Adaptive Trait Variation in Seedlings of Rare Endemic Mexican Spruce Provenances under Nursery Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Determination of Climatic Variables
2.3. Determination of Edaphic Variables
2.4. Weight of 1000 Seeds, Survival and Growth Rate of Seedlings
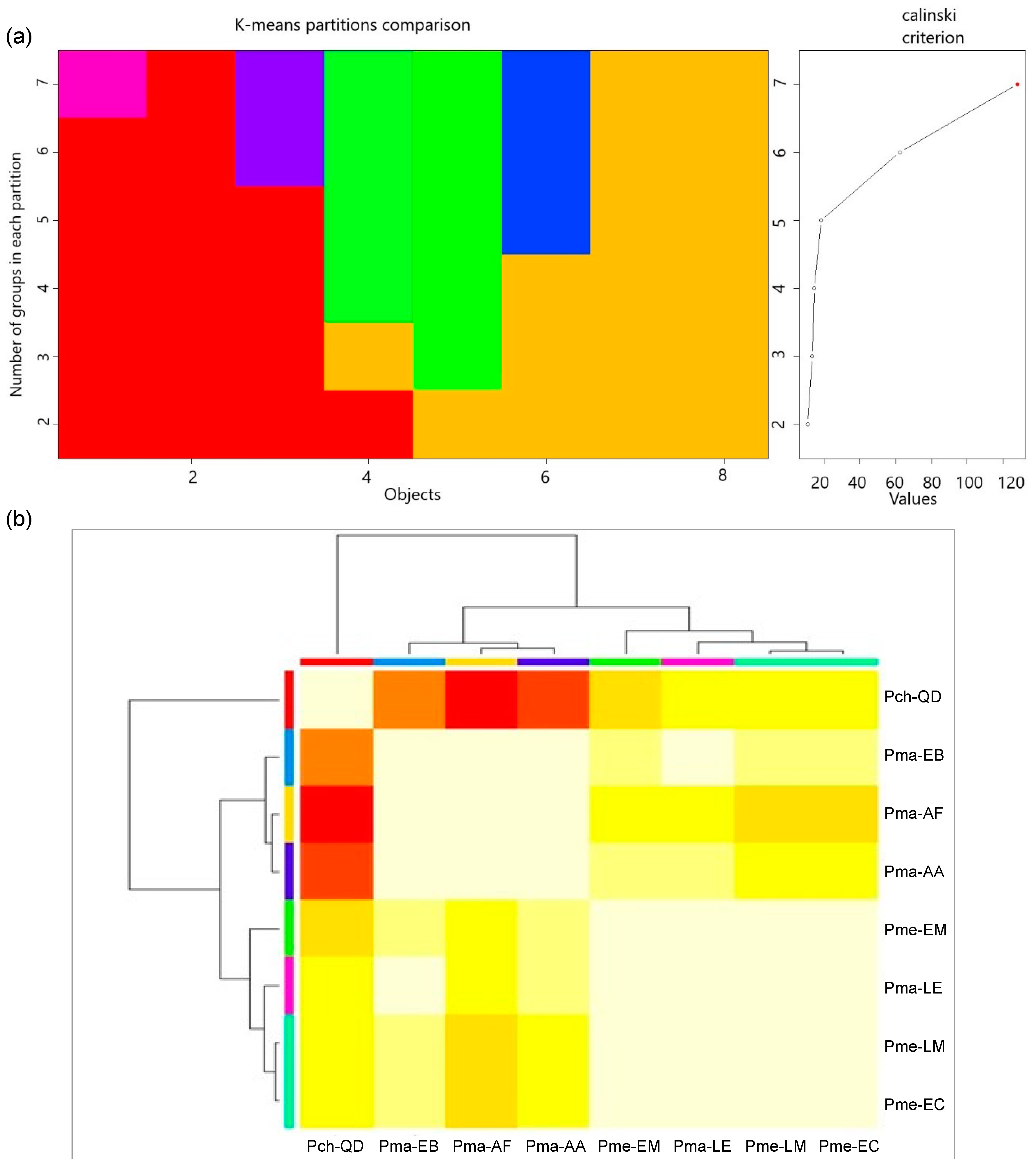
2.5. Classification by Cluster Analysis
2.6. Pairwise Multiple Comparisons of Mean Rank Sums and Spearman’s Correlations
2.7. Heritability of Picea martinezii and Picea mexicana
3. Results
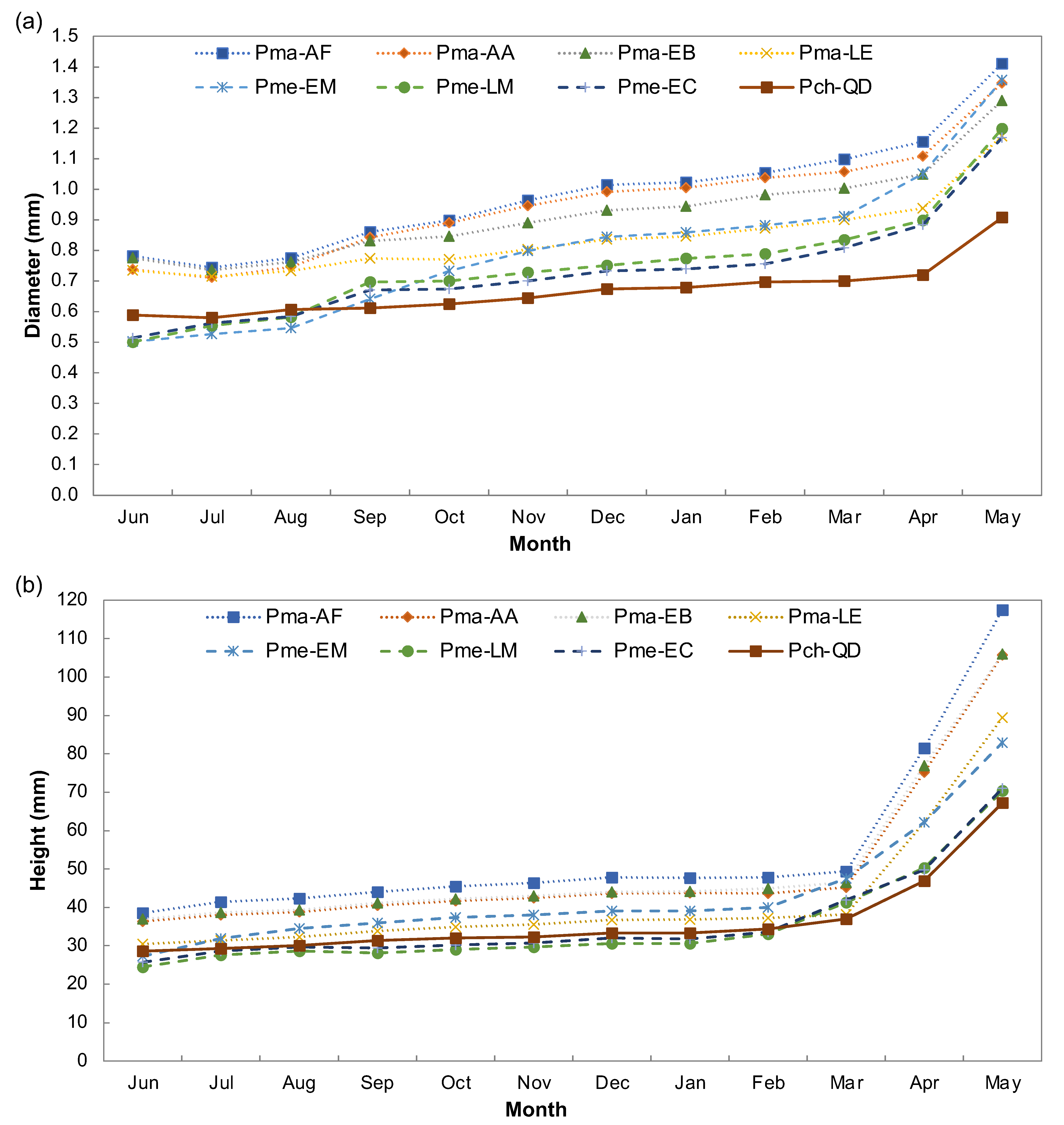
3.1. Provenance Differences in Quantitative Traits
3.2. Associations between Environmental Variables and Phenotypic Traits
3.3. Heritability (Within-Provenance) of Picea martinezii and P. mexicana Growth Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Callaham, R.Z. Provenance research: Investigation of genetic diversity associated with geography. In Unasylua; Food and Agriculture Organization of the United Nations: Rome, Italy, 1963; Volume 18, 12p. Available online: https://www.fs.usda.gov/research/treesearch/32867 (accessed on 7 April 2023).
- Gregorius, H.-R.; Bergmann, F.; Wehenkel, C. Analysis of biodiversity across levels of biological organization: A problem of defining traits. Perspect. Plant Ecol. Evol. Syst. 2003, 5, 209–218. [Google Scholar] [CrossRef]
- Wehenkel, C.; Bergmann, F.; Gregorius, H.R. Is there a trade-off between species diversity and genetic diversity in forest tree communities? Plant Ecol. 2006, 185, 151–161. [Google Scholar] [CrossRef]
- Bergmann, F.; Gregorius, H.R.; Kownatzki, D.; Wehenkel, C. Different diversity measures and genetic traits reveal different species genetic diversity relationships: A case study in forest tree communities. Silvae Genet. 2013, 62, 25–37. [Google Scholar] [CrossRef]
- Lamy, T.; Laroche, F.; David, P.; Massol, F.; Jarne, P. The contribution of species–genetic diversity correlations to the understanding of community assembly rules. Oikos 2017, 126, 759–771. [Google Scholar] [CrossRef]
- Grattapaglia, D.; Silva-Junior, O.B.; Resende, R.T.; Cappa, E.P.; Müller, B.S.F.; Tan, B.; Isik, F.; Ratcliffe, B.; El-Kassaby, Y.A. Quantitative genetics and genomics converge to accelerate forest tree breeding. Front. Plant Sci. 2018, 9, 1693. [Google Scholar] [CrossRef]
- Guries, R.P. Forest genetics and forest tree breeding. In Introduction to Forest Science; John Wiley and Sons Inc.: New York, NY, USA, 1990; pp. 98–118. [Google Scholar] [CrossRef]
- Matyas, C. Climatic adaptation of trees: Rediscovering provenance tests. Euphytica 1996, 92, 45–54. [Google Scholar] [CrossRef]
- Kramer, K.; Degen, B.; Buschbom, J.; Hickler, T.; Thuiller, W.; Sykes, M.T.; de Winter, W. Modelling exploration of the future of European beech (Fagus sylvatica L.) under climate change—Range, abundance, genetic diversity and adaptive response. For. Ecol. Manag. 2010, 259, 2213–2222. [Google Scholar] [CrossRef]
- Gömöry, D.; Longauer, R.; Hlásny, T.; Pacalaj, M.; Strmeň, S.; Krajmerová, D. Adaptation to common optimum in different populations of Norway spruce (Picea abies Karst.). Eur. J. For. Res. 2012, 131, 401–411. [Google Scholar] [CrossRef]
- Wicherts, J.M.; Johnson, W. Group differences in the heritability of items and test scores. Proc. R. Soc. B Biol. Sci. 2009, 276, 2675–2683. [Google Scholar] [CrossRef]
- O’Brien, E.K.; Manzanec, R.A.; Krauss, S.L. Provenance variation of ecologically important traits of forest trees: Implications for restoration. J. Appl. Ecol. 2007, 44, 583–593. [Google Scholar] [CrossRef]
- Roach, D.A. Variation in seed and seedling size in Anthoxanthum odoratum. Am. Midl. Nat. 1987, 117, 258–264. [Google Scholar] [CrossRef]
- Winn, A.A. Ecological and evolutionary consequences of seed size in Prunella vulgaris. Ecology 1988, 69, 1537–1544. [Google Scholar] [CrossRef]
- Biere, A. Parental effects in Lychnis flos-cuculi. I: Seed size, germination and seedling performance in a controlled environment. J. Evol. Biol. 1991, 4, 447–465. [Google Scholar] [CrossRef]
- Platenkamp, G.A.J.; Shaw, R.G. Environmental and genetic maternal effects on seed characters in Nemophila menziesii. Evolution 1993, 47, 540–555. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, M.; Liang, X.; Xu, M.; Liu, X.; Zhang, Y.; Liu, X.; Liu, J.; Gao, Y.; Qu, S.; et al. Quantitative trait loci for seed size variation in cucurbits–a review. Front. Plant Sci. 2020, 11, 304. [Google Scholar] [CrossRef]
- Wehenkel, C.; Vargas-Hernández, J.J.; Flores-López, C.; Quiñones Pérez, C.Z. Genética de las piceas en México. In Las Piceas (Picea, Pinaceae) de México; González-Elizondo, M.S., Wehenkel, C., Eds.; Comisión Nacional Forestal: Zapopan, Mexico, 2022; pp. 56–68. [Google Scholar]
- Ledig, F.T.; Mápula-Larreta, M.; Bermejo-Velázquez, B.; Reyes-Hernández, V.; Flores-López, C.; Capó-Arteaga, M.A. Locations of endangered spruce populations in Mexico and the demography of Picea chihuahuana. Madroño 2000, 47, 71–88. [Google Scholar]
- Dominguez-Guerrero, I.K.; Mariscal-Lucero, S.; Hernández-Díaz, J.C.; Heinze, B.; Prieto-Ruiz, J.A.; Wehenkel, C. Discrimination of Picea chihuahuana Martínez populations on the basis of numerous dendrometric, climatic and edaphic traits and genetic diversity. PeerJ 2017, 5, e3452. [Google Scholar] [CrossRef]
- Mendoza-Maya, E.; Gómez-Pineda, E.; Sáenz-Romero, C.; Hernández-Díaz, J.C.; López-Sánchez, C.A.; Vargas-Hernández, J.J.; Prieto-Ruíz, J.Á.; Wehenkel, C. Assisted migration and the rare endemic plant species: The case of two endangered Mexican spruces. PeerJ 2022, 10, e13812. [Google Scholar] [CrossRef]
- Lockwood, J.D.; Aleksić, J.M.; Zou, J.; Wang, J.; Liu, J.; Renner, S.S. A new phylogeny for the genus Picea from plastid, mitochondrial, and nuclear sequences. Mol. Phylogenet. Evol. 2013, 69, 717–727. [Google Scholar] [CrossRef]
- Ledig, F.T.; Jacob-Cervantes, V.; Hodgskiss, P.D.; Eguiluz-Piedra, T. Recent evolution and divergence among populations of a rare Mexican endemic, Chihuahua spruce, following Holocene climatic warming. Evolution 1997, 51, 1815–1827. [Google Scholar] [CrossRef]
- Ledig, F.T.; Bermejo-Velázquez, B.; Hodgskiss, P.D.; Johnson, D.R.; Flores-López, C.; Jacob-Cervantes, V. The mating system and genic diversity in Martinez spruce, an extremely rare endemic of Mexico’s Sierra Madre Oriental: An example of facultative selfing and survival in interglacial refugia. Can. J. For. Res. 2000, 30, 1156–1164. [Google Scholar] [CrossRef]
- Ledig, F.T.; Hodgskiss, P.D.; Jacob-Cervantes, V. Genetic diversity, mating system, and conservation of a Mexican subalpine relict, Picea mexicana Martínez. Conserv. Genet. 2002, 3, 113–122. [Google Scholar] [CrossRef]
- Clausen, J.; Keck, D.; Hiesey, W. Experimental Studies on the Nature of Species. I. Effects of Varied Environments on Western North American Plants; Carnegie Institution of Washington: Washington, DC, USA, 1940. [Google Scholar]
- Kim, E.; Donohue, K. Local adaptation and plasticity of Erysimum capitatum to altitude: Its implications for responses to climate change. J. Ecol. 2013, 101, 796–805. [Google Scholar] [CrossRef]
- Anderson, J.T.; Wadgymar, S.M. Climate change disrupts local adaptation and favours upslope migration. Ecol. Lett. 2020, 23, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Maya, E.; Espino-Espino, J.; Quiñones-Pérez, C.Z.; Flores-López, C.; Wehenkel, C.; Vargas-Hernández, J.J.; Sáenz-Romero, C. Propuesta de conservación de tres especies mexicanas de Picea en peligro de extinción. Rev. Fitotec. Mex. 2015, 38, 235–247. [Google Scholar] [CrossRef]
- Ledig, F.T.; Hodgskiss, P.D.; Krutovskii, K.V.; Neale, D.B.; Eguiluz-Piedra, T. Relationships among the Spruces (Picea, Pinaceae) at Southwestern North America. Syst. Bot. 2004, 29, 275–295. [Google Scholar] [CrossRef]
- Rehfeldt, G.E. A Spline Model of Climate for the Western United States; General Technical Report RMRS-GTR-165; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2004. [Google Scholar] [CrossRef]
- Hutchinson, M.F. Continent-wide data assimilation using thin plate smoothing splines. In Data Assimilation Systems; Jasper, E.D., Ed.; Meteorology: Melbourne, Australia, 1991; pp. 104–113. Available online: https://www.researchgate.net/publication/247765032_The_Application_of_Thin_Plate_Smoothing_Splines_to_Continent-Wide_Data_Assimilation (accessed on 7 April 2023).
- Hutchinson, M.F. ANUSPLIN, Version 4.3; Centre for Resource and Environmental Studies: St Michael, Barbados; The Australian National University: Canberra, Australia, 2004.
- Sáenz-Romero, C.; Guzmán-Reyna, R.R.; Rehfeldt, G.E. Altitudinal genetic variation among Pinus oocarpa populations in Michoacán, Mexico: Implications for seed zoning, conservation, tree breeding and global warming. For. Ecol. Manag. 2006, 229, 340–350. [Google Scholar] [CrossRef]
- Castellanos, J.Z.; Uvalle-Bueno, J.X.; Aguilar-Santelises, A. Manual de Interpretación de Análisis de Suelos y Agua; Instituto para la Innovación Tecnológica en la Agricultura: Celaya, Mexico, 2000; 226p. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate (No. 939); US Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Baker, A.S. Colorimetric determination of nitrate in soil and plant extracts with brucine. J. Agric. Food Chem. 1967, 15, 802–806. [Google Scholar] [CrossRef]
- León, A.R.; Aguilar, A.S. Materia orgánica. In Análisis Químico para Evaluar la Fertilidad del Suelo; Aguilar, A., Etchevers, J.D., Castellanos, J.Z., Eds.; Publicación Especial No. 1; Sociedad Mexicana de la Ciencia del Suelo: Chapingo, Mexico, 1987; pp. 85–91. [Google Scholar]
- Vázquez-Alarcón, A.; Aguilar-Noh, A. Prácticas del Curso Química de Suelos; Universidad Autónoma Chapingo, Departamento de Suelos: Chapingo, México, 2020; 125p, Available online: https://www.academia.edu/41715851/QU%C3%8DMICA_DE_SUELOS_MANUAL_DE_PR%C3%81CTICAS (accessed on 8 April 2023).
- Mualem, Y. A new model for predicting the hydraulic conductivity of unsaturated porous media. Water Resour. Res. 1976, 12, 513–522. [Google Scholar] [CrossRef]
- Herbert, V.F. Prácticas de Relaciones Agua-Suelo-Planta-Atmósfera; Universidad Autónoma Chapingo: Chapingo, Mexico, 1992; 167p. [Google Scholar]
- Bodenhofer, U.; Palme, J.; Melkonian, C.; Kothmeier, A. APCluster: An R package for affinity propagation clustering. Bioinformatics 2022, 27, 2463–2464. Available online: https://academic.oup.com/bioinformatics/article/27/17/2463/224402 (accessed on 7 April 2023). [CrossRef]
- Hartigan, J.A.; Wong, M.A. Algorithm AS 136: A k-means clustering algorithm. Applied Statistics. J. R. Stat. Soc. Ser. C (Appl. Stat.) 1979, 28, 100–108. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier Science B.V.: Amsterdam, The Netherlands, 1998. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. Available online: http://www.R-project.org/ (accessed on 7 April 2023).
- Pohlert, T.; Pohlert, M.T. Package ‘pmcmr’. R Package Version; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Hauke, J.; Kossowski, T. Comparison of values of Pearson’s and Spearman’s correlation coefficients on the same sets of data. Quaest. Geogr. 2011, 30, 87–93. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R News 2002, 2, 18–22. Available online: https://CRAN.R-project.org/doc/Rnews/ (accessed on 7 April 2023).
- Wickham, H.; Chang, W.; Wickham, M.H. Package ‘ggplot2’. Computer Software Manual. 2013. Available online: http://cran.r-project.org/web/packages/ggplot2/ggplot2.pdf (accessed on 4 September 2019).
- SAS Institute. SAS/STAT Guide for Personal Computers; SAS Institute: Cary, NC, USA, 1985; 378p, Available online: https://books.google.com.mx/books/about/SAS_STAT_Guide_for_Personal_Computers_Ve.html?id=EY4pAQAAMAAJ&redir_esc=y (accessed on 7 April 2023).
- Stroup, W.W.; Milliken, G.A.; Claassen, E.A.; Wolfinger, R.D. SAS System for Mixed Models; SAS Institute: Cary, NC, USA, 2018; Available online: https://support.sas.com/content/dam/SAS/support/en/books/sas-for-mixed-models-an-introduction/68787_excerpt.pdf (accessed on 7 April 2023).
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics; Addison Wesley Longman Limited: Harlow, UK, 1989. Available online: https://vulms.vu.edu.pk/Courses/GEN733/Downloads/Introduction%20to%20Quantitative%20Genetic-DS%20Falconer.pdf (accessed on 7 April 2023).
- Sorensen, F.C.; White, T.L. Effect of natural inbreeding on variance structure in tests of wind-pollination Douglas-fir progenies. For. Sci. 1988, 34, 102–118. Available online: https://www.fs.usda.gov/research/treesearch/4986 (accessed on 7 April 2023).
- Macel, M.; Lawson, C.S.; Mortimer, S.R.; Smilauerova, M.; Bischoff, A.; Crémieux, L.; Dolezal, J.; Edwards, A.R.; Lanta, V.; Bezemer, T.M.; et al. Climate vs. soil factors in local adaptation of two common plant species. Ecology 2007, 88, 424–433. [Google Scholar] [CrossRef]
- Roach, D.A.; Wulff, R.D. Maternal effects in plants. Annu. Rev. Ecol. Syst. 1987, 18, 209–235. [Google Scholar] [CrossRef]
- Rehfeldt, G.E. Adaptation of Picea engelmannii populations to the heterogeneous environments of the Intermountain West. Can. J. Bot. 1994, 72, 1197–1208. [Google Scholar] [CrossRef]
- Oleksyn, J.; Modrzýnski, J.; Tjoelker, M.G.; Zytkowiak, R.; Reich, P.; Karolewski, P. Growth and physiology of Picea abies populations from elevational transects: Common garden evidence for altitudinal ecotypes and cold adaptation. Funct. Ecol. 2002, 12, 573–590. [Google Scholar] [CrossRef]
- Islam, M.A.; Macdonald, S.E. Ecophysiological adaptations of black spruce (Picea mariana) and tamarack (Larix laricina) seedlings to flooding. Trees 2004, 18, 35–42. [Google Scholar] [CrossRef]
- Andersen, U.S.; Prado Córdova, J.; Nielsen, U.; Olsen, C.; Nielsen, C.; Sørensen, M.; Kollmann, J. Conservation through utilization: A case study of the vulnerable Abies guatemalensis in Guatemala. Oryx 2008, 42, 206–213. [Google Scholar] [CrossRef]
- Castellanos-Acuña, D.; Vance-Borland, K.W.; St. Clair, J.B.; Hamann, A.; López-Upton, J.; Gómez-Pineda, E.; Ortega-Rodríguez, J.M.; Sáenz-Romero, C. Climate-based seed zones for Mexico: Guiding reforestation under observed and projected climate change. New For. 2018, 49, 297–309. [Google Scholar] [CrossRef]
- Konôpková, A.; Pšidová, E.; Kurjak, D.; Stojnić, S.; Petrík, P.; Fleischer, P., Jr.; Kučerová, J.; Ježík, M.; Petek, A.; Gömöry, D.; et al. Photosynthetic performance of silver fir (Abies alba) of different origins under suboptimal growing conditions. Funct. Plant Biol. 2020, 47, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Petrík, P.; Grote, R.; Gömöry, D.; Kurjak, D.; Petek-Petrik, A.; Lamarque, L.J.; Sliacka Konôpková, A.; Mukarram, M.; Debta, H.; Fleischer, P., Jr. The Role of Provenance for the Projected Growth of Juvenile European Beech under Climate Change. Forests 2022, 14, 26. [Google Scholar] [CrossRef]
- Pickles, B.J.; Twieg, B.D.; O’Neill, G.A.; Mohn, W.W.; Simard, S.W. Local adaptation in migrated interior Douglas-fir seedlings is mediated by ectomycorrhizas and other soil factors. New Phytol. 2015, 207, 858–871. [Google Scholar] [CrossRef]
- Loha, A.; Tigabu, M.; Teketay, D.; Lundkvist, K.; Fries, A. Provenance variation in seed morphometric traits, germination, and seedling growth of Cordia africana Lam. New For. 2006, 32, 71–86. [Google Scholar] [CrossRef]
- Leal-Sáenz, A.; Waring, K.M.; Menon, M.; Cushman, S.A.; Eckert, A.; Flores-Rentería, L.; Hernández-Díaz, J.C.; López-Sánchez, C.A.; Martínez-Guerrero, J.H.; Wehenkel, C. Morphological differences in Pinus strobiformis across latitudinal and elevational gradients. Front. Plant Sci. 2020, 11, 559697. [Google Scholar] [CrossRef]
- Núñez-Farfán, J.; Careaga, S.A.; Fornoni, J.; Ruiz-Montoya, L.; Valverde, P. La evolución de la plasticidad fenotípica. Rev. Espec. Cienc. Químico-Biol. 2003, 6, 16–24. [Google Scholar]
- Bucholz, E.R.; Waring, K.M.; Kolb, T.E.; Swenson, J.K.; Whipple, A.V. Water relations and drought response of Pinus strobiformis. Can. J. For. Res. 2020, 50, 905–916. [Google Scholar] [CrossRef]
- Ghalambor, C.K.; McKay, J.K.; Carroll, S.P.; Reznick, D.N. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 2007, 21, 394–407. [Google Scholar] [CrossRef]


| Species | Code | Location Municipality, State | Population Size | Latitude (N) | Longitude (W) | Elevation (m) | N |
|---|---|---|---|---|---|---|---|
| Picea chihuahuana | Pch-QD | Quebrada de los Durán, Chiqueros, Guanaceví, Durango | 2628 | 26°08′48″ | 106°22′53″ | 2570 | 10 |
| Picea martinezii | Pma-AF | Agua Fría, Aramberri, Nuevo León | 2769 | 24°02′17″ | 99°42′39″ | 1820 | 20 |
| Pma-AA | Agua Alardín, Aramberri, Nuevo León | 84,498 | 24°02′34″ | 99°44′04″ | 2120 | 30 | |
| Pma-EB | El Butano, Ejido La Trinidad, Montemorelos, Nuevo León | 1253 | 25°10′41″ | 100°07′37″ | 2180 | 30 | |
| Pma-LE | La Encantada, Ejido La Encantada, Zaragoza, Nuevo León | 712 | 23°53′24″ | 99°47′30″ | 2378 | 12 | |
| Picea mexicana | Pme-EM | El Mohinora, Ejidos El Tule y Portugal, Guadalupe y Calvo, Chihuahua | 11,383 | 25°11′55″ | 100°21′52″ | 3113 | 30 |
| Pme-LM | La Marta, Felipe de la Peña, Arteaga, Coahuila | 17,728 | 25°57′41″ | 107°02′32″ | 3494 | 29 | |
| Pme-EC | El Coahuilón, Ejido Nuncio, Arteaga, Coahuila | 2253 | 25°14′51″ | 100°21′17″ | 3528 | 28 |
| Climatic Variable | Minimum | Maximum | Mean | SE * | |
|---|---|---|---|---|---|
| Long | Longitude (degree) | −99.71 | −107.04 | −101.69 | 0.75 |
| Lat | Latitude (degrees) | 20.71 | 26.14 | 24.39 | 0.26 |
| Elev | Elevation (m) | 1820 | 3528 | 2644 | 223.00 |
| Mat | Mean annual temperature (°C) | 8.5 | 15.6 | 12.4 | 0.70 |
| Map | Mean annual precipitation (mm) | 649 | 1106 | 826 | 32.00 |
| Gsp | Growing season precipitation, April to September (mm) | 485 | 790 | 630 | 21.00 |
| Mtcm | Mean temperature in the coldest month (°C) | 3.7 | 11.6 | 8.5 | 0.80 |
| Mmin | Mean minimum temperature in the coldest month (°C) | −5.9 | 4.5 | 1.1 | 1.00 |
| Mtwm | Mean temperature in the warmest month (°C) | 12.5 | 18.5 | 15.4 | 0.80 |
| Mmax | Mean maximum temperature in the warmest month (°C) | 19.4 | 25.6 | 23.0 | 0.83 |
| Sday | Julian date of the last spring freezing (days) | 39 | 184 | 89 | 14.22 |
| Fday | Julian date of the first autumn freezing (days) | 268 | 348 | 302 | 11.29 |
| Ffp | Length of the frost-free period (days) | 74 | 298 | 200 | 25.93 |
| Dd5 | Degree-days above 5 °C | 1540 | 3873 | 2766 | 259.20 |
| Gsdd5 | Degree-days above 5 °C in the frost-free period | 489 | 3408 | 2058 | 339.60 |
| D100 | Julian date when the sum of degree-days above 5 °C reaches 100 | 15 | 71 | 31.5 | 4.38 |
| DD0 | Degree-days below 0 °C (based on mean monthly temperature) | 0 | 45 | 7.5 | 1.60 |
| Mmindd0 | Degree-days below 0 °C (based on mean minimum monthly temperature) | 29 | 1162 | 316 | 91.84 |
| Smrpb | Summer precipitation balance: (Jul + Aug + Sep)/(Apr + May + Jun) | 1.29 | 4.77 | 2.39 | 0.32 |
| Smrsprpb | Summer/Spring precipitation balance: (Jul + Aug)/(Apr + May) | 1.26 | 13.14 | 4.84 | 1.32 |
| Sprp | Spring precipitation (Apr + May) (mm) | 26 | 129 | 94 | 11.36 |
| Smrp | Summer precipitation (Jul + Aug) (mm) | 163 | 486 | 275 | 20.57 |
| Winp | Winter precipitation (Nov + Dec + Jan + Feb) (mm) | 76 | 228 | 113 | 8.88 |
| Soil Variable | Minimum | Maximum | Mean | SE * | |
|---|---|---|---|---|---|
| EC | Electrical conductivity (dS/m) | 0.47 | 1.07 | 0.84 | 0.07 |
| NO3 | Nitrate (kg/ha) | 86 | 628 | 261 | 63 |
| P | Phosphorus (mg/kg) | 9.76 | 75.74 | 37.47 | 7.11 |
| OM | Organic matter (%) | 5.2 | 24.7 | 13.5 | 2.6 |
| %CaCO3 | Calcium carbonate (%) | 0 | 1.18 | 0.28 | 0.20 |
| %Sat | Percent saturation (%) | 48.0 | 104.2 | 70.7 | 6.7 |
| %Sand | Sand (%) | 22.2 | 61.4 | 44.1 | 5.6 |
| %Silt | Silt (%) | 25.3 | 58 | 38.7 | 4.1 |
| %Clay | Clay (%) | 7.6 | 26.6 | 17.1 | 2.1 |
| Den | Density (g/cm3) | 0.54 | 7.80 | 1.66 | 0.93 |
| pH | pH | 4.59 | 7.62 | 6.06 | 0.42 |
| Ca | Calcium (mg/kg) | 180 | 10,624 | 4784 | 1129 |
| Mg | Magnesium (mg/kg) | 123 | 528 | 263 | 49 |
| Na | Sodium (mg/kg) | 40.8 | 86.5 | 56.5 | 2.6 |
| K | Potassium (mg/kg) | 666 | 2475 | 1574 | 163 |
| Fe | Iron (mg/kg) | 31.2 | 521 | 234 | 52 |
| Zn | Zinc (mg/kg) | 3.0 | 118.1 | 19.7 | 14.9 |
| Mn | Manganese (mg/kg) | 18.9 | 171.7 | 59.0 | 17.5 |
| Cu | Copper (mg/kg) | 0.16 | 4.67 | 3.31 | 0.25 |
| CEC | Cation exchange capacity (meq/100 g soil) | 11.4 | 56.7 | 27.7 | 5.8 |
| HC | Hydraulic conductivity (cm/h) | 3.7 | 137.1 | 29.7 | 16.3 |
| Species | Provenance Code | SW (SE) * (g) | PN ** | No. of Seeds Sown | Emerged Seedlings (%) | Survival after 12 Months (%) |
|---|---|---|---|---|---|---|
| Picea chihuahuana | Pch-QD | 8.3 (NA) | NA | 93 | 93.5 | 79.2 |
| P. martinezii | Pma-AF | 24.4 (0.013) | 20 | 618 | 86.4 | 96.7 |
| Pma-AA | 22.0 (0.0065) | 30 | 930 | 90.8 | 97.6 | |
| Pma-EB | 23.0 (0.014) | 30 | 930 | 89.5 | 93.5 | |
| Pma-LE | 20.0 (0.00027) | 12 | 375 | 82.9 | 93.7 | |
| P. mexicana | Pme-EM | 4.9 (0.00072) | 30 | 929 | 95.5 | 87.6 |
| Pme-LM | 5.1 (0.00072) | 29 | 899 | 91.4 | 90.4 | |
| Pme-EC | 4.9 (0.003) | 28 | 867 | 94.8 | 91.3 |
| Pro * | D (mm) | H (mm) | Pma- AF | Pma-AA | Pma- EB | Pma- LE | Pme- EM | Pme- LM | Pme-EC |
|---|---|---|---|---|---|---|---|---|---|
| Pch-QD | 0.9 | 67 | 5, 50 (2 × 10−16 7.2 × 10−14) | 4, 38 (2 × 10−16 9.2 × 10−14) | 4, 39 (8.2 × 10−14 1.1 × 10−13) | 3, 22 (1.1 × 10−7 2.2 × 10−5) | 5, 15 (7.2 × 10−14 0.003) | 3, 3 (1 × 10−10 0.996) | 3, 4 (1.1 × 10−8 0.996) |
| Pma-AF | 1.4 | 117 | 1, 12 (0.008 0.001) | 1, 11 (1.1 × 10−11 0.0006) | 2, 28 (2 × 10−16 7.9 × 10−14) | 0, 35 (1.9 × 10−5 2 × 10−16) | 2, 47 (2 × 10−16 2 × 10−16) | 2, 46 (2 × 10−16 2 × 10−16) | |
| Pma-AA | 1.4 | 105 | 0, 1 (0.0007 1.000) | 1, 16 (5.9 × 10−14 1.5 × 10−11) | 1, 23 (0.7568 2 × 10−16) | 1, 35 (1.8 × 10−14 2 × 10−16) | 1, 34 (2 × 10−16 2 × 10−16) | ||
| Pma-EB | 1.3 | 106 | 1, 17 (2.6 × 10−9 6.8 × 10−11) | 1, 24 (0.1625 2 × 10−16) | 1, 36 (9.2 × 10−11 2 × 10−16) | 1, 35 (6.5 × 10−14 2 × 10−16) | |||
| Pma-LE | 1.2 | 89 | 2, 7 (8.2 × 10−14 0.172) | 0, 19 (0.916 9.5 × 10−14) | 0, 18 (1.00 9.6 × 10−14) | ||||
| Pme-EM | 1.4 | 82 | 2, 12 (6.6 × 10−14 5.3 × 10−13) | 2, 11 (1.2 × 10−14 5.8 × 10−13) | |||||
| Pme-LM | 1.2 | 70 | 0, 1 (0.681 1.000) | ||||||
| Pme-EC | 1.2 | 71 |
| Phenotypes | Variables * | rs | p Value | |
|---|---|---|---|---|
| Including Pch—QD | Height | O.M. | 0.90 | 0.0045 |
| Smrp | −0.83 | 0.011 | ||
| Elev | −0.83 | 0.015 | ||
| SW | 0.78 | 0.027 | ||
| Survival | Mmin | 0.98 | 0.00008 | |
| Long | −0.96 | 0.0001 | ||
| Ffp | 0.95 | 0.0011 | ||
| Fday | 0.95 | 0.0011 | ||
| Smrpb | −0.92 | 0.0011 | ||
| Sday | −0.93 | 0.0022 | ||
| Mtcm | 0.93 | 0.0022 | ||
| Mmindd0 | −0.88 | 0.0022 | ||
| Height | 0.81 | 0.021 | ||
| Seed weight | Elev | −0.90 | 0.004 | |
| Mat | 0.90 | 0.0045 | ||
| Smrp | −0.87 | 0.0045 | ||
| Mtwm | 0.90 | 0.0045 | ||
| Dd5 | 0.90 | 0.0045 | ||
| Map | −0.85 | 0.0072 | ||
| Smrsprpb | −0.84 | 0.0093 | ||
| Smrpb | −0.84 | 0.0093 | ||
| Height | 0.72 | 0.027 | ||
| Excluding Pch—QD | Height | %Silt | −0.99 | 0.00001 |
| Elev | −0.96 | 0.0027 | ||
| SW | 0.89 | 0.012 | ||
| Mmax | 0.85 | 0.016 | ||
| Potassium | 0.85 | 0.016 | ||
| O.M | 0.86 | 0.023 | ||
| Seed weight | Smrp | −0.954 | 0.0008 | |
| Smrpb | −0.900 | 0.0056 | ||
| Smrsprpb | −0.928 | 0.0067 | ||
| %Silt | −0.86 | 0.011 | ||
| Map | −0.89 | 0.012 | ||
| Mmax | 0.85 | 0.016 | ||
| Mtcm | 0.86 | 0.023 | ||
| Mat | 0.86 | 0.023 | ||
| Elev | −0.86 | 0.023 | ||
| D100 | −0.86 | 0.023 |
| Species | Seedling Growth Trait | Percentage of Provenance-Related Variation * | h2i | h2f |
|---|---|---|---|---|
| Picea martinezii | Diameter | 14.5 | 0.19 ± 0.55 | 0.64 |
| Height | 13.4 | 0.21 ± 0.35 | 0.65 | |
| Picea mexicana | Diameter | 8.1 | 0.47 ± 0.72 | 0.83 |
| Height | 14.9 | 0.55 ± 0.60 | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wehenkel, C.; Torres-Valverde, J.M.; Hernández-Díaz, J.C.; Mendoza-Maya, E.; Carrillo-Parra, A.; Solis-González, S.; López-Upton, J. Adaptive Trait Variation in Seedlings of Rare Endemic Mexican Spruce Provenances under Nursery Conditions. Forests 2023, 14, 790. https://doi.org/10.3390/f14040790
Wehenkel C, Torres-Valverde JM, Hernández-Díaz JC, Mendoza-Maya E, Carrillo-Parra A, Solis-González S, López-Upton J. Adaptive Trait Variation in Seedlings of Rare Endemic Mexican Spruce Provenances under Nursery Conditions. Forests. 2023; 14(4):790. https://doi.org/10.3390/f14040790
Chicago/Turabian StyleWehenkel, Christian, José Marcos Torres-Valverde, José Ciro Hernández-Díaz, Eduardo Mendoza-Maya, Artemio Carrillo-Parra, Santiago Solis-González, and Javier López-Upton. 2023. "Adaptive Trait Variation in Seedlings of Rare Endemic Mexican Spruce Provenances under Nursery Conditions" Forests 14, no. 4: 790. https://doi.org/10.3390/f14040790
APA StyleWehenkel, C., Torres-Valverde, J. M., Hernández-Díaz, J. C., Mendoza-Maya, E., Carrillo-Parra, A., Solis-González, S., & López-Upton, J. (2023). Adaptive Trait Variation in Seedlings of Rare Endemic Mexican Spruce Provenances under Nursery Conditions. Forests, 14(4), 790. https://doi.org/10.3390/f14040790






