Wood Decay Fungi Associated with Galleries of the Emerald Ash Borer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inoculum Preparation
2.2. Ash Wood Substrate
2.3. Microcosm Preparation
2.4. Loss of Weight Evaluation and Data Analysis
2.5. Micromorphological Studies
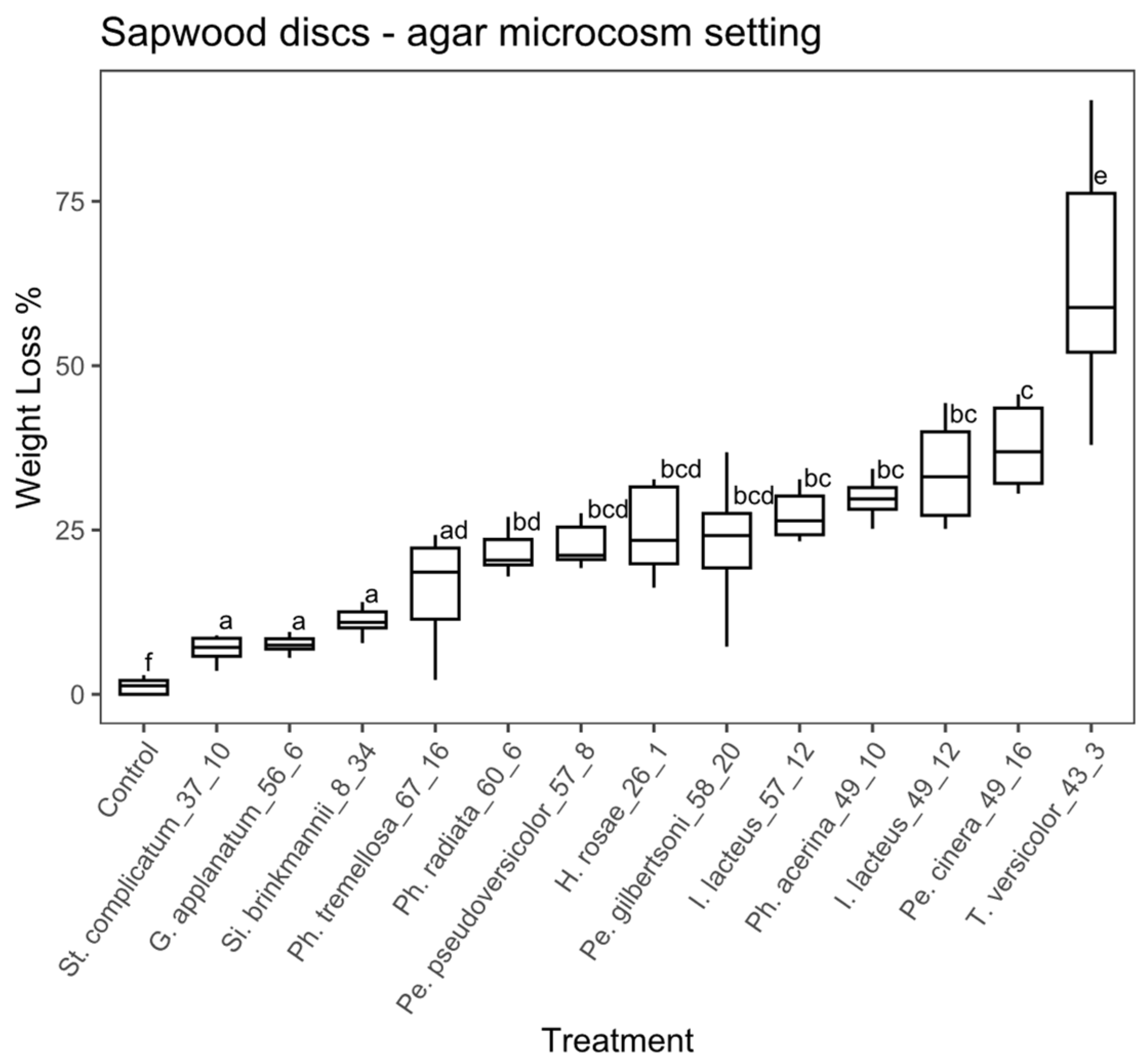
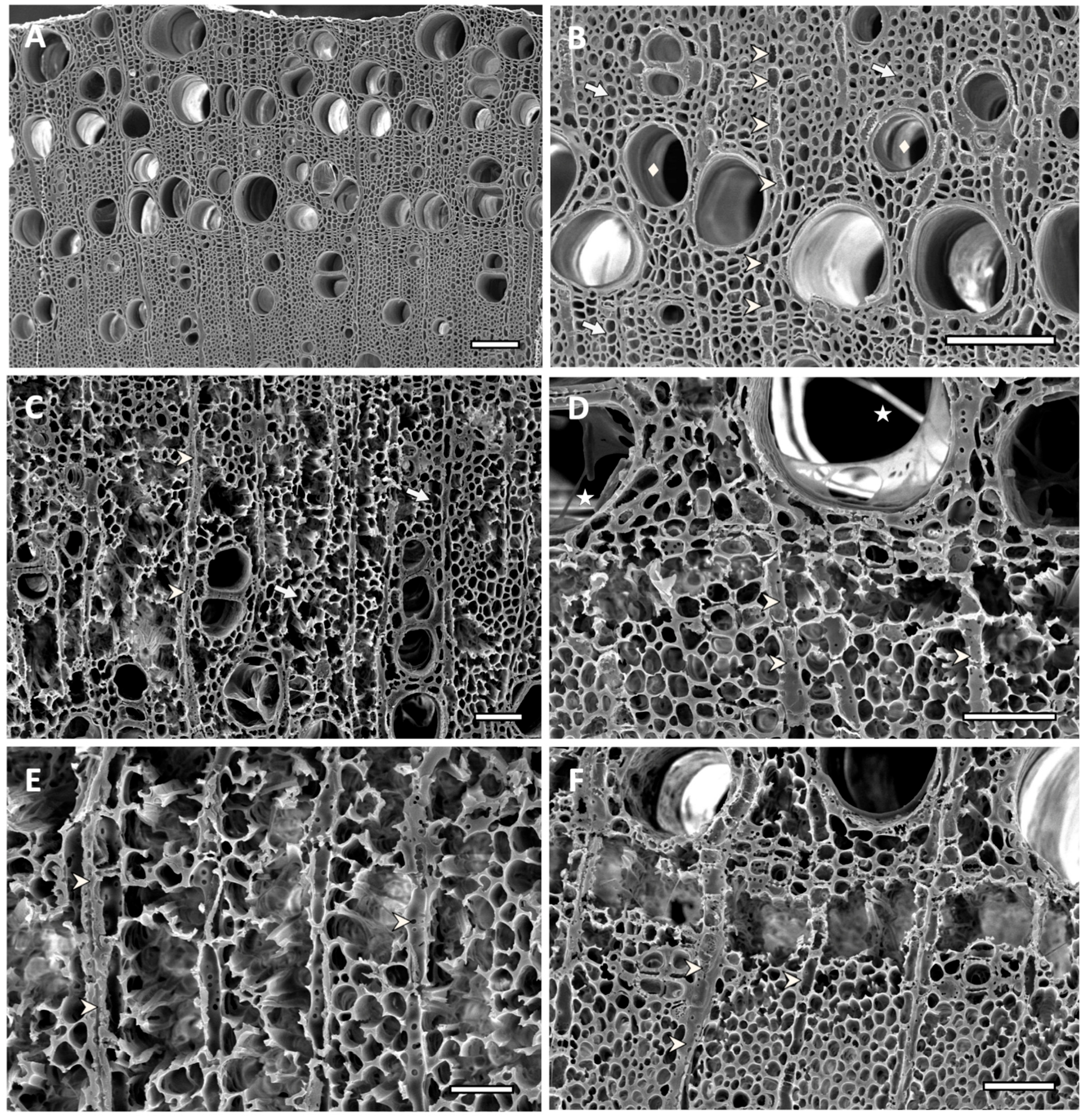
3. Results
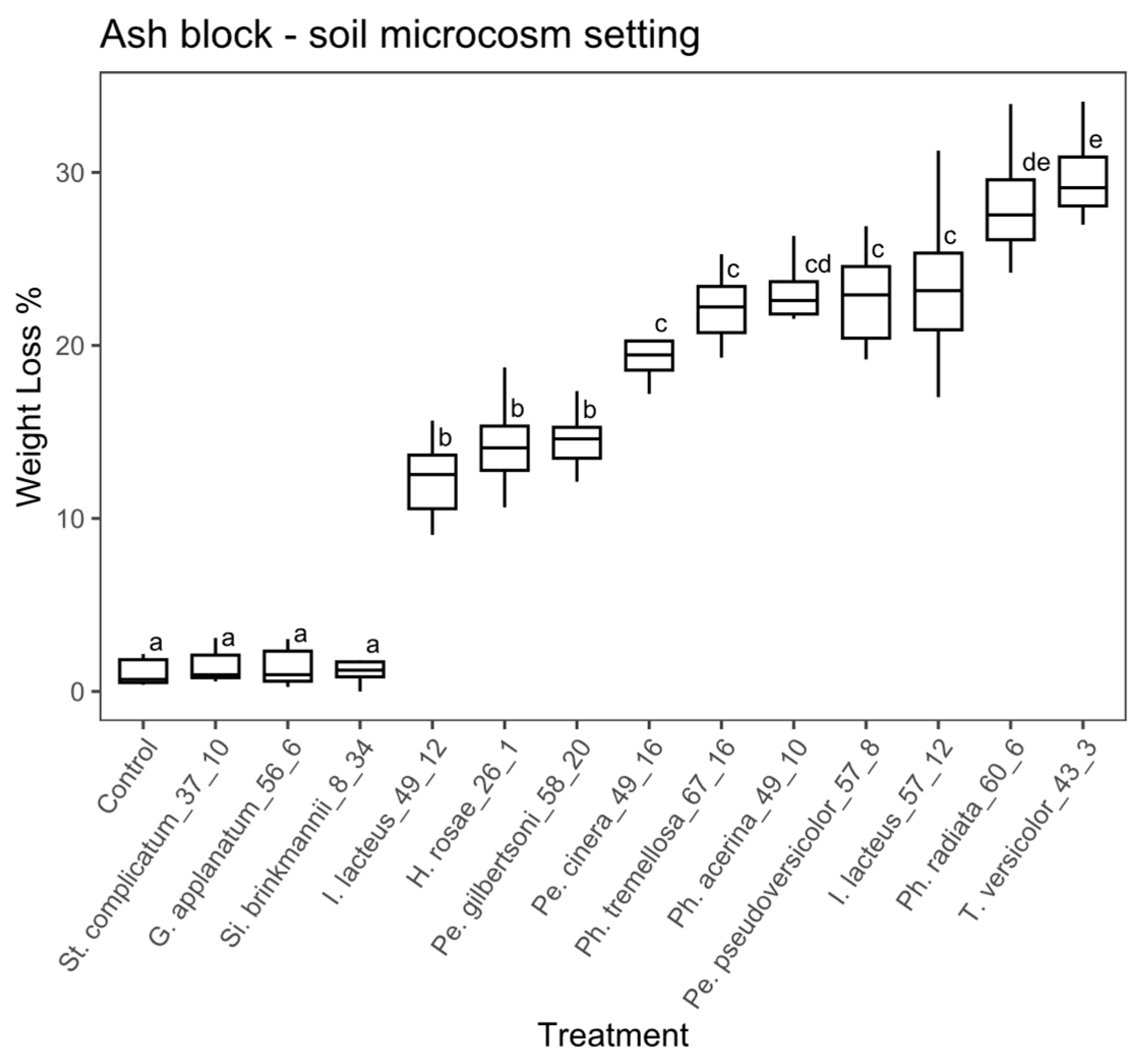
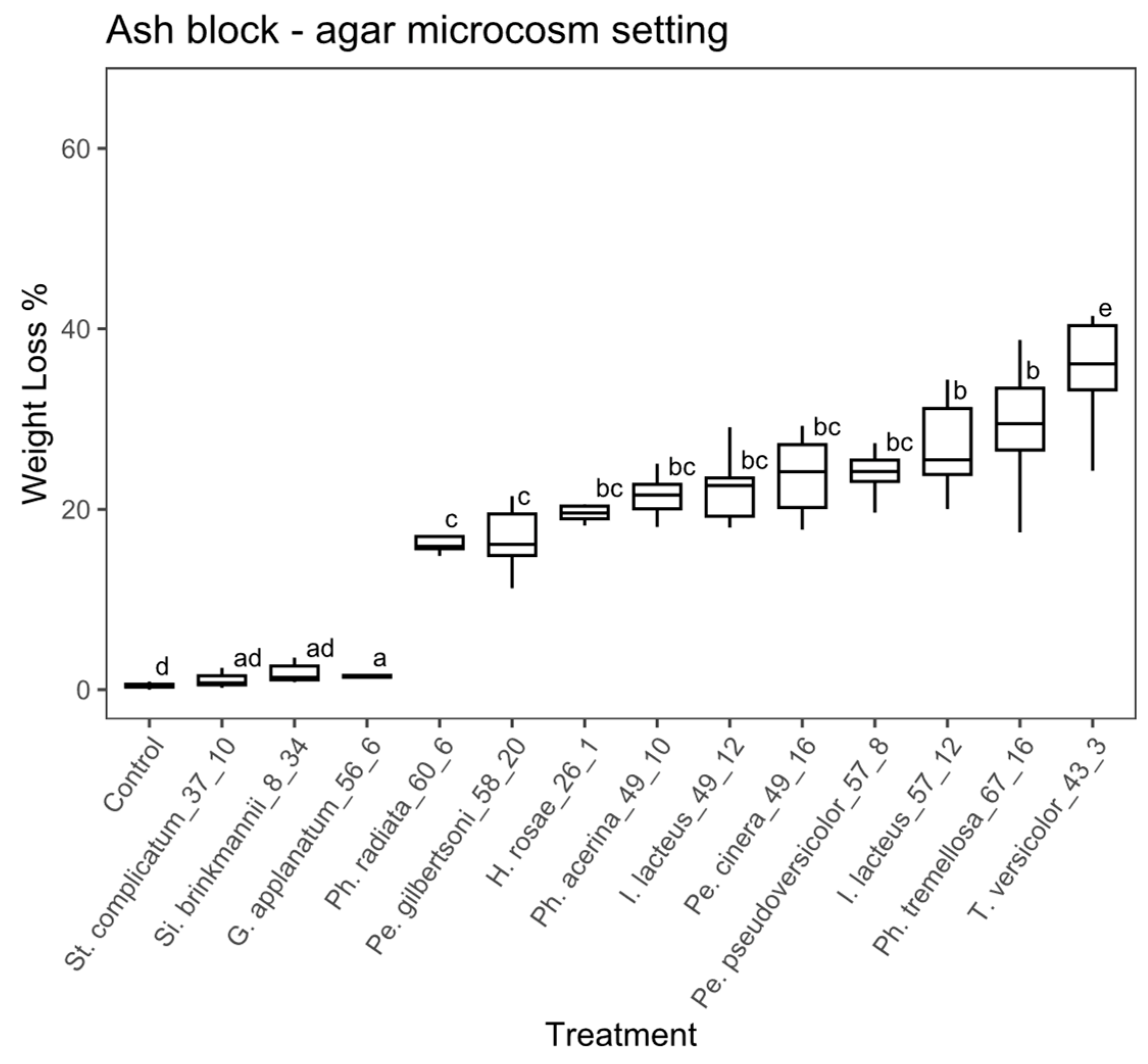
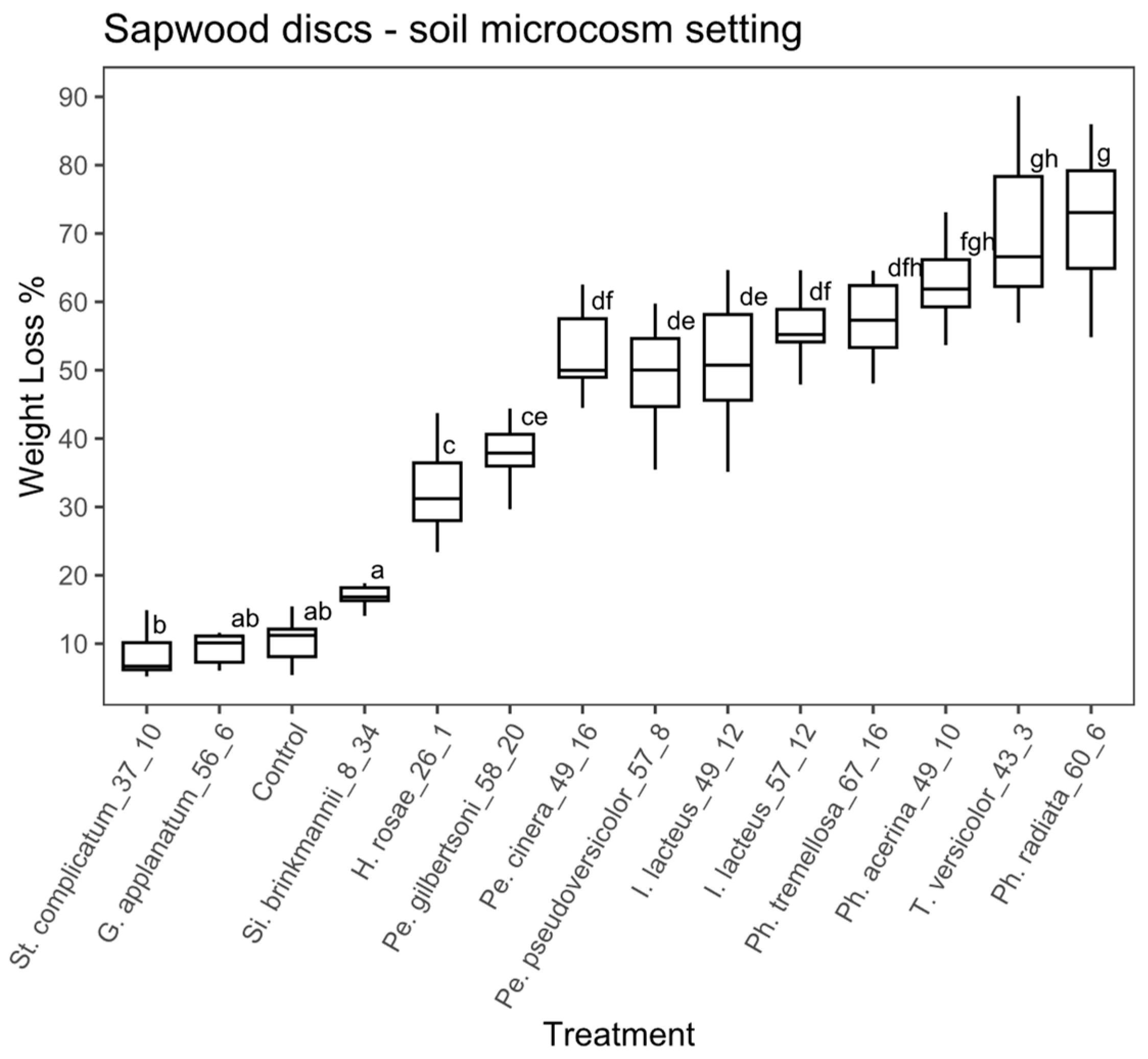
3.1. Weight Loss under Different Experimental Settings
Micromorphological Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCullough, D.G. Challenges, Tactics and Integrated Management of Emerald Ash Borer in North America. For. Int. J. For. Res. 2019, 93, 197–211. [Google Scholar] [CrossRef]
- Aukema, J.E.; Leung, B.; Kovacs, K.; Chivers, C.; Britton, K.O.; Englin, J.; Frankel, S.J.; Haight, R.G.; Holmes, T.P.; Liebhold, A.M.; et al. Economic Impacts of Non-Native Forest Insects in the Continental United States. PLoS ONE 2011, 6, e24587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herms, D.A.; Gandhi, K.J.; Smith, A.; Cardina, J.; Knight, K.S.; Herms, C.P.; Long, R.P.; McCullough, D.G. Ecological Impacts of Emerald Ash Borer in Forests of Southeast Michigan. In Proceedings of the 20th US Department of Agriculture Interagency Research Forum on Invasive Species 2009, Annapolis, MD, USA, 13–16 January 2009; McManus, K., Gottschalk, K.W., Eds.; US Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2009. General Technical Report NRS-P-51. pp. 36–37. [Google Scholar]
- Klooster, W.S.; Gandhi, K.J.; Long, L.C.; Perry, K.I.; Rice, K.B.; Herms, D.A. Ecological Impacts of Emerald Ash Borer in Forests at the Epicenter of the Invasion in North America. Forests 2018, 9, 250. [Google Scholar] [CrossRef] [Green Version]
- Herms, D.A.; McCullough, D.G. Emerald Ash Borer Invasion of North America: History, Biology, Ecology, Impacts, and Management. Annu. Rev. Entomol. 2014, 59, 13–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacs, K.F.; Haight, R.G.; McCullough, D.G.; Mercader, R.J.; Siegert, N.W.; Liebhold, A.M. Cost of Potential Emerald Ash Borer Damage in U.S. Communities, 2009–2019. Ecol. Econ. 2010, 69, 569–578. [Google Scholar] [CrossRef]
- Sydnor, T.D.; Bumgardner, M.; Subburayalu, S. Community Ash Densities and Economic Impact Potential of Emerald Ash Borer (Agrilus planipennis) in Four Midwestern States. AUF 2011, 37, 84–89. [Google Scholar] [CrossRef]
- Rebek, E.J.; Herms, D.A.; Smitley, D.R. Interspecific Variation in Resistance to Emerald Ash Borer (Coleoptera: Buprestidae) Among North American and Asian Ash (Fraxinus Spp.). Environ. Entomol. 2008, 37, 242–246. [Google Scholar] [CrossRef] [Green Version]
- Rigsby, C.M.; Showalter, D.N.; Herms, D.A.; Koch, J.L.; Bonello, P.; Cipollini, D. Physiological Responses of Emerald Ash Borer Larvae to Feeding on Different Ash Species Reveal Putative Resistance Mechanisms and Insect Counter-Adaptations. J. Insect Physiol. 2015, 78, 47–54. [Google Scholar] [CrossRef]
- Villari, C.; Herms, D.A.; Whitehill, J.G.A.; Cipollini, D.; Bonello, P. Progress and Gaps in Understanding Mechanisms of Ash Tree Resistance to Emerald Ash Borer, a Model for Wood-boring Insects That Kill Angiosperms. New Phytol. 2016, 209, 63–79. [Google Scholar] [CrossRef]
- Knight, K.S.; Brown, J.P.; Long, R.P. Factors Affecting the Survival of Ash (Fraxinus Spp.) Trees Infested by Emerald Ash Borer (Agrilus planipennis). Biol. Invasions 2013, 15, 371–383. [Google Scholar] [CrossRef]
- Persad, A.B.; Siefer, J.; Montan, R.; Kirby, S.; Rocha, O.J.; Redding, M.E.; Ranger, C.M.; Jones, A.W. Effects of Emerald Ash Borer Infestation on the Structure and Material Properties of Ash Trees. Arboric. Urban For. 2013, 39, 11–16. [Google Scholar] [CrossRef]
- Jennings, D.E.; Taylor, P.B.; Duan, J.J. The Mating and Oviposition Behavior of the Invasive Emerald Ash Borer (Agrilus planipennis), with Reference to the Influence of Host Tree Condition. J. Pest Sci. 2014, 87, 71–78. [Google Scholar] [CrossRef]
- Cappaert, D.; McCullough, D.G.; Poland, T.M.; Siegert, N.W. Emerald Ash Borer in North America: A Research and Regulatory Challenge. Am. Entomol. 2005, 51, 152–165. [Google Scholar] [CrossRef] [Green Version]
- Lyons, D.; Jones, G.; Wainio-Keizer, K. Biology and Phenology of the Emerald Ash Borer, Agrilus planipennis . In Proceedings of the XV U.S. Department of Agriculture Interagency Research Forum on Gypsy Moth and Other Invasive Species 2004, Annapolis, MD, USA, 13–16 January 2004. [Google Scholar]
- Bauer, L.; Haack, R.A.; Miller, D.L.; Petrice, T.R. Emerald Ash Borer Life Cycle. In Proceedings of the Emerald Ash Borer Research and Technology Development Meeting, Port Huron, MI, USA, 30 September–1 October 2003; Mastro, V., Reardon, R., Eds.; U.S. Forest Service, Forest Health Technology Enterprise Team: Morgantown, WV, USA, 2004. FHTET 2004-02. [Google Scholar]
- Kolka, R.K.; D’Amato, A.W.; Wagenbrenner, J.W.; Slesak, R.A.; Pypker, T.G.; Youngquist, M.B.; Grinde, A.R.; Palik, B.J. Review of Ecosystem Level Impacts of Emerald Ash Borer on Black Ash Wetlands: What Does the Future Hold? Forests 2018, 9, 179. [Google Scholar] [CrossRef] [Green Version]
- Held, B.W.; Simeto, S.; Rajtar, N.N.; Cotton, A.J.; Showalter, D.N.; Bushley, K.E.; Blanchette, R.A. Fungi Associated with Galleries of the Emerald Ash Borer. Fungal Biol. 2021, 125, 551–559. [Google Scholar] [CrossRef]
- Rajtar, N.N.; Held, B.W.; Blanchette, R.A. Fungi from Galleries of the Emerald Ash Borer Produce Cankers in Ash Trees. Forests 2021, 12, 1509. [Google Scholar] [CrossRef]
- Song, Z.; Kennedy, P.G.; Liew, F.J.; Schilling, J.S. Fungal Endophytes as Priority Colonizers Initiating Wood Decomposition. Funct. Ecol. 2017, 31, 407–418. [Google Scholar] [CrossRef]
- Eriksson, K.-E.L.; Blanchette, R.A.; Ander, P. Microbial and Enzymatic Degradation of Wood and Wood Components; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-46687-8. [Google Scholar]
- Riley, R.; Salamov, A.A.; Brown, D.W.; Nagy, L.G.; Floudas, D.; Held, B.W.; Levasseur, A.; Lombard, V.; Morin, E.; Otillar, R.; et al. Extensive Sampling of Basidiomycete Genomes Demonstrates Inadequacy of the White-Rot/Brown-Rot Paradigm for Wood Decay Fungi. Proc. Natl. Acad. Sci. USA 2014, 111, 9923–9928. [Google Scholar] [CrossRef] [Green Version]
- Blanchette, R.A. Delignification by Wood-Decay Fungi. Annu. Rev. Phytopathol. 1991, 29, 381–403. [Google Scholar] [CrossRef]
- Schilling, J.S.; Kaffenberger, J.T.; Liew, F.J.; Song, Z. Signature Wood Modifications Reveal Decomposer Community History. PLoS ONE 2015, 10, e0120679. [Google Scholar] [CrossRef]
- Anulewicz, A.C.; Mccullough, D.; Cappaert, D. Emerald Ash Borer (Agrilus planipennis) Density and Canopy Dieback in Three North American Ash Species. Arboric. Urban For. 2007, 33, 338–349. [Google Scholar] [CrossRef]
- Poland, T.M.; Chen, Y.; Koch, J.; Pureswaran, D. Review of the Emerald Ash Borer (Coleoptera: Buprestidae), Life History, Mating Behaviours, Host Plant Selection, and Host Resistance. Can. Entomol. 2015, 147, 252–262. [Google Scholar] [CrossRef] [Green Version]
- Tanis, S.R.; McCullough, D.G. Differential Persistence of Blue Ash and White Ash Following Emerald Ash Borer Invasion. Can. J. For. Res. 2012, 42, 1542–1550. [Google Scholar] [CrossRef]
- Raupp, M.J.; Cumming, A.B.; Raupp, E.C. Street Tree Diversity in Eastern North America and Its Potential for Tree Loss to Exotic Borers. Arboric. Urban For. 2006, 32, 297–304. [Google Scholar] [CrossRef]
- 2019 Minnesota State Agency Emerald Ash Borer Report|Minnesota Environmental Quality Board. Available online: https://www.eqb.state.mn.us/2019-minnesota-state-agency-emerald-ash-borer-report (accessed on 24 October 2022).
- Community Tree Inventories|The UFOR Nursery & Lab. Available online: https://trees.umn.edu/outreach/community-tree-inventories (accessed on 24 October 2022).
- Coetzee, M.P.A.; Marincowitz, S.; Muthelo, V.G.; Wingfield, M.J. Ganoderma Species, Including New Taxa Associated with Root Rot of the Iconic Jacaranda mimosifolia in Pretoria, South Africa. IMA Fungus 2015, 6, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Paterson, R.R.M. Ganoderma Disease of Oil Palm-A White Rot Perspective Necessary for Integrated Control. Crop Prot. 2007, 26, 1369–1376. [Google Scholar] [CrossRef] [Green Version]
- Mang, S.M.; Marcone, C.; Maxim, A.; Camele, I. Investigations on Fungi Isolated from Apple Trees with Die-Back Symptoms from Basilicata Region (Southern Italy). Plants 2022, 11, 1374. [Google Scholar] [CrossRef]
- Archer, K.; Nicholas, D.D.; Schultz, T.P. Screening of Wood Preservatives: Comparison of the Soil-Block, Agar-Block, and Agar-Plate Tests. For. Prod. J. 1995, 45, 86. [Google Scholar]
- Kuo, C.-J.; Kimsey, M.; Page-Dumroese, D.S.; Kirker, G.; Fu, A.Q.; Cai, L. Investigating Soil Effects on Outcomes of a Standardized Soil–Block Test. For. Prod. J. 2022, 72, 140–146. [Google Scholar] [CrossRef]
- Wiejak, A.; Francke, B. Testing and Assessing Method for the Resistance of Wood-Plastic Composites to the Action of Destroying Fungi. Materials 2021, 14, 697. [Google Scholar] [CrossRef]
- Wazny, J.; Cookson, L.J. Comparison of the Agar-Block and Soil-Block Methods Used for Evaluation of Fungitoxic Value of Wood Preservatives. Folia For. Polonica. Ser. B-Drzew. 1995, 25, 73–82. [Google Scholar]
- Schilling, J.S.; Jacobson, K.B. Agar-Block Microcosms for Controlled Plant Tissue Decomposition by Aerobic Fungi. JoVE 2011, 48, 2283. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Graves, S.; Piepho, H.-P.; Dorai-Raj, L.S. MultcompView: Visualizations of Paired Comparisons; R Project: Vienna, Austria, 2019. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation; R Project: Vienna, Austria, 2022. [Google Scholar]
- Wickham, H. Tidyverse: Easily Install and Load the Tidyverse; R Project: Vienna, Austria, 2022. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests; R Project: Vienna, Austria, 2022. [Google Scholar]
- Kassambara, A. Ggpubr: Ggplot2 Based Publication Ready Plots; R Project: Vienna, Austria, 2022. [Google Scholar]
- Held, B.W.; Blanchette, R.A. Deception Island, Antarctica, Harbors a Diverse Assemblage of Wood Decay Fungi. Fungal Biol. 2017, 121, 145–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajpai, P. Hardwood Anatomy. In Biermann’s Handbook of Pulp and Paper; Elsevier: Amsterdam, The Netherlands, 2018; pp. 153–208. ISBN 978-0-12-814240-0. [Google Scholar]
- Gartner, B.L. Plant Stems: Physiology and Functional Morphology; Physiological Ecology; Academic Press: San Diego, CA, USA, 1995; ISBN 978-0-08-053908-9. [Google Scholar]
- Panshin, A.J.; Zeeuw, C.D. Textbook of Wood Technology: Structure, Identification, Uses, and Properties of the Commercial Woods of the United States and Canada; McGraw-Hill: New York, NY, USA, 1970; ISBN 978-0-07-048440-5. [Google Scholar]
- Blanchette, R.A.; Obst, J.R.; Hedges, J.I.; Weliky, K. Resistance of Hardwood Vessels to Degradation by White Rot Basidiomycetes. Can. J. Bot. 1988, 66, 1841–1847. [Google Scholar] [CrossRef]
- Schwarze, F.W.M.R. Wood Decay under the Microscope. Fungal Biol. Rev. 2007, 21, 133–170. [Google Scholar] [CrossRef]
- Thurner, M.; Beer, C.; Crowther, T.; Falster, D.; Manzoni, S.; Prokushkin, A.; Schulze, E.-D. Sapwood Biomass Carbon in Northern Boreal and Temperate Forests. Glob. Ecol. Biogeogr. 2019, 28, 640–660. [Google Scholar] [CrossRef] [Green Version]
- Zabel, R.A.; Morrell, J.J. Natural Decay Resistance (Wood Durability). In Wood Microbiology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 455–470. ISBN 978-0-12-819465-2. [Google Scholar]
- Boddy, L. Fungal Community Ecology and Wood Decomposition Processes in Angiosperms: From Standing Tree to Complete Decay of Coarse Woody Debris. Ecol. Bull. 2001, 49, 43–56. [Google Scholar] [CrossRef]
- Schwarze, F.W.M.R.; Fink, S.; Deflorio, G. Resistance of Parenchyma Cells in Wood to Degradation by Brown Rot Fungi. Mycol. Prog. 2003, 2, 267–274. [Google Scholar] [CrossRef]
- Boddy, L.; Rayner, A.D.M. Ecological Roles of Basidiomycetes Forming Decay Communities in Attached Oak Branches. New Phytol. 1983, 93, 77–88. [Google Scholar] [CrossRef]
- Chen, Y.; Ciaramitaro, T.; Poland, T.M. Moisture Content and Nutrition as Selection Forces for Emerald Ash Borer Larval Feeding Behaviour. Ecol. Entomol. 2011, 36, 344–354. [Google Scholar] [CrossRef]
- Baietto, M.; Wilson, A.D. Relative in Vitro Wood Decay Resistance of Sapwood from Landscape Trees of Southern Temperate Regions. HortScience 2010, 45, 401–408. [Google Scholar] [CrossRef] [Green Version]
- Scheffer, T.C. Natural Resistance of Wood to Microbial Deterioration. Annu. Rev. Phytopathol. 1966, 4, 147–168. [Google Scholar] [CrossRef]
- Blanchette, R.A. Degradation of the Lignocellulose Complex in Wood. Can. J. Bot. 1995, 73, 999–1010. [Google Scholar] [CrossRef]
- Gilbertson, R.L.; Ryvarden, L. North American Polypores. Vol. I. Abortiporus-Lindtneria; Fungiflora A/S: Oslo, Norway, 1986. [Google Scholar]
- Gilbertson, R.L.; Ryvarden, L. North American Polypores Vol. 2. Megasporoporia-Wrightoporia; Fungiflora A/S: Oslo, Norway, 1987; pp. 437–885. [Google Scholar]
- Bari, E.; Nazarnezhad, N.; Kazemi, S.M.; Tajick Ghanbary, M.A.; Mohebby, B.; Schmidt, O.; Clausen, C.A. Comparison between Degradation Capabilities of the White Rot Fungi Pleurotus ostreatus and Trametes versicolor in Beech Wood. Int. Biodeterior. Biodegrad. 2015, 104, 231–237. [Google Scholar] [CrossRef]
- Bari, E.; Daryaei, M.G.; Karim, M.; Bahmani, M.; Schmidt, O.; Woodward, S.; Tajick Ghanbary, M.A.; Sistani, A. Decay of Carpinus betulus Wood by Trametes versicolor—An Anatomical and Chemical Study. Int. Biodeterior. Biodegrad. 2019, 137, 68–77. [Google Scholar] [CrossRef]
- Singh, P.; Sulaiman, O.; Hashim, R.; Peng, L.C.; Singh, R.P. Evaluating Biopulping as an Alternative Application on Oil Palm Trunk Using the White-Rot Fungus Trametes versicolor . Int. Biodeterior. Biodegrad. 2013, 82, 96–103. [Google Scholar] [CrossRef]
- Blanchette, R.A. Screening Wood Decayed by White Rot Fungi for Preferential Lignin Degradation. Appl. Environ. Microbiol. 1984, 48, 647–653. [Google Scholar] [CrossRef] [Green Version]
- Blanchette, R.A.; Reid, I.D. Ultrastructural Aspects of Wood Delignification by Phlebia (Merulius) Tremellosus . Appl. Environ. Microbiol. 1986, 52, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Maillard, F.; Schilling, J.; Andrews, E.; Schreiner, K.M.; Kennedy, P. Functional Convergence in the Decomposition of Fungal Necromass in Soil and Wood. FEMS Microbiol. Ecol. 2020, 96, fiz209. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, R.; Párraga, M.; Navarrete, J.; Carrasco, I.; de la Vega, E.; Ortiz, M.; Herrera, P.; Jurgens, J.A.; Held, B.W.; Blanchette, R.A. Investigations of Biodeterioration by Fungi in Historic Wooden Churches of Chiloé, Chile. Microb. Ecol. 2014, 67, 568–575. [Google Scholar] [CrossRef]
- Son, E.; Kim, J.-J.; Lim, Y.W.; Au-Yeung, T.T.; Yang, C.Y.H.; Breuil, C. Diversity and Decay Ability of Basidiomycetes Isolated from Lodgepole Pines Killed by the Mountain Pine Beetle. Can. J. Microbiol. 2011, 57, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Potvin, L.R.; Richter, D.L.; Jurgensen, M.F.; Dumroese, R.K. Association of Pinus banksiana Lamb. and Populus tremuloides Michx. Seedling Fine Roots with Sistotrema brinkmannii (Bres.) J. Erikss. (Basidiomycotina). Mycorrhiza 2012, 22, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, T.; Bilański, P. Fungi Detected in the Previous Year’s Leaf Petioles of Fraxinus excelsior and Their Antagonistic Potential against Hymenoscyphus fraxineus . Forests 2021, 12, 1412. [Google Scholar] [CrossRef]
- Schilling, J.S.; Kaffenberger, J.T.; Held, B.W.; Ortiz, R.; Blanchette, R.A. Using Wood Rot Phenotypes to Illuminate the “Gray” Among Decomposer Fungi. Front. Microbiol. 2020, 11, 1288. [Google Scholar] [CrossRef] [PubMed]
- Boddy, L.; Griffith, G.S. Role of Endophytes and Latent Invasion in the Development of Decay Communities in Sapwood of Angiospermous Trees. Sydowia 1989, 41, 41–73. [Google Scholar]
- Boddy, L.; Hiscox, J.; Gilmartin, E.C.; Johnston, S.R.; Heilmann-Clausen, J. Chapter 12 Wood Decay Communities in Angiosperm Wood. In The Fungal Community; Dighton, J., White, J.F., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 169–190. [Google Scholar]
- Gilmartin, E.C.; Jusino, M.A.; Pyne, E.J.; Banik, M.T.; Lindner, D.L.; Boddy, L. Fungal Endophytes and Origins of Decay in Beech (Fagus sylvatica) Sapwood. Fungal Ecol. 2022, 59, 101161. [Google Scholar] [CrossRef]
- Cline, L.C.; Schilling, J.S.; Menke, J.; Groenhof, E.; Kennedy, P.G. Ecological and Functional Effects of Fungal Endophytes on Wood Decomposition. Funct. Ecol. 2018, 32, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Parfitt, D.; Hunt, J.; Dockrell, D.; Rogers, H.J.; Boddy, L.; Griffith, G.W. Do All Trees Carry the Seeds of Their Own Destruction? PCR Reveals Numerous Wood Decay Fungi Latently Present in Sapwood of a Wide Range of Angiosperm Trees. Fungal Ecol. 2010, 3, 338–346. [Google Scholar] [CrossRef]
- Jacobsen, R.M.; Kauserud, H.; Sverdrup-Thygeson, A.; Bjorbækmo, M.M.; Birkemoe, T. Wood-Inhabiting Insects Can Function as Targeted Vectors for Decomposer Fungi. Fungal Ecol. 2017, 29, 76–84. [Google Scholar] [CrossRef]
- Seibold, S.; Müller, J.; Baldrian, P.; Cadotte, M.W.; Štursová, M.; Biedermann, P.H.W.; Krah, F.-S.; Bässler, C. Fungi Associated with Beetles Dispersing from Dead Wood—Let’s Take the Beetle Bus! Fungal Ecol. 2019, 39, 100–108. [Google Scholar] [CrossRef]
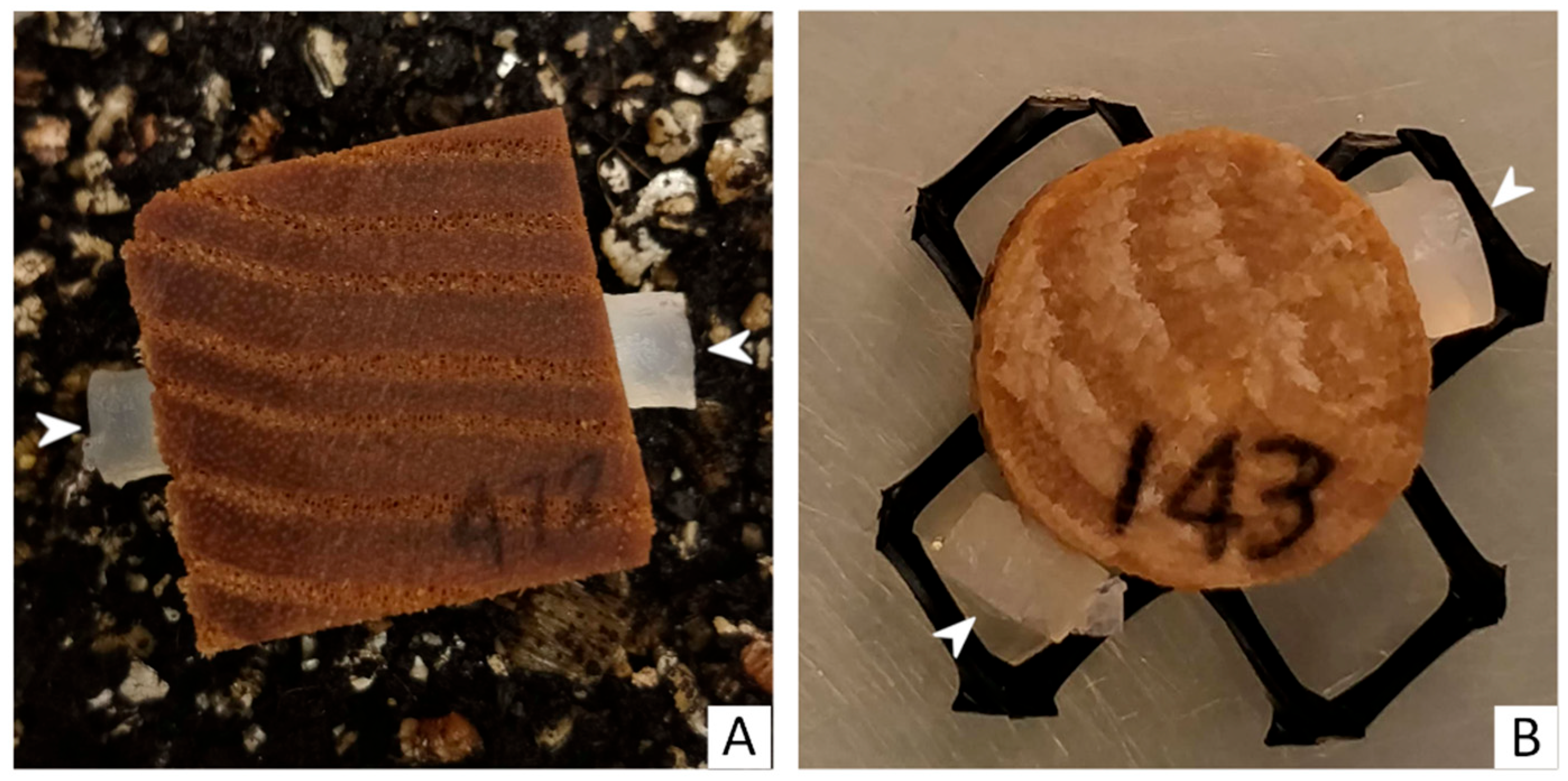


| Strain ID | Species | GenBank |
|---|---|---|
| EAB 8-34 | Sistotrema brinkmannii (Bres.) J. Erikss. | MT777396 |
| EAB 26-1 | Hyphodermella rosae (Bres.) Nakasone | MT777404 |
| EAB 37-10 | Stereum complicatum (Fr.) Fr. | MT777419 |
| EAB 43-3 | Trametes versicolor (L.) Lloyd | MT777401 |
| EAB 49-10 | Phlebia acerina Peck | MT777416 |
| EAB 49-12 | Irpex lacteus (Fr.) Fr. | MT777397 |
| EAB 49-16 | Peniophora cinerea (Pers.) Cooke | MT777398 |
| EAB 56-6 | Ganoderma applanatum (Pers.) Pat. | MT777403 |
| EAB 57-12 | Irpex lacteus (Fr.) Fr. | MT777397 |
| EAB 58-20 | Peniophora gilbertsonii Boidin | MT777400 |
| EAB 60-6 | Phlebia radiata Fr. | MT777402 |
| EAB 67-16 | Phlebia tremellosa (Schrad.) Nakasone & Burds. | MT777399 |
| EAB 57-8 | Peniophora pseudoversicolor Boidin | MT777405 |
| Treatment | Experimental Setting | ||||
|---|---|---|---|---|---|
| Strain ID | Species | Block Soil | Block Agar | Disc Soil | Disc Agar |
| --- | Control | 1.1 ± 0.7 | 0.5 ± 0.3 | 10.5 ± 3.2 | 1.2 ± 1.2 |
| EAB 8-34 | Sistotrema brinkmannii | 1.1 ± 0.7 | 1.9 ± 0.8 | 17.4 ± 2.5 | 11.5 ± 2.5 |
| EAB 26-1 | Hyphodermella rosae | 14.3 ± 2.5 | 19.8 ± 2.7 | 33.5 ± 8.5 | 25 ± 6.3 |
| EAB 37-10 | Stereum complicatum | 1.5 ± 0.9 | 1.1 ± 11.2 | 8.4 ± 3.3 | 6.9 ± 1.8 |
| EAB 43-3 | Trametes versicolor | 29.6 ± 2.2 | 37.7 ± 0 | 70.1 ± 11 | 63.2 ± 18.4 |
| EAB 49-10 | Phlebia acerina | 23.2 ± 1.8 | 21.5 ± 2.1 | 63.7 ± 7.9 | 29.3 ± 3.8 |
| EAB 49-12 | Irpex lacteus | 12.2 ± 2.1 | 22.3 ± 3.5 | 51 ± 9.6 | 34.1 ± 7.4 |
| EAB 49-16 | Peniophora cinerea | 20.5 ± 3.6 | 23.9 ± 4.2 | 52.7 ± 6.3 | 35.4 ± 10.9 |
| EAB 56-6 | Ganoderma applanatum | 1.9 ± 2.2 | 3.1 ± 3.7 | 9.3 ± 2.2 | 7.5 ± 1.2 |
| EAB 57-12 | Irpex lacteus | 22.9 ± 5 | 26.9 ± 5.1 | 56.2 ± 5.9 | 27.2 ± 3.5 |
| EAB 57-8 | Peniophora pseudoversicolor | 22.8 ± 2.7 | 23.7 ± 2.9 | 49 ± 7.8 | 23.7 ± 5.3 |
| EAB 58-20 | Peniophora gilbertsoni | 14.5 ± 1.5 | 16.9 ± 3.2 | 39.1 ± 7.6 | 23 ± 8.4 |
| EAB 60-6 | Phlebia radiata | 28.2 ± 3 | 17 ± 3.4 | 72.2 ± 9.9 | 22.1 ± 4.2 |
| EAB 67-16 | Phlebia tremellosa | 22.7 ± 2.9 | 27.8 ± 8.7 | 58.9 ± 9.9 | 16.3 ± 7.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simeto, S.; Held, B.W.; Blanchette, R.A. Wood Decay Fungi Associated with Galleries of the Emerald Ash Borer. Forests 2023, 14, 576. https://doi.org/10.3390/f14030576
Simeto S, Held BW, Blanchette RA. Wood Decay Fungi Associated with Galleries of the Emerald Ash Borer. Forests. 2023; 14(3):576. https://doi.org/10.3390/f14030576
Chicago/Turabian StyleSimeto, Sofía, Benjamin W. Held, and Robert A. Blanchette. 2023. "Wood Decay Fungi Associated with Galleries of the Emerald Ash Borer" Forests 14, no. 3: 576. https://doi.org/10.3390/f14030576






