. We found that many functional traits decreased at low latitudes, probably to minimize heat and water loss in hotter environments. Furthermore, many traits and leaf N and P decreased at higher elevations, while phenolic compounds increased, indicating that plants employed a more conservative growth strategy and possessed higher chemical defenses to cope with harsher environmental conditions. Whether the trait variation observed here is due to phenotypic plasticity or genetic adaptation is a promising avenue for future studies. In summary, our study provides an understanding of in-situ population variation in the growth and defense traits of three Eucalyptus species widely used in forestry within the introduced range in southern China, which has implications for the spread of these species, particularly under climate warming.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14050936/s1, Table S1: Morphological characteristics of the three study species; Table S2: Mixed-effects model of the effects of species, latitude, altitude, and interactions on all traits of all species; Table S3: Mixed-effects model showing the effects of species, MAT, MAP, and interactions on all traits of all species; Figure S1: Contour plots illustrating the effects of altitude and latitude, and their interactions on all traits in E. grandis; Figure S2: Contour plots illustrating the effects of altitude and latitude, and their interactions on all traits in E. saligna; Figure S3: Contour plots illustrating the effects of altitude and latitude, and their interactions on all traits in E. robusta; Figure S4: Relationship between leaf length, petiole length, and area of each species, and latitude and altitude; Figure S5: Differences in leaf length, petiole length, and area across locations of each species; Figure S6: Contour plots illustrating the effects of MAP and MAT, and their interactions on all traits in E. grandis; Figure S7: Contour plots illustrating the effects of MAP and MAT, and their interactions on all traits in E. saligna; Figure S8: Contour plots illustrating the effects of MAP and MAT, and their interactions on all traits in E. robusta; Figure S9: Relationship between leaf length, petiole length, and area of each species, and MAT and MAP; Figure S10: Relationship between chemical defense traits of each species and latitude and altitude; Figure S11: Relationship between chemical defense traits of each species and MAT and MAP.
Author Contributions
Conceptualization—S.R.; study design—S.R., J.S.H.W. and H.L.; funding—S.R.; sampling—H.L., J.S.H.W., J.L. and M.R.A.; supervision—S.R., J.S.H.W. and D.D.; data management—H.L. and J.Z.; analysis—H.L.; writing original draft—H.L. and S.R.; editing drafts—S.R., M.R. and J.S.H.W.; reviewing—all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Jiangsu University Research Foundation (20JDG055) and National Natural Science Foundation of China (32001087). J.S.H.W. was supported by the Jiangsu Natural Science Foundation (BK20200905).
Data Availability Statement
All data generated and analyzed during this study are included in this article.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Binggeli, P. A taxonomic, biogeographical and ecological overview of invasive woody plants. J. Veg. Sci. 1996, 7, 121–124. [Google Scholar] [CrossRef]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from Global Invasive Species Database. In Encyclopedia of Biological Invasions; Simberloff, D., Rejmanek, M., Eds.; University of California Press: Berkeley, CA, USA, 2011; pp. 715–716. [Google Scholar]
- Richardson, D.M.; Rejmánek, M. Trees and shrubs as invasive alien species—A global review. Divers. Distrib. 2011, 17, 788–809. [Google Scholar] [CrossRef]
- Saihanna, S.; Tanaka, T.; Okamura, Y.; Kusumoto, B.; Shiono, T.; Hirao, T.; Kubota, Y.; Murakami, M. A paradox of latitudinal leaf defense strategies in deciduous and evergreen broadleaved trees. Ecol. Res. 2018, 33, 1011–1017. [Google Scholar] [CrossRef]
- Dong, N.; Prentice, I.C.; Wright, I.J.; Evans, B.J.; Togashi, H.F.; Caddy-Retalic, S.; McInerney, F.A.; Sparrow, B.; Leitch, E.; Lowe, A.J. Components of leaf-trait variation along environmental gradients. New Phytol. 2020, 228, 82–94. [Google Scholar] [CrossRef]
- Read, Q.D.; Moorhead, L.C.; Swenson, N.G.; Bailey, J.K.; Sanders, N.J. Convergent effects of elevation on functional leaf traits within and among species. Funct. Ecol. 2014, 28, 37–45. [Google Scholar] [CrossRef]
- Moreira, X.; Castagneyrol, B.; Abdala-Roberts, L.; Berny-Mier y Teran, J.C.; Timmermans, B.G.H.; Bruun, H.H.; Covelo, F.; Glauser, G.; Rasmann, S.; Tack, A.J.M. Latitudinal variation in plant chemical defences drives latitudinal patterns of leaf herbivory. Ecography 2018, 41, 1124–1134. [Google Scholar] [CrossRef]
- Gong, H.; Cui, Q.; Gao, J. Latitudinal, soil and climate effects on key leaf traits in northeastern China. Glob. Ecol. Conserv. 2020, 22, e00904. [Google Scholar] [CrossRef]
- Ackerly, D.D.; Reich, P.B. Convergence and correlations among leaf size and function in seed plants: A comparative test using independent contrasts. Am. J. Bot. 1999, 86, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Valladares, F.; Bastias, C.C.; Godoy, O.; Granda, E.; Escudero, A. Species coexistence in a changing world. Front. Plant Sci. 2015, 6, 866. [Google Scholar] [CrossRef]
- De Frenne, P.; Graae, B.J.; Rodríguez-Sánchez, F.; Kolb, A.; Chabrerie, O.; Decocq, G.; De Kort, H.; De Schrijver, A.; Diekmann, M.; Eriksson, O.; et al. Latitudinal gradients as natural laboratories to infer species’ responses to temperature. J. Ecol. 2013, 101, 784–795. [Google Scholar] [CrossRef]
- Carboni, M.; Calderon-Sanou, I.; Pollock, L.; Violle, C.; DivGrass, C.; Thuiller, W. Functional traits modulate the response of alien plants along abiotic and biotic gradients. Glob. Ecol. Biogeogr. 2018, 27, 1173–1185. [Google Scholar] [CrossRef]
- Thuiller, W.; Richardson, D.M.; Rouget, M.; Procheş, Ş.; Wilson, J.R.U. Interactions between environment, species traits, and human uses describe patterns of plant invasions. Ecology 2006, 87, 1755–1769. [Google Scholar] [CrossRef]
- Davidson, A.M.; Jennions, M.; Nicotra, A.B. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett. 2011, 14, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Abhilasha, D.; Quintana, N.; Vivanco, J.; Joshi, J. Do allelopathic compounds in invasive Solidago canadensis s.l. restrain the native European flora? J. Ecol. 2008, 96, 993–1001. [Google Scholar] [CrossRef]
- Aguilera, N.; Becerra, J.; Guedes, L.M.; Villaseñor-Parada, C.; González, L.; Hernández, V. Allelopathic effect of the invasive Acacia dealbata Link (Fabaceae) on two native plant species in south-central Chile. Gayana Bot. 2015, 72, 231–239. [Google Scholar] [CrossRef]
- Westerband, A.C.; Knight, T.M.; Barton, K.E. Intraspecific trait variation and reversals of trait strategies across key climate gradients in native Hawaiian plants and non-native invaders. Ann. Bot. 2021, 127, 553–564. [Google Scholar] [CrossRef]
- Seipel, T.; Alexander, J.M.; Daehler, C.C.; Rew, L.J.; Edwards, P.J.; Dar, P.A.; McDougall, K.; Naylor, B.; Parks, C.; Pollnac, F.W.; et al. Performance of the herb Verbascum thapsus along environmental gradients in its native and non-native ranges. J. Biogeogr. 2015, 42, 132–143. [Google Scholar] [CrossRef]
- Sumathi, M.; Yasodha, R. Microsatellite resources of Eucalyptus: Current status and future perspectives. Bot. Stud. 2014, 55, 73. [Google Scholar] [CrossRef]
- Chen, W.; Zou, Y.; Dang, Y.; Sakai, T. Spatial distribution and dynamic change monitoring of Eucalyptus plantations in China during 1994–2013. Trees 2021, 36, 405–414. [Google Scholar] [CrossRef]
- Wang, J.; Deng, Y.; Li, D.; Liu, Z.; Wen, L.; Huang, Z.; Jiang, D.; Lu, Y. Soil aggregate stability and its response to overland flow in successive Eucalyptus plantations in subtropical China. Sci. Total Environ. 2022, 807, 151000. [Google Scholar] [CrossRef]
- Huoran, W.; Zeping, J.; Hong, Y. Australian trees grown in China as exotics. Trop. Geogr. 1994, 14, 29. [Google Scholar]
- Chen, S.X.; Wu, Z.H.; Li, Z.H.; Xie, Y.J.; Li, T.H.; Zhou, Q.Y.; Arnold, R. Selection of Species for Solid Wood Production in Southern China. J. Trop. Sci. 2010, 22, 308–316. [Google Scholar]
- Xie, Y.J. Study on Eucalyptus Selection Objectives and Current Situation of Genetic Resources in China. Eucalypt. Sci. Technol. 2012, 29, 8. [Google Scholar]
- Zheng, J.Q.; Chen, S.X. A discussion on utilization of Eucalyptusin China. Eucalypt. Sci. Technol. 2017, 34, 42–46. [Google Scholar]
- Huang, M.; Ge, X.; Shi, H.; Tong, Y.; Shi, J. Prediction of current and future potential distributions of the Eucalyptus pest Leptocybe invasa (Hymenoptera: Eulophidae) in China using the CLIMEX model. Pest. Manag. Sci. 2019, 75, 2958–2968. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Xu, J.; Li, G.; Liu, W. Site Classification of Eucalyptus urophylla × Eucalyptus grandis Plantations in China. Forests 2020, 11, 871. [Google Scholar] [CrossRef]
- Qu, Z.; Liu, B.; Ma, Y.; Sun, H. Differences in bacterial community structure and potential functions among Eucalyptus plantations with different ages and species of trees. Appl. Soil. Ecol. 2020, 149, 103515. [Google Scholar] [CrossRef]
- Calviño-Cancela, M.; Rubido-Bará, M. Invasive potential of Eucalyptus globulus: Seed dispersal, seedling recruitment and survival in habitats surrounding plantations. For. Ecol. Manag. 2013, 305, 129–137. [Google Scholar] [CrossRef]
- Xu, Y.; Li, C.; Zhu, W.; Wang, Z.; Wu, L.; Du, A. Effects of enrichmemt planting with native tree species on bacterial community structure and potential impact on Eucalyptus plantations in southern China. J. For. Res. 2022, 33, 1349–1363. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, P.; Huang, Y.; Zhu, L.; Ni, G.; Zhao, X.; Huang, Z. Hydrologic balance, net primary productivity and water use efficiency of the introduced exotic Eucalyptus grandis × Eucalyptus urophylla plantation in south-western China. J. Plant. Ecol. 2019, 12, 982–992. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Lan, J.; Tongway, D.; Freudenberger, D. An Environmental Impact Assessment of Different Management Regimes in Eucalypt Plantations in Southern China Using Landscape Function Analysis. J. Sustain. For. 2020, 41, 526–536. [Google Scholar] [CrossRef]
- Ben Ghnaya, A.; Hamrouni, L.; Amri, I.; Ahoues, H.; Hanana, M.; Romane, A. Study of allelopathic effects of Eucalyptus erythrocorys L. crude extracts against germination and seedling growth of weeds and wheat. Nat. Prod. Res. 2016, 30, 2058–2064. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.W.; Ai, Y.B.; Liu, Y.H. Variations in leaf functional traits along the altitude gradient of Pinus tabuliformis and its environmental explanations in Beijing Songshan Mountain. J. Beijing For. Univ. 2021, 43, 47–55. [Google Scholar]
- Le Bagousse-Pinguet, Y.; Gross, N.; Maestre, F.T.; Maire, V.; de Bello, F.; Fonseca, C.R.; Kattge, J.; Valencia, E.; Leps, J.; Liancourt, P. Testing the environmental filtering concept in global drylands. J. Ecol. 2017, 105, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, Y.; Wei, W. Variations of leaf typical shrub and herb species along a climate gradient in arid areas of Northwest China. Chin. J. Ecol. 2021, 40, 3769–3777. [Google Scholar]
- Zhang, X.; He, X.; Gao, J.; Wang, L. Latitudinal and climate effects on key plant traits in Chinese forest ecosystems. Glob. Ecol. Conserv. 2019, 17, e00527. [Google Scholar] [CrossRef]
- Roa-Fuentes, L.L.; Templer, P.H.; Campo, J. Effects of precipitation regime and soil nitrogen on leaf traits in seasonally dry tropical forests of the Yucatan Peninsula, Mexico. Oecologia 2015, 179, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Cornelissen, J.H.; Falster, D.S.; Garnier, E.; Hikosaka, K.; Lamont, B.B.; Lee, W.; Oleksyn, J.; Osada, N.; et al. Assessing the generality of global leaf trait relationships. New Phytol. 2005, 166, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Gao, J. Soil and climatic drivers of plant SLA (specific leaf area). Glob. Ecol. Conserv. 2019, 20, 696. [Google Scholar] [CrossRef]
- Fan, X.; Yan, X.; Qian, C.; Bachir, D.G.; Yin, X.; Sun, P.; Ma, X.F. Leaf size variations in a dominant desert shrub, Reaumuria soongarica, adapted to heterogeneous environments. Ecol. Evol. 2020, 10, 10076–10094. [Google Scholar] [CrossRef]
- Yates, M.J.; Anthony Verboom, G.; Rebelo, A.G.; Cramer, M.D. Ecophysiological significance of leaf size variation in Proteaceae from the Cape Floristic Region. Funct. Ecol. 2010, 24, 485–492. [Google Scholar] [CrossRef]
- Tian, M.; Yu, G.; He, N.; Hou, J. Leaf morphological and anatomical traits from tropical to temperate coniferous forests: Mechanisms and influencing factors. Sci. Rep. 2016, 6, 19703. [Google Scholar] [CrossRef] [PubMed]
- Guerin, G.R.; Wen, H.; Lowe, A.J. Leaf morphology shift linked to climate change. Biol. Lett. 2012, 8, 882–886. [Google Scholar] [CrossRef]
- Castro-Díez, P.; Puyravaud, J.P.; Cornelissen, J.H.C. Leaf structure and anatomy as related to leaf mass per area variation in seedlings of a wide range of woody plant species and types. Oecologia 2000, 124, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Sterner, R.W.; Gorokhova, E.; Fagan, W.F.; Markow, T.A.; Cotner, J.B.; Harrison, J.F.; Hobbie, S.E.; Odell, G.M.; Weider, L.J. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 2000, 3, 540–550. [Google Scholar] [CrossRef]
- Zhao, N.; Yu, G.; He, N.; Xia, F.; Wang, Q.; Wang, R.; Xu, Z.; Jia, Y. Invariant allometric scaling of nitrogen and phosphorus in leaves, stems, and fine roots of woody plants along an altitudinal gradient. J. Plant. Res. 2016, 129, 647–657. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Z.; Xing, W.; Liu, G. Plasticity in latitudinal patterns of leaf N and P of Oryza rufipogon in China. Plant. Biol. 2014, 16, 917–923. [Google Scholar] [CrossRef]
- Gershenzon, J.; Ullah, C. Plants protect themselves from herbivores by optimizing the distribution of chemical defenses. Proc. Natl. Acad. Sci. USA 2022, 119, e2120277119. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Jaros, A.; Lee, G.; Mozola, C.; Weir, Q.; Salminen, J.P. Hydrolyzable tannins as “quantitative defenses”: Limited impact against Lymantria dispar caterpillars on hybrid poplar. J. Insect. Physiol. 2009, 55, 297–304. [Google Scholar] [CrossRef]
- Lewinsohn, T.M.; Roslin, T. Four ways towards tropical herbivore megadiversity. Ecol. Lett. 2008, 11, 398–416. [Google Scholar] [CrossRef]
- Coley, P.D.; Aide, T.M. Comparison of herbivory and plant defenses in temperate and tropical broad-leaved forests. In Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions; Price, P.W., Lewinsohn, T.M., Fernandes, G.W., Benson, W.W., Eds.; Wiley: New York, NY, USA, 1991; pp. 25–49. [Google Scholar]
- Dobzhansky, T. Evolution in the tropics. Am. Sci. 1950, 38, 209–221. [Google Scholar]
- Moles, A.T.; Bonser, S.P.; Poore, A.G.B.; Wallis, I.R.; Foley, W.J. Assessing the evidence for latitudinal gradients in plant defence and herbivory. Funct. Ecol. 2011, 25, 380–388. [Google Scholar] [CrossRef]
- Zhang, N.; Tonsor, S.J.; Traw, M.B. A geographic cline in leaf salicylic acid with increasing elevation in Arabidopsis thaliana. Plant. Signal. Behav. 2015, 10, e992741. [Google Scholar] [CrossRef] [PubMed]
- Zidorn, C.; Schubert, B.; Stuppner, H. Altitudinal differences in the contents of phenolics in flowering heads of three members of the tribe Lactuceae (Asteraceae) occurring as introduced species in New Zealand. Biochem. Syst. Ecol. 2005, 33, 855–872. [Google Scholar] [CrossRef]
- Körner, C. Alpine Plant Life; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar] [CrossRef]
- Benson, D.; Mcdougall, L.J.C. Ecology of Sydney plant species. Part 6. Dicotyledon family Myrtaceae. Cunninghamia 1998, 5, 808–987. [Google Scholar]
- Brooker, M.; Slee, A.; Connors, J.; Duffy, S.; West, J. EUCLID: Eucalypts of Australia; CSIRO Publishing: Collingwood, Australia, 2006. [Google Scholar]
- Núñez, C.E. Morphological study of fibers of four trees of Eucalyptus saligna implanted in Concordia, Entre Rios, Argentina. Rev. Cienc. Tecnol. 2014, 22, 40–44. [Google Scholar]
- Martins, F.B.; Silva, J.C.d.; Streck, E.N.A. Estimating base temperature for leaf appearence rate and phyllochron in two eucaliptus species seedling phase. Rev. Árvore. 2007, 31, 373–381. [Google Scholar]
- Estrada, C.E.; Ramírez, M.A. Tasa de descuento y rotación forestal: El caso del Eucalyptus Saligna. Lect. Econ. 2010, 73, 149–164. [Google Scholar]
- Ogunwande, I.A.; Olawore, N.O.; Adeleke, K.A.; Konig, W.A. Chemical composition of the essential oils from the leaves of three Eucalyptus species growing in Nigeria. J. Essent. Oil. Res. 2011, 15, 297–301. [Google Scholar] [CrossRef]
- Lyu, H.L.; Luo, C.L.; Shi, Y.; Zhao, Y.W.; Peng, Z.B.; Yu, S.J.; Lan, J.; Wang, J.Z. Effects of culture conditions on cuttage rooting of Eucalyptus grandis. Guangxi For. Sci. 2020, 49, 241–244. [Google Scholar]
- Rao, H.X.; Peng, X.H.; Luo, X.Q.; Chen, L.; Jiang, L.Y. Softwood cutting from tissue culture shoots of Eucalyputus saligna. J. China Forestry Sci. Technol. 2010, 24, 104–106. [Google Scholar]
- Tan, B.T. Study on tissue culture and industrialized breeding technolique of Eucalyptus saligna. Hunan For. Sci. Technol. 2009, 36, 9–11. [Google Scholar]
- Gao, Y.; Zhang, J.; Tang, H.; Liu, N.; Li, G.; Yue, D. The characteristics of the complete chloroplast genome for Eucalyptus robusta (Myrtaceae). Mitochondrial. DNA B Resour. 2021, 6, 3517–3518. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhou, R.H.; Yu, F.Y.; Dong, H.J.; Chen, C.L.; Yu, J.; Hao, J.F. Dynamic changes of undergrowth species diversity and biomass of Eucalyptus robusta plantations at different ages. Bull. Bot. Res. 2021, 41, 496–505. [Google Scholar]
- Jian, Y.Q.; Wang, Y.; Huang, X.J.; Li, G.Q.; Zhao, B.X.; Guo, Q.Y.; Ye, W.C. Two new euglobals from the leaves of Eucalyptus robusta. J. Asian Nat. Prod. Res. 2012, 14, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.A.; Gaertner, M.; Robertson, M.P.; Richardson, D.M. The prognosis for Ailanthus altissima (Simaroubaceae; tree of heaven) as an invasive species in South Africa; insights from its performance elsewhere in the world. S. Afr. J. Bot. 2017, 112, 283–289. [Google Scholar] [CrossRef]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant ecological strategies: Some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Lusk, C.H.; Contreras, O.; Figueroa, J. Growth, biomass allocation and plant nitrogen concentration in Chilean temperate rainforest tree seedlings: Effects of nutrient availability. Oecologia 1996, 109, 49–58. [Google Scholar] [CrossRef]
- Wright, I.J.; Westoby, M. Cross-species relationships between seedling relative growth rate, nitrogen productivity and root vs. leaf function in 28 Australian woody species. Funct. Ecol. 2000, 14, 97–107. [Google Scholar] [CrossRef]
- Grime, J.P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Wright, I.J.; Westoby, M. Differences in seedling growth behaviour among species: Trait correlations across species, and trait shifts along nutrient compared to rainfall gradients. J. Ecol. 1999, 87, 85–97. [Google Scholar] [CrossRef]
- Mattson, W.J., Jr. Herbivory in relation to plant nitrogen content. Ann. Rev. Ecol. Syst. 1980, 11, 119–161. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Diaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global climatic drivers of leaf size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, W.; Zhang, Y. Differences in leaf traits of Spartina alterniflora between native and invaded habitats: Implication for evolution of alien species competitive ability increase. Ecol. Indic. 2022, 138, 108799. [Google Scholar] [CrossRef]
- Wright, I.J.; Ackerly, D.D.; Bongers, F.; Harms, K.E.; Ibarra-Manriquez, G.; Martinez-Ramos, M.; Mazer, S.J.; Muller-Landau, H.C.; Paz, H.; Pitman, N.C.A.; et al. Relationships among ecologically important dimensions of plant trait variation in seven neotropical forests. Ann. Bot. 2007, 99, 1003–1015. [Google Scholar] [CrossRef]
- Penuelas, J.; Sardans, J.; Llusia, J.; Owen, S.M.; Silva, J.; Niinemets, U. Higher allocation to low cost chemical defenses in invasive species of Hawaii. J. Chem. Ecol. 2010, 36, 1255–1270. [Google Scholar] [CrossRef]
- Henn, J.J.; Yelenik, S.; Damschen, E.I. Environmental gradients influence differences in leaf functional traits between native and non-native plants. Oecologia 2019, 191, 397–409. [Google Scholar] [CrossRef]
- Wei, M.; Wang, S.; Wu, B.D.; Jiang, K.; Zhou, J.W.; Wang, C.Y. Variability of leaf functional traits of invasive tree Rhus typhina L. in North China. J. Cent. South Univ. 2020, 27, 155–163. [Google Scholar] [CrossRef]
- Chen, Z.Z.; Ge, J.L.; Zhao, C.M.; Shen, G.Z.; Xu, W.T.; Xie, Z.Q. Leaf functional traits and correlations in three zonal forests in eastern China. Plant. Sci. J. 2020, 38, 347–359. [Google Scholar]
- Ackerly, D.; Knight, C.; Weiss, S.; Barton, K.; Starmer, K. Leaf size, specific leaf area and microhabitat distribution of chaparral woody plants: Contrasting patterns in species level and community level analyses. Oecologia 2002, 130, 449–457. [Google Scholar] [CrossRef]
- Guo, W.H.; Wang, H.; Yu, M.K.; Wu, T.G.; Han, Y. Latitude variation mechanism of leaf traits of Metasequoia glyptostroboides in eastern coastal China. Chin. J. Appl. Ecol. 2017, 28, 772–778. [Google Scholar]
- Betway, K.R.; Hollister, R.D.; May, J.L.; Oberbauer, S.F.; Botta-Dukát, Z. Species-specific trends and variability in plant functional traits across a latitudinal gradient in northern Alaska. J. Veg. Sci. 2021, 32, e13040. [Google Scholar] [CrossRef]
- Wang, R.; Yu, G.; He, N.; Wang, Q.; Zhao, N.; Xu, Z. Latitudinal variation of leaf morphological traits from species to communities along a forest transect in eastern China. J. Geogr. Sci. 2016, 26, 15–26. [Google Scholar] [CrossRef]
- Guo, Z.; Lin, H.; Chen, S.; Yang, Q. Altitudinal patterns of leaf traits and leaf allometry in bamboo Pleioblastus amarus. Front. Plant. Sci. 2018, 9, 1110. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Fan, R.; Niklas, K.J.; Zhong, Q.; Yang, F.; Li, M.; Chen, X.; Sun, M.; Cheng, D. “Diminishing returns” in the scaling of leaf area vs. dry mass in Wuyi Mountain bamboos, Southeast China. Am. J. Bot. 2017, 104, 993–998. [Google Scholar] [CrossRef]
- Sun, M.; Su, T.; Zhang, S.B.; Li, S.F.; Anberree-Lebreton, J.; Zhou, Z.K. Variations in leaf morphological traits of Quercus guyavifolia (Fagaceae) were mainly influenced by water and ultraviolet irradiation at high elevations on the Qinghai-Tibet Plateau, China. Int. J. Agric. Biol. 2016, 18, 266–273. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Jiang, K.; Liu, J. Differences in leaf functional traits and allelopathic effects on seed germination and growth of Lactuca sativa between red and green leaves of Rhus typhina. S. Afr. J. Bot. 2017, 111, 17–22. [Google Scholar] [CrossRef]
- Shi, W.; Wang, G.; Han, W. Altitudinal variation in leaf nitrogen concentration on the eastern slope of Mount Gongga on the Tibetan Plateau, China. PLoS ONE 2012, 7, e44628. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.B.; Malhi, Y.; Torres, I.C.; Metcalfe, D.B.; van de Weg, M.J.; Meir, P.; Silva-Espejo, J.E.; Huasco, W.H. Nutrient limitation in rainforests and cloud forests along a 3000-m elevation gradient in the Peruvian Andes. Oecologia 2013, 172, 889–902. [Google Scholar] [CrossRef]
- Raich, J.W.; Russell, A.E.; Vitousek, P.M. Primary productivity and ecosystem development along an elevational gradient on Mauna Loa, Hawai‘i. Ecology 1997, 78, 707–721. [Google Scholar]
- Vitousek, P.M.; Aplet, G.; Turner, D.; Lockwood, J.J. The Mauna Loa environmental matrix: Foliar and soil nutrients. Oecologia 1992, 89, 372–382. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Kerkhoff, A.J.; Swenson, N.G.; Enquist, B.J. Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. New Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef]
- Abdala-Roberts, L.; Covelo, F.; Parra-Tabla, V.; Teran, J.; Mooney, K.A.; Moreira, X. Intra-specific latitudinal clines in leaf carbon, nitrogen, and phosphorus and their underlying abiotic correlates in Ruellia Nudiflora. Sci. Rep. 2018, 8, 596. [Google Scholar] [CrossRef] [PubMed]
- Top, S.M.; Preston, C.M.; Dukes, J.S.; Tharayil, N. Climate influences the content and chemical composition of foliar tannins in green and senesced tissues of Quercus rubra. Front. Plant. Sci. 2017, 8, 423. [Google Scholar] [CrossRef]
- Kraus, T.E.C.; Dahlgren, R.A.; Zasoski, R.J. Tannins in nutrient dynamics of forest ecosystems—A review. Plant. Soil. 2003, 256, 41–66. [Google Scholar] [CrossRef]
- Malisch, C.S.; Salminen, J.P.; Kolliker, R.; Engstrom, M.T.; Suter, D.; Studer, B.; Luscher, A. Drought effects on proanthocyanidins in sainfoin (Onobrychis viciifolia Scop.) are dependent on the plant’s ontogenetic stage. J. Agric. Food. Chem. 2016, 64, 9307–9316. [Google Scholar] [CrossRef]
- Monschein, M.; Jaindl, K.; Buzimkić, S.; Bucar, F. Content of phenolic compounds in wild populations of Epilobium angustifolium growing at different altitudes. Pharm. Biol. 2015, 53, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Abdala-Roberts, L.; Galman, A.; Petry, W.K.; Covelo, F.; de la Fuente, M.; Glauser, G.; Moreira, X. Interspecific variation in leaf functional and defensive traits in oak species and its underlying climatic drivers. PLoS ONE 2018, 13, e0202548. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; DeWalt, S.J.; Siemann, E.; Rogers, W.E. Differences in cold hardiness between introduced populations of an invasive tree. Biol. Invasions 2012, 14, 2029–2038. [Google Scholar] [CrossRef]
- Frei, E.R.; Ghazoul, J.; Matter, P.; Heggli, M.; Pluess, A.R. Plant population differentiation and climate change: Responses of grassland species along an elevational gradient. Glob. Chang. Biol. 2014, 20, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Sexton, J.P.; McKay, J.K.; Sala, A. Plasticity and genetic diversity may allow saltcedar to invade cold climates in North America. Ecol. Appl. 2002, 12, 1652–1660. [Google Scholar] [CrossRef]
- Yeh, P.J.; Price, T.D. Adaptive phenotypic plasticity and the successful colonization of a novel environment. Am. Nat. 2004, 164, 531–542. [Google Scholar] [CrossRef]
- Gallagher, R.V.; Randall, R.P.; Leishman, M.R. Trait differences between naturalized and invasive plant species independent of residence time and phylogeny. Conserv. Biol. 2015, 29, 360–369. [Google Scholar] [CrossRef]
- Geng, Y.P.; Pan, X.Y.; Xu, C.Y.; Zhang, W.J.; Li, B.; Chen, J.K.; Lu, B.R.; Song, Z.P. Phenotypic plasticity rather than locally adapted ecotypes allows the invasive alligator weed to colonize a wide range of habitats. Biol. Invasions 2007, 9, 245–256. [Google Scholar] [CrossRef]
- Yu-Peng, G.; Wen-Ju, Z.; Bo, L.; Jia-Kuan, C.J.B.S. Phenotypic plasticity and invasiveness of alien plants. Biodivers. Sci. 2004, 12, 447. [Google Scholar]
- Jain, S.K.; Martins, P.S. Ecological genetics of the colonizing ability of rose clover (Trifolium hirtum ALL.). Am. J. Bot. 1979, 66, 361–366. [Google Scholar] [CrossRef]
- Barrett, S.J.E.; Richardson, H.J. Genetic Attributes of Invading Species. In Ecology of Biological Invasions; Groves, R.H., Rurdon, J.J., Eds.; Academy of Science: Canberra, Australia, 1986; pp. 21–30. [Google Scholar]
- Jones, R.C.; Nicolle, D.; Steane, D.A.; Vaillancourt, R.E.; Potts, B.M. High density, genome-wide markers and intra-specific replication yield an unprecedented phylogenetic reconstruction of a globally significant, speciose lineage of Eucalyptus. Mol. Phylogenet. Evol. 2016, 105, 63–85. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.; van der Merwe, M.; Wilson, P.G.; Kooyman, R.M.; Rossetto, M. Managing the risk of genetic swamping of a rare and restricted tree. Conserv. Genet. 2019, 20, 1113–1131. [Google Scholar] [CrossRef]
- Griffin, A.; Burgess, I.; Wolf, L. Patterns of natural and manipulated hybridisation in the genus Eucalyptus L’hérit. -1 A Review. Aust. J. Bot. 1988, 36, 41–66. [Google Scholar] [CrossRef]
- Arnold, R.; Xie, Y.; Luo, J.; Wang, H.; Midgley, S.J. A tale of two genera: Exotic Eucalyptus and Acacia species in China. 2. Plantation resource development. Int. For. Rev. 2020, 22, 153–168. [Google Scholar] [CrossRef]
- Godoy, O.; Saldaña, A.; Fuentes, N.; Valladares, F.; Gianoli, E. Forests are not immune to plant invasions: Phenotypic plasticity and local adaptation allow Prunella vulgaris to colonize a temperate evergreen rainforest. Biol. Invasions 2011, 13, 1615–1625. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends. Plant. Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).