The Effects of Monthly Rainfall and Temperature on Flowering and Fruiting Intensities Vary within and among Selected Woody Species in Northwestern Ethiopia
Abstract
1. Introduction
2. Materials and Methods
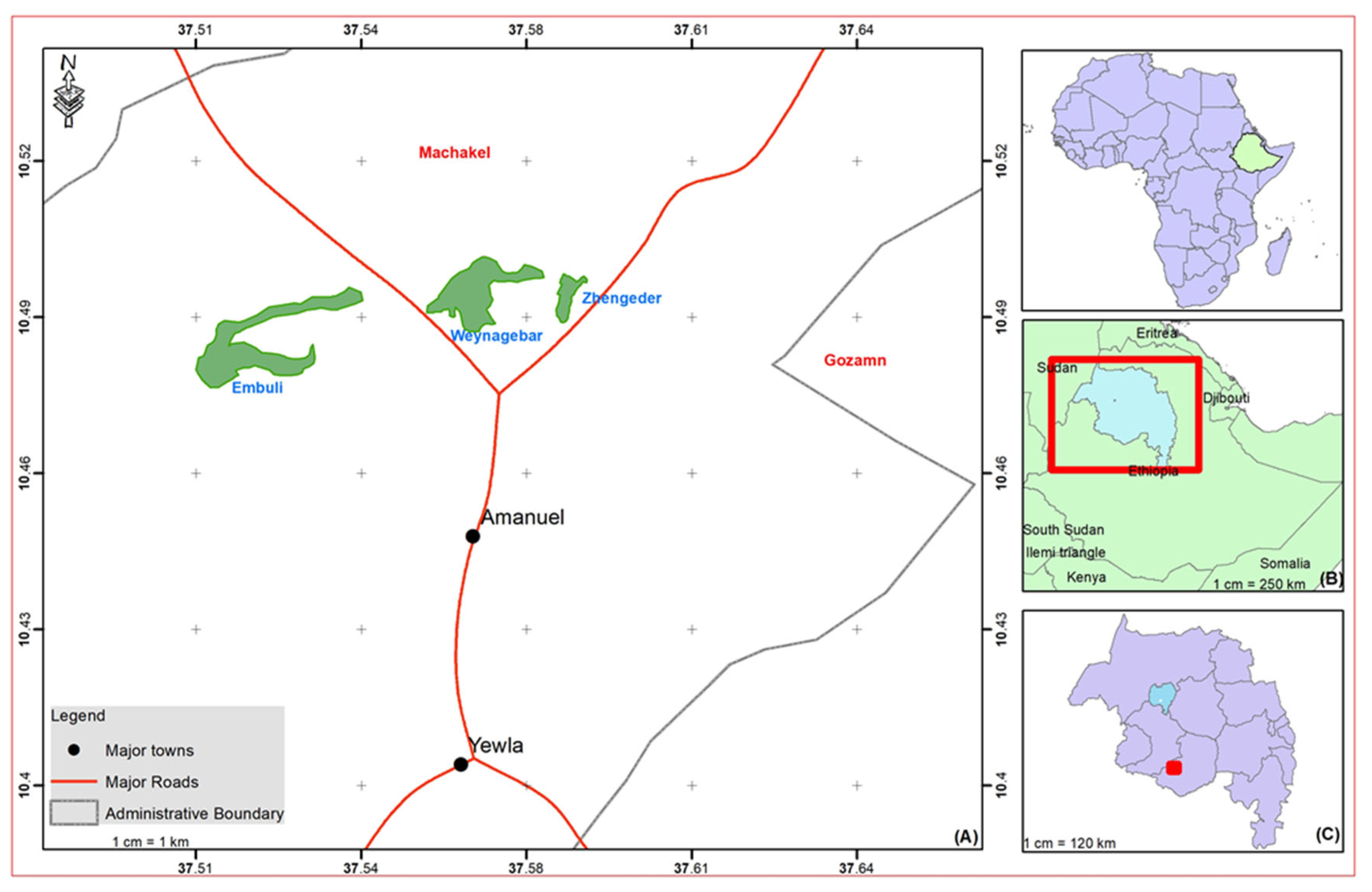
2.1. Study Area
2.2. Study Species
2.3. Data Collection
2.4. Data Analyses
3. Results
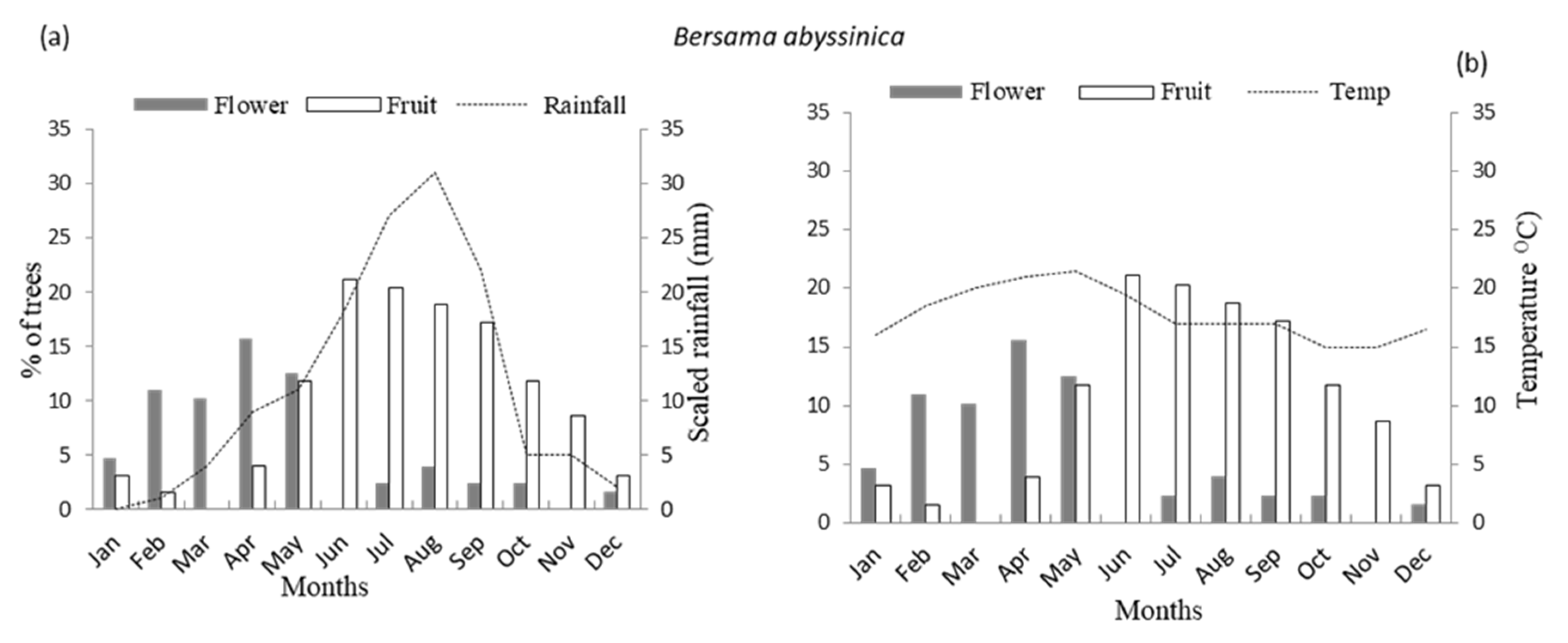
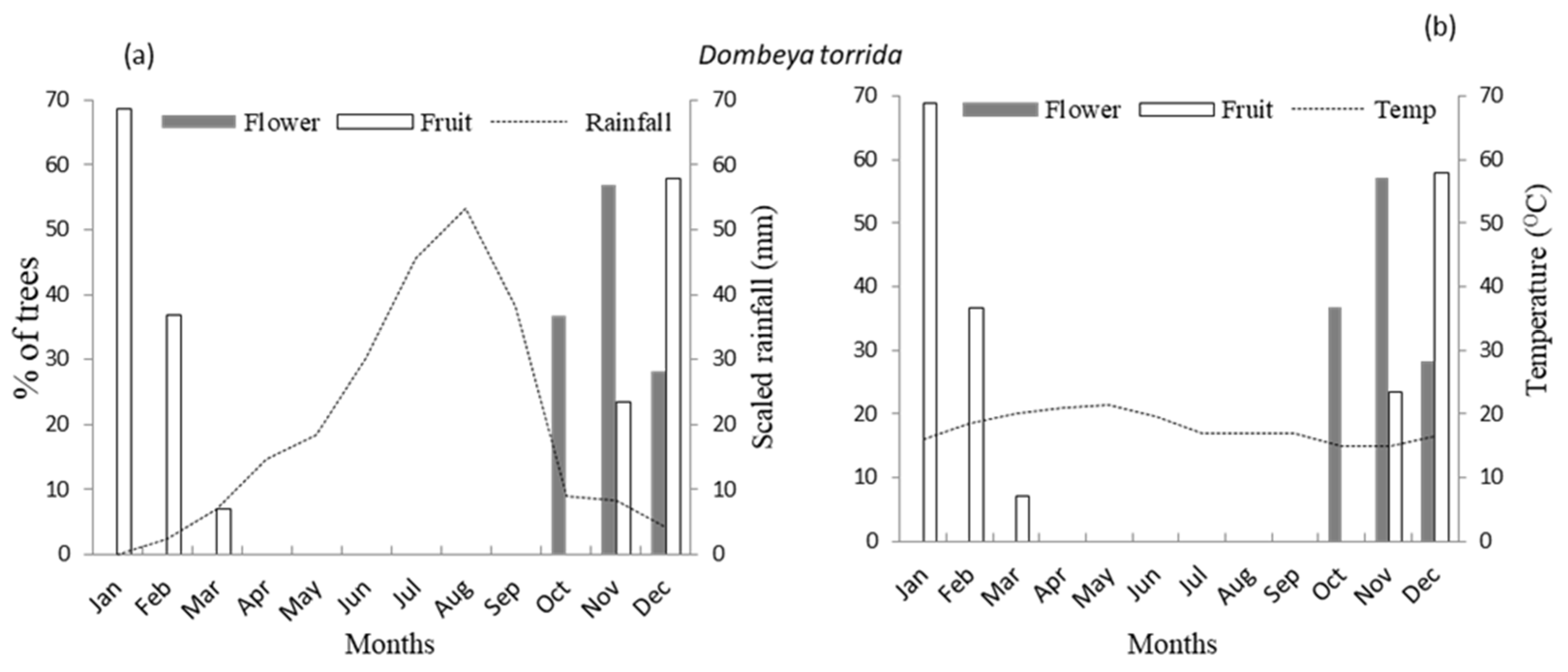
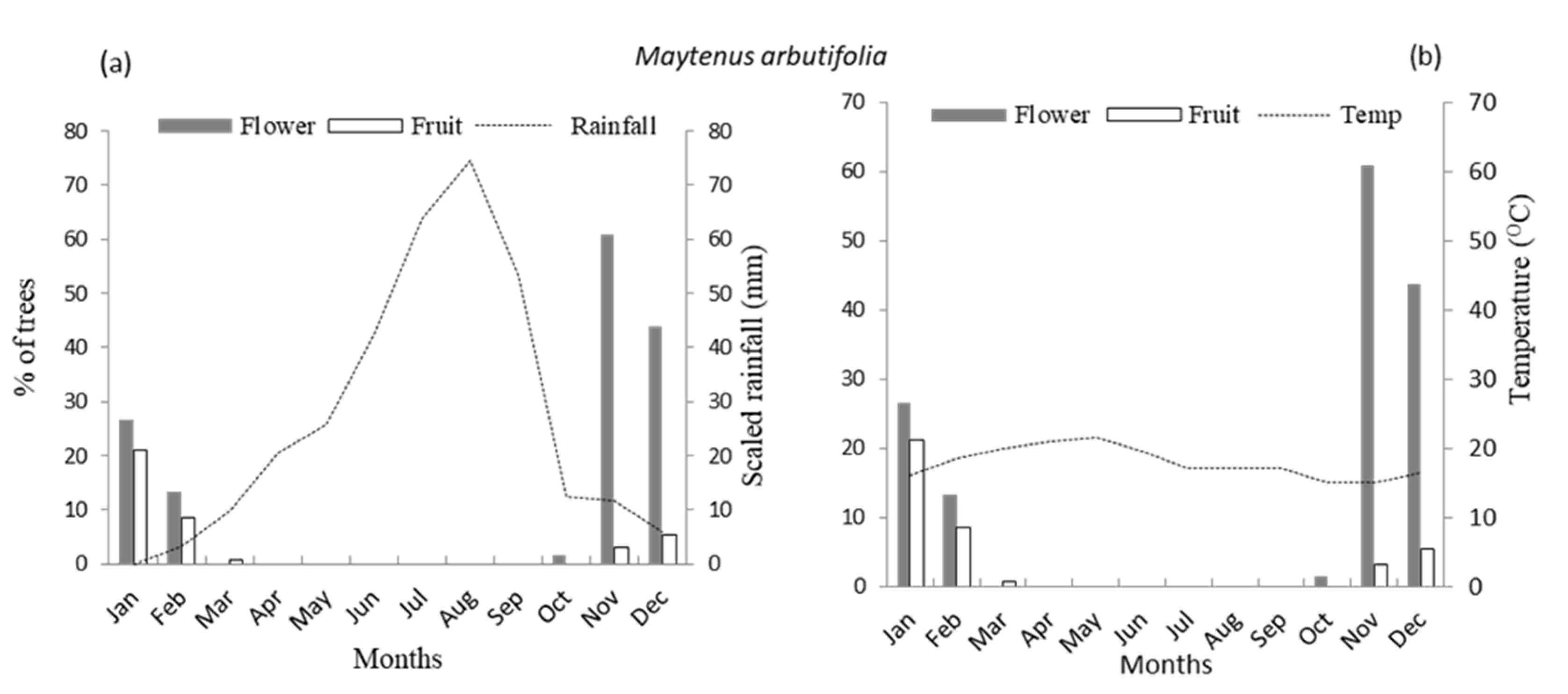
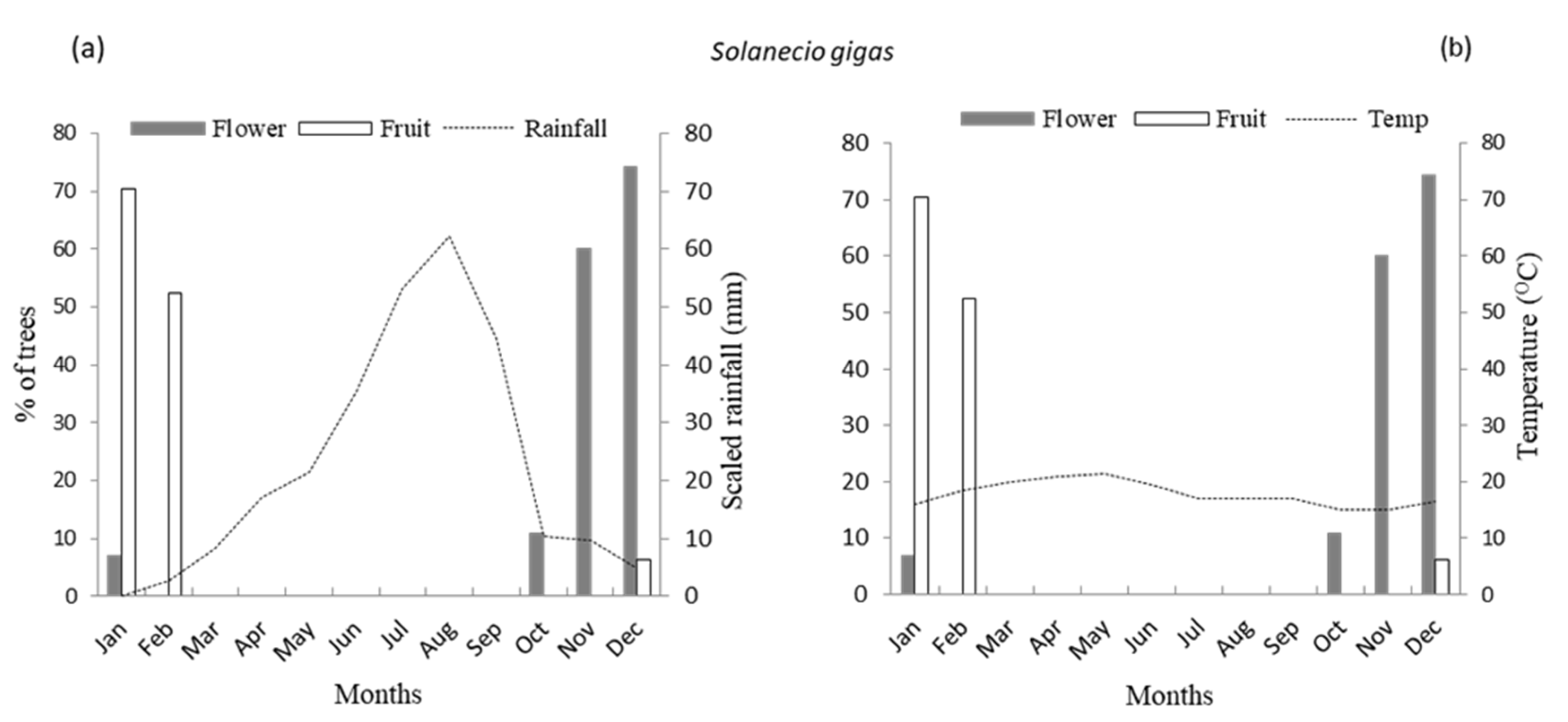
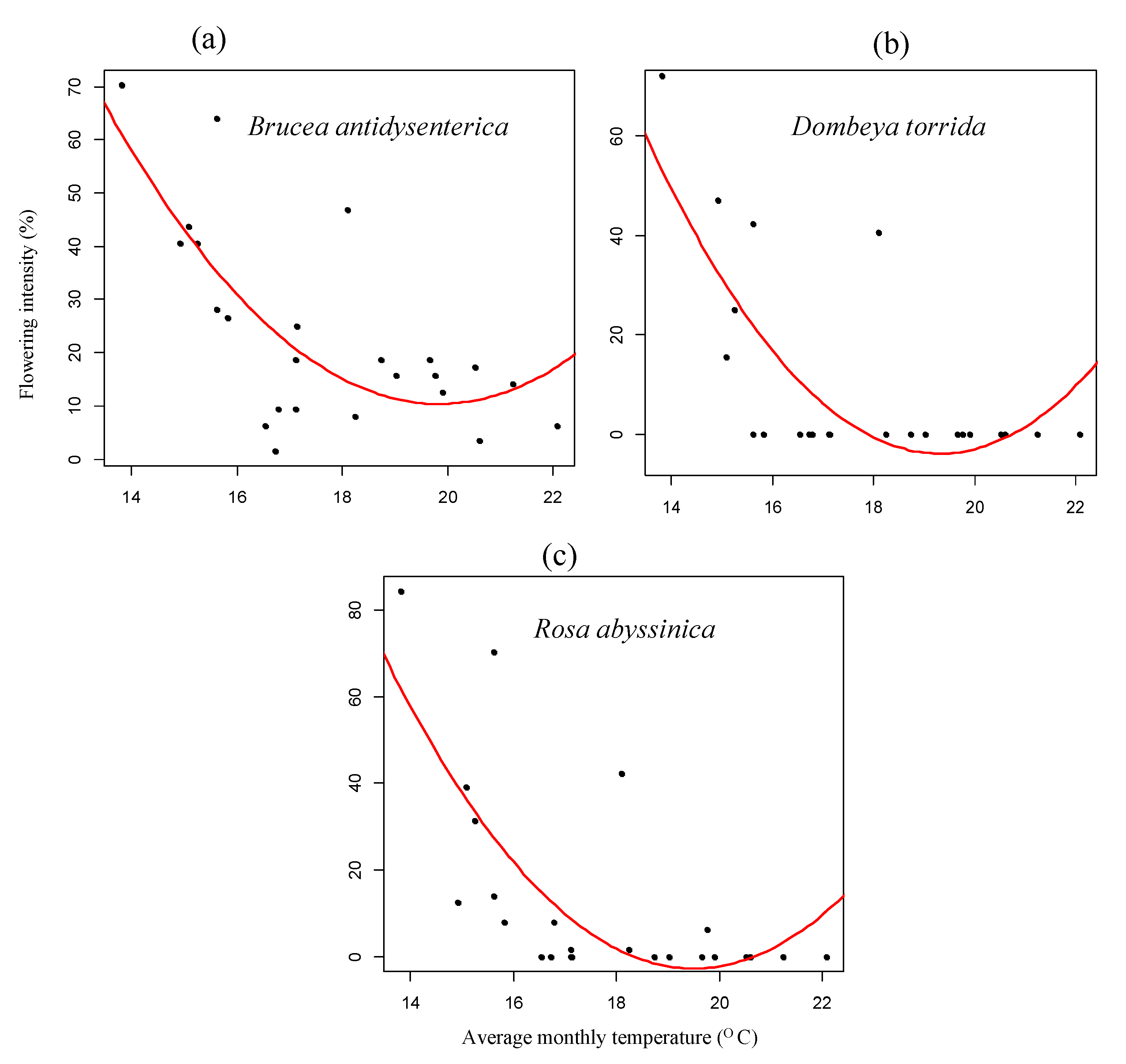
3.1. Flowering Phenology
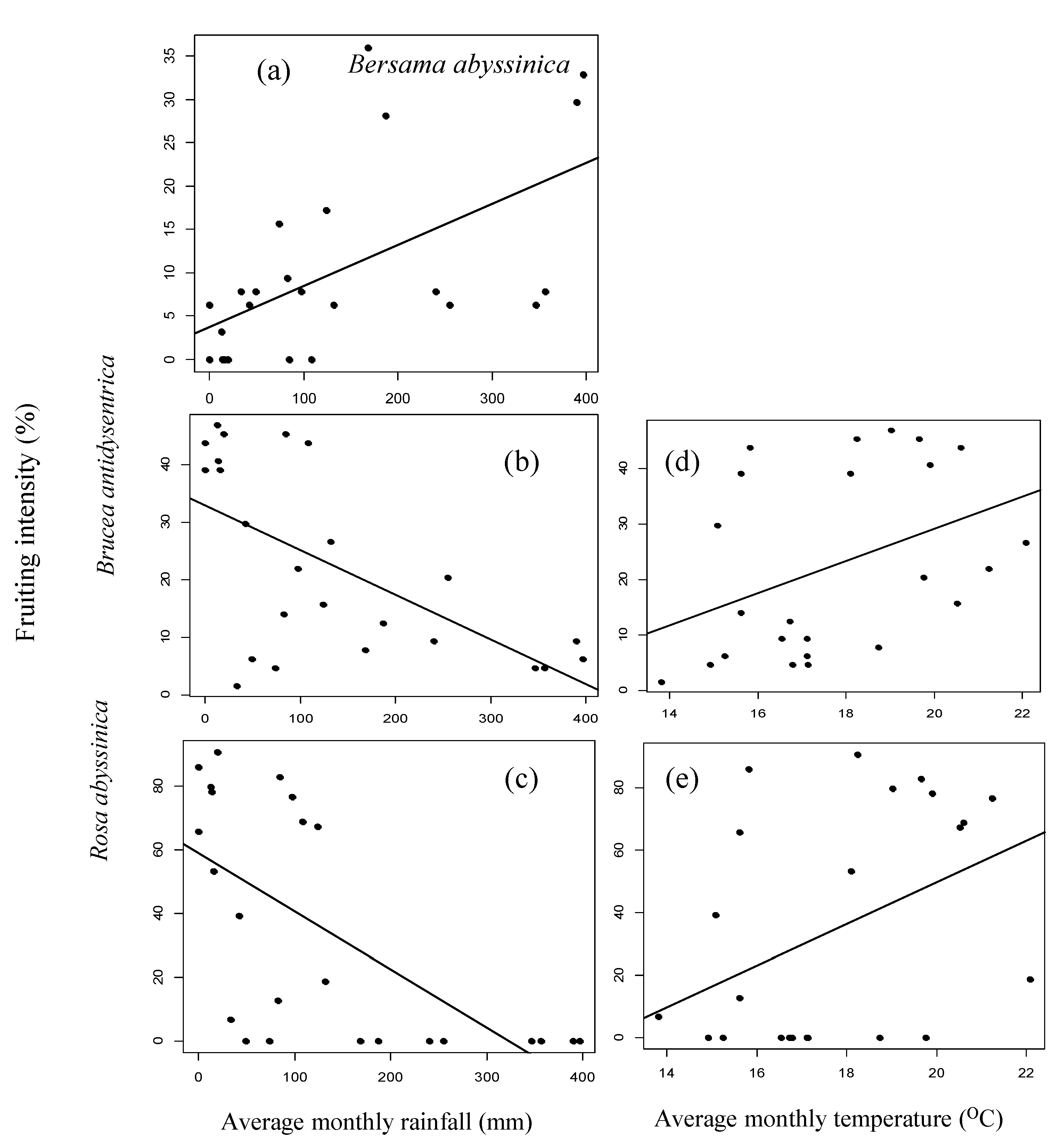
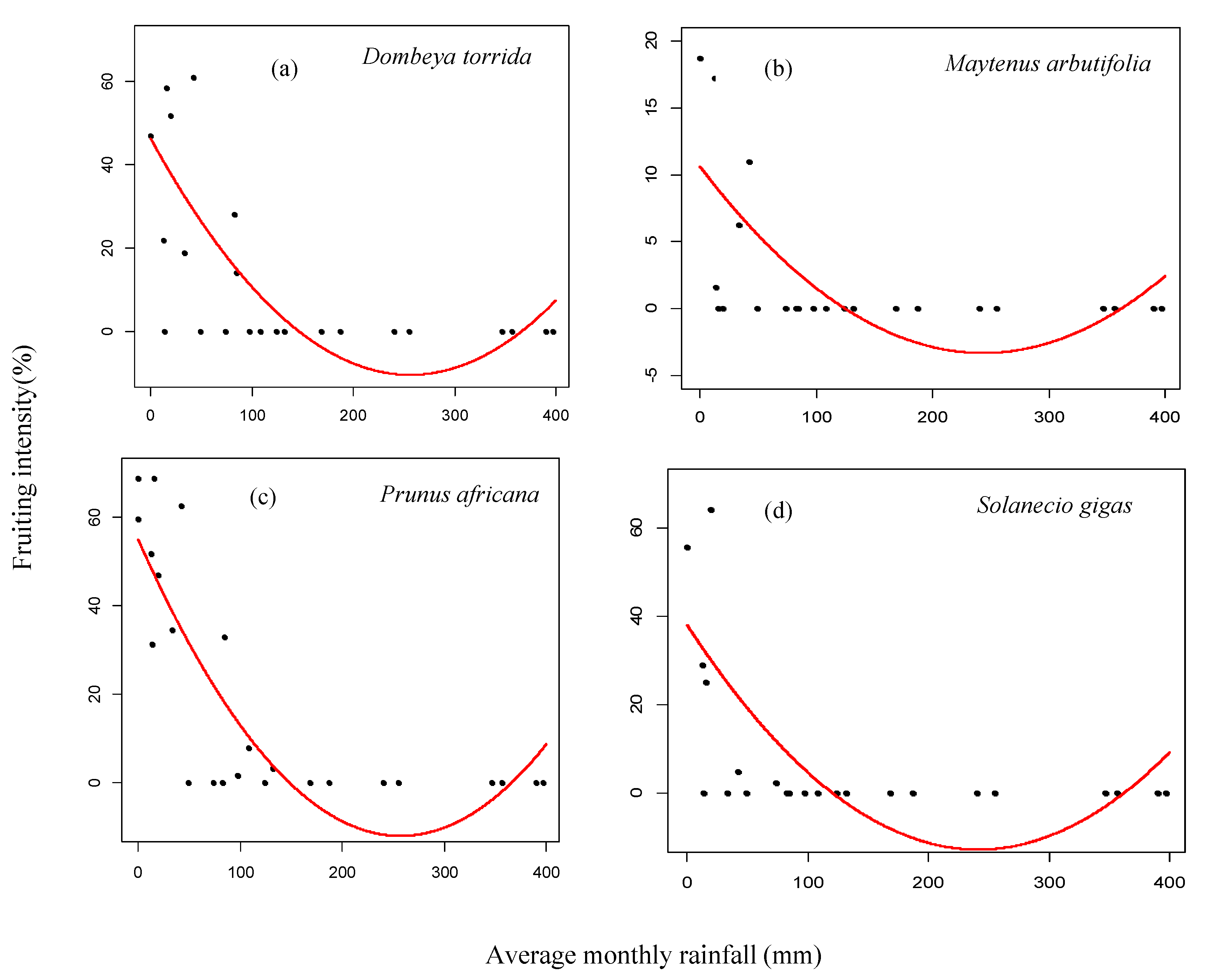
3.2. Fruiting Phenology
4. Discussion
4.1. Patterns of Monthly Rainfall and Temperature on Flowering Phenology of Woody Species
4.2. Patterns of Monthly Rainfall and Temperature on Fruiting Phenology of Woody Species
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwartz, M.D. Introduction. In Phenology: An Integrative Environmental Science; Schwartz, M.D., Ed.; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 2003; pp. 3–7. [Google Scholar]
- Denny, E.G.; Gers, K.L.; Miller-Rushing, A.J.; Tierney, G.L.; Crimmins, T.M.; Enquist, C.A.F.; Guertin, P.; Rosemartin, A.H.; Schwartz, M.D.; Thomas, K.A.; et al. Standardized phenology monitoring methods to track plant and animal activity for science and resource management applications. Int. J. Biometeorol. 2014, 58, 591–601. [Google Scholar] [CrossRef]
- Sanchez-Azofeifa, A.; Kalacska, M.E.; Quesada, M.; Stoner, K.E.; Jorge, A.; Lob, J.A.; Arroyo-Mora, P. Tropical Dry Climates. In Phenology: An Integrative Environmental Science; Schwartz, M.D., Ed.; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 2003; pp. 121–138. [Google Scholar]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kuebler, K.; Bissolli, P.; Braslavska, O.G.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Glob. Chang. Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Tang, J.; Körner, C.; Muraoka, H.; Piao, S.; Shen, M.; Thackeray, S.J.; Yang, X. Emerging opportunities and challenges in phenology: A review. Ecosphere 2016, 7, e01436. [Google Scholar] [CrossRef]
- Zhao, M.; Peng, C.; Xiang, W.; Deng, X.; Tian, D.; Zhou, X.; Yu, G.; He, H.; Zhao, Z. Plant phenological modeling and its application in global climate change research: Overview and future challenges. Environ. Rev. 2012, 21, 1–14. [Google Scholar] [CrossRef]
- IPCC. Climate Change: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Fenner, M. The phenology of growth and reproduction in plants. Perspect. Plant Ecol. Evol. Syst. 1998, 1, 78–91. [Google Scholar] [CrossRef]
- Willis, C.G.; Ellwood, E.R.; Primack, R.B.; Davis, C.C.; Pearson, K.D.; Gallinat, A.S.; Yost, J.M.; Nelson, G.; Mazer, S.J.; Rossington, N.L.; et al. Old Plants, New Tricks: Phenological Research Using Herbarium Specimens. Trends Ecol. Evol. 2017, 32, 531–546. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Moza, M.K.; Bhatnagar, A.K. Phenology and climate change. Curr. Sci. 2005, 9, 243–244. [Google Scholar]
- Williams, J.W.; Jackson, S.T.; Kutzbacht, J.E. Projected distributions of novel and disappearing climates by 2100 AD. Proc. Natl. Acad. Sci. USA 2007, 104, 5738–5742. [Google Scholar] [CrossRef] [PubMed]
- Dejene, T.; Mohamed, O.; Yilma, Z.; Eshete, A. Phenology of leaf, flower and fruits of Boswellia neglecta and Commiphora myrrha in Borena Zone, Southeastern Ethiopia. Hort Flora Res. Spectr. 2016, 5, 269–274. [Google Scholar]
- Shiferaw, W.; Bekele, T.; Demissew, S.; Aynekulu, E. Phenology of the alien invasive plant species Prosopis juliflora in arid and semi-arid areas in response to climate variability and some perspectives for its control in Ethiopia. Pol. J. Ecol. 2020, 68, 37–46. [Google Scholar] [CrossRef]
- Lobo, J.; Quesada, M.; Stoner, K.E.; Fuchs, E.J.; Herrerias-Diego, Y.; Rojas, J.; Saborio, G. Factors affecting phenological patterns of Bombaceaous trees in seasonal forests in Costa Rica and Mexico. Am. J. Bot. 2003, 90, 1054–1063. [Google Scholar] [CrossRef]
- Chuine, I.; Beaubien, E.G. Phenology is a major determinant of tree species range. Ecol. Lett. 2001, 4, 500–510. [Google Scholar] [CrossRef]
- Borchert, R.; Robertson, K.; Schwartz, M.D.; Williams-Linera, G. Phenology of temperate trees in tropical climates. Int. J. Biometeorol. 2005, 50, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Igboabuchi, N.A.; Echereme, C.B.; Ekwealor, K.U. Phenology in plants: Concepts and uses. Int. J. Sci. Res. Methodol. 2018, 11, 8–24. [Google Scholar]
- Van Schaik, C.; Terborgh, W.J.; Wright, J.S. The phenology of tropical forests: Adaptive significance and consequences for primary consumers. Annu. Rev. Ecol. Syst. 1993, 24, 353–377. [Google Scholar] [CrossRef]
- Newstrom, L.E.; Frankie, G.W.; Baker, H.G. A new classification for plant phenology based on flowering patterns in lowland tropical rainforest trees at La Selva, Costa Rica. Biotropica 1994, 26, 141–159. [Google Scholar] [CrossRef]
- Sakai, S. Phenological diversity in tropical forests. Popul. Ecol. 2001, 43, 77–86. [Google Scholar] [CrossRef]
- Sakai, S.; Kitajima, K. Tropical phenology: Recent advances and perspectives. Ecol. Res. 2019, 34, 50–54. [Google Scholar] [CrossRef]
- Inouye, D.W.; Wielgolaski, F.E. High altitude climates. In Phenology: An Integrative Environmental Science; Schwartz, M.D., Ed.; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 2003; pp. 75–92. [Google Scholar]
- Tesfaye, G.; Teketay, D.; Fetene, M.; Beck, E. Phenology of seven indigenous tree species in a dry Afromontane forest, southern Ethiopia. Trop. Ecol. 2011, 52, 229–241. [Google Scholar]
- Parmesan, C. Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Glob. Chang. Biol. 2007, 13, 1860–1872. [Google Scholar] [CrossRef]
- Mosissa, D. Flowering and Fruiting Phenology of Some Forest Plant Species in the Remnants of Combretum—Terminalia Woodlands of Western Ethiopia. Acta Sci. Agric. 2019, 3, 126–132. [Google Scholar] [CrossRef]
- Friis, I.; Demissew, S.; Breugel, P.V. Atlas of potential vegetation of Ethiopia. In Biologiske Skrifter; The Royal Danish Academy of Sciences and Letters: Copenhagen, Denmark, 2010; Volume 58, p. 315. [Google Scholar]
- Wube, A.A.; Franz Bucar, F.; Asres, K.; Gibbons, S.; Rattray, L.; Croft, S.L. Antimalarial Compounds from Kniphofia foliosa roots. Phytother. Res. 2005, 19, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Teketay, D.; Senbeta, F.; Maclachlan, M.; Bekele, M.; Barklund, P. Edible Wild Plants in Ethiopia; Teketay, D., Ed.; Addis Ababa University Press: Addis Ababa, Ethiopia, 2010. [Google Scholar]
- Lulekal, E.; Asfaw, A.; Kelbessa, K.; Van Damme, P. Linking Ethnobotany, Herbaria and Flora to Conservation: The Case of Four Angiosperm Families at the National Herbarium of Ethiopia. J. East Afr. Nat. Hist. 2012, 101, 99–125. [Google Scholar] [CrossRef]
- Anza, M.; Worku, F.; Libsu, S.; Mamo, F.; Endale, M. Phytochemical Screening and Antibacterial Activity of Leaves Extract of Bersama abyssinica. J. Adv. Bot. Zool. 2015, 3, 2348–7313. [Google Scholar]
- Meragiaw, M.; Asfaw, Z.; Argaw, M. The Status of Ethnobotanical Knowledge of Medicinal Plants and the Impacts of Resettlement in Delanta, Northwestern Wello, Northern Ethiopia. Hindawi 2016, 2016, 5060247. [Google Scholar] [CrossRef] [PubMed]
- Dagnachew, S.; Teketay, D.; Demissew, S.; Hawas, T.; Kindu, M. Herbarium-based study of phenology of twelve indigenous 563 and endemic species from Ethiopia. S. Afr. J. Bot. 2022, 150, 260–274. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 14 July 2022).
- Ehrlén, J. Selection on flowering time in a life-cycle context. Oikos 2015, 124, 92–101. [Google Scholar] [CrossRef]
- Morellato, L.P.C.; Talora, D.C.; Takahasi, A.; Bencke, C.C.; Romera, E.C.; Zipparro, V.B. Phenology of Atlantic rain forest trees: A comparative study. Biotropica 2000, 32, 811–823. [Google Scholar] [CrossRef]
- Abrahamczyk, S.; Kluge, J.; Gareca, Y.; Reichle, S.; Kessler, M. The Influence of Climatic Seasonality on the Diversity of Different Tropical Pollinator Groups. PLoS ONE 2011, 6, e27115. [Google Scholar] [CrossRef]
- Cortés-Flores, J.; Hernández-Esquivel, K.; González-Rodríguez, A.; Ibarra-Manríquez, G. Flowering phenology, growth forms, and pollination syndromes in tropical dry forest species: Influence of phylogeny and abiotic factors. Am. J. Bot. 2017, 104, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Bendix, J.; Homeier, J.; Ortiz, E.C.; Emck, P.; Breckle, S.W.; Beck, E. Seasonality of weather and tree phenology in a tropical evergreen mountain rainforest. Int. J. Biometeorol. 2006, 50, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Frankie, W.G.; Baker, G.H.; Opler, A.P. Comparative phenological studies of trees in tropical wet and dry forests in the lowlands of Costa Rica. J. Ecol. 1974, 62, 881–919. [Google Scholar] [CrossRef]
- Justiniano, M.J.; Fredericksen, T.S. Phenology of tree species in Bolivian dry forests. Biotropica 2000, 32, 276–281. [Google Scholar] [CrossRef]
- Malizia, L.R. Seasonal fluctuations of birds, fruits, and flowers in a subtropical forest of Argentina. Condor 2001, 103, 45–61. [Google Scholar] [CrossRef]
- Berlin, E.K.; Pratt, K.T.; Simon, C.J.; Kowalsky, R.J. Plant phenology in a Cloud Forest on the Island of Maui, Hawaii. Biotropica 2000, 32, 90–99. [Google Scholar] [CrossRef]
- Janzen, D.H. Synchronization of sexual reproduction of trees within the dry season in Central America. Evolution 1967, 21, 620–637. [Google Scholar] [CrossRef]
- Bawa, K.S.; Kang, H.; Grayum, M.H. Relationship among time, frequency and duration of flowering in tropical rain forest trees. Am. J. Bot. 2003, 90, 877–887. [Google Scholar] [CrossRef]
- Anderson, D.P.; Nordheim, V.E.; Moermond, C.T.; Bi, Z.B.G.; Boesch, C. Factors influencing tree phenology in Tai National Park, Cote d’Ivoire. Biotropica 2005, 37, 631–641. [Google Scholar] [CrossRef]
- Teketay, D. Seed Ecology and Regeneration in Dry Afromontane Forests of Ethiopia. Ph.D. Thesis, Swedish University of Agricultural Sciences, Umeå, Sweden, 1996. [Google Scholar]
- Burger, W.C. Flowering periodicity at four levels in eastern Ethiopia. Biotropica 1974, 6, 38–42. [Google Scholar] [CrossRef]
- Tesfaye, G.; Teketay, D.; Fetene, M.; Beck, E. Regeneration of seven indigenous tree species in a dry Afromontane forest, southern Ethiopia. Flora 2010, 205, 135–143. [Google Scholar] [CrossRef]




| No | Species | Variable | Estimate | Standard Error | F-Value | Adjusted R2 | p-Value |
|---|---|---|---|---|---|---|---|
| 1 | Bersama abyssinica | Temp | 1.551 | 0.588 | 6.958 | 0.21 | 0.015 |
| RF | −0.0127 | 0.010 | 4.368 | 0.23 | 0.22 | ||
| 2 | Maytenus arbutifolia | Temp | −4.942 | 1.518 | 9.414 | 0.423 | 0.004 |
| RF | −0.073 | 0.026 | 9.414 | 0.423 | 0.011 | ||
| 3 | Prunus africana | Temp | −4.330 | 1.983 | 4.768 | 0.14 | 0.04 |
| RF | −0.031 | 0.034 | 2.763 | 0.13 | 0.38 | ||
| 4 | Solaneciogigas | Temp | −4.71 | 2.12 | 3.413 | 0.17 | 0.036 |
| RF | −0.065 | 0.034 | 4.554 | 0.236 | 0.07 |
| Species | Factor | Estimates | Standard Error | F-Values | Adjusted R2 | p-Values |
|---|---|---|---|---|---|---|
| Brucea antidysentrica | T | −55.57 | 20.45 | 12.09 | 0.49 | 0.01 |
| T2 | 1.4 | 0.57 | 0.02 | |||
| RF | −0.1177 | 0.11 | 2.41 | 0.11 | 0.278 | |
| RF2 | 0.00015 | 0.0002 | 0.576 | |||
| Dombeya torrida | T | −72.75 | 22.03 | 12.03 | 0.48 | 0.003 |
| T2 | 1.88 | 0.61 | 0.006 | |||
| RF | −0.008 | 0.12 | 1.461 | 0.03 | 0.498 | |
| RF2 | 0.000074 | 0.0003 | 0.807 | |||
| Rosa abyssinica | T | −77.97 | 26.1 | 11.51 | 0.47 | 0.007 |
| T2 | 1.998 | 0.73 | 0.01 | |||
| RF | 0.131 | 0.1372 | 1.649 | 0.05 | 0.350 | |
| RF2 | 0.0001 | 0.00035 | 0.617 |
| No | Species | Variable | Estimate | Standard Error | F-Values | Adjusted R2 | p-Values |
|---|---|---|---|---|---|---|---|
| 1 | Bersama abyssinica | Temp | −0.809 | 0.858 | 5.534 | 0.28 | 0.356 |
| RF | 0.047 | 0.015 | 10.23 | 0.29 | 0.004 | ||
| 2 | Brucea antidysenterica | Temp | 3.019 | 1.07 | 12.78 | 0.51 | 0.010 |
| RF | −0.079 | 0.019 | 12.78 | 0.51 | <0.001 | ||
| 3 | Rosa abyssinica | Temp | 6.97 | 2.25 | 15.85 | 0.56 | 0.005 |
| RF | −0.185 | 0.039 | 15.85 | 0.56 | <0.001 |
| Species | Factors | Estimates | Standard Error | F-Values | Adjusted R2 | p-Values |
|---|---|---|---|---|---|---|
| Dombeya torrida | RF | −0.44 | 0.11 | 11.37 | 0.47 | <0.001 |
| RF2 | 0.0008 | 0.0003 | 0.007 | |||
| T | 8.1143 | 39.3815 | 1.45 | 0.04 | 0.839 | |
| T2 | −0.3365 | 1.0942 | 0.761 | |||
| Maytenus arbutifolia | RF | −0.1444 | 0.03285 | 8.0 | 0.38 | 0.002 |
| RF2 | 0.00024 | 0.000083 | 0.01 | |||
| T | −4.42234 | 10.57080 | 1.20 | 0.02 | 0.680 | |
| T2 | 0.09619 | 0.29371 | 0.747 | |||
| Prunus africana | RF | −0.52 | 0.088 | 26.64 | 0.69 | <0.001 |
| RF2 | 0.001 | 0.0002 | <0.001 | |||
| T | 7.0066 | 41.4899 | 0.45 | −0.05 | 0.868 | |
| T2 | −0.2589 | 1.1528 | 0.824 | |||
| Solanecio gigas | RF | −0.42 | 0.11 | 8.73 | 0.40 | 0.001 |
| RF2 | 0.0009 | 0.0003 | 0.006 | |||
| T | 31.068 | 38.17 | 0.74 | −0.02 | 0.425 | |
| T2 | −0.9198 | 1.0605 | 0.396 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dagnachew, S.; Teketay, D.; Demissew, S.; Awas, T.; Lemessa, D. The Effects of Monthly Rainfall and Temperature on Flowering and Fruiting Intensities Vary within and among Selected Woody Species in Northwestern Ethiopia. Forests 2023, 14, 541. https://doi.org/10.3390/f14030541
Dagnachew S, Teketay D, Demissew S, Awas T, Lemessa D. The Effects of Monthly Rainfall and Temperature on Flowering and Fruiting Intensities Vary within and among Selected Woody Species in Northwestern Ethiopia. Forests. 2023; 14(3):541. https://doi.org/10.3390/f14030541
Chicago/Turabian StyleDagnachew, Sinework, Demel Teketay, Sebsebe Demissew, Tesfaye Awas, and Debissa Lemessa. 2023. "The Effects of Monthly Rainfall and Temperature on Flowering and Fruiting Intensities Vary within and among Selected Woody Species in Northwestern Ethiopia" Forests 14, no. 3: 541. https://doi.org/10.3390/f14030541
APA StyleDagnachew, S., Teketay, D., Demissew, S., Awas, T., & Lemessa, D. (2023). The Effects of Monthly Rainfall and Temperature on Flowering and Fruiting Intensities Vary within and among Selected Woody Species in Northwestern Ethiopia. Forests, 14(3), 541. https://doi.org/10.3390/f14030541






