Abstract
Rates of biodiversity loss remain high, threatening the life support system upon which all human life depends. In a case study, a novel biodiversity composite index (BCI) in line with the Convention on Biological Diversity is established in Tyrol, Austria, based on available national forest inventory and forest typing data. Indicators are referenced by ecological modeling, protected areas, and unmanaged forests using a machine learning approach. Our case study displays an average biodiversity rating of 57% out of 100% for Tyrolean forests. The respective rating for ecosystem diversity is 49%; for genetic diversity, 53%; and for species diversity, 71%. Coniferous forest types are in a more favorable state of preservation than deciduous and mixed forests. The BCI approach is transferable to Central European areas with forest typing. Our objective is to support the conservation of biodiversity and provide guidance to regional forest policy. BCI is useful to set restoration priorities, reach conservation targets, raise effectiveness of financial resources spent on biodiversity conservation, and enhance Sustainable Forest Management.
1. Introduction
Biodiversity loss is one of the greatest ecological challenges of our time [1] Biodiversity plays a crucial role in biological processes, provision of ecosystem services, and stability of forest ecosystems [2,3,4,5]. With current rates of biodiversity loss [6,7], forest multifunctionality and productivity are decreasing at an accelerating rate [8].
Evaluating biodiversity is a highly complex task [9,10]. Additionally, biodiversity indicators are still criticized for poor indicator–indicandum relationships [11,12,13,14]. Following Heink and Kowarik [15], an indicator is of major relevance for a given issue, e.g., assessment of a specific impact for conservation policy (tree diameter and age classes), while an indicandum is the indicated phenomenon (old-growth forests). Although the relationship to the indicandum may not be fully understood yet, we will refer to these metrics as “indicators” in the following.
Due to weak correlations with the indicandum, indicator species concepts have not been successful [12,13], while concepts for forest genetic monitoring are missing in Europe [16]. Policymakers, forest managers, and scientists are facing severe knowledge gaps while having to decide which and how to choose and aggregate biodiversity indicators [17,18,19,20] as well as defining baselines.
Structures, processes, and taxonomic groups are currently used as ecological indicators [15]. Our study applies metrics of structural diversity relevant to forest biodiversity based on scientific evidence. Structural diversity concepts indicate potential habitat quality, niche differentiation, structural complexity [7], and other sources of forest biodiversity [18], e.g., for umbrella species [21] and bird species [22]. There is broad scientific evidence for positive relationships between measures of forest structural variety and elements of biodiversity [23,24,25].
On large spatial and temporal scales, the availability of reliable data sets is a limiting factor for biodiversity assessments and monitoring [9,10]. Without sound biodiversity monitoring and reporting systems, natural resources get overexploited or marginalized in decision-making [26]. Gaps in biodiversity monitoring may contribute to the lack of success in biodiversity policy implementation [16]. This may be one of the main reasons why, despite international conventions and large financial efforts [27], current rates of biodiversity loss remain high, threatening the life support system upon which all human life depends [28].
There are three biodiversity indicator sets internationally accepted, developed by the European Environment Agency, Biodiversity Indicators Partnership, and Ministerial Conference on the Protection of Forests in Europe. All of them cannot be used to judge, compare, or predict consequences of forest management for forest biodiversity at the regional level.
Understanding ecological impacts of forest management practices on biodiversity and associated ecosystem processes is essential for developing Sustainable Forest Management approaches [29,30]. Some forest ecosystem services can work in synergy whereas others, such as biodiversity and intensive timber production, are hardly compatible [31]. This policy–policy conflict is one of the most acknowledged trade-offs related to forest management [32,33,34]. Sustainable Forest Management is characterized by taking consequences of operational decisions for biodiversity into consideration [35], which is very difficult to achieve for forest enterprises. Unambiguous and practical concepts to define and measure forest biodiversity relevant to scale and purpose are needed [36,37].
Selecting appropriate indicators is particularly challenging using forest inventory data which originally were designed for forest resource management purposes [18]. Main impacts of forest management on forest biodiversity are changes in forest structure, species composition [38,39], and forest genetic resources. It is therefore reasonable to monitor changes in these determinants [40,41].
Large-scale forest inventories have rarely been used for biodiversity assessments [42,43]. However, forest inventories proved their potential to overcome data deficits on large spatial and temporal scales [21,25,41,44,45]. Major advantages of inventory-based biodiversity assessments are the repeated measurements which detect temporal changes [10] at low additional costs [45,46] for a high number of attributes, forest types, sample sizes, and scales [10,41]. In the long term, changes in biodiversity may even be related to forest management [41] and forest policy measures, which makes it highly reasonable to choose biodiversity indicators based on existing forest inventory data. Forest typing models cannot solely be used for tree species selection under various climate warming scenarios. An Austrian case study demonstrates the great potential of forest typing models and machine learning for conservation planning and policy guidance.
In this study, a novel biodiversity composite index (BCI) to assess forest biodiversity of the federal autonomous province of Tyrol, Austria, is presented. BCI was created in the Interreg-project “BioΔ4” and was designed to be transferable to neighboring Central European regions in, e.g., Austria, Italy, and Germany. The basic assumption of BCI is that forests of high naturalness can maintain biodiversity best on large temporal and spatial scales. BCI targets heterogeneity and levels of diversity evolving naturally (or nature identical) at a forest stand to conserve overall forest biodiversity on a landscape scale. BCI logic structure is in line with the Convention on Biological Diversity (CBD). It follows the Convention’s internationally accepted definition of biodiversity, stating that “biodiversity is the variability among living organisms from all sources including, inter alia, terrestrial, marine and other aquatic ecosystems and the ecological complexes of which they are part; this includes diversity within species, between species, and of ecosystems” (CBD, 1992). In line with the CBD, we define ecosystems as “a dynamic complex of plant, animal and micro-organism communities and their non-living environment interacting as a functional unit.” In our case study, BCI “ecosystem diversity” indicates the variations in forest ecosystems within the geographical location Tyrol.
Our objective was to create a stand-scale biodiversity index assembled from indicators which (1) are based on available data sets, (2) are based on high scientific evidence relevant to biodiversity, and (3) equally consider ecosystem, species, and genetic diversity. The new approach can be repeated cost-efficiently in each forest inventory period.
BCI provides quantitative aggregation and simplification of ecological information which can help policy makers to implement biodiversity policies and distribute conservation funding, e.g., for ecosystem restoration. Ranking of forest types on four levels and high-resolution spatial maps of forest diversity with BCI can support decision-making in biodiversity conservation (e.g., target forest types, target regions, ecosystems, species or genetic restoration, conservation priorities, etc.) and evaluate effectiveness of financial resources spent on ecosystem restoration and Sustainable Forest Management (e.g., cost-benefit-analysis). As a minimum requirement, we recommend a future positive BCI trend on all levels as a quantitative goal for regional to national forest policy in order to halt the loss of biological diversity and meet strategic CBD targets.
2. Material
2.1. Forests of Tyrol
Tyrol has a size of 12,684 km2 and is located in the Eastern Alps. The territory is separated into two parts, namely North Tyrol and East Tyrol (Figure 1). It ranges from 500 to 3800 m above sea level and shows an inner alpine mountainous climate with subcontinental traits. The 520,000 ha of alpine coniferous forests are characterized by dense vegetation in combination with cold climate leading to acidic, thick organic soil horizons [47]. Total stock levels are about 114 M. m3 (328 m3/ha) with annual growth rates of 2.2 M. m3 [48]. With 57.6% tree species abundance, Norway spruce (Picea abies) is predominant [48].
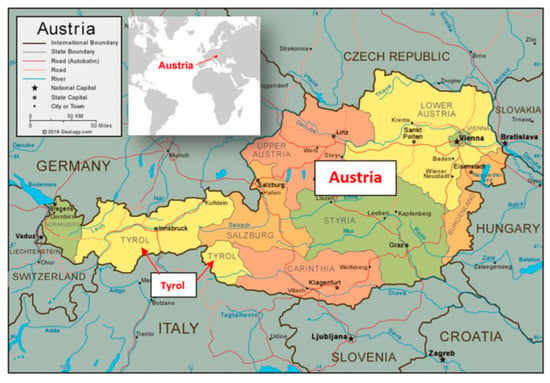
Figure 1.
Study area. Maps of the study area Tyrol, which is located in Austria (47°41′47.30″ N, 13°20′44.64″ E), Central Europe. The map was taken from https://geology.com/world/austria-satellite-image.shtml (accessed 6 March 2023).
Designated protective forests (e.g., forests protecting infrastructure and settlements from natural hazards) can be found on 48% of the total forest area [48]. Forest regeneration deficits in Tyrolean protective forests have repeatedly been reported [48,49]. Severe game impact on forest regeneration can be found on 57% of the forest area [48]. Dead wood levels account for 10.8% of the living stand volume [48].
2.2. Data Sets
This case study combines field-based measures and lidar-derived approaches using data sets provided by the Austrian Research Centre for Forests (AFI (Austrian Forest Inventory), AUPICMAP study (Geographic-genetic map of the Austrian Norway spruce population), Austrian Planting Statistics, and Nature Forest Reserves), and by the Tyrolean Regional Government (Forest typing project, vegetation surveys, TIRIS (Tyrolean Spatial Information System)). Data processing is done in R, QGIS, and python (Table 1). Reference values for the dead wood levels are supplied by protected areas, e.g., the National parks “Hohe Tauern” and “Berchtesgaden’’. For other biodiversity indicators, reference values can be found in earlier scientific studies [48,50,51,52,53].

Table 1.
Data provision and processing. Twelve biodiversity indicators are established based on data sets provided by the Austrian Research Centre for Forests, the Tyrolean Regional Government, and national park managements.
Biodiversity assessment is performed on 1162 Austrian Forest Inventory subplots. The AFI uses a permanent foursome grid sampling with a grid size of 3.89 km (1 AFI plot ≙ 4 AFI sub plots). Biodiversity indicators are assessed on the AFI subplot level. A detailed AFI field sampling manual, calculation methods, and theoretical background can be found in Hauk and Schadauer [54]. High-resolution forest typing of Tyrol based on ecological modeling was performed in 2019. Considering terrain models, geological models, climate models, expert knowledge, and field data [53], ecological modeling demarcates forest types on small scales (Figure 2A).
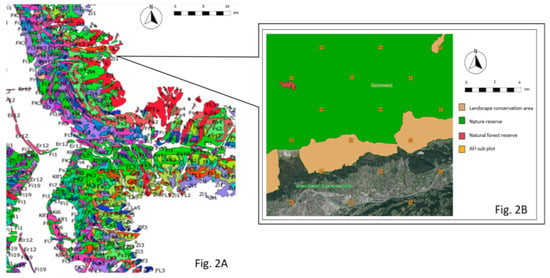
Figure 2.
Processing inventory data with QGIS (47°24′59.99″ N, 11°27′59.99″ E). (A) Excerpt of the QGIS forest typing [53]. Different colors and abbreviations indicate the forest types. (B) Forest typing data (e.g., beige and green objects) is overlaid with TIRIS (e.g., orthophoto), AFI (orange dots), and reference area (red polygon) data.
2.3. Assignment of AFI Plots to Forest Typing
Firstly, forest typing data is spatially overlaid with TIRIS, AFI, AUPICMAP, and reference area data in QGIS version 3.16 LTR (Figure 2B). Secondly, all AFI subplots outside the forest typing objects are excluded from analysis. Thirdly, if AFI subplots lay outside of the forest typing objects but contain field data; they are assigned manually to the forest type with the closest air-line distance by photo referencing (Figure 3). Biodiversity assessment of Tyrol is based on 1162 AFI subplots and 1521 vegetation survey plots. Forest inventory data and vegetation surveys are assigned to 82 forest types [53] and 223,628 QGIS objects. A total of 347 AFI subplots were excluded in the case study.
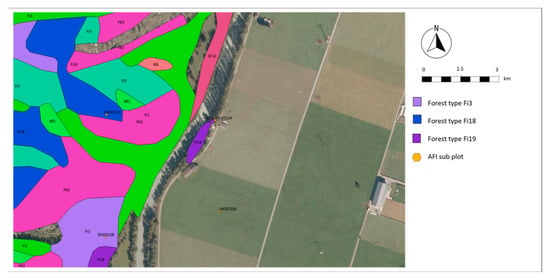
Figure 3.
Assigning AFI plots to forest types (47°24′59.99″ N, 1°27′59.99″ E). AFI plots (orange dots, numerical codes) are assigned to forest types (colored areas, alpha-numerical codes) in QGIS by position (e.g., 04303108 to Fi3) or photo referencing (e.g., 04303124 to Fi19). AFI subplots without AFI data located outside of forest type objects are excluded from analysis (e.g., 04303100).
3. Methods
Following McElhinny et al. [55], we collected all data sets available for Tyrol and the neighboring countries, quantified all stand attributes, identified a logical structure, defined a set of indicators according to the CBD definition of biodiversity, and combined these attributes into an additive biodiversity index.
Assessments of BCI can be done using one out of four levels, namely species, ecosystem, genetic, and biodiversity. In line with Grabherr et al. [50], indicators can assume ratings between zero (lowest) and 9.0 (highest) points. Following McElhinny et al. [55], outcomes are expressed as percentage (0%–100%) to ease interpretation.
On the one hand, rare but ecologically highly valuable traits (bonus indicators) may compensate for a lower level of common forest traits (biodiversity indicators). On the other hand, missing but rarely occurring forest traits are not rated disadvantageous and BCI does not benchmark against a particular scale of temporal variation [56]. Among available data sets, we favored quantitative and high-resolution measurements of high scientific value and large temporal scales in the choice between biodiversity and bonus indicators (e.g., “management constraints” is a biodiversity indicator, “planting intensity” is a bonus indicator).
3.1. Ecosystem Diversity
Ecosystem, species, and genetic diversity assessment considers three biodiversity indicators and one bonus indicator, respectively. If at least two out of four (>50%) indicators are rated, AFI subplots are included. The indication of 100% is always adapted to the maximum number of points possible under the current number of indicators at the AFI subplot (e.g., 100% = 27.0 points if three biodiversity indicators could be rated with up to 9.0 points; or 100% = 18.0 points, if two biodiversity indicators could be rated with up to 9.0 points).
assesses the deviation of the actual tree layer structure (AFI) from an expected, site-specific layer structure (forest typing). rewards differentiation of successional stages on small scales and late forest successional phases. considers dead wood quantity () and quality (). Dead wood quantity is assessed by comparing actual quantities (AFI) to reference values in protected areas and within the AFI data set (Figure 4A,B). rewards shrub layers established naturally in certain forest types, late stand ages, and large tree diameter breast heights.
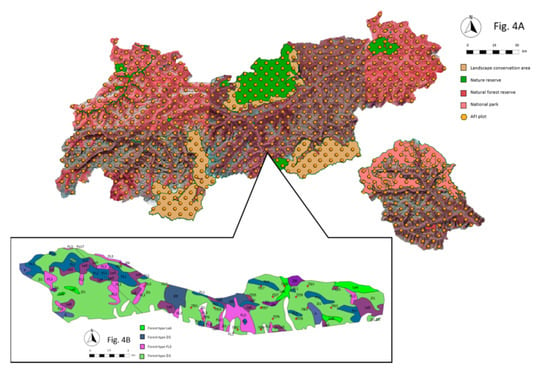
Figure 4.
Dead wood reference levels in protected areas (47°15′13.468″ N, 11°36′5.353″ E). (A) AFI plots (orange dots) within protected areas (green, beige, and salmon-colored polygons) and protected area inventories are used as reference levels for dead wood quantity . (B) In addition, nature forest reserves and protected area inventories are surveyed. Within a forest type, the respectively highest dead wood quantity out of all inventory data sets is compared to the actual subplot level.
3.2. Species Diversity
is based on a target-performance comparison between actual (AFI) and potential (forest typing data) tree species composition. evaluates the naturalness of species composition of the ground vegetation and their ecosystem disturbance indicating value. assesses if the actual humus form (AFI) deviates from the expected ones (forest typing). rewards extensive game impact on forest regeneration.
3.3. Genetic Diversity
characterizes forest gap structure by calculating a surface balance between forest and non-forest area (Figure 5). evaluates genetic diversity of the predominant tree species, Norway spruce, by computing intraspecific haplotype distance to reference populations. considers inclination and distance to forest road systems of a forest site to estimate probability of extensive forest management. evaluates the probability of tree species to contain a native gene pool by examining their planting intensity. uses varying branching types of Norway spruce as a proxy for detecting genetically allochthonous plant material.
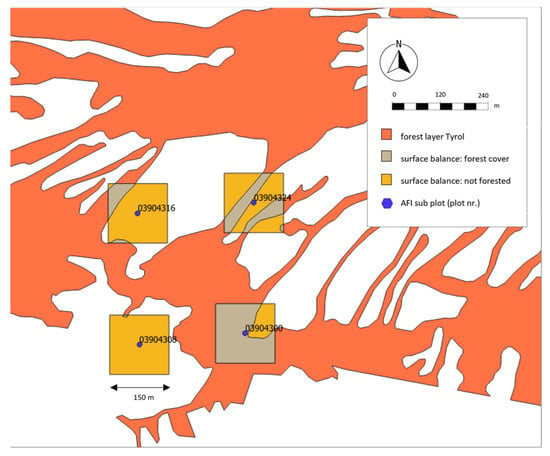
Figure 5.
Assessment of surface balance (47°15′34.7724″ N, 11°24′1.3500″ E). Squares with side length 150 m are used to compute surface balance around AFI subplots (e.g., plot nr. 03904308) between forest (light orange polygons) and non-forest area (grey polygons) in QGIS.
3.4. Biodiversity
BCI considers nine biodiversity indicators and three bonus indicators (Figure 6). Indicator ratings (0–9.0 points) are aggregated on the AFI subplot level. BCI is computed by addition of indicators and levels of diversity without weighting, which makes the concept transferable and easy to adapt. If at least six out of twelve (>50%) indicators are rated, the AFI subplots are included. Rare forest types containing less than three AFI subplots (n = 14) are not considered in BCI assessment.
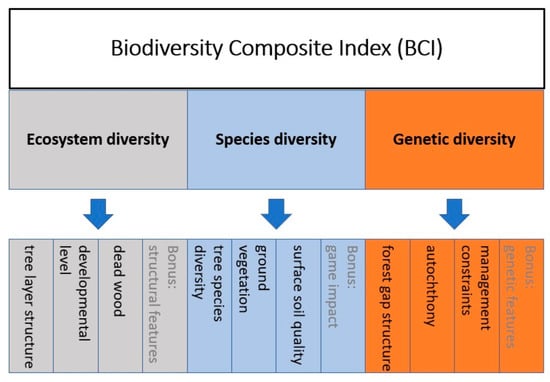
Figure 6.
BCI index structure. The modular structure equally considers ecosystem, species, and genetic diversity with three biodiversity indicators (black font color) and one bonus indicator (gray font color), respectively. Indicators are aggregated without weighting. Abbreviations are listed in the Appendix A (Table A1).
From a methodological point of view, BCI can be seen as an enhancement of the studies of Grabherr et al. [50] and Geburek et al. [57]. The framework follows classic niche theories [58,59,60] which explain co-existence of species with unique species traits and ecological niches varying in space and time. Consequently, species cannot be interchanged easily in a community. In the sense of Whittaker [61], BCI targets high beta-diversity levels to conserve overall forest biodiversity.
The choice of indicators relevant to biodiversity needs to be legitimated [15]. Scientific evidence for the relation between the diversity metric (indicator) and indicandum is provided in the Appendix A (Table A2), and detailed description of indicator evaluation can be found in the Electronic Supplementary Material. Following Virkkala [62], Brin et al. [63], and Gao et al. [14], indicators are selected from data sets available according to logical inference and by referring to other studies of high statistical validity.
3.5. Predictive Modeling with R Randomforest
In line with Bitterlich [64], Lappi and Bailey [65], and Sterba [66], evaluation outcomes are aggregated on the stratum level. With the help of the training data set (AFI subplots; n = 1162 data points with BCI ratings) and machine learning, R randomForest predicts biodiversity levels of 223,628 forest patches (QGIS polygons).
We applied the bagging classification algorithm randomForest in R, which is a group of regression trees made from random selection of samples of the training data [67]. Every random forest in this study is composed of 500 regression trees. For every regression tree in the forest, a training set is drawn from the sample plots, using bootstrap aggregating (bagging). The decision tree is built by rule-based splitting of the bagging sample into subsets, maximizing the variance between the subsets [68]. At each split in the learning process, a random subset of impact variables is used [69]. The splitting process is repeated recursively on each derived subset until (i) the subset has identical values with the target variable or (ii) the splitting no longer adds value to the prediction [70]. The mean value of the target variable within a final subset (leaf of the decision tree) is used as the conditional prediction of the target variable for a corresponding combination of impact variables [68].
For the application of a high-precision data-mining machine learning algorithm, we created polygon centroids of all forest type areas in QGIS as a prediction data set (Figure 7A). Predictive model performance is improved by adding the variables forest type (forest typing), altitude (Copernicus V1.1 DEM), geographical coordinates (TIRIS), and forest type groups (Appendix A Table A3) to the training and prediction data set. Model fit is controlled by additionally repredicting the training set and comparing prediction with R randomForest training data. The standard deviance between training and prediction data is 0%–19%. High deviances of 19% occur seldom in case extraordinary low values in the training set are repredicted.
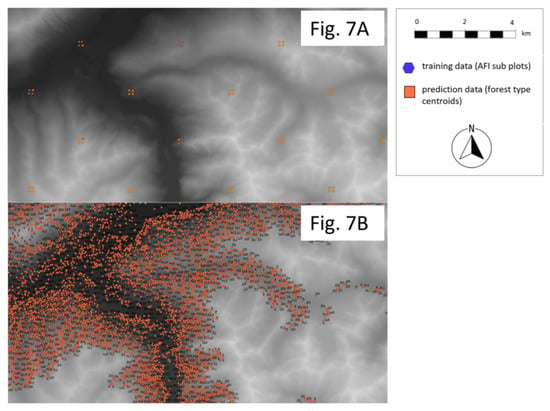
Figure 7.
The training and prediction data set in R randomForest (47°15′34.7724″ N, 11°24′1.3500″ E). (A) The training set of AFI subplots. (B) The prediction set of forest typing polygon centroids for modeling ecosystem, species, genetic diversity, and biodiversity with R randomForest.
In the next step, we assigned centroid values of the prediction set to the polygons to create maps of Tyrol (Figure 7B). Overall, we applied four prediction models, as BCI indicators can be aggregated on the level of species, ecosystem, genetic, and biodiversity. For our case study, we considered the area-weighted mean of the forest area objects in QGIS in high resolution.
To illustrate the prediction outcomes, forest area coloring was done in five classes (0%–20%, 20%–40%, 40%–60%, 60%–80%, 80%–100%) in QGIS. For the additional creation of spatial. jpg maps of Tyrolean forest diversity for the Tyrolean Regional Government, we applied cube spline interpolation in SAGA GIS. Before running the final models, we tested the model approach several times, performing probability checks using solely data of the smallest political district of Tyrol (‘Innsbruck’, forest area 37 km2).
4. Results
BCI spatial area assessments can be interpreted on the level of diversity of species and ecosystem, genetic diversity, and biodiversity. Our study displays an average biodiversity rating of 57% (area-weighted mean of forest area) for Tyrol. The respective rating of ecosystem diversity is 49%; of genetic diversity, 53%; and of species, 71% (Figure 8).
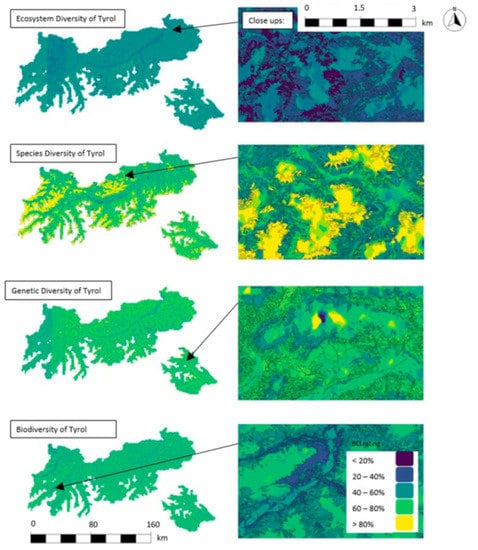
Figure 8.
Maps of forest ecosystem, species, genetics, and biodiversity (47°15′13.468″ N, 11°36′5.353″ E). High-resolution maps of forest ecosystem, species, genetics, and biodiversity of Tyrolean forests. Outcomes are displayed in five classes (0%–20%, 21%–40%, 41%–60%, 61%–80%, 81%–100%).
BCI outcomes display high spatial heterogeneity on small scale. The divergence between valleys (e.g., “Inntal”) and higher alpine areas in all models is evident through their darker coloring (i.e., lower BCI ratings). This effect is most pronounced for species diversity (Figure 8, left side).
Indicators with high average ratings in the biodiversity assessment are “autochthony” (94%), “tree layer structure” (94%), and “game impact” (91%). Indicators with low average ratings are “structural features” (19%), “management constraints” (36%), and “tree species diversity” (46%).
Indicators available most frequently at the 1507 AFI subplots studied are “forest gap structure” (1507 AFI sub plots), “forest vegetation” (1454 plots), and “structural features” (1004 plots). Indicators with low availability are “autochthony” (520 plots), “surface soil quality” (836 plots), and “tree layer structure” (943 plots).
High-altitude areas received higher BCI ratings than low elevation areas, which is in line with forest type evaluation. Surveying model outcomes on the level of the forest type (Table 2), it can be concluded that coniferous forests in Tyrol are in a more favorable state of preservation and can maintain biodiversity with higher probability than broad-leaved and mixed forests.

Table 2.
BCI evaluation outcomes on the forest type level.
High ratings indicate that the forest type is in a more favorable state of preservation and can maintain a certain aspect of biodiversity with higher probability. On the contrary, low ratings display a less favorable state of preservation. They may indicate the need for active management to conserve certain aspects of forest biodiversity.
High ecosystem diversity ratings are displayed by the forest types of “Overlay humus-carbonate larch-stone pine forest” (68%), “Subalpine dry silicate larch-spruce forest” (65%), and “Subalpine dry carbonate (larch) spruce forest” (64%). In contrast, models indicate low ecosystem diversity ratings in “Moist basic (gray alder) maple-ash mixed forest” (40%), “Colline grey alder riparian forest” (38%), and “Silicate hardwood spruce-fir forest” (37%).
In the case study, forest types of high species diversity ratings are “Subalpine coniferous avalanche sites” (100%), “Mountain pine, green alder, hardwood scrub, scrubby areas” (98%), and “Subalpine basic larch-spruce forest” (89%). Low species diversity ratings are assigned to “Warm carbonate oak-ash-lime forest” (44%), “Rich loam-deciduous beech forest” (43%), and “Fresh silicate lime-ash-pedunculate oak forest” (27%).
Our models indicate high genetic diversity ratings for “Subalpine dry silicate larch-spruce forest” (82%), “Cool carbonate steep slope larch-pine forest” (82%), and “Overlay humus-carbonate larch-stone pine forest” (78%). Low genetic diversity ratings are found in “Colline grey alder riparian forest” (49%), “Fresh clay beech forest with conifers” (49%), and “Montane grey alder riparian forest” (40%). Highest probability for autochthony of the Norway spruce populations is detected in the Central and Eastern parts of Northern Tyrol. For detailed outcomes, please consider the Supplementary Material.
Overall, high biodiversity ratings can be found in “Subalpine dry silicate larch-spruce forest” (74%), “Subalpine coniferous avalanche sites” (72%), and “Overlay humus-carbonate larch-stone pine forest” (72%). On the contrary, low biodiversity ratings are in “Colline grey alder riparian forest” (46%), “Montane grey alder riparian forest” (45%), and “Fresh silicate lime-ash-pedunculate oak forest” (42%).
5. Discussion
5.1. Approach and Biodiversity Indicator Choice
The BCI approach differs substantially from the way other authors identified, weighted, and scored indicators. As we chose indicators based on inventory data availability and scientific literature, we forwent performing a principal component analysis to test for redundancy, such as in McElhinny et al. [55] and Storch et al. [41]. In line with LaRue et al. [71] and Ette et al. [72], we expect the BCI indicators to be intercorrelated and neither ecologically nor statistically independent.
Some indicators can be a proxy for more than one level of biodiversity which, based on scientific knowledge, might seem difficult to assign, e.g., on the one hand, the availability of about 25 m3/ha of dead wood is an important quantitative threshold value for many endangered species [73,74]. On the other hand, general positive correlations between dead wood volume and wood-living fungi species, dead wood volume, and saproxylic species diversity, and between dead wood diversity and saproxylic species diversity are found for Europe in a meta-study [14]. However, this does not endanger assessment quality. In our case study, dead wood quantity is an ecological diversity indicator. Ratings are rising linearly with the share of reference levels (see Supplementary Material). If species diversity were targeted instead, ratings other than linear ones might be more appropriate.
Weighting as a final step in aggregation would have a major impact on the results. Nevertheless, respecting the limited knowledge about ecological communities, biological interactions, and genetic diversity in forests, putting weights to biodiversity indicators reveals more about the study authors and scientific community than substantially reaching an assessment that is closer to the true status of biological diversity. We agree with Okland [75] and Storch et al. [41] that indicator weighting is only reasonable for monitoring certain taxonomic groups with known correlations to specific habitat quality requirements. In line with McElhinny et al. [55], we expected weighting to probably subjoin more subjectivity to the BCI without providing additional insights.
5.2. Compare Study Outcomes
Spatial comparison within Tyrol shows that forest areas of high elevation tend to have higher BCI ratings compared to valleys in all models. Coniferous forests are in a more favorable state of preservation and can maintain biodiversity with higher probability than broad-leaved and mixed forests. In Austria, natural or semi-natural forests are mainly stocked in the subalpine, inner parts of the Alps and are characterized by a dominance of coniferous tree species [50]. In Tyrol, only 13% of the area is suitable for permanent settlement [76], which puts high pressure on ecosystems of low elevations such as broad-leaved and mixed forests. This effect is most pronounced in a species diversity model which also shows highest assessment heterogeneity on small spatial scales. BCI can be used to regionally define conservation targets, e.g., ecosystem restoration of forest types (‘Silicate hardwood spruce-fir forest”) in regions with below-average BCI performance (e.g., low elevation sites), or to regionally promote a particular level of biodiversity in a specific area (e.g., measures for ecosystem diversity such as retention trees).
It is not possible for us to directly compare our case study outcomes with other biodiversity assessments [10,41,55,77], due to unavailable indicator values in Tyrol (e.g., bark diversity, hollow trees, litter dry weight, litter decomposition, tree age, vegetation cover), different scales [57], and different study purposes [77]. However, there is partial agreement in the choice of indicators such as perennial species richness [55,77], natural regeneration [41,57,77], standing and lying dead wood [41,55,57,77], old growth trees [10,41,55,57,77], genetic diversity of Norway spruce [57], forest fragmentation [57,77], and tree species frequency [10]. Benchmarking based on vegetation types can also be found in Parkes et al. [77]. The choice of indicators in this study largely corresponds to a meta-study of Gao et al. [14], who demonstrated that the biodiversity indicators chosen most frequently in 142 European ecological studies are dead wood volume, age of canopy trees, vascular plant species, tree canopy cover, decay classes, and dead wood diversity.
5.3. Advantages and Disadvantages
Major advantages of the BCI approach are easy transferability, cost-efficient long-term monitoring of forest policy measures, and the logical indicator structure in line with the CBD. BCI can be used as a conservation planning tool to halt biodiversity loss on the national scale. With our state-of-the-art data pre- and postprocessing, BCI sticks very close to the policy-relevant definition of biodiversity in the CBD, which 183 member countries agreed on in 1992. We provide a new option to assess biodiversity based on available national forest inventory and forest typing data. Outcomes can be interpreted on three levels (diversity of ecosystem, species diversity, and genetic diversity) and aggregated to assess forest biodiversity in high resolution on varying spatial scales. By not weighting indicators, the framework remains easy to adapt to neighboring regions in Central Europe.
Quantitative aggregation and simplification of ecological spatial information may help policy makers and conservationists to implement biodiversity policies and assign conservation funding, e.g., for ecosystem restoration. The ranking of forest types and high-resolution spatial maps of forest diversity can support decision-making in biodiversity conservation (e.g., target forest types, target regions, conservation priorities, and ecosystem-, species-, or genetic restoration measures) and retrospectively evaluate effectiveness of financial resources spent on ecosystem restoration and nature conservation. Additionally, effects of different forest management measures on biodiversity can be assessed per forest type and used to advance Sustainable Forest Management. Within one forest inventory period, performing cost-benefit analyses of, e.g., biodiversity conservation efforts, forest management practices, forest road building, regional forest policy funding, and Sustainable Forest Management measures will be made available. Quantifying forest biodiversity with BCI allows targeted management of a landscape’s biodiversity and distributed biodiversity values. BCI can be used as a measurable, objective, and quantitative guidance for regional forest and conservation policy using the first BCI assessment as a baseline minimum.
However, the BCI concept could not overcome all weaknesses of forest inventory-based approaches described in Storch et al. [41], e. g., large-scale forest inventory design may not capture small areas like nature reserves well enough and very rare forest types must be excluded from the analysis. Plot measures may not be representative for the forest stand and most biodiversity aspects can only be addressed through surrogates. Additionally, most genetic diversity indicators focus on the major tree species of Tyrol, Norway spruce, as data for other species are not available. The indicators “autochthony”, “tree layer structure”, and “game impact” display high ratings in the BCI assessment. For upcoming BCI assessments, these indicator evaluations should be revised based on experience gained from the Tyrolean case study. Applying BCI, error propagation of forest typing models can possibly occur. Nevertheless, by using ecological modeling, referencing indicators by forest type, employing GIS data such as orthophotos, and machine learning, we were able to advance reliability and spatial resolution of forest biodiversity assessments.
6. Conclusions
Assessing biodiversity is highly complex. The intention of BCI is to aggregate and simplify ecological information in a surrogate approach, advance forest-inventory based assessments, and monitor all levels of forest biodiversity in line with the CBD.
In the case study, average ecosystem diversity is 49%, species diversity is 71%, genetic diversity is 53%, and biodiversity is 57%. In Tyrol, coniferous forests are in a better state of preservation and can maintain biodiversity with higher probability than broad- leaved and mixed forests. These findings, next to rankings of forest types and high-resolution spatial maps of forest biodiversity, can be used to advance land use policies, forest management, nature conservation, and landscape planning in Austria, e.g., by cost-benefit analysis. The approach is transferable to neighboring regions with forest-typing, e.g., in Germany, Italy, and Austria.
For Tyrol, we highly recommend a second BCI assessment within six years to solve the baseline problem, monitor temporal and spatial changes, detect trends in forest biodiversity, and evaluate effects of forest management and biodiversity conservation. BCI can give objective guidance and feedback to forest policy to counteract the biodiversity crisis. We recommend a future positive BCI trend on all levels as a quantitative goal for regional forest policy to meet strategic CBD targets.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14040709/s1.
Author Contributions
Conceptualization, J.-S.E.; Methodology, J.-S.E.; Software, J.-S.E. and M.S.; Validation, J.-S.E.; Formal analysis, J.-S.E.; Investigation, J.-S.E.; Resources, T.G.; Data curation, J.-S.E.; Writing—original draft, J.-S.E.; Writing—review & editing, J.-S.E.; Visualization, J.-S.E.; Supervision, T.G.; Project administration, J.-S.E. and T.G.; Funding acquisition, J.-S.E. and T.G. All authors have read and agreed to the published version of the manuscript.
Funding
Parts of the study were funded by the European Union (Interreg-project). Open access funding was provided by the Austrian Research Centre for Forests (BFW). Besides, no funding has been received.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is not publicly available due to third party policies. Restrictions apply to the availability of forest inventory data. Data was obtained from the protected area managements, the Tyrolean Regional Government, and the Austrian Research Centre for Forests.
Conflicts of Interest
The authors declare no conflict of interest.
List of Acronyms
| AFI | Austrian Forest Inventory |
| AUPICMAP | Geographic-genetic map of the Austrian Norway spruce population |
| BCI | Biodiversity composite index |
| BI | Biodiversity Indicator |
| BONUS | Bonus Indicator |
| CBD | Convention on Biological Diversity |
| TIRIS | Tyrolean Spatial Information System |
| QGIS | Quantum-Geographic Information System |
Appendix A

Table A1.
Abbreviations of indicators.
Table A1.
Abbreviations of indicators.
| Ecosystem Diversity | |
|---|---|
| Biodiversity indicator: Tree layer structure | |
| Biodiversity indicator: Developmental level | |
| Biodiversity indicator: Dead wood | |
| Biodiversity indicator: Dead wood I: Quantity | |
| Biodiversity indicator: Dead wood II: Quality | |
| Bonus indicator: Structural features | |
| Bonus indicator: Structural features I: Shrub cover | |
| Bonus indicator: Structural features II: Stand age | |
| Bonus indicator: Structural features III: Diameter breast height | |
| Species diversity | |
| Biodiversity indicator: Tree species diversity | |
| Biodiversity indicator: Ground vegetation | |
| Biodiversity indicator: Surface soil quality | |
| Bonus indicator: Game impact | |
| Genetic diversity | |
| Biodiversity indicator: Forest gap structure | |
| Biodiversity indicator: Autochthony | |
| Biodiversity indicator: Management constraints | |
| Biodiversity indicator: Management constraints I: Inclination | |
| Biodiversity indicator: Management constraints II: Distance to forest road | |
| Bonus indicator: Genetic features | |
| Bonus indicator: Genetic features I: Natural Regeneration | |
| Bonus indicator: Genetic features II: Phenology |

Table A2.
Indicative value of diversity indicators. Surrogates for forest biodiversity and scientific evidence for their indicative value.
Table A2.
Indicative value of diversity indicators. Surrogates for forest biodiversity and scientific evidence for their indicative value.
| Indicators | Scientific Evidence |
|---|---|
| Ecosystem diversity indication | |
| tree layer structure (s. s., naturalness of tree layer composition) | Structural spatial diversity increases resource partitioning of light use among species [78,79,80] and indicates the number of niches occurring vertically and horizontally within the canopy [22]. Greater overlap of crowns indicates a greater use of niche space for light in the canopy [37,81] and is a measure of ecological niche space size [71]. Tree layer structure is a proxy for forest management intensity [50]. Heterogenous vegetation heights are associated with greater ecosystem function [71]. |
| developmental level (s. s., diversity of the developmental stages) | Variation of tree dimension can be used as a proxy for habitat quality or biotope trees [10] and related macro- and microhabitats [82]. Forest age differentiation indicates high niche supply and affects community composition [83]. Late successional stages are proxies for ecosystem productivity [84], biotic resistance to invasion [85], and light absorption [80]. The developmental level can be a hint towards management intensity [50]. The indicator approach is based on mosaic cycle concepts [86,87] and niche theory [88,89]. |
| dead wood (s. s., dead wood quantity and quality) | Dead wood promotes forest biodiversity [74,90,91,92,93,94,95,96]. It provides habitat, shelter, growth substratum, and nutrition for various organisms, e.g., bryophytes, saproxylic insects, and fungi [96,97,98,99]. Coarse woody debris supports numerous forest ecosystem functions [100], e.g., nutrient cycling [101,102]. Occurrence of coarse woody debris may indicate forest ecosystem processes such as mortality, ingrowth, competition [103], and ecosystem disturbance [104]. |
| structural features (s. s., shrub cover stand age, and diameter breast height) | Structural diversity is a proxy for structural complexity, potential habitat variability, and niche differentiation for umbrella species [21,105]. The occurrence of shrub species can be an important contribution to maintain forest biodiversity [106]. Shrub and tree height is a proxy for vertical stratification of niche space [71], e.g., for birds [22]. Mean canopy height indicates the number of niches filled within the ecosystem volume [107]. Canopy tree age was found to correlate positively with epiphytic lichen diversity [14]. Large tree diameters indicate high potential for tree-related habitats [108]. |
| Species diversity indication | |
| tree species diversity (s. s., naturalness of tree species composition) | There is high scientific evidence for a positive correlation between tree species diversity and the number of bird [109], ground beetle [110,111], arthropod [112], and ground vegetation species [110,113]. Tree species abundance can be used as a proxy for species diversity of, e.g., saproxylic beetles, bryophytes, lichens, fungi, and arthropods [114,115,116,117]. |
| ground vegetation (s. s., naturalness of plant species composition, disturbance indication) | Plant species diversity indicates partitioning of resource use between species [118]. Native plant species diversity is a proxy for the number of different niche spaces filled by native plant species [119]. |
| surface soil quality (s. s., divergence from the expected humus form) | Most species diversity of Europe can be found in the soil ecosystems [16]. Humus form is one of the regulating factors for the composition of species communities [120,121,122,123]. The diversity of zoological groups linearly correlates to soil pH value and humus type [124]. There is high evidence for the relevance of humus type for forest biodiversity and overall species diversity [2,120,124,125,126]. Slight changes in physico-chemical components can lead to great changes in soil biota communities [127]. |
| Game impact (s. s., extensive game impact on forest regeneration) | There is broad consensus on the relevance of tree browsing for forest biodiversity [128,129,130]. Severe herbivore impact leads to tree species segregation, lacking regeneration, and disturbed forest succession [131]. |
| Genetic diversity indication | |
| forest gap structure (s. s., surface balance forest–non forest area) | Forest fragmentation is a serious threat to genetic diversity [132,133,134,135]. Fragmentation subdivides populations into small units and imposes barriers to migration, which is an important driver for extinction [136]. Fragmentation can erode neutral and adaptive genetic diversity and lowers effective population sizes and genetic variability [137,138]. It promotes genetic drift and inbreeding depression [139]. Habitat fragmentation may affect adaptive potential of populations and their fitness level negatively [135,140]. Susceptibility to habitat fragmentation and habitat split is highly species-specific [138]. Dispersal ability, migration, habitat availability, and range of environmental tolerance is decisive for genetic consequences for species, populations, and individuals [138,141,142]. Allelic richness is most vulnerable to habitat fragmentation with rare gene expressions preferentially being eliminated [135,139]. |
| autochthony (s. s., genetic distance between populations of Norway spruce) | Autochthonous populations show small-scale genetic differentiation and local adaption of tree species [143,144], promoting tree population differentiation [145,146]. Negative effects of allochthonous seed sources are maladaptation to the local environment, intraspecific hybridization (introgression), cryptic invasion, and other unintended effects on associated species which can be seen as environmental risks [144,147]. Genetic variability of introduced forest reproductive material tends to be considerably lower compared to local populations [148]. Artificial transfer of genetic information, e.g., by using forest reproductive material, tends to degrade forest genetic structures [149]. |
| Management constraints (s. s., inclination, and distance to forest road) | Main drivers of extinction are of anthropogenic origin [150,151]. Forest management may affect forest genetic resources through changes in genetic drift, mating systems, fertility, and species migration [147,152]. It can lead to the loss of rare and localized alleles [153,154]. Silviculture influences the major evolutionary forces of selection, genetic drift, and gene flow [136,155,156,157]. Forest management can affect mating systems, genetic variation and population structure of forest trees [158,159], lowers effective population sizes [155,160], and impacts the adaptive potential of forests [159]. |
| Genetic features (s. s., tree planting intensity, phenology of Norway spruce, and crown structure) | Choice of reproductive forest material has probably the most significant impact on the genetic diversity of forest trees in Europe [161]. Possible negative effects of long-distance seed transfer on genetic diversity are described in Kremer [147] and Carnus et al. [162]. Hybridization of local and non-local genotypes may affect genetic population structure negatively through outbreeding depression, introgression, demographic invasion, introduction of diseases, and genetic erosion [144,147]. Branching types of Norway spruce may be used as a hint to detect genetically allochthonous plant material [163]. Lower stand density affects pollen and seed dispersal positively and promotes pollen dispersal [164] and pollen densities [165,166]. Decreasing tree density is likely to increase wind turbulences, and pollen and seed long-distance dispersal [164,167,168]. |

Table A3.
Assignment to forest type groups. Assignment of forest types [53] to forest type groups [50].
Table A3.
Assignment to forest type groups. Assignment of forest types [53] to forest type groups [50].
| Forest Type Groups [50] | Forest Types Assigned [53] |
|---|---|
| Mountain pine and scrub forest communities | Bu10, Ge1, Ge8, Ge9, Lat2, Lat3, Lat4, Lh8, k |
| Carbonate-rich subalpine pine and larch forests | Fs5, Ki20, La1, La2, La4, La6, Lat1, Zi2, Zi3, Zi6 |
| Carbonate-rich montane mixed spruce-coniferous forests | Fi5, Fi6, Fi7, Fi8, Fi10, Fi13, Fi14, Fi18, Fi20, Fi23, Fi25, Fs13, FT3, FT9, FT13, FT15, FT18, FT19, FT20, LhT2 |
| Silicate-rich spruce-fir forests | Fi2, Fi3, Fi4, Fi9, Fi11, Fi19, Fi22, FT1, FT2, FT5, FT8, FT10, FT12 |
| Moist coniferous and birch forests (including bog edge forests) | Fi16, Fi17, FT7, FT22, Ki21, Fs11, Fs14, Fs18 |
| Mixed pine forests on carbonate | Ki1, Ki2, Ki3, Ki17, Ki18, Ki19, LhK3 |
| Silicate (spruce-fir) beech forests | Bu5, FT17, Ftb2, Ftb4, Ftb11, Ftb12, LhT1, TB2 |
| Mixed maple and ash forests | Bu4, Ei1, Lh4, Lh6, Lh9, Lh16, Lh17, Lh18 |
| Lime and mixed lime forests | Ei3, Ei4, Lh1, Lh2, Lh3, Lh11, Lh13, LhB2 |
| Base-rich dry beech forests | Bu3, Bu7, Fkb1, Ftb20 |
| Brown soil (spruce-fir) beech forests | Ftb3 |
| Downy oak forests | MH2 |
| Oak and oak-pine forests | Ei2, Ei7, Ei12, Ki4, Ki15 |
| Willow communities | Er4, Er11, Er12 |
| Hard riparian forests | Fi21, Ki9, Lh12, Lh14 |
| Stream-accompanying alder-ash forests | Er2, Er3, Er7, Er8 |
| Fresh carbonate (spruce-fir) beech forests | Bu1, Bu17, FT16, Ftb1, Ftb6, Ftb7, Ftb10, Ftb13, Ftb 14, Ftb16, LhB1, LhB3, TB1 |
| High-altitude beech forests with maple | Bu11, Bu20, Ftb8 |
| Subalpine coniferous forests on silicate | Fi12, Fs1, Fs2, Fs3, Fs4, Fs10, Fs12, Fs17, La5, La7, Zi1, Zi4, Zi5 |
| Gray alder forests | Er1, Er5, Lh5, Lh21, Er13 |
| Pine forests on silicate | FT21, Ki6, Ki7, Ki16, La3 |
| Carbonate-rich subalpine coniferous mixed spruce forests | Fi1, Fs6, Fs7, Fs8, Fs9, FT6 |
| Block forest, rubble, and rock sites on carbonate (newly established) | FK1, FK2, Fkb2, FL2, Lh10, Klf2, LhK2, Ki23, Zlf4 |
| Block forest, rubble, and rock sites on silicate (newly established) | FK3, Fkb3, FL1, Klf1, LhK1, Zlf3, Ki5, Lh7, FL3 |
References
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S. Planetary Boundaries: Exploring the Safe Operating Space for Humanity. Ecology and Society 14, 32. Available online: http://www.ecologyandsociety.org/vol14/iss2/art32/ (accessed on 1 March 2023).
- Hooper, D.U.; Chapin, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of Biodiversity on Ecosystem Functioning: A Consensus of Current Knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Balvanera, P.; Pfisterer, A.B.; Buchmann, N.; He, J.-S.; Nakashizuka, T.; Raffaelli, D.; Schmid, B. Quantifying the Evidence for Biodiversity Effects on Ecosystem Functioning and Services. Ecol. Lett. 2006, 9, 1146–1156. [Google Scholar] [CrossRef]
- Mace, G.M.; Norris, K.; Fitter, A.H. Biodiversity and Ecosystem Services: A Multilayered Relationship. Trends Ecol. Evol. 2012, 27, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Gamfeldt, L.; Snäll, T.; Bagchi, R.; Jonsson, M.; Gustafsson, L.; Kjellander, P.; Ruiz-Jaen, M.C.; Froberg, M.; Stendahl, J.; Philipson, C.D.; et al. Higher Levels of Multiple Ecosystem Services Are Found in Forests with More Tree Species. Nat. Commun. 2013, 4, 1340. [Google Scholar] [CrossRef]
- Reich, P.B.; Tilman, D.; Isbell, F.; Mueller, K.; Hobbie, S.E.; Flynn, D.F.B.; Eisenhauer, N. Impacts of Biodiversity Loss Escalate Through Time as Redundancy Fades. Science 2012, 336, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C. Biodiversity Loss and Its Impact on Humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Crowther, T.W.; Picard, N.; Wiser, S.; Zhou, M.; Alberti, G.; Schulze, E.-D.; McGuire, A.D.; Bozzato, F.; Pretzsch, H.; et al. Positive Biodiversity-Productivity Relationship Predominant in Global Forests. Science 2016, 354, aaf8957. [Google Scholar] [CrossRef]
- Purvis, A.; Hector, A. Getting the Measure of Biodiversity. Nature 2000, 405, 212–219. [Google Scholar] [CrossRef]
- Heym, M.; Uhl, E.; Moshammer, R.; Dieler, J.; Stimm, K.; Pretzsch, H. Utilising Forest Inventory Data for Biodiversity Assessment. Ecol. Indic. 2020, 121, 107196. [Google Scholar] [CrossRef]
- Ferris, R.; Humphrey, J.W. A Review of Potential Biodiversity Indicators for Application in British Forests. Forestry 1999, 72, 313–328. [Google Scholar] [CrossRef]
- Margules, C.; Pressey, R.L.; Williams, P.H. Representing Biodiversity: Data and Procedures for Identifying Priority Areas for Conservation. J. Biosci. 2002, 27, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Duelli, P.; Obrist, M.K. Biodiversity Indicators: The Choice of Values and Measures. Agric. Ecosyst. Environ. 2003, 98, 87–98. [Google Scholar] [CrossRef]
- Gao, T.; Nielsen, A.B.; Hedblom, M. Reviewing the Strength of Evidence of Biodiversity Indicators for Forest Ecosystems in Europe. Ecol. Indic. 2015, 57, 420–434. [Google Scholar] [CrossRef]
- Heink, U.; Kowarik, I. What Criteria Should Be Used to Select Biodiversity Indicators? Biodivers. Conserv. 2010, 19, 3769–3797. [Google Scholar] [CrossRef]
- Ette, J.S.; Geburek, T. Why European Biodiversity Reporting Is Not Reliable. AMBIO 2021, 50, 929–941. [Google Scholar] [CrossRef]
- Yoccoz, N.G.; Nichols, J.D.; Boulinier, T. Monitoring of Biological Diversity in Space and Time. Trends Ecol. Evol. 2001, 16, 446–453. [Google Scholar] [CrossRef]
- McElhinny, C.; Gibbons, P.; Brack, C.; Bauhus, J. Forest and Woodland Stand Structural Complexity: Its Definition and Measurement. For. Ecol. Manag. 2005, 218, 1–24. [Google Scholar] [CrossRef]
- Katzner, T.; Milner-Gulland, E.J.; Bragin, E. Using Modelling to Improve Monitoring of Structured Populations: Are We Collecting the Right Data? Conserv. Biol. 2007, 21, 241–252. [Google Scholar] [CrossRef]
- Jones, J.P.; Collen, B.; Atkinson, G.; Baxter, P.W.; Bubb, P.; Illian, J.B.; Katzner, T.; Keane, A.; Loh, J.; Mcdonald-Madden, E.; et al. The Why, What, and How of Global Biodiversity Indicators beyond the 2010 Target. Conserv. Biol. 2010, 25, 450–457. [Google Scholar] [CrossRef]
- Müller, J.; Pöllath, J.; Moshammer, R.; Schröder, B. Predicting the Occurrence of Middle Spotted Woodpecker Dendrocopos Medius on a Regional Scale, Using Forest Inventory Data. For. Ecol. Manag. 2009, 257, 502–509. [Google Scholar] [CrossRef]
- MacArthur, R.H.; MacArthur, J.W. On Bird Species Diversity. Ecology 1961, 42, 594–598. [Google Scholar] [CrossRef]
- Begon, M.; Harper, J.L.; Townsend, C.R. Ökologie, Individuen, Populationen und Lebensgemeinschaften [Ecology, Indi-Viduals, Populations, and Communities]; Birkhäuser Publishers: Basel, Switzerland, 1991. [Google Scholar]
- McNally, R.; Parkinson, A.; Horrocks, G.; Conole, L.; Tzaros, C. Relationships between Terrestrial Vertebrate Diversity, Abundance, and Availability of Coarse Woody Debris on South-Eastern Australian Floodplains. Biol. Conserv. 2001, 99, 191–205. [Google Scholar] [CrossRef]
- Winter, S.; Chirici, G.; McRoberts, R.E.; Hauk, E.; Tomppo, E. Possibilities for Harmonizing National Forest Inventory Data for Use in Forest Biodiversity Assessments. Int. J. Environ. Res. Public Health 2008, 81, 33–44. [Google Scholar] [CrossRef]
- Norton, B.G. Improving ecological communication: The role of ecologists in environmental policy formation. Ecol. Appl. 1998, 8, 350–364. [Google Scholar] [CrossRef]
- Waldron, A.; Miller, D.C.; Redding, D.; Mooers, A.; Kuhn, T.S.; Nibbelink, N.; Roberts, J.T.; Tobias, J.A.; Gittleman, J.L. Reductions in Global Biodiversity Loss Predicted from Conservation Spending. Nature 2017, 551, 364–367. [Google Scholar] [CrossRef]
- Science for Environment Policy: Ecosystem Services and Biodiversity; Bristol, UK. 2015. Available online: https://ec.europa.eu/environment/integration/research/newsalert/pdf/ecosystem_services_biodiversity_IR11_en.pdf (accessed on 1 March 2023).
- Kusumoto, B.; Shiono, T.; Miyoshi, M.; Maeshiro, R.; Fujii, S.; Kuuluvainen, T.; Kubota, Y. Functional Response of Plant Communities to Clearcutting: Management Impacts Differ between Forest Vegetation Zones. J. Appl. Ecol. 2015, 52, 171–180. [Google Scholar] [CrossRef]
- Henneron, L.; Aubert, M.; Bureau, F.; Dumas, Y.; Ningre, F.; Perret, S.; Richter, C.; Balandier, P.; Chauvat, M. Forest Management Adaptation to Climate Change: A Cornelian Dilemma between Drought Resistance and Soil Macro-Detritivore Functional Diversity. J. Appl. Ecol. 2015, 52, 913–927. [Google Scholar] [CrossRef]
- Pohjanmies, T.; Eyvindson, K.; Triviño, M.; Mönkkönen, M. More Is More? Forest Management Allocation at Different Spatial Scales to Mitigate Conflicts between Ecosystem Services. Landsc. Ecol. 2017, 32, 2337–2349. [Google Scholar] [CrossRef]
- Boscolo, M.; Vincent, J.R. Nonconvexities in the Production of Timber, Biodiversity, and Carbon Sequestration. J. Environ. Econ. Manag. 2003, 46, 251–268. [Google Scholar] [CrossRef]
- Duncker, P.S.; Barreiro, S.M.; Hengeveld, G.M.; Lind, T.; Mason, W.L.; Ambrozy, S.; Spiecker, H. Classification of Forest Management Approaches: A New Conceptual Framework and Its Applicability to European Forestry. Ecol. Soc. 2012, 17, 51. [Google Scholar] [CrossRef]
- Eyvindson, K.; Repo, A.; Mönkkönen, M. Mitigating Forest Biodiversity and Ecosystem Service Losses in the Era of Biobased Economy. For. Policy Econ. 2018, 92, 119–127. [Google Scholar] [CrossRef]
- UNECE; FAO. State of Europe’s Forests 2020. Status and Trends in Sustainable Forest Management in Europe; Liaison Unit Bratislava: Bratislava, Slovakia, 2020. [Google Scholar]
- Sarkar, S.; Margules, C. Operationalizing Biodiversity for Conservation Planning. J. Biosci. 2002, 27, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Williams, J. Metrics for Assessing the Biodiversity Values of Farming Systems and Agricultural Landscapes. Pac. Conserv. Biol. 2004, 10, 145–163. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Margules, C.R.; Botkin, D.B. Indicators of Biodiversity for Ecologically Sustainable Forest Management. Conserv. Biol. 2000, 14, 941–950. [Google Scholar] [CrossRef]
- Kuuluvainen, T. Forest Management and Biodiversity Conservation Based on Natural Ecosystem Dynamics in Northern Europe: The Complexity Challenge. AMBIO 2009, 38, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Taboada, Á.; Tárrega, R.; Calvo, L.; Marcos, E.; Marcos, J.A.; Salgado, J.M. Plant and Carabid Beetle Species Diversity in Relation to Forest Type and Structural Heterogeneity. Eur. J. For. Res. 2008, 129, 31–45. [Google Scholar] [CrossRef]
- Storch, F.; Dormann, C.F.; Bauhus, J. Quantifying forest structural diversity based on large-scale inventory data: A new approach to support biodiversity monitoring. J. Ecosyst. Ecography 2018, 5, 34. [Google Scholar] [CrossRef]
- Kändler, G. Bestockungsinventur Auf den Stichproben der Bodenzustandserhebung 2006 [Stocking Inventory on the Soil Condition Survey Samples 2006]. FVA Einblick 2006, 2, 11–12. [Google Scholar]
- Polley, H. Monitoring in Wäldern: Die Bundeswaldinventur und Verknüpfungen für Naturschutzfragen [Forest Monitoring: National Forest Inventory and Links for Nature Conservation Issues]; Thünen Institute for Forest Ecosystems: Braunschweig, Germany; Naturschutz und Biologische Vielfalt: Vienna, Austria, 2010; Volume 83, pp. 65–78. [Google Scholar]
- Chirici, G.; Winter, S.; McRoberts, R.E. National Forest Inventories: Contributions to Forest Biodiversity Assessments; Springer Publishers: Dordrecht, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Corona, P.; Chirici, G.; McRoberts, R.E.; Winter, S.; Barbati, A. Contribution of Large-Scale Forest Inventories to Biodiversity Assessment and Monitoring. For. Ecol. Manag. 2011, 262, 2061–2069. [Google Scholar] [CrossRef]
- Corona, P.; Köhl, M.; Marchetti, M. Advances in Forest Inventory for Sustainable Forest Management and Biodiversity Monitoring; Springer Publishers: Dordrecht, The Netherlands, 2003. [Google Scholar] [CrossRef]
- Spiecker, H. Silvicultural Management in Maintaining Biodiversity and Resistance of Forests in Europe—Temperate Zone. J. Environ. Manag. 2003, 67, 55–65. [Google Scholar] [CrossRef]
- BFW. Ergebnisse der österreichischen Waldinventur (ÖWI) 2007–2009. [Results of the Austrian Forest Inventory (ÖWI) 2007–2009]; Austrian Research Centre for Forests: Vienna, Austria, 2011; Available online: https://bfw.ac.at/030/pdf/1818_pi24.pdf (accessed on 1 March 2023).
- Government, T.R. Waldzustandsinventur [Forest Condition Inventory]. Available online: https://www.tirol.gv.at/umwelt/wald/waldzustand/waldberichte/ (accessed on 1 March 2023).
- Grabherr, G.; Koch, G.; Kirchmeir, H.; Reiter, K. Hemerobie österreichischer Waldökosysteme [Hemeroby of Austrian Forest Ecosystems]; Wagner University Press: Innsbruck, Austria, 1998. [Google Scholar]
- Raab, S.; Feller, S.; Uhl, E.; Schäfer, A.; Ohrner, G. Aktuelle Holzernteverfahren Am Hang [Temporary Forest Harvesting Techniques on Slopes]; LWF Wissen: 2002; Volume 36. Available online: https://www.lwf.bayern.de/service/publikationen/lwf_wissen/064166/index.php (accessed on 1 March 2023).
- Geburek, T.; Schweinzer, K. Simulationsstudie Fichte: Daten Des AUPICMAP Projekts [A Simulation Study on Norway Spruce: Data of the AUPICMAP Project]. Austrian Research Centre for Forests: Vienna, Austria, unpublished.
- Hotter, M.; Simon, A.; Vacik, H.; Wallner, M.; Simon, A. Waldtypisierung Tirol [Forest Typing Tyrol]; Tyrolean Regional Government: Innsbruck, Austria. Available online: https://www.tirol.gv.at/umwelt/wald/schutzwald/waldtypisierung/waldtypenhandbuch/ (accessed on 1 March 2023).
- Hauk, E.; Schadauer, K. Instruktionen für die Feldarbeiten der österreichischen Waldinventur 2007–2009 [Field Work Manual of the Austrian Forest Inventory 2007–2009]. Available online: https://www.bfw.gv.at/instruktion-feldarbeit-oesterreichische-waldinventur/ (accessed on 1 March 2023).
- McElhinny, C.; Gibbons, P.; Brack, C. An Objective and Quantitative Methodology for Constructing an Index of Stand Structural Complexity. For. Ecol. Manag. 2006, 235, 54–71. [Google Scholar] [CrossRef]
- Landres, P.B.; Morgan, P.; Swanson, F.J. Overview of the Use of Natural Variability Concepts in Managing Ecological Systems. Ecol. Appl. 1999, 9, 1179–1188. [Google Scholar] [CrossRef]
- Geburek, T.; Milasowszky, N.; Frank, G.; Konrad, H.; Schadauer, K. The Austrian Forest Biodiversity Index: All in One. Ecol. Indic. 2010, 10, 753–761. [Google Scholar] [CrossRef]
- MacArthur, R.H. Geographical Ecology: Patterns in the Distribution of Species; Princeton University Press: Princeton, NJ, USA, 1972. [Google Scholar]
- Tilman, D. Resource Competition and Community Structure; Princeton University Press: Princeton, NJ, USA, 1982. [Google Scholar]
- Gause, G.F. The Struggle for Existence; Dover Publication: London, UK, 2019. [Google Scholar]
- Whittaker, R.H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 1960, 30, 279–338. [Google Scholar] [CrossRef]
- Virkkala, R. Why study woodpeckers? The significance of woodpeckers in forest ecosystems. Ann. Zool. Fennici. 2006, 43, 82–85. Available online: https://www.jstor.org/stable/23735920 (accessed on 1 March 2023).
- Brin, A.; Meredieu, C.; Piou, D.; Brustel, H.; Jactel, H. Changes in Quantitative Patterns of Dead Wood in Maritime Pine Plantations over Time. For. Ecol. Manag. 2008, 256, 913–921. [Google Scholar] [CrossRef]
- Bitterlich, W. Die Winkelzählprobe [Angle Count Sampling]. Forstwiss. Cent. 1952, 71, 215–225. [Google Scholar] [CrossRef]
- Lappi, J.; Bailey, R.L. Estimation of the Diameter Increment Function or Other Tree Relations Using Angle-Count Samples. For. Sci. 1987, 33, 725–739. [Google Scholar] [CrossRef]
- Sterba, H. Diversity Indices Based on Angle Count Sampling and Their Interrelationships When Used in Forest Inventories. Forestry 2008, 8, 587–597. [Google Scholar] [CrossRef]
- Breiman, N.L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer Publishing: New York, NY, USA, 2002. [Google Scholar]
- Ho, T.K. The Random Subspace Method for Constructing Decision Forests. IEEE Trans. Pattern Anal. Mach. Intell. 1998, 20, 832–844. [Google Scholar] [CrossRef]
- Quinlan, J.R. Induction of Decision Trees. Mach. Learn. 1986, 1, 81–106. [Google Scholar] [CrossRef]
- LaRue, E.; Hardiman, B.; Elliott, J.; Fei, S. Structural Diversity as a Predictor of Ecosystem Function. Environ. Res. Lett. 2019, 14, 114011. [Google Scholar] [CrossRef]
- Ette, J.S.; Ritter, T.; Vospernik, S. Insights in Forest Structural Diversity Indices with Machine Learning: What Is Indicated? Biodivers. Conserv. 2023. [Google Scholar] [CrossRef]
- Bütler, R.; Schläpfer, R. Wie viel Totholz braucht der Wald [How much dead wood does a forest need]? Schweiz. Z. Forstwes. 2004, 155, 31–37. [Google Scholar] [CrossRef]
- Müller, J.; Bussler, H.; Utschick, H. Wie viel Totholz braucht der Wald? Ein wissenschaftsbasiertes Konzept gegen Artenschwund in den Totholzzönosen [How much deadwood does the forest need? A science-based concept against species loss in coenoses of dead wood]. Nat. Landsch. 2007, 39, 165–170. [Google Scholar]
- Okland, B. Unlogged Forests: Important Sites for Preserving the Diversity of Mycetophilids (Diptera: Sciaroidea). Biol. Conserv. 1996, 76, 297–310. [Google Scholar] [CrossRef]
- Government, T.R. Schutzwald in Tirol: Landesschutzwaldkonzept 2000 [Protective Forests of Tyrol: State Protection Forest Concept 2000]; Tyrolean Regional Government: Innsbruck, Austria. Available online: https://www.tirol.gv.at/umwelt/wald/schutzwald/landesschutzwaldkonzept/ (accessed on 1 March 2023).
- Parkes, D.; Greame, N.; Chea, D. Assessing the Quality of Native Vegetation: The ‘Habitat Hectares’ Approach. Ecol. Manag. Restor. 2003, 4, 29–38. [Google Scholar] [CrossRef]
- Kohyama, T. Size-Structured Tree Populations in Gap-Dynamic Forest: The Forest Architecture Hypothesis for the Stable Coexistence of Species. J. Ecol. 1993, 81, 131–143. [Google Scholar] [CrossRef]
- Yachi, S.; Loreau, J. Does complementary resource use enhance ecosystem function? A model of light competition in plant communities. Ecol. Lett. 2006, 10, 54–62. [Google Scholar] [CrossRef]
- Atkins, J.W.; Fahey, R.T.; Hardiman, B.H.; Gough, C.M. Forest Canopy Structural Complexity and Light Absorption Rela-Tionships at the Subcontinental Scale. J. Geophys. Res. Biogeosci. 2018, 123, 1387–1405. [Google Scholar] [CrossRef]
- Zheng, L.T.; Chen, H.Y.H.; Yan, E.R. Tree Species Diversity Promotes Litterfall Productivity through Crown Complementarity in Subtropical Forests. J. Ecol. 2019, 107, 1852–1861. [Google Scholar] [CrossRef]
- Larrieu, L.; Cabanettes, A.; Brin, A.; Bouget, C.; Deconchat, M. Tree Microhabitats at the Stand Scale in Montane Beech–Fir Forests: Practical Information for Taxa Conservation in Forestry. Eur. J. For. Res. 2013, 133, 355–367. [Google Scholar] [CrossRef]
- Gossner, M.M.; Schall, P.; Ammer, C.; Ammer, U.; Engel, K.; Schubert, H.; Simon, U.; Utschick, H.; Weisser, W.W. Forest Management Intensity Measures as Alternative to Stand Properties for Quantifying Effects on Biodiversity. Ecosphere 2014, 5, 113. [Google Scholar] [CrossRef]
- Hardiman, B.S.; Bohrer, G.; Gough, C.M.; Vogel, C.S.; Curtis, P.S. The Role of Canopy Structural Complexity in Wood Net Primary Production of a Maturing Northern Deciduous Forest. Ecology 2011, 92, 1818–1827. [Google Scholar] [CrossRef]
- Iannone, B.V.; Potter, K.M.; Hamil, K.A.D.; Huang, W.; Zhang, H.; Guo, Q.; Oswalt, C.M.; Woodall, C.W.; Fei, S. Evidence of Biotic Resistance to Invasions in Forests of the Eastern USA Landscape. Ecology 2016, 31, 85–99. [Google Scholar] [CrossRef]
- Remmert, H. Sukzessionen im Klimax-System [Successions in the climax system]. Verh. Ges. Okol. 1987, 16, 27–34. [Google Scholar]
- Remmert, H. Das Mosaik-Zyklus-Konzept und seine Bedeutung für den Naturschutz: Eine Übersicht [The mosaic cycle concept and its relevance to conservation: An overview]. Congr. Rep. Lauf. Semin. 1989, 5, 5–15. [Google Scholar]
- Rosenzweig, M.L. Species Diversity in Space and Time; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Chase, J.M.; Leibold, M.A. Ecological Niches: Linking Classical and Contemporary Approaches; University of Chicago Press: Chicago, IL, USA, 2003. [Google Scholar] [CrossRef]
- Ohlson, M.; Söderström, L.; Hörnberg, G.; Zackrisson, O.; Hermansson, J. Habitat Qualities versus Long-Term Continuity as Determinants of Biodiversity in Boreal Old-Growth Swamp Forests. Biol. Conserv. 1997, 81, 221–231. [Google Scholar] [CrossRef]
- Siitonen, J.; Martikainen, P.; Punttila, P.; Rauh, J. Coarse Woody Debris and Stand Characteristics in Mature, Managed and Boreal Mesic Forests in Southern Finland. For. Ecol. Manag. 2000, 128, 211–225. [Google Scholar] [CrossRef]
- Kappes, H.; Topp, W. Emergence of Coleoptera from Deadwood in a Managed Broadleaved Forest in Central Europe. Biodivers. Conserv. 2004, 13, 1905–1924. [Google Scholar] [CrossRef]
- Persiani, A.M.; Audisio, P.; Lunghini, D.; Maggi, O.; Granito, V.M.; Biscaccianti, A.B.; Chiavetta, U.; Marchetti, M. Linking Taxonomical and Functional Biodiversity of Saproxylic Fungi and Beetles in Broad-Leaved Forests in Southern Italy with Varying Management Histories. Plant Biosyst. 2010, 144, 250–261. [Google Scholar] [CrossRef]
- Rondeux, J.; Sanchez, C. Review of Indicators and Field Methods for Monitoring Biodiversity within National Forest Inventories: Core Variable Dead Wood. Environ. Monit. Assess. 2009, 164, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Brin, A.; Bouget, C.; Brustel, H.; Jactel, H. Diameter of Downed Woody Debris Does Matter for Saproxylic Beetle Assemblages in Temperate Oak and Pine Forests. J. Insect Conserv. Divers. 2011, 15, 653–669. [Google Scholar] [CrossRef]
- Lassauce, A.; Paillet, Y.; Jactel, H.; Bouget, C. Deadwood as a Surrogate for Forest Biodiversity: Meta-Analysis of Correlations between Deadwood Volume and Species Richness of Saproxylic Organisms. Ecol. Indic. 2011, 11, 1027–1039. [Google Scholar] [CrossRef]
- Harmon, M.E.; Franklin, J.F.; Swanson, F.J.; Sollins, P.; Gregory, S.V.; Lattin, J.D.; Anderson, N.H.; Cline, S.P.; Aumen, N.G.; Sedell, J.R.; et al. Ecology of Coarse Woody Debris in Temperate Ecosystems. Adv. Ecol. Res. 1986, 15, 133–302. [Google Scholar] [CrossRef]
- Blasi, C.; Marchetti, M.; Chiavetta, U.; Aleffi, M.; Audisio, P.; Azzella, M.M.; Brunialti, G.; Capotorti, G.; Del Vico, E.; Lattanzi, E.; et al. Multi-taxon and Forest Structure Sampling for Identification of Indicators and Monitoring of Old-growth Forest. Plant Biosyst. 2010, 144, 160–170. [Google Scholar] [CrossRef]
- Stokland, J.N.; Siitonen, J.; Jonsson, B.G. Biodiversity in Dead Wood; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Cornwell, W.K.; Cornelissen, J.H.C.; Allison, S.D.; Bauhus, J.; Eggleton, P.; Preston, C.M.; Scarff, F.; Weedon, J.T.; Wirth, C.; Zanne, A.E. Plant Traits and Wood Fates across the Globe: Rotted, Burned, or Consumed? Glob. Chang. Biol. 2009, 15, 2431–2449. [Google Scholar] [CrossRef]
- Litton, C.M.; Raich, J.W.; Ryan, M.G. Carbon Allocation in Forest Ecosystems. Glob. Chang. Biol. 2007, 13, 2089–2109. [Google Scholar] [CrossRef]
- Kahl, T.; Mund, M.; Bauhus, J.; Schulze, E.D. Dissolved Organic Carbon from European Beech Logs: Patterns of Input to and Retention by Surface Soil. Ecoscience 2012, 19, 364–373. [Google Scholar] [CrossRef]
- Svensson, J.S.; Jeglum, J.K. Structure and Dynamics of an Undisturbed Old-Growth Norway Spruce Forest on the Rising Bothnian Coastline. For. Ecol. Manag. 2001, 15, 67–79. [Google Scholar] [CrossRef]
- Schliemann, S.A.; Bockheim, J.G. Methods for Studying Treefall Gaps: A Review. For. Ecol. Manag. 2011, 7, 1143–1151. [Google Scholar] [CrossRef]
- Zahner, V.; Sikora, L.; Pasinelli, G. Heart Rot as a Key Factor for Cavity Tree Selection in the Black Woodpecker. For. Ecol. Manag. 2012, 271, 98–103. [Google Scholar] [CrossRef]
- Paul, M.; Hinrichs, T.; Janßen, A.; Schmitt, H.P.; Soppa, B. Forstliche Genressourcen in Deutschland: Konzepte zur Erhaltung und nachhaltigen Nutzung forstlicher Genressourcen in der Bundesrepublik Deutschland [Forest Genetic Re-sources of Germany: Concepts for Maintenance and Sustainable Use of Forest Genetic Resources in Germany]; Bundesministerium für Ernährung, Landwirtschaft und Verbraucherschutz: Bonn, Germany, 2000. [Google Scholar]
- Currie, D.J.; Mittelbach, G.G.; Cornell, H.V.; Field, R.; Guégan, F.; Hawkins, B.A.; Kaufman, D.M.; Kerr, J.T.; Oberdorff, T.; O’Brien, E.; et al. Predictions and Tests of Climate-based Hypotheses of Broad-scale Variation in Taxonomic Richness. Ecol. Lett. 2004, 7, 1121–1134. [Google Scholar] [CrossRef]
- Hilmo, O.; Holien, H.; Hytteborn, H.; Ely-Aastrup, H. Richness of Epiphytic Lichens in Differently Aged Picea abies Plantations Situated in the Oceanic Region of Central Norway. Lichenologist 2009, 41, 97–108. [Google Scholar] [CrossRef]
- Baguette, M.; Deceuninck, B.; Muller, Y. Effects of spruce afforestation on bird community dynamics in a native broad-leaved forest area. Acta Oecologica 1994, 15, 275–288. Available online: http://hdl.handle.net/2078.1/48606 (accessed on 1 March 2023).
- Fahy, O.; Gormally, M. A Comparison of Plant and Carabid Beetle Communities in Irish Oak Woodland with a Nearby Conifer Plantation and Clear-Felled Site. For. Ecol. Manag. 1998, 110, 263–273. [Google Scholar] [CrossRef]
- Magura, T.B.; Tothmeresz, M.; Bordan, Z. Effects of nature management practices on carabid assemblages (Coleoptera: Carabidae) in a non-native plantation. Biol. Conserv. 2000, 93, 95–102. [Google Scholar] [CrossRef]
- Chey, V.K.; Holloway, J.D.; Speight, M.R. Diversity of Moths in Forest Plantations and Natural Forests in Sabah. Bull. Entomol. Res. 1997, 87, 371–385. [Google Scholar] [CrossRef]
- Humphrey, J.W.; Ferris, R.; Jukes, M.R.; Peace, A.J. The Potential Contribution of Conifers Plantations to the UK Biodiversity Action Plan. Bot. J. Scotl. 2002, 54, 49–62. [Google Scholar] [CrossRef]
- Uliczka, H.; Angelstam, P. Occurrence of Epiphytic Macrolichens in Relation to Tree Species and Age in Managed Boreal Forest. Ecography 1999, 22, 396–405. [Google Scholar] [CrossRef]
- Brändle, M.; Brandl, R. Species Richness of Insects and Mites on Trees: Expanding Southwood. J. Anim. Ecol. 2001, 70, 41–504. [Google Scholar] [CrossRef]
- Berglund, H.; O’Hara, R.B.; Jonsson, B.G. Quantifying Habitat Requirements of Tree-Living Species in Fragmented Boreal Forests with Bayesian Methods. Conserv. Biol. 2009, 23, 1127–1137. [Google Scholar] [CrossRef]
- Ulyshen, M.D. Arthropod Vertical Stratification in Temperate Deciduous Forests: Implications for Conservation-Oriented Management. For. Ecol. Manag. 2011, 261, 1479–1489. [Google Scholar] [CrossRef]
- Silvertown, J. Plant coexistence and the niche. Trends Ecol. Evol. 2004, 19, 605–611. [Google Scholar] [CrossRef]
- Turnbull, L.A.; Isbell, F.; Purves, D.W.; Loreau, M.; Hector, A. Understanding the Value of Plant Diversity for Ecosystem Function through Niche Theory. Philos. Trans. R. Soc. B 2016, 283, 20160536. [Google Scholar] [CrossRef]
- Ponge, J.F. Humus Forms in Terrestrial Ecosystems: A Framework to Biodiversity. Soil Biol. Biochem. 2003, 35, 935–945. [Google Scholar] [CrossRef]
- Paquin, P.; Coderre, D. Changes in Soil Macroarthropod Communities in Relation to Forest Maturation through Three Successional Stages in the Canadian Boreal Forest. Oecologia 1997, 112, 104–111. [Google Scholar] [CrossRef]
- Peltier, A.; Ponge, J.-F.; Jordana, R.; Arino, A. Humus Forms in Mediterranean Scrublands with Aleppo Pine. Soil Sci. Soc. Am. J. 2001, 65, 884–896. [Google Scholar] [CrossRef]
- Ponge, J.F. Biocenoses of Collembola in atlantic temperate grass-woodland ecosystems. Pedobiologia 1993, 37, 223–244. [Google Scholar]
- Salmon, S.; Artuso, N.; Frizzera, L.; Zampedri, R. Relationships between Soil Fauna Communities and Humus Forms: Response to Forest Dynamics and Solar Radiation. Soil Biol. Biochem. 2008, 40, 1707–1715. [Google Scholar] [CrossRef]
- Schäfer, M.; Schauermann, J. The Soil Fauna of Beech Forests: Comparison between a Mull and a Moder Soil. Pedobiologia 1991, 34, 299–314. [Google Scholar]
- Salmon, S.; Mantel, J.; Frizzera, L.; Zanella, A. Changes in humus forms and soil animal communities in two developmental phases of Norway spruce on an acidic substrate. For. Ecol. Manag. 2006, 237, 47–56. [Google Scholar] [CrossRef]
- Cassagne, N.; Bal-Serin, M.C.; Gers, C.; Gauquelin, T. Changes in Humus Properties and Collembolan Communities Following the Replanting of Beech Forests with Spruce. Pedobiologia 2004, 48, 267–276. [Google Scholar] [CrossRef]
- Gill, R.M.A. A Review of Damage by Mammals on North Temperate Forests III: Impact on Trees and Forests. Forestry 1992, 65, 363–388. [Google Scholar] [CrossRef]
- Pastor, J.; Moen, R.A.; Cohen, Y. Spatial Heterogeneities, Carrying Capacity, and Feedbacks in Animal-Landscape Interactions. J. Mammal. 1997, 78, 1040–1052. [Google Scholar] [CrossRef]
- Reimoser, F. Steering the Impacts of Ungulates on Temperate Forest. J. Nat. Conserv. 2003, 10, 243–252. [Google Scholar] [CrossRef]
- Reimoser, F.; Reimoser, S.; Klansek, E. Wildlebensräume: Habitatqualität, Wildschadensanfälligkeit, Bejagbarkeit [Wild-Life Areas: Habitat Quality, Susceptability for Game Damage, Huntability]; Zentralstelle Österreichischer Landesjagdverbände: Vienna, Austria, 2006; Available online: https://wildlife.reimoser.info/special.php (accessed on 1 March 2023).
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Henle, K.; Lindenmayer, D.B.; Margules, C.R.; Saunders, D.A.; Wissel, C. Species Survival in Fragmented Landscapes: Where Are We Now? Biodivers. Conserv. 2004, 13, 1–8. [Google Scholar] [CrossRef]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat Fragmentation and Its Lasting Impact on Earth’s Ecosystems. Sci. Adv. 2015, 1, 1500052. [Google Scholar] [CrossRef] [PubMed]
- Dobeš, C.; Konrad, H.; Geburek, T. Potential Population Genetic Consequences of Habitat Fragmentation in Central European Forest Trees and Associated Understorey Species—An Introductory Survey. Diversity 2017, 9, 9. [Google Scholar] [CrossRef]
- Ledig, F.T. Human impacts on genetic diversity in forest ecosystems. Oikos 1992, 63, 87–108. [Google Scholar] [CrossRef]
- Johansson, M.; Primmer, C.R.; Merila, J. Does Habitat Fragmentation Reduce Fitness and Adaptability? A Case Study of the Common Frog (Rana Temporaria). Mol. Ecol. 2007, 16, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Dixo, M.; Metzger, J.P.; Morgante, J.S.; Zamudio, K.R. Habitat Fragmentation Reduces Genetic Diversity and Connectivity among Toad Populations in the Brazilian Atlantic Coastal Forest. Biol. Conserv. 2009, 142, 1560–1569. [Google Scholar] [CrossRef]
- Amos, W.; Harwood, J. Factors Affecting Levels of Genetic Diversity in Natural Populations. Philos. Trans. R. Soc. B 1998, 353, 177–186. [Google Scholar] [CrossRef]
- Sgro, C.M.; Lowe, A.J.; Hoffmann, A.A. Building Evolutionary Resilience for Conserving Biodiversity under Climate Change. Evol. Appl. 2011, 4, 326–337. [Google Scholar] [CrossRef]
- Andersen, L.W.; Fog, K.; Damgaard, C. Habitat Fragmentation Causes Bottlenecks and Inbreeding in the European Tree Frog (Hyla arborea). Proc. Biol. Sci. 2004, 271, 1293–1302. [Google Scholar] [CrossRef]
- Peakall, R.; Lindenmayer, D. Genetic Insights into Population Recovery Following Experimental Perturbation in a Fragmented Landscape. Biol. Conserv. 2006, 132, 520–532. [Google Scholar] [CrossRef]
- Kleinschmit, J.R.G.; Kownatzki, D.; Gregorius, H.R. Adaptational characteristics of autochthonous populations—Consequences for provenance delineation. For. Ecol. Manag. 2004, 197, 213–224. [Google Scholar] [CrossRef]
- Mijnsbrugge, K.; Bischoff, A.; Smith, B. A Question of Origin: Where and How to Collect Seed for Ecological Restoration. Basic Appl. Ecol. 2010, 11, 300–311. [Google Scholar] [CrossRef]
- Worrell, R. A Comparison between European Continental and British Provenances of Some British Native Trees: Growth, Survival and Stem Form. For. Int. J. For. Res. 1992, 65, 253–280. [Google Scholar] [CrossRef]
- Jones, A.T.; Hayes, M.J.; Sackville Hamilton, N. 2001 The Effect of Provenance on the Performance of Crataegus monogyna in hedges. J. Appl. Ecol. 2001, 38, 952–962. [Google Scholar] [CrossRef]
- Kremer, A. 2001 Genetic Risks in Forestry. In Risk Management and Sustainable Forestry; EFI Proceedings No. 45; EFI: Joensuu, Finland, 2001. [Google Scholar]
- Laikre, L.; Ryman, N. Effects on Intraspecific Biodiversity from Harvesting and Enhancing Natural Populations. AMBIO 1996, 25, 504–509. Available online: http://www.jstor.org/stable/4314530 (accessed on 1 March 2023).
- Jansen, S.; Konrad, H.; Geburek, T. The Extent of Historic Translocation of Norway Spruce Forest Reproductive Material in Europe. Ann. For. Sci. 2017, 74, 56–72. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global Biodiversity Scenarios for the Year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global Effects of Land Use on Local Terrestrial Biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef]
- Buiteveld, J.; Vendramin, G.G.; Leonardi, S.; Kamer, K.; Geburek, T. Genetic Diversity and Differentiation in European Beech (Fagus sylvatica L.) Stands Varying in Management History. For. Ecol. Manag. 2007, 247, 98–106. [Google Scholar] [CrossRef]
- Buchert, G.P.; Rajora, O.P.; Hood, J.V. Effects of Harvesting on Genetic Diversity in Old-Growth Eastern White Pine in Ontario, Canada. Conserv. Biol. 1997, 11, 747–758. [Google Scholar] [CrossRef]
- Rajora, O.P.; Rahnam, M.H.; Buchert, G.P.; Dancik, B.P. Microsatellite DNA Analysis of Genetic Effects of Harvesting in Old-Growth Eastern White Pine (Pinus strobus) in Ontario, Canada. Mol. Ecol. 2000, 9, 330–348. [Google Scholar] [CrossRef]
- Finkeldey, R.; Ziehe, M. Genetic implications of silvicultural regimes. For. Ecol. Manag. 2004, 197, 231–244. [Google Scholar] [CrossRef]
- Ratnam, W.; Rajora, O.P.; Finkeldey, R.; Aravanopoulos, F.; Bouvet, J.-M.; Vaillancourt, R.E.; Kanashiro, M.; Fady, B.; Tomita, M.; Vinson, C. Genetic Effects of Forest Management Practices: Global Synthesis and Perspectives. For. Ecol. Manag. 2014, 333, 52–65. [Google Scholar] [CrossRef]
- Kavaliauskas, D.; Fussi, B.; Westergren, M.; Aravanopoulos, F.; Finzgar, D.; Baier, R.; Alizoti, P.; Bozic, G.; Avramidou, E.; Konnert, M.; et al. The Interplay between Forest Management Practices, Genetic Monitoring, and Other Long-Term Monitoring Systems. Forests 2018, 9, 133. [Google Scholar] [CrossRef]
- Paffetti, D.; Travaglini, D.; Buonamici, A.; Nocentini, S.; Vendramin, G.G.; Giannini, R.; Vettori, C. The Influence of Forest Management on Beech (Fagus sylvatica L.) Stand Structure and Genetic Diversity. For. Ecol. Manag. 2012, 284, 34–44. [Google Scholar] [CrossRef]
- Aravanopoulos, F.A. Do Silviculture and Forest Management Affect the Genetic Diversity and Structure of Long-Impacted Forest Tree Populations? Forests 2018, 9, 355. [Google Scholar] [CrossRef]
- Oliver, T.H.; Heard, M.S.; Isaac, N.J.B.; Roy, D.B.; Procter, D.; Eigenbrod, F.; Freckleton, R.P.; Hector, A.L.; Orme, C.D.L.; Petchey, O.; et al. Biodiversity and resilience of ecosystem functions. Trends Ecol. Evol. 2015, 30, 673–684. [Google Scholar] [CrossRef]
- Geburek, T.; Konrad, H. Why the Conservation of Genetic Resources Has Not Worked. Conserv. Biol. 2008, 22, 267–274. [Google Scholar] [CrossRef]
- Carnus, J.M.; Parrotta, J.; Brockerhoff, E.; Arbez, M.; Jactel, H. Planted Forests and Biodiversity. J. For. 2006, 104, 65–77. [Google Scholar] [CrossRef]
- Geburek, T.; Robitschek, K.; Milasowszky, N. A Tree of Many Faces: Why Are There Different Crown Types in Norway Spruce (Picea abies [L.] Karst.)? Flora 2008, 203, 126–133. [Google Scholar] [CrossRef]
- Hardy, J.O. How Fat Is the Tail? Heredity 2009, 103, 437–438. [Google Scholar] [CrossRef]
- Smouse, P.E.; Sork, V.L. Measuring Pollen Flow in Forest Trees: An Exposition of Alternative Approaches. For. Ecol. Manag. 2004, 197, 21–38. [Google Scholar] [CrossRef]
- Bacles, C.F.E.; Burczyk, J.; Lowe, A.J.; Ennos, R.A. Historical and Contemporary Mating Patterns in Remnant Populations of the Forest Tree Fraxinus excelsior L. Evolution 2005, 59, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Digiovanni, F.; Kevan, P.G. Factors Affecting Pollen Dynamics and Its Importance to Pollen Contamination: A Review. Ca-Nadian J. For. Res. 1991, 21, 1155–1170. [Google Scholar] [CrossRef]
- Salzer, K. Wind- and Bird-Mediated Gene Flow in Pinus Cembra: Effects on Spatial Genetic Structure and Potential Close-Relative Inbreeding. Ph.D. Thesis, University of Zurich, Zurich, Switzerland, 2011. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



