Abstract
The increased frequency of climate change-induced droughts poses a survival challenge for forest trees, particularly for the common beech (Fagus sylvatica L.). Drought conditions adversely affect water supply and nutrient uptake, yet there is limited understanding of the intricate interplay between nutrient availability and drought stress on the physiology, growth, and biomass accumulation in young trees. We aimed to address this knowledge gap by examining the effects of irrigation and fertilisation and their interaction with various parameters in common beech saplings, including foliar and root N, P, and K concentrations; height and diameter increments; and aboveground and belowground biomass production. Our findings revealed that a higher fertilisation dose increased nutrient availability, also partially mitigating immediate drought impacts on foliar N concentrations. Also, higher fertilisation supported the post-drought recovery of foliar phosphorus levels in saplings. Prolonged drought affected nitrogen and potassium foliar concentrations, illustrating the lasting physiological impact of drought on beech trees. While drought-stressed beech saplings exhibited reduced height increment and biomass production, increased nutrient availability positively impacted root collar diameters. These insights have potential implications for forest management practices, afforestation strategies, and our broader understanding of the ecological consequences of climate change on forests.
1. Introduction
The recent increase in extreme drought and heatwave events has had a severe impact on many forest ecosystems worldwide [1], and the severity and frequency of such extreme climate events in the near future will be a significant limiting factor for tree growth and survival at least for boreal and temperate areas [2]. The current and projected adverse effects of climate change on the stability, structure and biodiversity of forest ecosystems in Europe present risks to essential ecosystem services and functions such as water protection, biodiversity, carbon sequestration, and timber production [3,4,5,6]. Given that common beech (Fagus sylvatica L.) is an economically and ecologically important species in Europe, with a total area of beech-dominated forests covering between 14 and 15 million hectares [7], its survival and ability to withstand changing climate conditions are of great importance. Despite its relatively high plasticity [8,9,10], common beech prefers a temperate climate with mild winters and humid summers [8,11,12], while a pronounced continental (cold/dry) climate restricts its distribution [13,14,15,16].
The latest research shows that recent droughts have seriously impacted beech forests [17,18,19]. Depleted soil moisture conditions during drought can significantly affect tree physiology, affecting tree growth and mortality rates [20,21].
In common beech, drought was found to trigger various physiological processes, including decreased leaf water potential, turgor loss, reduced transpiration, and photosynthesis [14,22,23,24]. This may lead to an alteration of anatomical characteristics, affecting factors such as xylem sap flow and other physiological performances, ultimately causing a reduction in the rate of photosynthesis, decreasing stomatal conductance [25], and constraining nutrient uptake and assimilation [26,27].
During drought, the reduction of nutrient availability from the soil can promote impairment of the plant’s nutritional status and its general functioning [28]; for instance, lower foliar and root phosphorus and potassium concentrations in beech can lower its biomass production [29].
On the other hand, plants that are able to restore their nutrient uptake and allocation efficiently will more successfully re-establish their physiological functions [30,31] following drought. Besides physiological processes, drought significantly affects morphological traits in beech.
Drought-exposed beech saplings exhibit reduced height, stem diameter [32], fine-root biomass and root growth [33,34,35]. Reduced root growth and function, as well as altered nutrient allocation patterns, can also affect the uptake of essential elements, such as nitrogen and phosphorus [36]. The negative impact of drought is particularly significant for afforestation projects with young beech trees [37], although in some cases beech saplings have successfully adapted to changed climate conditions [38]. The adaptive strategies of beech saplings are very complex, depending on drought severity [3], duration [39] and soil nutrient availability [27], influencing different functional [40] and morphological adaptive traits [41]. Conditions of low and high nutrient availability have different impacts on tree drought resilience. Trees growing in conditions of low nutrient availability could be more drought resistant due to better-developed root systems, reduced aboveground biomass and smaller vessel diameter, reducing cavitation risk [30,42]. However, poor beech nutritional status may hinder drought endurance [28], potentially causing carbon starvation and impeding tree recovery [30,42]. Conversely, high nutrient availability can increase vulnerability to carbon starvation or hydraulic failure during drought, which is associated with higher biomass production and cavitation risk [30,43,44,45]. On the other hand, an adequate nutrient supply boosts water use efficiency, nutrient uptake, and distribution during drought, leading to quicker post-drought recovery [30].
One of the ways to increase nutrient availability in the soil, and consequently enhance nutrient uptake by plants even under drought conditions is the application of mineral fertilisers [46,47]. The effects of increased nutrient availability on different physiological and morphological traits such as root functioning, gas exchange, growth, and foliar nitrogen were investigated in different tree species [31,48,49,50], yet there is no general consensus on the possible role of nutrition in alleviating the negative effects of drought in trees.
Therefore, the objective of this study was to investigate the interaction between nutrient availability and drought on nutrition, growth, and biomass accumulation in beech saplings during drought and after drought release. Additionally, the research aimed to explore the connection between nutrient status and the potential for recovery and assess the prolonged effects of drought.
We hypothesised that a higher dose of fertilisation would:
- I.
- Increase nutrient availability, partially mitigating the negative effects of drought on nutrition by maintaining adequate foliar concentrations of nitrogen, phosphorus, and potassium during drought.
- II.
- Alleviate the prolonged effect of drought on the foliar concentrations, growth, and biomass production of beech saplings by maintaining them at the level of regularly watered saplings.
2. Materials and Methods
2.1. Experimental Design and Treatments
The greenhouse experiment was set up in the nursery of the Croatian Forest Research Institute, Jastrebarsko, Croatia (45°40′03″ N, 15°38′26″ E). A total of 1120 one-year-old potted common beech saplings were transplanted into 6 litre square pots in a substrate of 1:3 sand: peat and placed in an open-sided greenhouse equipped with roof-blocking precipitation, an automated drip irrigation system (Irritrol Total Control® with six stations, Irritrol Systems, Riverside, CA, USA), and polyethylene shade nets with a light permeability of 50% (Figure S1).
The experiment was set up in April 2020 as a Latin square with four different treatments: WH—regular watering, high dose of fertiliser, DH—induced drought, high dose of fertiliser, WL—regular watering, low dose of fertiliser, and DL—induced drought, low dose of fertiliser. Each treatment had 8 replicates, with 35 randomly selected pots/saplings per replicate (Figure S2). The timeline of measurements and sampling is shown in Figure 1.
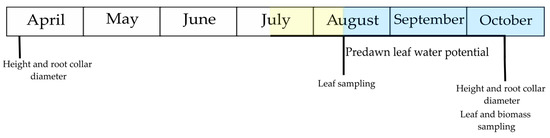
Figure 1.
Timeline of measurements and sampling. The yellow square depicts the induced drought period, the blue line depicts the start of rewatering and the blue square depicts the post-drought period.
Controlled-release fertiliser (Osmocote Exact Standard 5–6 M, 8.6% NH4-N and 6.4% NO3-N, 9% P, 12% K, 2% MgO, 0.47% Fe, 0.07% Mn, 0.06% Cu, 0.03% Zn, 0.02% B, and 0.02% Mo) was mixed into the substrate in two different doses: 2.0 g/L for low-dose fertilisation and 4.0 g/L treatment for high-dose fertilisation, i.e., low and optimal fertiliser dose for growing potted beech saplings [51]. For the first eight weeks of the experiment, all saplings were well-watered to absorb fertiliser and recover from transplant shock.
The saplings in regularly watered treatments (WH, WL) received 1 L of water once a week throughout the experiment (April–October). The amount of 1 litre of water per week/pot was calculated as one sixth of the mean difference between the weight of six pots filled with substrate and watered to field capacity and the weight of the same pots one week later, which was performed at the beginning of the experiment.
Saplings in the drought treatments (DH, DL) were not watered for 32 days during the induced drought period (14 July–14 August), i.e., until the first visible symptoms of drought stress (wilting leaves) were found on most plants treated by drought.
2.2. Response Parameters
Sapling water status was determined by predawn leaf water potential (Ψpd) measurements. They were performed weekly from the beginning of the induced drought (Figure 1) on four randomly selected plants from each replicate of each treatment (128 saplings in total), which were excluded from foliar and biomass sampling. Ψpd was measured on one mature leaf per sapling, utilising a Scholander pressure chamber (Pressure Chamber Instruments Model 600, PMS Instrument Company, Albany, OR, USA). Samples for the foliar analysis of nitrogen (N), phosphorus (P), and potassium (K) were collected during the induced drought period just before rewatering and again in the post-drought period, 9 weeks after rewatering. A medium-sized leaf was sampled from the upper part of each sapling in each replicate. A composite sample was made of all leaves sampled in 1 replicate—altogether 32 samples, 4 samples per replicate. Samples were dried at 105 °C to a constant mass and then milled (Fritsch Pulverisette 14 Mill, Fritsch GmbH Manufacturers of Laboratory Instruments, Idar-Oberstein, Germany) [52]. The concentration of total N was determined on an elemental analyser (Leco CNS 2000, LECO Corporation, St. Joseph, MI, USA), P was determined using a UV/VIS spectrophotometer (UVS-2700 LaboMed Inc., Los Angeles, CA, USA) [52], and K on the atomic absorption spectrometer (Perkin-Elmer Aanalyst 700, PerkinElmer Inc., Waltham, MA, USA) [52], following digestion with a combination of HNO3 and H2O2 in a microwave oven (Milestone Ethos One, Milestone Srl, Sorisole, Italy). Foliar nutrient concentrations were compared with available reference values for beech [53]. The temporal change of N, P and K foliar concentrations between drought and post-drought periods within each treatment was calculated using the following formula:
Temporal change = foliar concentration (post drought period) − foliar concentration (drought period)
The height (measured from the root collar to the top) and diameter of the root collar of all saplings were measured before leaves emerged (hstart, dstart) and at the end of the experiment (hend, dend) using a measuring rod and a digital calliper, respectively. Because of initial differences in height and root collar diameter, relative height increment (ih) and relative root collar diameter increment (id) were calculated using the following formula:
ih = (hend − hstart/hstart) × 100, id = (dend − dstart/dstart) × 100
At the end of the experiment (October 2020) and before the onset of autumnal yellowing, three average-sized saplings were sampled from each replicate (96 saplings in total) to determine aboveground (leaves and stems) and belowground (coarse roots > 2 mm diameter, fine roots < 2 mm diameter) biomass. The roots were washed from substrate residues and separated into coarse roots and fine roots. Leaves, stems, and coarse and fine roots were dried to constant mass and weighed, resulting in the following measurements: leaf biomass (LB), stem biomass (SB), coarse-root biomass (CRB), fine-root biomass (FRB), aboveground biomass (AGB), belowground biomass (BGB), and total biomass (TB). A composite sample was made of fine roots from each replicate and then ground in a Fritsch Pulverisette 14 Mill [52]. The concentration of total N, P and K were determined as described earlier for foliar concentrations.
We considered different effects of irrigation and fertilisation treatments based on values from final leaf/biomass sampling: a prolonged effect of drought was considered when drought treatments had lower values in comparison with values recorded in regularly watered treatments, always comparing the same level of fertilisation.
Recovery was considered only for foliar nutrient concentrations if there were no differences between drought and regularly watered treatments. Here, two separate sampling campaigns allowed us to crosscheck the presumed recovery in the post-drought period against the effects at the peak of drought.
2.3. Statistical Analysis
All statistical analyses were performed with R-studio, version 4.1.1 [54]. Data normality and residual homogeneity were checked for all data before analysis.
To determine differences in foliar concentrations between treatments (WH, DH, WL, DL) during each period, we used the pairwise comparisons t-test with Holm–Bonferroni correction. The temporal change of N, P and K foliar concentrations was analyzed with a one-way analysis of variance to determine significant differences between periods within each treatment.
Differences among individual treatments (WH, DH, WL, DL) for biomass and fine roots N, P and K concentrations were assessed by using analysis of variance (ANOVA) followed by a Tukey post hoc test. In addition, results were tested for an influence of the main factors “irrigation” and “fertilisation,” as well as their interactions using two-way ANOVA.
In the case of non-normally distributed data (height and root collar diameter increment), the Kruskal–Wallis test was followed by the Dunn multiple comparisons test.
3. Results
3.1. Water Status of Saplings
Drought-treated saplings (DH and DL treatments) maintained predawn leaf water potentials (Ψpd) at the same level as regularly watered saplings (WH and WL treatments) during the first three days of the drought period (Figure 2). A week later, the Ψpd of the DH and DL saplings had already become significantly lower than in WH and WL. The values of Ψpd in drought-treated saplings continued to decrease afterwards, reaching mean values of −2.1 MPa (±0.2 SE) in DH and −1.8 MPa (±0.2 SE) in DL in mid-August (day 32 of induced drought).
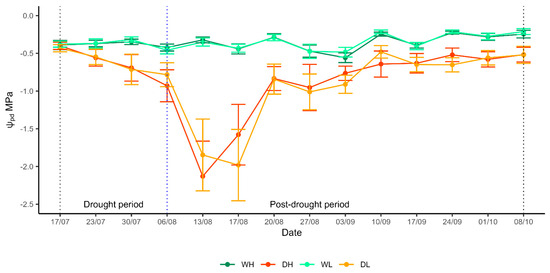
Figure 2.
Seasonal pattern of predawn leaf water potential (Ψpd), in the drought and post-drought period for each treatment; regular watering and high dose of fertiliser (WH), regular watering and low dose of fertiliser (WL), induced drought and high dose of fertiliser (DH) and induced drought and low dose of fertiliser (DL). The first vertical dotted line (black) indicates the beginning of the drought, the second vertical dotted line (blue) indicates the time point of rewatering and the beginning of the post-drought period, and the third vertical dotted line (black) indicates the end of the experiment. Dots indicate mean values and vertical bars indicate ± confidence intervals.
After rewatering, the Ψpd of drought-treated saplings began to gradually increase. At the end of the experiment, 78% of saplings in DH and 94% of saplings in DL treatment had recovered their Ψpd to values to close to, but never fully matching, the Ψpd of WH and WL saplings. Saplings in the WH and WL treatments maintained their Ψpd between −0.5 MPa and −0.2 MPa (mean value −0.3 MPa ± 0.01 SE) throughout the experiment.
3.2. Nutritional Status of Saplings
3.2.1. Foliar Concentrations
Foliar nitrogen (N) concentrations were within or above the normal range for beech [53] both in the drought and the post-drought period (Figure 3A), but concentrations were significantly lower in drought-treated saplings compared to regularly watered plants. In the drought period, a significant effect of fertilisation was observed in both drought-treated and regularly watered saplings (Table S1), resulting in higher foliar N concentrations in saplings treated with a high fertiliser dose. In the post-drought period, foliar N concentrations were lower in drought-treated saplings than in regularly watered saplings indicating the prolonged effect of drought in both fertilisation treatments. The influence of fertilisation was not as pronounced as during the drought period, with no significant differences established between fertilisation treatments. Foliar N concentrations in drought treatments decreased significantly in the post-drought period but remained within the normal range (Table 1).
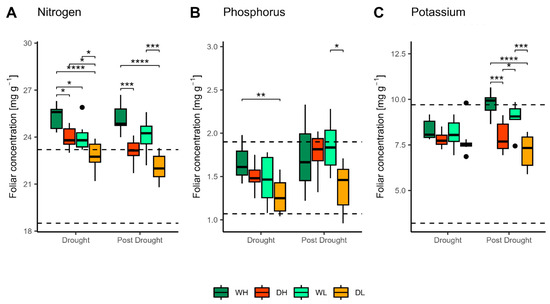
Figure 3.
Nitrogen (A), phosphorus (B) and potassium (C) foliar concentrations in the drought and post-drought period, for each treatment; regular watering and high dose of fertiliser (WH), regular watering and low dose of fertiliser (WL), induced drought and high dose of fertiliser (DH) and induced drought and low dose of fertiliser (DL). Levels of significance: * p < 0.05; ** p < 0.01; *** p < 0.001, **** p < 0.0001. Dotted lines represent an optimal range of foliar N, P and K concentrations for common beech according to [53].

Table 1.
Temporal change of foliar concentrations of N, P, K, between the drought and post-drought period calculated by formula: foliar concentration (post drought)—foliar concentration (drought) within each treatment: regular watering and high dose of fertiliser (WH), regular watering and low dose of fertiliser (WL), induced drought and high dose of fertiliser (DH) and induced drought and low dose of fertiliser (DL).
Both in the drought and the post-drought period, foliar phosphorus (P) concentrations (Figure 3B) were in the normal range, according to [53]. The most significant difference between treatments was observed during the drought period, with P concentrations in the WH treatment being significantly higher than in the DL treatment, indicating a significant influence of both drought and fertilisation (Figure 3C, Table S1). In the post-drought period, the only significant differences in foliar P values were established between DL and WL treatments. Foliar P concentrations in the DH saplings did not differ from those of the WH and WL saplings in either period. P concentrations generally increased in the post-drought period, but this was significant only in low-fertilised saplings, indicating partial recovery (Table 1).
Foliar potassium (K) concentrations of DH, WL, and DL were mostly within the normal range [53] (Figure 3C). No significant effect of drought or fertilisation was found on foliar K concentrations in the drought period; significant differences were established only in the post-drought period, with WH concentrations suggesting a luxury supply of K (Table S1). Foliar K concentrations were significantly reduced in saplings previously exposed to drought compared to regularly watered saplings, indicating the prolonged effect of drought on foliar K and the importance of water for K nutrition in beech (Table S1). After drought release, increased foliar K concentrations were observed for regularly watered treatments, but not in the induced drought treatments (Table 1).
3.2.2. Fine-Root Nutrient Concentrations
N concentrations in fine-roots were affected only by fertilisation (Table S2), while P and K fine-root concentrations were affected by both irrigation and fertilisation. Saplings in high-fertilisation treatments had significantly higher root N concentrations than low-fertilisation treatments (Table 2). P concentrations were progressively diminishing in the order WH > DH > WL > DL. For K, only DL treatment values were different (lower) from the rest (Table 2). We did not observe any prolonged effect of drought on fine-root nutrient concentrations.

Table 2.
Mean values ± SD for fine-root concentrations of nitrogen (N), phosphorus (P) and potassium (K) in each treatment; regular watering and high dose of fertiliser (WH), regular watering and low dose of fertiliser (WL), induced drought and high dose of fertiliser (DH) and induced drought and low dose of fertiliser (DL).
3.3. Growth Responses
The relative height increment (ih) of WH saplings was significantly higher than the ih of saplings in all other treatments, while no difference in ih was noted between DH and DL saplings (Figure 4). Regularly watered saplings had higher ih values than drought-treated saplings. The prolonged effect of drought was found for saplings in both fertilisation treatments. At the end of the experiment, the difference in total height was quite apparent between the WH and other treatments, although this was not significant between WH and WL. Absolute height and root collar diameter values are shown in Table 3. Contrary to ih, we found that drought-exposed saplings treated with a high fertiliser dose (DH) had higher relative root collar diameter increment (id) values than drought-exposed saplings treated with a low fertiliser dose (DL). There was no significant difference between the id of DH and WL saplings, indicating a partial compensatory effect of fertilisation vs. adequate water supply. As expected, and similar to height increment, saplings in the WH treatment had the highest id. A prolonged drought effect was established for both growth parameters and in both fertilisation levels.
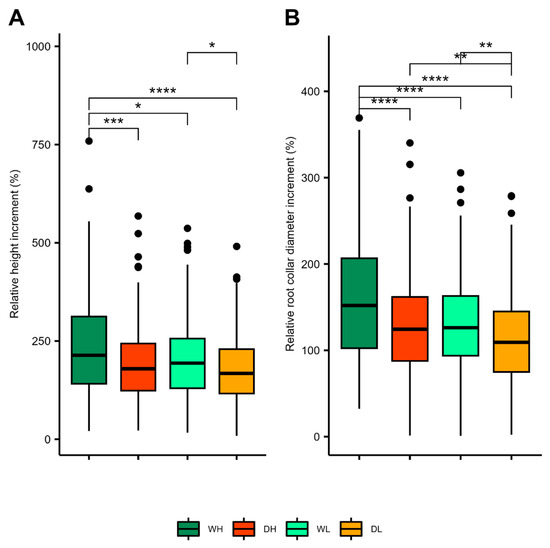
Figure 4.
(A) Relative height increment (ih) and (B) relative root collar diameter increment (id) in each treatment; regular watering and high dose of fertiliser (WH), regular watering and low dose of fertiliser (WL), induced drought and high dose of fertiliser (DH) and induced drought and low dose of fertiliser (DL). Levels of significance in differences among treatments are indicated as: * p < 0.05; ** p < 0.01; *** p < 0.001, **** p < 0.0001.

Table 3.
Height (h) and root collar diameter (d) of saplings measured at the start (hstart, dstart) and at the end (hend, dend) of the experiment in each treatment; regular watering and high dose of fertiliser (WH), regular watering and low dose of fertiliser (WL), induced drought and high dose of fertiliser (DH) and induced drought and low dose of fertiliser (DL).
3.4. Biomass Responses
Total biomass (TB) production was strongly affected by irrigation (Table 4), but the prolonged effect of drought was found only for the high-fertilisation treatment (Table 5). The production of aboveground biomass (AGB) was affected by both irrigation and fertilisation, as well as their interaction (Table 4), resulting in the highest aboveground biomass production in WH saplings (Table 5). The dry biomass of both stem (SB) and leaves (LB) in WH was also significantly higher than in the other treatments (Table 5). No significant effects of irrigation or fertilisation were found on the belowground biomass (BGB) (Table 4). However, the belowground biomass of WH saplings was the highest and significantly different from DH, which had the lowest belowground biomass. We found similar relations for coarse-root biomass (CRB) (Table 5). A high dose of fertilisation affected irrigated beech saplings by stimulating aboveground biomass production, while the belowground biomass was not affected by higher fertilisation dose regardless of the irrigation regime (Table 5). Fine-root biomass production was similar in all treatments (Table 5), with no significant effects of either irrigation or fertilisation (Table 4). Belowground to aboveground biomass ratio differed only between WH and DL, while DH and WL treatments had similar ratios of around 1:1 (Table 5).

Table 4.
Main effects of irrigation (regular watering vs. induced drought), fertilisation (high dose vs. low dose of fertiliser) and their interaction (irrigation × fertilisation) on dry biomass parameters and allometric relationships, at the end of the experiment, calculated with two-way ANOVA. F values for the factors and their interactions are shown.

Table 5.
Mean values ± SD for parameters of dry biomass (g) and its allometric relationship in each treatment; regular watering and high dose of fertiliser (WH), regular watering and low dose of fertiliser (WL), induced drought and high dose of fertiliser (DH) and induced drought and low dose of fertiliser (DL).
4. Discussion
4.1. Water Status of Saplings during Drought and in the Post-Drought Period
The level of drought stress during the experiment was documented by measuring Ψpd. While regularly watered saplings did not experience drought stress, drought-treated beech saplings in both fertilisation treatments experienced severe stress, as indicated by Ψpd values comparable to those associated with a severe water deficit in beech saplings [23] and the onset of native embolism in mature beech trees [18].
Although juvenile beech saplings typically recover Ψpd levels within one [55] to several days after drought events [23,56], our study uncovered an incomplete recovery, indicating potential non-reversible losses in hydraulic conductance. The persistence of drought effects may be attributed to embolisms formed during water stress, which may not dissolve after rewatering [57,58,59]. Drought-induced alterations in anatomical characteristics, including xylem vessel size, structure, sap flow, and whole-plant hydraulic conductivity, significantly impact a plant’s ability to cope with water scarcity [45,60,61]. These anatomical changes, coupled with physiological responses like reduced photosynthesis and stomatal conductance, collectively contribute to the overall vulnerability of plants to the destructive effects of drought [25].
However, we did not observe more serious damage linked to drought-induced xylem embolism to the plant tissues, such as defoliation [62] or enhanced mortality [18,63]. The Ψpd value of −0.5 MPa at the end of our experiment indicated a partial recovery of the water status of saplings. Based on these findings, we anticipated finding differences between regularly watered and drought-treated saplings for other investigated parameters in terms of either prolonged drought effects or recovery, underscoring the importance of considering both physiological and anatomical responses to understand a plant’s resilience to drought stress.
4.2. Nutritional Status of Saplings in the Drought Period
We recorded a positive effect of fertilisation on nitrogen, phosphorus, and potassium foliar concentrations, with both fertiliser doses enabling optimal foliar nutritional status of common beech saplings during drought. Higher dose of fertiliser significantly increased N and P concentrations, indicating increased nutrient availability. In agreement with our first hypothesis, higher nutrient availability showed a significant impact on reducing the immediate effect of drought on foliar concentrations, but only for nitrogen. Nevertheless, a high dose of fertiliser did not help keep N concentrations in drought-treated saplings at the same level as in regularly watered saplings, which is in agreement with Ouyang et al. [48], who state that drought-stressed saplings cannot benefit from the additional availability of inorganic N. While the drought effect was significant for both N and P, foliar N concentrations were affected comparatively more. This is in line with the results of Netzer et al. [64], who stated that foliar N reflects current drought stress. Multiple studies confirmed an impaired soil-borne uptake of nitrogen during drought, affecting foliar nutrient concentrations [26,65]. During periods of drought, the ability to absorb nutrients containing nitrogen from the soil is generally diminished as a result of the closure of stomata, reduced transpiration, and the mobility of nutrients [66]. However, saplings in our DH treatment did have significantly higher N concentrations in leaves in comparison with low-fertilised, drought-treated saplings, demonstrating the positive effect of fertilisation on foliar N even in drought conditions; this is in accordance with the finding that effective nitrogen nutrition shows the ability to alleviate water stress in crops by sustaining metabolic processes even at low tissue water potential [67]. Also, there is a possibility that higher nitrogen availability prior to drought led to higher foliar N concentrations during drought, as the plants were able to draw on their N reserves to sustain their growth and function. This possibility can be inferred from high N foliar values that were recorded at the peak of the drought, even in DL saplings. Drought also causes a reduction in P absorption and transport in plants, as phosphate ions move through soils primarily through diffusion, and if the water content in the soil decreases, P mobility also decreases [68]. We expected to see some reduction in foliar P with drought; however, at this point, significant differences were only found between the high-fertilisation regularly watered treatment and the low-fertilisation drought treatment. Contrary to our results, a German study that simulated summer drought decreased foliar P concentrations in common beech saplings, while N concentrations were unaffected [69]. Overall, we found that relatively small differences in foliar concentrations between fertilisation treatments could have been the result of the fact that both levels of fertilisation lead to satisfactory sapling nutrition, which may have also influenced the sapling drought response. The effects could have been stronger if the foliar nutrient status of the low-fertilisation treatment had been closer to deficiency.
4.3. Nutritional Status of Saplings in the Post-Drought Period
Nine weeks after restoring regular watering, foliar P concentrations in high-fertilised saplings matched those in regularly watered saplings, supporting the hypothesis that a high fertiliser dose promotes recovery. Previous findings indicate that the application of P can improve drought tolerance, though it is not clear if P has a direct physiological effect on a plant’s drought resistance or an indirect nutritional effect on root growth and thus enhanced soil water uptake [70]. A study by da Silva et al. [66] demonstrated that the addition of phosphorus can mitigate the negative impact of drought stress on yield. In our results, we may suspect this to be the reason for the fact that the root collar diameter increment percentage was higher in the drought-treated/high-fertilised saplings than in the drought-treated/low-fertilised saplings.
Saplings in the low-fertilisation treatment have not been able to sufficiently recover their P levels, indicating a prolonged drought effect. The slow diffusion of phosphorus in soils, compounded by reduced mobility during drought [69,71,72], can lead to increased energy demand for phosphorus uptake in plants [69,73]. Prolonged drought stress may consequently hinder energy metabolism and impede vegetative growth, highlighting the critical role of phosphorus dynamics in plant response to drought [69].
The prolonged effect of drought was also noted in N and K foliar concentrations, regardless of the fertilisation dose, pointing to the long-term effects of drought on beech physiology, in addition to the immediate drought-related effects that plants can mostly remedy based on their reserves. The foliar K concentrations in the post-drought phase displayed significant dependence on water availability, mirroring the findings of a study by da Silva et al. [66]. Interestingly, this effect was present only as the prolonged drought effect, while it was not recorded in the drought period. Some studies have shown that higher levels of K fertilisation may allow crop plants [74,75] to better tolerate water stress, but this has not been confirmed for beech so far.
4.4. Fine-Root Nutrient Concentrations
Similar to our results, Leberecht et al. [76] found no effect of drought on root N in beech saplings. We found that a prolonged drought effect was only relevant for K fine-root concentrations in the low-fertilisation treatment, also showing that higher concentrations of K can help alleviate the effects of drought. This corresponds to the findings of Peuke and Rennenberg [29], who reported decreases in root K of drought-stressed beech saplings and no effect of drought on nitrogen root concentrations. On the other hand, the lack of a prolonged effect of drought for other nutrients, as well as K in HF treatment, may indicate a rapid recovery of beech roots after the drought. During seasonal drought, beech roots largely reflect a “fast” strategy, meaning that beech has an adaptable fine-root system that uses a fast mobilisation of internal storages for new fine-root growth in order to ensure sustained resource uptake [77].
4.5. Effects of Drought and Fertilisation on Sapling Growth and Biomass Partitioning
The growth response of beech saplings to drought was found to be twofold: while drought caused a decreased height increment where fertilisation could not even out the loss to height growth caused by the lack of water, increased nutrient availability had a positive effect on the root collar diameter increment of drought-exposed saplings.
The stimulating effect of increased fertiliser doses on the height and root collar diameter of beech saplings has been documented in previous studies [51,78]. As root collar diameter was shown to predict the survival of young saplings after transplanting [79], we can assume that this effect of fertilisation on root collar diameter can help mitigate the negative effects of drought on the success of afforestation with beech saplings. In contrast, we may expect problems with sapling growth and survival due to drought being more frequent in nutrient-poor soils. In our case, although increased nutrient availability had a positive effect on the root collar diameter increment of drought-exposed saplings, it did not promote diameter growth to the point of fully reducing the effects of drought; the prolonged drought effect was recorded for both height and diameter increments and in both fertiliser treatments. These findings are contrary to our hypothesis that higher fertilisation will alleviate the prolonged effect of drought on growth and biomass production in beech saplings.
Comparisons between saplings with different watering and fertilisation treatments (WH and DL) revealed a lower belowground to aboveground biomass ratio for WH saplings. Trees were shown to invest more assimilates underground after drought [80], increasing root depth and root-to-shoot ratios [81]. Plants in drought conditions generally invest less in the stem and leaves to reduce water loss [82,83]. On the other hand, tree species with a higher investment in leaves and stems tend to maintain stomatal opening and resist stem embolism during a drought event [84], increasing their mortality risk. Our findings align with the concept that plants adjust their biomass allocation in response to changes in nutrient availability and drought conditions [31,85,86,87].
Although drought typically reduces growth and shifts biomass partitioning to fine-root biomass [88], our study noted an increase in belowground biomass and coarse-root biomass in response to drought, possibly due to the relatively short duration of the drought event and sufficient nutrient availability. Some studies indicate that moderate drought tends to increase fine-root biomass, while long-lasting or extreme drought may have the opposite effect [22,33,81].
The absence of differences in fine-root biomass among treatments may also signify a rapid growth response of fine roots after drought, supporting the concept of ephemeral roots [33]. These thin, ephemeral roots shed during drought and are capable of regrowth upon soil rewetting, providing beech trees with a competitive advantage by enabling rapid proliferation when resources become available [77].
5. Conclusions
Our study emphasises the importance of maintaining optimal nutrient availability for the nutritional status and growth of beech saplings during drought, also shedding light on possible adaptive strategies. Although the benefits of high nutrient availability to combat drought stress seem to be limited, fertilisation played a crucial role in the recovery of phosphorus levels after drought, which is important in the context of a general decline of foliar phosphorus concentrations in Europe. The prolonged drought effect on nitrogen and potassium foliar concentrations also emphasises the enduring physiological impact of drought on beech trees.
Our findings contribute various insights into beech nutrient responses and growth patterns, expanding our understanding of the impact of climate change on beech trees. Regarding the effects of drought on beech saplings such as reduced height increment and biomass production, we propose the adequate fertilisation of saplings to mitigate the physiological consequences of drought, ensuring a larger root collar diameter as a quality measure and a prerequisite for successful afforestation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14122445/s1, Table S1. Main effects of irrigation (regular watering vs. induced drought), fertilisation (high dose vs. low dose of fertiliser) and its interaction (irrigation × fertilisation) on nitrogen (N), phosphorus (P) and potassium (K) foliar concentration in the drought and post-drought period, as calculated with two-way ANOVA. F values for the factors and their interactions are shown; Table S2. Main effects of irrigation (regular watering vs. induced drought), fertilisation (high dose vs. low dose of fertiliser) and its interaction (irrigation × fertilisation) on nitrogen (N), phosphorus (P) and potassium (K) root concentration, at the end of the experiment., as calculated with two-way ANOVA. F values for the factors and their interactions are shown; Figure S1. Pot experiment in the greenhouse; Figure S2. Experimental design layout of the greenhouse pot experiment using a latin square arrangement.
Author Contributions
Conceptualisation, M.M., I.S. (Ivan Seletković), K.S. and N.P.; data curation, M.M. and M.O.; formal analysis, M.M., M.O. and M.J.; funding acquisition, I.S. (Ivan Seletković) and N.P.; investigation, M.M., I.S. (Ivan Seletković), M.O., M.Š., I.S. (Ivana Sirovica), I.Z., R.B. and N.P.; methodology, M.M., I.S. (Ivan Seletković), M.O., K.S. and N.P.; project administration, M.M., I.S. (Ivan Seletković), M.O. and N.P.; resources, N.P.; supervision, K.S. and N.P.; visualisation, M.M.; writing—original draft, M.M., I.S. (Ivan Seletković), M.O., M.J., K.S. and N.P.; writing—review and editing, M.M., I.S. (Ivan Seletković), M.O., M.J., K.S., M.S., A.G., M.Š., I.S. (Ivana Sirovica), R.B. and N.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been fully supported by the Croatian Science Foundation under the project VitaClim (IP-2018-01-5222). The work of doctoral student Mia Marušić has been supported by the “Young researchers’ career development project–training of doctoral students” of the Croatian Science Foundation. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of Croatian Science Foundation.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We highly appreciate the work of researchers and technicians of the Croatian Forest Research Institute who participated in the measurements and laboratory analysis for this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Frei, E.R.; Gossner, M.M.; Vitasse, Y.; Queloz, V.; Dubach, V.; Gessler, A.; Ginzler, C.; Hagedorn, F.; Meusburger, K.; Moor, M.; et al. European Beech Dieback after Premature Leaf Senescence during the 2018 Drought in Northern Switzerland. Plant Biol. 2022, 24, 1132–1145. [Google Scholar] [CrossRef] [PubMed]
- Babst, F.; Bouriaud, O.; Poulter, B.; Trouet, V.; Girardin, M.P.; Frank, D.C. Twentieth Century Redistribution in Climatic Drivers of Global Tree Growth. Sci. Adv. 2019, 5, eaat4313. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A Global Overview of Drought and Heat-Induced Tree Mortality Reveals Emerging Climate Change Risks for Forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Hartmann, H.; Bastos, A.; Das, A.J.; Esquivel-Muelbert, A.; Hammond, W.M.; Martínez-Vilalta, J.; McDowell, N.G.; Powers, J.S.; Pugh, T.A.M.; Ruthrof, K.X.; et al. Climate Change Risks to Global Forest Health: Emergence of Unexpected Events of Elevated Tree Mortality Worldwide. Annu. Rev. Plant Biol. 2022, 73, 673–702. [Google Scholar] [CrossRef]
- Bredemeier, M. Forest Management and the Water Cycle: An Ecosystem-Based Approach; Bredemeier, M., Cohen, S., Godbold, D.L., Lode, E., Pichler, V., Schleppi, P., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2011; ISBN 978-90-481-9834-4. [Google Scholar]
- Glatthorn, J.; Annighöfer, P.; Balkenhol, N.; Leuschner, C.; Polle, A.; Scheu, S.; Schuldt, A.; Schuldt, B.; Ammer, C. An Interdisciplinary Framework to Describe and Evaluate the Functioning of Forest Ecosystems. Basic Appl. Ecol. 2021, 52, 1–14. [Google Scholar] [CrossRef]
- Brunet, J.; Fritz, Ö.; Richnau, G. Biodiversity in European Beech Forests—A Review with Recommendations for Sustainable Forest Management. Ecol. Bull. 2010, 53, 77–94. [Google Scholar]
- Leuschner, C.; Ellenberg, H. Ecology of Central European Forests; Springer: Cham, Switzerland, 2017; Volume I, ISBN 9783319430409. [Google Scholar]
- Wang, F.; Israel, D.; Ramírez-Valiente, J.A.; Sánchez-Gómez, D.; Aranda, I.; Aphalo, P.J.; Robson, T.M. Seedlings from Marginal and Core Populations of European Beech (Fagus sylvatica L.) Respond Differently to Imposed Drought and Shade. Trees Struct. Funct. 2020, 35, 53–67. [Google Scholar] [CrossRef]
- Stojnić, S.; Orlović, S.; Miljković, D.; Galić, Z.; Kebert, M.; von Wuehlisch, G. Provenance Plasticity of European Beech Leaf Traits under Differing Environmental Conditions at Two Serbian Common Garden Sites. Eur. J. For. Res. 2015, 134, 1109–1125. [Google Scholar] [CrossRef]
- Seletković, Z.; Tikvić, I.; Prpić, B. Ekološka Konstitucija Obične Bukve. In Obična Bukva (Fagus sylvatica L.) u Hrvatskoj; Matić, S., Ed.; Akademija šumarskih znanosti: Zagreb, Republic of Croatia, 2003. [Google Scholar]
- Pretzsch, H.; Hilmers, T.; Uhl, E.; Bielak, K.; Bosela, M.; del Rio, M.; Dobor, L.; Forrester, D.I.; Nagel, T.A.; Pach, M.; et al. European Beech Stem Diameter Grows Better in Mixed than in Mono-Specific Stands at the Edge of Its Distribution in Mountain Forests. Eur. J. For. Res. 2021, 140, 127–145. [Google Scholar] [CrossRef]
- Bolte, A.; Czajkowski, T.; Cocozza, C.; Tognetti, R.; De Miguel, M.; Pšidová, E.; Ditmarová, L.; Dinca, L.; Delzon, S.; Cochard, H.; et al. Desiccation and Mortality Dynamics in Seedlings of Different European Beech (Fagus sylvatica L.) Populations under Extreme Drought Conditions. Front. Plant Sci. 2016, 7, 751. [Google Scholar] [CrossRef]
- Gessler, A.; Keitel, C.; Kreuzwieser, J.; Matyssek, R.; Seiler, W.; Rennenberg, H. Potential Risks for European Beech (Fagus sylvatica L.) in a Changing Climate. Trees Struct. Funct. 2007, 21, 1–11. [Google Scholar] [CrossRef]
- Meier, I.C.; Leuschner, C. Belowground Drought Response of European Beech: Fine Root Biomass and Carbon Partitioning in 14 Mature Stands across a Precipitation Gradient. Glob. Chang. Biol. 2008, 14, 2081–2095. [Google Scholar] [CrossRef]
- Leuschner, C. Drought Response of European Beech (Fagus sylvatica L.)—A Review. Perspect. Plant Ecol. Evol. Syst. 2020, 47, 125576. [Google Scholar] [CrossRef]
- Schuldt, B.; Ruehr, N.K. Responses of European Forests to Global Change-Type Droughts. Plant Biol. 2022, 24, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Walthert, L.; Ganthaler, A.; Mayr, S.; Saurer, M.; Waldner, P.; Walser, M.; Zweifel, R.; von Arx, G. From the Comfort Zone to Crown Dieback: Sequence of Physiological Stress Thresholds in Mature European Beech Trees across Progressive Drought. Sci. Total Environ. 2021, 753, 141792. [Google Scholar] [CrossRef] [PubMed]
- Martinez del Castillo, E.; Zang, C.S.; Buras, A.; Hacket-Pain, A.; Esper, J.; Serrano-Notivoli, R.; Hartl, C.; Weigel, R.; Klesse, S.; Resco de Dios, V.; et al. Climate-Change-Driven Growth Decline of European Beech Forests. Commun. Biol. 2022, 5, 163. [Google Scholar] [CrossRef] [PubMed]
- Rukh, S.; Sanders, T.G.M.; Krüger, I.; Schad, T.; Bolte, A. Distinct Responses of European Beech (Fagus sylvatica L.) to Drought Intensity and Length—A Review of the Impacts of the 2003 and 2018–2019 Drought Events in Central Europe. Forests 2023, 14, 248. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Klein, T.; Bartlett, M.; Sack, L.; Pellegrini, A.F.A.; Choat, B.; Jansen, S. Meta-Analysis Reveals That Hydraulic Traits Explain Cross-Species Patterns of Drought-Induced Tree Mortality across the Globe. Proc. Natl. Acad. Sci. USA 2016, 113, 5024–5029. [Google Scholar] [CrossRef]
- Zang, U.; Goisser, M.; Grams, T.E.E.; Häberle, K.H.; Matyssek, R.; Matzner, E.; Borken, W.; Epron, D. Fate of Recently Fixed Carbon in European Beech (Fagus sylvatica) Saplings during Drought and Subsequent Recovery. Tree Physiol. 2014, 34, 29–38. [Google Scholar] [CrossRef]
- Arend, M.; Sever, K.; Pflug, E.; Gessler, A.; Schaub, M. Seasonal Photosynthetic Response of European Beech to Severe Summer Drought: Limitation, Recovery and Post-Drought Stimulation. Agric. For. Meteorol. 2016, 220, 83–89. [Google Scholar] [CrossRef]
- Rennenberg, H.; Loreto, F.; Polle, A.; Brilli, F.; Fares, S.; Beniwal, R.S.; Gessler, A. Physiological Responses of Forest Trees to Heat and Drought. Plant Biol. 2006, 8, 556–571. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Reum Han, A.; Han, A.; Kim, H.S. Responses to Drought Stress in Prunus sargentii and Larix kaempferi Seedlings Using Morphological and Physiological Parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- Gessler, A.; Keitel, C.; Nahm, M.; Rennenberg, H.; Geßler, A.; Keitel, C.; Nahm, M.; Rennenberg, H. Water Shortage Affects the Water and Nitrogen Balance in Central European Beech Forests. Plant Biol. 2004, 6, 289–298. [Google Scholar] [CrossRef]
- Salehi, M.; Walthert, L.; Zimmermann, S.; Waldner, P.; Schmitt, M.; Schleppi, P.; Liechti, K.; Ahmadi, M.; Zahedi Amiri, G.; Brunner, I.; et al. Leaf Morphological Traits and Leaf Nutrient Concentrations of European Beech Across a Water Availability Gradient in Switzerland. Front. For. Glob. Chang. 2020, 3, 19. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Gessler, A. Global Climate Change and Tree Nutrition: Influence of Water Availability. Tree Physiol. 2010, 30, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Peuke, A.D.; Rennenberg, H. Impacts of Drought on Mineral Macro- and Microelements in Provenances of Beech (Fagus sylvatica L.) Seedlings. Tree Physiol. 2011, 31, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Gessler, A.; Schaub, M.; McDowell, N.G. The Role of Nutrients in Drought-Induced Tree Mortality and Recovery. New Phytol. 2016, 214, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Schönbeck, L.; Li, M.H.; Lehmann, M.M.; Rigling, A.; Schaub, M.; Hoch, G.; Kahmen, A.; Gessler, A. Soil Nutrient Availability Alters Tree Carbon Allocation Dynamics during Drought. Tree Physiol. 2021, 41, 697–707. [Google Scholar] [CrossRef]
- Thiel, D.; Kreyling, J.; Backhaus, S.; Beierkuhnlein, C.; Buhk, C.; Egen, K.; Huber, G.; Konnert, M.; Nagy, L.; Jentsch, A. Different Reactions of Central and Marginal Provenances of Fagus sylvatica to Experimental Drought. Eur. J. For. Res. 2014, 133, 247–260. [Google Scholar] [CrossRef]
- Leuschner, C.; Backes, K.; Hertel, D.; Schipka, F.; Schmitt, U.; Terborg, O.; Runge, M. Drought Responses at Leaf, Stem and Fine Root Levels of Competitive Fagus sylvatica L. and Quercus petraea (Matt.) Liebl. Trees in Dry and Wet Years. For. Ecol. Manag. 2001, 149, 33–46. [Google Scholar] [CrossRef]
- Ruehr, N.K.; Offermann, C.A.; Gessler, A.; Winkler, J.B.; Ferrio, J.P.; Buchmann, N.; Barnard, R.L. Drought Effects on Allocation of Recent Carbon: From Beech Leaves to Soil CO2 Efflux. New Phytol. 2009, 184, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Zang, U.; Goisser, M.; Häberle, K.H.; Matyssek, R.; Matzner, E.; Borken, W. Effects of Drought Stress on Photosynthesis, Rhizosphere Respiration, and Fine-Root Characteristics of Beech Saplings: A Rhizotron Field Study. J. Plant Nutr. Soil Sci. 2014, 177, 168–177. [Google Scholar] [CrossRef]
- Bista, D.R.; Heckathorn, S.A.; Jayawardena, D.M.; Mishra, S.; Boldt, J.K. Effects of Drought on Nutrient Uptake and the Levels of Nutrient-Uptake Proteins in Roots of Drought-Sensitive and -Tolerant Grasses. Plants 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-Wide Reduction in Primary Productivity Caused by the Heat and Drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Varsamis, G.; Papageorgiou, A.C.; Merou, T.; Takos, I.; Malesios, C.; Manolis, A.; Tsiripidis, I.; Gailing, O. Adaptive Diversity of Beech Seedlings under Climate Change Scenarios. Front. Plant Sci. 2019, 9, 1918. [Google Scholar] [CrossRef]
- Gessler, A.; Bottero, A.; Marshall, J.; Arend, M. The Way Back: Recovery of Trees from Drought and Its Implication for Acclimation. New Phytol. 2020, 228, 1704–1709. [Google Scholar] [CrossRef]
- González de Andrés, E.; Rosas, T.; Camarero, J.J.; Martínez-Vilalta, J. The Intraspecific Variation of Functional Traits Modulates Drought Resilience of European Beech and Pubescent Oak. J. Ecol. 2021, 109, 3652–3669. [Google Scholar] [CrossRef]
- Zang, U.; Goisser, M.; Meyer, N.; Häberle, K.H.; Borken, W. Chemical and Morphological Response of Beech Saplings (Fagus sylvatica L.) to an Experimental Soil Drought Gradient. For. Ecol. Manag. 2021, 498, 119569. [Google Scholar] [CrossRef]
- Coomes, D.A.; Jenkins, K.L.; Cole, L.E.S. Scaling of Tree Vascular Transport Systems along Gradients of Nutrient Supply and Altitude. Biol. Lett. 2007, 3, 86–90. [Google Scholar] [CrossRef]
- Mitchell, P.J.; O’Grady, A.P.; Tissue, D.T.; White, D.A.; Ottenschlaeger, M.L.; Pinkard, E.A. Drought Response Strategies Define the Relative Contributions of Hydraulic Dysfunction and Carbohydrate Depletion during Tree Mortality. New Phytol. 2013, 197, 862–872. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of Plant Survival and Mortality during Drought: Why Do Some Plants Survive While Others Succumb to Drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Scoffoni, C.; Albuquerque, C.; Brodersen, C.R.; Townes, S.V.; John, G.P.; Cochard, H.; Buckley, T.N.; McElrone, A.J.; Sack, L. Leaf Vein Xylem Conduit Diameter Influences Susceptibility to Embolism and Hydraulic Decline. New Phytol. 2017, 213, 1076–1092. [Google Scholar] [CrossRef]
- Geremew, A.; Carson, L.; Woldesenbet, S.; Carpenter, C.; Peace, E.; Weerasooriya, A. Interactive Effects of Organic Fertilizers and Drought Stress on Growth and Nutrient Content of Brassica juncea at Vegetative Stage. Sustainability 2021, 13, 13948. [Google Scholar] [CrossRef]
- Schönbeck, L.; Gessler, A.; Schaub, M.; Rigling, A.; Hoch, G.; Kahmen, A.; Li, M.-H. Soil Nutrients and Lowered Source:Sink Ratio Mitigate Effects of Mild but Not of Extreme Drought in Trees. Environ. Exp. Bot. 2020, 169, 103905. [Google Scholar] [CrossRef]
- Ouyang, S.-N.; Gessler, A.; Saurer, M.; Hagedorn, F.; Gao, D.-C.; Wang, X.-Y.; Schaub, M.; Li, M.-H.; Shen, W.-J.; Schönbeck, L. Root Carbon and Nutrient Homeostasis Determines Downy Oak Sapling Survival and Recovery from Drought. Tree Physiol. 2021, 41, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ouyang, S.; Gessler, A.; Wang, X.; Na, R.; He, H.S.; Wu, Z.; Li, M.H. Root Carbon Resources Determine Survival and Growth of Young Trees Under Long Drought in Combination with Fertilization. Front. Plant Sci. 2022, 13, 929855. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.F.; Mateus, N.S.; Rosário, M.O.; Garcez, T.B.; Mazzafera, P.; Lavres, J. Enhancing Potassium Content in Leaves and Stems Improves Drought Tolerance of Eucalyptus Clones. Physiol. Plant. 2021, 172, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Potočić, N.; Seletković, I.; Čater, M.; Ćosić, T.; Mario, Š.; Vedriš, M.; Šango, M.; Vedriš, M. Ekofiziološki Odziv Suncu Izloženih Sadnica Obične Bukve (Fagus sylvatica L.) Pri Različitim Razinama Gnojidbe. Sumar. List 2009, 133, 289–300. [Google Scholar]
- Rautio, P.; Fürst, A.; Stefan, K.; Raitio, H.; Bartels, U. Part XII: Sampling and Analysis of Needles and Leaves. In UNECE ICP Forests Programme Co-Ordinating Centre (ed.): Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; Thünen Institute of ForestEcosystems: Eberswalde, Germany, 2022; p. 19 + Annex. [Google Scholar]
- Mellert, K.H.; Göttlein, A. Comparison of New Foliar Nutrient Thresholds Derived from van Den Burg’s Literature Compilation with Established Central European References. Eur. J. For. Res. 2012, 131, 1461–1472. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 12 December 2022).
- Pflug, E.E.; Buchmann, N.; Siegwolf, R.T.W.; Schaub, M.; Rigling, A.; Arend, M. Resilient Leaf Physiological Response of European Beech (Fagus sylvatica L.) to Summer Drought and Drought Release. Front. Plant Sci. 2018, 9, 187. [Google Scholar] [CrossRef]
- Tognetti, R.; Johnson, J.D.; Michelozzi, M. The Response of European Beech (Fagus sylvatica L.) Seedlings from Two Italian Populations to Drought and Recovery. Trees 1995, 9, 348–354. [Google Scholar] [CrossRef]
- Johnson, K.M.; Jordan, G.J.; Brodribb, T.J. Wheat Leaves Embolized by Water Stress Do Not Recover Function upon Rewatering. Plant. Cell Environ. 2018, 41, 2704–2714. [Google Scholar] [CrossRef]
- Tomasella, M.; Nardini, A.; Hesse, B.D.; MacHlet, A.; Matyssek, R.; Häberle, K.H. Close to the Edge: Effects of Repeated Severe Drought on Stem Hydraulics and Non-Structural Carbohydrates in European Beech Saplings. Tree Physiol. 2019, 39, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.A.; Batz, T.A.; McAdam, S.A.M. Xylem Embolism Resistance Determines Leaf Mortality during Drought in Persea Americana. Plant Physiol. 2020, 182, 547. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.O.; An, J.Y.; Combalicer, M.S.; Chun, J.P.; Oh, S.K.; Park, B.B. Morpho-Anatomical Traits and Soluble Sugar Concentration Largely Explain the Responses of Three Deciduous Tree Species to Progressive Water Stress. Front. Plant Sci. 2021, 12, 738301. [Google Scholar] [CrossRef]
- Hesse, B.D.; Gebhardt, T.; Hafner, B.D.; Hikino, K.; Reitsam, A.; Gigl, M.; Dawid, C.; Häberle, K.-H.; Grams, T.E.E. Physiological Recovery of Tree Water Relations upon Drought Release—Response of Mature Beech and Spruce after Five Years of Recurrent Summer Drought. Tree Physiol. 2023, 43, 522–538. [Google Scholar] [CrossRef] [PubMed]
- Arend, M.; Link, R.M.; Zahnd, C.; Hoch, G.; Schuldt, B.; Kahmen, A. Lack of Hydraulic Recovery as a Cause of Post-Drought Foliage Reduction and Canopy Decline in European Beech. New Phytol. 2022, 234, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- García-Plazaola, J.I.; Becerril, J.M. Effects of Drought on Photoprotective Mechanisms in European Beech (Fagus sylvatica L.) Seedlings from Different Provenances. Trees Struct. Funct. 2000, 14, 485–490. [Google Scholar] [CrossRef]
- Netzer, F.; Thöm, C.; Celepirovic, N.; Ivankovic, M.; Alfarraj, S.; Dounavi, A.; Simon, J.; Herschbach, C.; Rennenberg, H. Drought Effects on C, N, and P Nutrition and the Antioxidative System of Beech Seedlings Depend on Geographic Origin. J. Plant Nutr. Soil Sci. 2016, 179, 136–150. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Uscola, M.; Jacobs, D.F. The Role of Stored Carbohydrates and Nitrogen in the Growth and Stress Tolerance of Planted Forest Trees. New For. 2015, 46, 813–839. [Google Scholar] [CrossRef]
- da Silva, E.; Nogueira, R.; da Silva, M.; de Albuquerque, M. Drought Stress and Plant Nutrition. Plant Stress 2011, 5, 32–41. [Google Scholar]
- Saud, S.; Fahad, S.; Yajun, C.; Ihsan, M.Z.; Hammad, H.M.; Nasim, W.; Amanullah; Arif, M.; Alharby, H. Effects of Nitrogen Supply on Water Stress and Recovery Mechanisms in Kentucky Bluegrass Plants. Front. Plant Sci. 2017, 8, 983. [Google Scholar] [CrossRef] [PubMed]
- Nye, P.H.; Tinker, P.B. Solute Movement in the Soil-Root System; Studies in Ecology; University of California Press: Oakland, CA, USA, 1977; ISBN 9780520034518. [Google Scholar]
- Peuke, A.D.; Rennenberg, H. Carbon, Nitrogen, Phosphorus, and Sulphur Concentration and Partitioning in Beech Ecotypes (Fagus sylvatica L.): Phosphorus Most Affected by Drought. Trees 2004, 18, 639–648. [Google Scholar] [CrossRef]
- Tariq, A.; Pan, K.; Olatunji, O.A.; Graciano, C.; Li, Z.; Sun, F.; Zhang, L.; Wu, X.; Chen, W.; Song, D.; et al. Phosphorous Fertilization Alleviates Drought Effects on Alnus Cremastogyne by Regulating Its Antioxidant and Osmotic Potential. Sci. Rep. 2018, 8, 5644. [Google Scholar] [CrossRef] [PubMed]
- Rausch, C.; Bucher, M. Molecular Mechanisms of Phosphate Transport in Plants. Planta 2002, 216, 23–37. [Google Scholar] [CrossRef]
- Goll, D.S.; Joetzjer, E.; Huang, M.; Ciais, P. Low Phosphorus Availability Decreases Susceptibility of Tropical Primary Productivity to Droughts. Geophys. Res. Lett. 2018, 45, 8231–8240. [Google Scholar] [CrossRef]
- Grossman, A.R.; Takahashi, H. Macronutrient Utilization by Photosynthetic Eukaryotes and the Fabric of Interactions. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 163–210. [Google Scholar] [CrossRef]
- Grzebisz, W.; Gransee, A.; Szczepaniak, W.; Diatta, J. The Effects of Potassium Fertilization on Water-Use Efficiency in Crop Plants. J. Plant Nutr. Soil Sci. 2013, 176, 355–374. [Google Scholar] [CrossRef]
- Martineau, E.; Domec, J.C.; Bosc, A.; Denoroy, P.; Fandino, V.A.; Lavres, J.; Jordan-Meille, L. The Effects of Potassium Nutrition on Water Use in Field-Grown Maize (Zea mays L.). Environ. Exp. Bot. 2017, 134, 62–71. [Google Scholar] [CrossRef]
- Leberecht, M.; Dannenmann, M.; Tejedor, J.; Simon, J.; Rennenberg, H.; Polle, A. Segregation of Nitrogen Use between Ammonium and Nitrate of Ectomycorrhizas and Beech Trees. Plant. Cell Environ. 2016, 39, 2691–2700. [Google Scholar] [CrossRef]
- Nikolova, P.S.; Bauerle, T.L.; Häberle, K.H.; Blaschke, H.; Brunner, I.; Matyssek, R. Fine-Root Traits Reveal Contrasting Ecological Strategies in European Beech and Norway Spruce During Extreme Drought. Front. Plant Sci. 2020, 11, 1211. [Google Scholar] [CrossRef] [PubMed]
- Bagherzadeh, A.; Brumme, R.; Beese, F. Biomass and Nutrients Allocation in Pot Cultured Beech Seedlings: Influence of Nitrogen Fertilizer. J. For. Res. 2008, 19, 263–270. [Google Scholar] [CrossRef]
- Jacobs, D.F.; Salifu, K.F.; Seifert, J.R. Relative Contribution of Initial Root and Shoot Morphology in Predicting Field Performance of Hardwood Seedlings. New For. 2005, 30, 235–251. [Google Scholar] [CrossRef]
- Hagedorn, F.; Joseph, J.; Peter, M.; Luster, J.; Pritsch, K.; Geppert, U.; Kerner, R.; Molinier, V.; Egli, S.; Schaub, M.; et al. Recovery of Trees from Drought Depends on Belowground Sink Control. Nat. Plants 2016, 2, 16111. [Google Scholar] [CrossRef] [PubMed]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How Tree Roots Respond to Drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K. Drought Stress in Plants: An Overview. In Plant Responses to Drought Stress; From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33. ISBN 978-3-642-32652-3. [Google Scholar]
- Lauri, P.-É.; Marceron, A.; Normand, F.; Dambreville, A.; Regnard, J.-L. Soil Water Deficit Decreases Xylem Conductance Efficiency Relative to Leaf Area and Mass in the Apple. J. Plant Hydraul. 2014, 1, e0003. [Google Scholar] [CrossRef]
- Chen, Z.; Li, S.; Wan, X.; Liu, S. Strategies of Tree Species to Adapt to Drought from Leaf Stomatal Regulation and Stem Embolism Resistance to Root Properties. Front. Plant Sci. 2022, 13, 926535. [Google Scholar] [CrossRef] [PubMed]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012; ISBN 9780123849052. [Google Scholar]
- Guasconi, D.; Manzoni, S.; Hugelius, G. Climate-Dependent Responses of Root and Shoot Biomass to Drought Duration and Intensity in Grasslands–a Meta-Analysis. Sci. Total Environ. 2023, 903, 166209. [Google Scholar] [CrossRef]
- Greenwood, D.J. Quantitative Theory and the Control of Soil Fertility. New Phytol. 1983, 94, 1–18. [Google Scholar] [CrossRef]
- van Hees, A. Growth and Morphology of Pedunculate Oak (Quercus robur L.) and Beech (Fagus sylvatica L.) Seedlings in Relation to Shading and Drought. Ann. Sci. For. 1997, 54, 9–18. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
