Trends in Atmospheric CO2 Fertilization Effects with Stand Age Based on Tree Rings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Annual Cycle Data
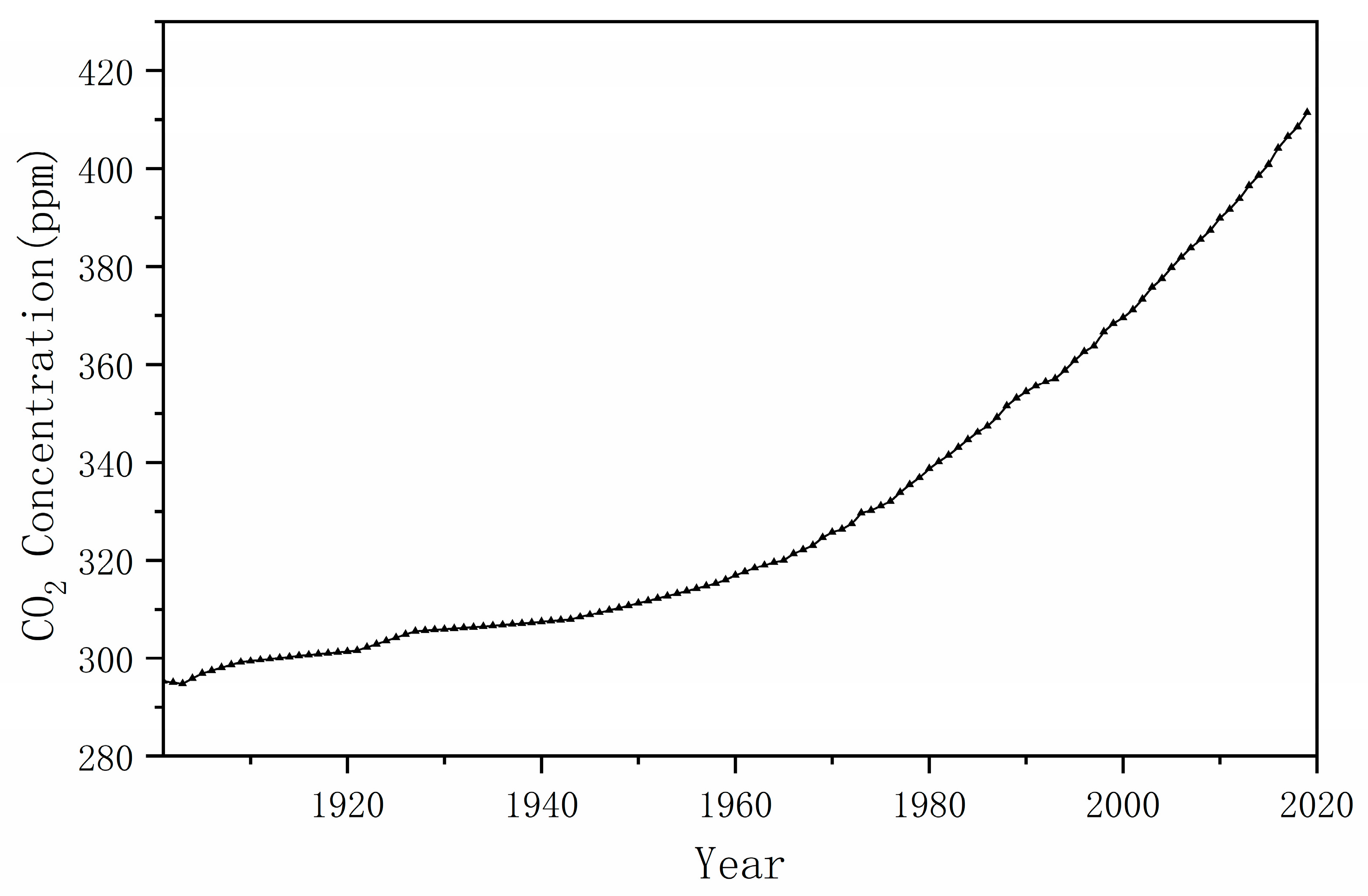
2.2. Atmospheric CO2 Concentration Data
2.3. Climate Data
2.4. Atmospheric Nitrogen Deposition Data
2.5. Koppen Climate Type Data
2.6. Data Processing
3. Results
3.1. Generalized Additive Mixed Model Results
3.2. Trends in CO2 Fertilization Effects with Stand Age
3.3. Differences in the Effects of CO2 Fertilization in Different Climatic Zones
3.4. Differences in CO2 Fertilization Effects across Forest Types
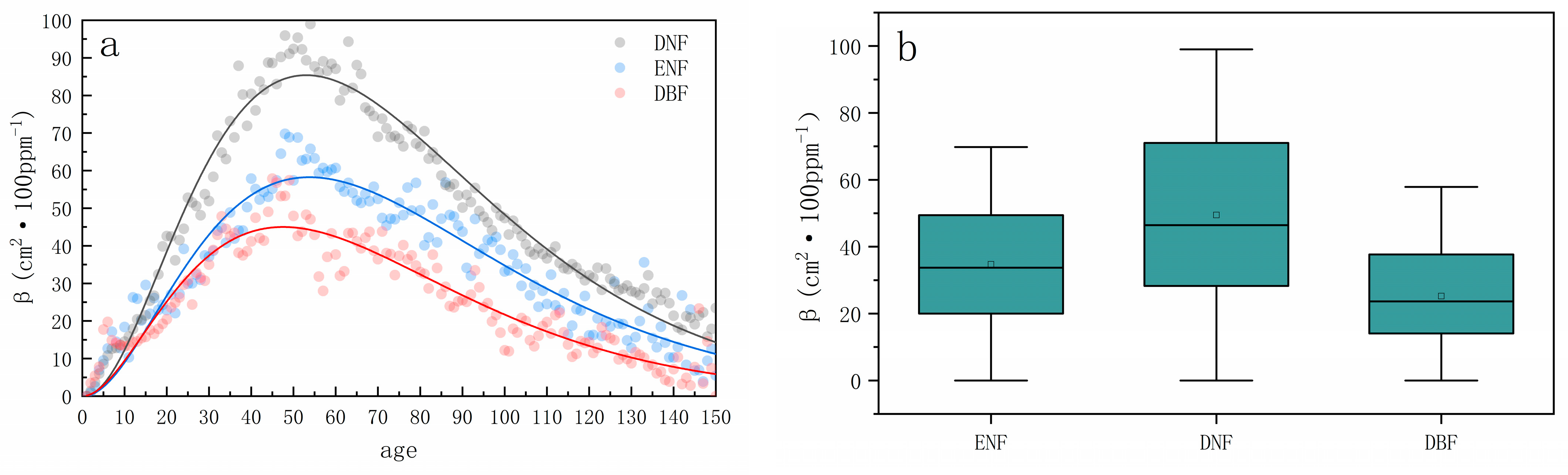
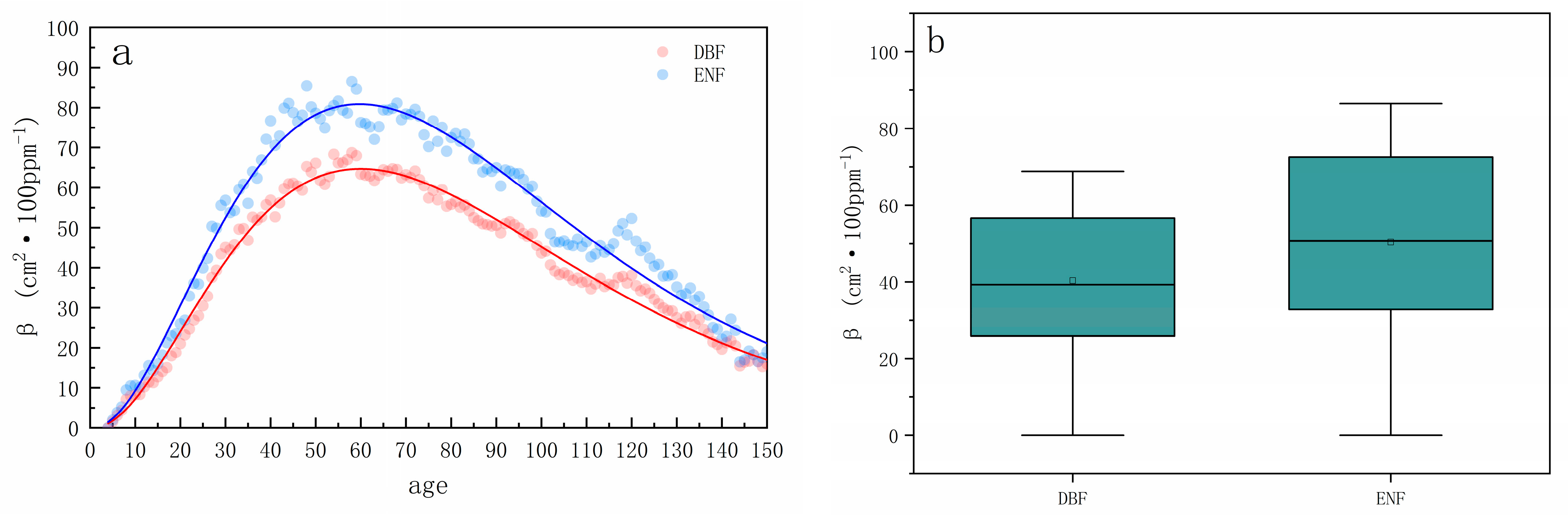

| Climatic Zone | Stand Type | Parameters | R2 | SSE | RMSE | RF | |||
|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | ||||||
| B (Arid) | ENF | 11.20 | 11.53 | 23.42 | 2.26 | 0.96 | 3492.39 | 4.89 | 146 |
| DNF | 9.69 | 9.92 | 24.96 | 2.15 | 0.89 | 4747.05 | 5.70 | 146 | |
| DBF | 9.44 | 8.64 | 23.45 | 2.03 | 0.87 | 3797.77 | 5.10 | 146 | |
| ENF | 13.75 | 12.52 | 27.93 | 1.99 | 0.96 | 4287.93 | 5.42 | 146 | |
| C (Temperate) | DBF | 10.01 | 9.42 | 27.72 | 2.04 | 0.97 | 725.73 | 2.23 | 146 |
| ENF | 11.41 | 12.18 | 30.35 | 1.67 | 0.88 | 5610.83 | 6.20 | 146 | |
| D (Continental) | DNF | 13.29 | 13.60 | 33.01 | 1.39 | 0.70 | 2244.25 | 12.40 | 146 |
| DBF | 10.67 | 11.54 | 34.43 | 1.55 | 0.93 | 2024.99 | 3.72 | 146 | |
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- MacCracken, M.C. Prospects for Future Climate Change and the Reasons for Early Action. J. Air Waste Manag. Assoc. 2008, 58, 735–786. [Google Scholar] [CrossRef] [PubMed]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Luke, G.; Hauck, J.; Le Corinne, Q.; Luijkx, I.T.; Olsen, A.; Peters, G.P.; et al. Global Carbon Budget 2022. Earth Syst. Sci. Data 2022, 14, 4811–4900. [Google Scholar] [CrossRef]
- He, M.; Piao, S.; Huntingford, C.; Xu, H.; Wang, X.; Bastos, A.; Cui, J.; Gasser, T. Amplified warming from physiological responses to carbon dioxide reduces the potential of vegetation for climate change mitigation. Commun. Earth Environ. 2022, 3, 160. [Google Scholar] [CrossRef]
- Gifford, L. See the Carbon through the Trees: Market-Based Climate Change Mitigation, Forest Carbon Offsets and the Uneven Power of Carbon Accounting. Ph.D. Thesis, University of Colorado, Boulder, CO, USA, 2018. [Google Scholar]
- Yue, C.; Ciais, P.; Cadule, P.; Thonicke, K.; van Leeuwen, T.T. Modelling the role of fires in the terrestrial carbon balance by incorporating SPITFIRE into the global vegetation model ORCHIDEE—Part 2: Carbon emissions and the role of fires in the global carbon balance. Geosci. Model Dev. 2015, 8, 1321–1338. [Google Scholar] [CrossRef]
- Gampe, D.; Zscheischler, J.; Reichstein, M.; O’Sullivan, M.; Smith, W.K.; Sitch, S.; Buermann, W. Increasing impact of warm droughts on northern ecosystem productivity over recent decades. Nat. Clim. Change 2021, 11, 772–779. [Google Scholar] [CrossRef]
- Piao, S.; Sitch, S.; Ciais, P.; Friedlingstein, P.; Peylin, P.; Wang, X.; Ahlström, A.; Anav, A.; Canadell, J.G.; Cong, N. Evaluation of terrestrial carbon cycle models for their response to climate variability and to CO2 trends. Glob. Change Biol. 2013, 19, 2117–2132. [Google Scholar] [CrossRef] [PubMed]
- Bacastow, R.; Keeling, C.D. Atmospheric carbon dioxide and radiocarbon in the natural carbon cycle: II. Changes from AD 1700 to 2070 as deduced from a geochemical model. In Proceedings of the Brookhaven Symposia in Biology, Upton, NY, USA, 16–18 May 1972; pp. 86–135. [Google Scholar]
- Huang, J.-G.; Bergeron, Y.; Denneler, B.; Berninger, F.; Tardif, J. Response of forest trees to increased atmospheric CO2. Crit. Rev. Plant Sci. 2007, 26, 265–283. [Google Scholar] [CrossRef]
- Bazzaz, F.A. The response of natural ecosystems to the rising global CO2 levels. Annu. Rev. Ecol. Syst. 1990, 21, 167–196. [Google Scholar] [CrossRef]
- Field, C.B.; Jackson, R.B.; Mooney, H.A. Stomatal responses to increased CO2: Implications from the plant to the global scale. Plant Cell Environ. 1995, 18, 1214–1225. [Google Scholar] [CrossRef]
- Norby, R.J.; Zak, D.R. Ecological lessons from free-air CO2 enrichment (FACE) experiments. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 181–203. [Google Scholar] [CrossRef]
- Jiang, G.; Qu, C. Photosynthetic response of six woody species to elevated CO2, in quercus liaotungensis forest in the beijing mountainous areas. Chin. J. Plant Ecol. 2000, 24, 204–208. [Google Scholar]
- Li, Z.; Chen, W.; Wei, J.; Keyimu, M.; Zhang, Y.; Zhang, S.; Wang, X. Tree-ring growth responses of Liaodong Oak (Quercus wutaishanica) to climate in the Beijing Dongling Mountain of China. Acta Ecol. Sin. 2021, 41, 27–37. [Google Scholar]
- Babst, F.; Alexander, M.R.; Szejner, P.; Bouriaud, O.; Klesse, S.; Roden, J.; Ciais, P.; Poulter, B.; Frank, D.; Moore, D.J. A tree-ring perspective on the terrestrial carbon cycle. Oecologia 2014, 176, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Fritts, H. Tree Rings and Climate; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Battipaglia, G.; Zalloni, E.; Castaldi, S.; Marzaioli, F.; Cazzolla-Gatti, R.; Lasserre, B.; Tognetti, R.; Marchetti, M.; Valentini, R.J. Long tree-ring chronologies provide evidence of recent tree growth decrease in a central African tropical forest. PLoS ONE 2015, 10, e0120962. [Google Scholar] [CrossRef] [PubMed]
- Cook, E.R.; Peters, K. The smoothing spline: A new approach to standardizing forest interior tree-ring width series for dendroclimatic studies. Tree-Ring Bull. 1981, 41, 45–53. [Google Scholar]
- Biondi, F.; Qeadan, F.J. A theory-driven approach to tree-ring standardization: Defining the biological trend from expected basal area increment. Tree-Ring Res. 2008, 64, 81–96. [Google Scholar] [CrossRef]
- Wu, X.; Shao, X. Status of dendroclimatological study and its prospects in China. Adv. Earth Sci. 1993, 8, 31–35. [Google Scholar]
- Chen, Z.; Zhang, X.; He, X.; Davi, N.K.; Li, L.; Bai, X. Response of radial growth to warming and CO2 enrichment in southern Northeast China: A case of Pinus tabulaeformis. Clim. Change 2015, 130, 559–571. [Google Scholar] [CrossRef]
- Geoff Wang, G.; Chhin, S.; Bauerle, W.L. Effect of natural atmospheric CO2 fertilization suggested by open-grown white spruce in a dry environment. Glob. Change Biol. 2006, 12, 601–610. [Google Scholar] [CrossRef]
- Huang, J.-G.; Zhang, Q.-B. Tree rings and climate for the last 680 years in Wulan area of northeastern Qinghai-Tibetan Plateau. Clim. Change 2007, 80, 369–377. [Google Scholar] [CrossRef]
- Graybill, D.A.; Idso, S.B. Detecting the aerial fertilization effect of atmospheric CO2 enrichment in tree-ring chronologies. Glob. Biogeochem. Cycles 1993, 7, 81–95. [Google Scholar] [CrossRef]
- Knapp, P.A.; Soulé, P.T.; Grissino-Mayer, H.D. Detecting potential regional effects of increased atmospheric CO2 on growth rates of western juniper. Glob. Change Biol. 2001, 7, 903–917. [Google Scholar] [CrossRef]
- Schweingruber, F.; Briffa, K.; Nogler, P. A tree-ring densitometric transect from Alaska to Labrador: Comparison of ring-width and maximum-latewood-density chronologies in the conifer belt of northern North America. Int. J. Biometeorol. 1993, 37, 151–169. [Google Scholar] [CrossRef]
- Girardin, M.P.; Bernier, P.Y.; Raulier, F.; Tardif, J.C.; Conciatori, F.; Guo, X.J. Testing for a CO2 fertilization effect on growth of Canadian boreal forests. J. Geophys. Res. Biogeosci. 2011, 116, G01012. [Google Scholar] [CrossRef]
- Zhao, S.; Pederson, N.; D’Orangeville, L.; HilleRisLambers, J.; Boose, E.; Penone, C.; Bauer, B.; Jiang, Y.; Manzanedo, R.D. The International Tree-Ring Data Bank (ITRDB) revisited: Data availability and global ecological representativity. J. Biogeogr. 2019, 46, 355–368. [Google Scholar] [CrossRef]
- Neftel, A.; Friedli, H.; Moor, E.; Lötscher, H.; Oeschger, H.; Siegenthaler, U.; Stauffer, B.J.T. Historical carbon dioxide record from the Siple Station ice core. J. Geophys. Res. Atmos. 1994, 93, 92093-0444. [Google Scholar]
- Girardin, M.P.; Bouriaud, O.; Hogg, E.H.; Kurz, W.; Zimmermann, N.E.; Metsaranta, J.M.; de Jong, R.; Frank, D.C.; Esper, J.; Büntgen, U. No growth stimulation of Canada’s boreal forest under half-century of combined warming and CO2 fertilization. Proc. Natl. Acad. Sci. USA 2016, 113, E8406–E8414. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Ju, W.; Chen, J.M.; Ciais, P.; Cescatti, A.; Sardans, J.; Janssens, I.A.; Wu, M.; Berry, J.A. Recent global decline of CO2 fertilization effects on vegetation photosynthesis. Science 2020, 370, 1295–1300. [Google Scholar] [CrossRef]
- Climatic Research Unit, Met Office. CRU TS Version 4.05. Available online: https://crudata.uea.ac.uk/cru/data/hrg/cru_ts_4.05/ (accessed on 20 June 2022).
- Hegglin, M.; Kinnison, D.; Lamarque, J.-F. CCMI nitrogen surface fluxes in support of CMIP6—Version 2.0. Version 20160706. Earth Syst. Grid Fed. Available online: https://www.wdc-climate.de/ui/cmip6?input=input4MIPs.CMIP6.CMIP.NCAR.NCAR-CCMI-2-0 (accessed on 20 June 2022).
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Amthor, J.S. Terrestrial higher-plant response to increasing atmospheric [CO2] in relation to the global carbon cycle. Glob. Change Biol. 1995, 1, 243–274. [Google Scholar] [CrossRef]
- Arp, W. Effects of source-sink relations on photosynthetic acclimation to elevated CO2. Plant Cell Environ. 1991, 14, 869–875. [Google Scholar] [CrossRef]
- Bowes, G. Facing the inevitable: Plants and increasing atmospheric CO2. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993, 44, 309–332. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Ceulemans, R.; Mousseau, M. Tansley Review No. 71 Effects of elevated atmospheric CO2 on woody plants. New Phytol. 1994, 127, 425–446. [Google Scholar] [CrossRef]
- Saxe, H.; Ellsworth, D.S.; Heath, J. Tree and forest functioning in an enriched CO2 atmosphere. New Phytol. 1998, 139, 395–436. [Google Scholar] [CrossRef]
- Körner, C. Plant CO2 responses: An issue of definition, time and resource supply. New Phytol. 2006, 172, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Norby, R.J.; Wullschleger, S.D.; Gunderson, C.A.; Johnson, D.W.; Ceulemans, R. Tree responses to rising CO2 in field experiments: Implications for the future forest. Plant Cell Environ. 1999, 22, 683–714. [Google Scholar] [CrossRef]
- Wang, X. Effects of species richness and elevated carbon dioxide on biomass accumulation: A synthesis using meta-analysis. Oecologia 2007, 152, 595–605. [Google Scholar] [CrossRef]
- Ryan, M.G.; Binkley, D.; Fownes, J.H. Age-related decline in forest productivity: Pattern and process. Adv. Ecol. Res. 1997, 27, 213–262. [Google Scholar]
- Ellsworth, D.S.; Anderson, I.C.; Crous, K.Y.; Cooke, J.; Drake, J.E.; Gherlenda, A.N.; Gimeno, T.E.; Macdonald, C.A.; Medlyn, B.E.; Powell, J.R. Elevated CO2 does not increase eucalypt forest productivity on a low-phosphorus soil. Nat. Commun. 2017, 7, 279–282. [Google Scholar] [CrossRef]
- Oren, R.; Ellsworth, D.S.; Johnsen, K.H.; Phillips, N.; Ewers, B.E.; Maier, C.; Schäfer, K.V.; McCarthy, H.; Hendrey, G.; McNulty, S.G. Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enriched atmosphere. Nature 2001, 411, 469–472. [Google Scholar] [CrossRef]
- Sigurdsson, B.D.; Medhurst, J.L.; Wallin, G.; Eggertsson, O.; Linder, S.J. Growth of mature boreal Norway spruce was not affected by elevated [CO2] and/or air temperature unless nutrient availability was improved. Tree Physiol. 2013, 33, 1192–1205. [Google Scholar] [CrossRef]
- Lloyd, J.; Farquhar, G. The CO2 dependence of photosynthesis, plant growth responses to elevated atmospheric CO2 concentrations and their interaction with soil nutrient status. I. General principles and forest ecosystems. Funct. Ecol. 1996, 10, 4–32. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Lichter, J. Limited carbon storage in soil and litter of experimental forest plots under increased atmospheric CO2. Nature 2001, 411, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Soulé, P.T.; Knapp, P.A. Radial growth rate increases in naturally occurring ponderosa pine trees: A late-20th century CO2 fertilization effect? Glob. Change Biol. 2006, 171, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, M.F.; Ineson, P.; Scott, A. Elevated CO2 reduces the nitrogen concentration of plant tissues. Glob. Change Biol. 1998, 4, 43–54. [Google Scholar] [CrossRef]
- Luo, Y.; Su, B.; Currie, W.S.; Dukes, J.S.; Finzi, A.; Hartwig, U.; Hungate, B.; McMurtrie, R.E.; Oren, R.; Parton, W.J. Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. BioScience 2004, 54, 731–739. [Google Scholar] [CrossRef]
- Norby, R.J.; Warren, J.M.; Iversen, C.M.; Medlyn, B.E.; McMurtrie, R.E. CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proc. Natl. Acad. Sci. USA 2010, 107, 19368–19373. [Google Scholar] [CrossRef] [PubMed]
- Loehle, C. Anomalous responses of plants to CO2 enrichment. Oikos 1995, 73, 181–187. [Google Scholar] [CrossRef]
- Long, S. Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations: Has its importance been underestimated? Plant Cell Environ. 1991, 14, 729–739. [Google Scholar] [CrossRef]
- Desjardins, R.; Gifford, R.; Nilson, T.; Greenwood, E.; Gifford, R. Interaction of carbon dioxide with growth-limiting environmental factors in vegetation productivity: Implications for the global carbon cycle. In Advances in Bioclimatology; Springer: Berlin/Heidelberg, Germany, 1992; pp. 24–58. [Google Scholar]
- Rowland-Bamford, A.; Baker, J.; Allen, L., Jr.; Bowes, G. Interactions of CO2 enrichment and temperature on carbohydrate accumulation and partitioning in rice. Environ. Exp. Bot. 1996, 36, 111–124. [Google Scholar] [CrossRef]
- Van Der Sleen, P.; Groenendijk, P.; Vlam, M.; Anten, N.P.; Boom, A.; Bongers, F.; Pons, T.L.; Terburg, G.; Zuidema, P.A. No growth stimulation of tropical trees by 150 years of CO2 fertilization but water-use efficiency increased. Nat. Geosci. 2015, 8, 24–28. [Google Scholar] [CrossRef]
- Hendrickson, L.; Ball, M.; Wood, J.; Chow, W.; Furbank, R.T. Low temperature effects on photosynthesis and growth of grapevine. Plant Cell Environ. 2004, 27, 795–809. [Google Scholar] [CrossRef]
- Sage, R.F.; Kubien, D.S. The temperature response of C3 and C4 photosynthesis. Plant Cell Environ. 2007, 30, 1086–1106. [Google Scholar] [CrossRef] [PubMed]
- Owensby, C.E.; Ham, J.M.; Knapp, A.K.; Auen, L.M. Biomass production and species composition change in a tallgrass prairie ecosystem after long-term exposure to elevated atmospheric CO2. Glob. Change Biol. 1999, 5, 497–506. [Google Scholar] [CrossRef]
- Curtis, P.S.; Wang, X. A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 1998, 113, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Piao, S.; Wang, K.; Wang, X.; Wang, T.; Ciais, P.; Chen, A.; Lian, X.; Peng, S.; Peñuelas, J.; et al. Temporal trade-off between gymnosperm resistance and resilience increases forest sensitivity to extreme drought. Nat. Ecol. Evol. 2020, 4, 1075–1083. [Google Scholar] [CrossRef]
- McGroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C:N:P stoichiometry in forests worldwide: Implications of terrestrial redfield-type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]
- Niklas, K.J. Plant allometry, leaf nitrogen and phosphorus stoichiometry, and interspecific trends in annual growth rates. Ann. Bot. 2006, 97, 155–163. [Google Scholar] [CrossRef]
- Hansen, J.; Beck, E. The fate and path of assimilation products in the stem of 8-year-old Scots pine (Pinus sylvestris L.) trees. Trees 1990, 4, 16–21. [Google Scholar] [CrossRef]
- Oribe, Y.; Funada, R.; Kubo, T. Relationships between cambial activity, cell differentiation and the localization of starch in storage tissues around the cambium in locally heated stems of Abies sachalinensis (Schmidt) Masters. Trees 2003, 17, 185–192. [Google Scholar] [CrossRef]
- Oberhuber, W.; Swidrak, I.; Pirkebner, D.; Gruber, A. Temporal dynamics of nonstructural carbohydrates and xylem growth in Pinus sylvestris exposed to drought. Can. J. For. Res. 2011, 41, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Kurepin, L.V.; Stangl, Z.R.; Ivanov, A.G.; Bui, V.; Mema, M.; Hüner, N.P.; Öquist, G.; Way, D.; Hurry, V. Contrasting acclimation abilities of two dominant boreal conifers to elevated CO2 and temperature. Plant Cell Environ. 2018, 41, 1331–1345. [Google Scholar] [CrossRef] [PubMed]
- Way, D.A.; Sage, R.F. Elevated growth temperatures reduce the carbon gain of black spruce [Picea mariana (Mill.) BSP]. Glob. Change Biol. 2008, 14, 624–636. [Google Scholar] [CrossRef]
- Way, D.A.; Sage, R.F. Thermal acclimation of photosynthesis in black spruce [Picea mariana (Mill.) BSP]. Plant Cell Environ. 2008, 31, 1250–1262. [Google Scholar] [CrossRef]
- Zhang, X.W.; Wang, J.R.; Ji, M.F.; Milne, R.I.; Wang, M.H.; Liu, J.-Q.; Shi, S.; Yang, S.-L.; Zhao, C.-M. Higher thermal acclimation potential of respiration but not photosynthesis in two alpine Picea taxa in contrast to two lowland congeners. PLoS ONE 2015, 10, e0123248. [Google Scholar] [CrossRef]
- Needham, J.F.; Chambers, J.; Fisher, R.; Knox, R.; Koven, C.D. Forest responses to simulated elevated CO2 under alternate hypotheses of size-and age-dependent mortality. Glob. Change Biol. 2020, 26, 5734–5753. [Google Scholar] [CrossRef]
- Yu, K.; Smith, W.K.; Trugman, A.T.; Condit, R.; Hubbell, S.P.; Sardans, J.; Peng, C.; Zhu, K.; Peñuelas, J.; Cailleret, M.; et al. Pervasive decreases in living vegetation carbon turnover time across forest climate zones. Proc. Natl. Acad. Sci. USA 2019, 116, 24662–24667. [Google Scholar] [CrossRef]
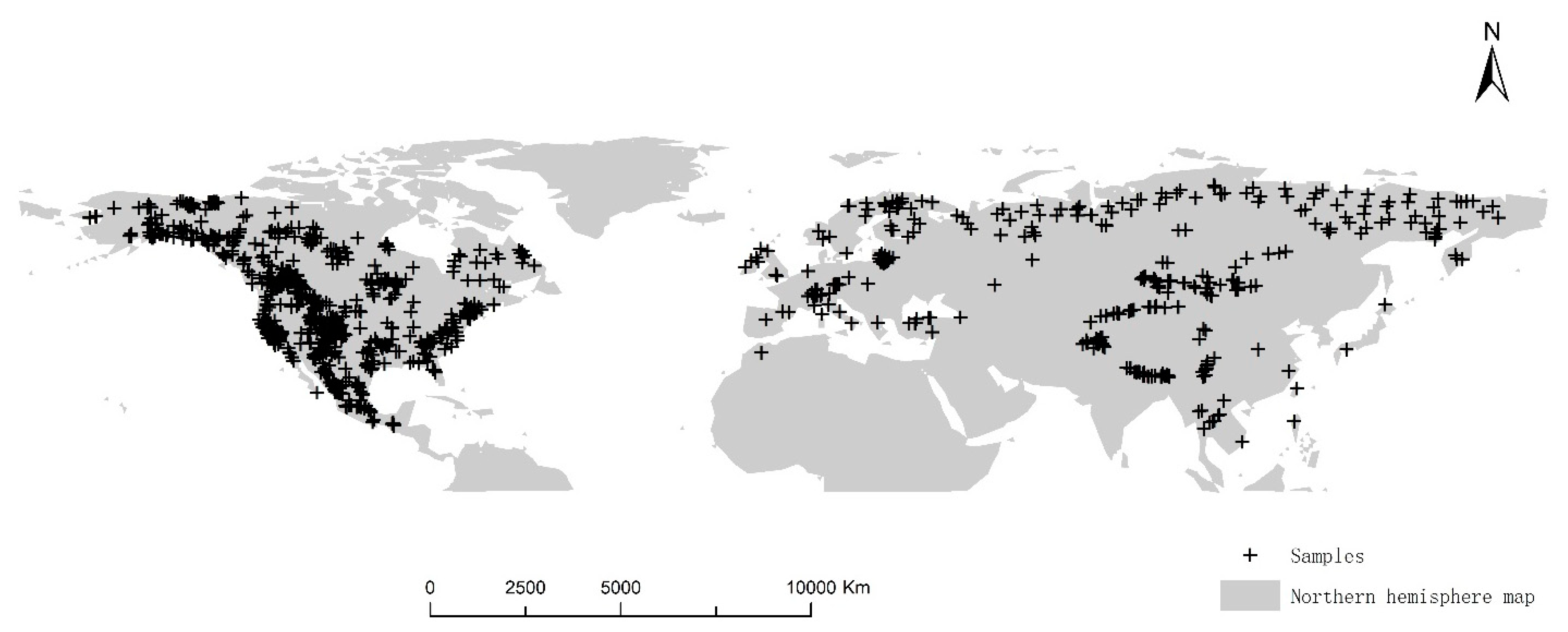

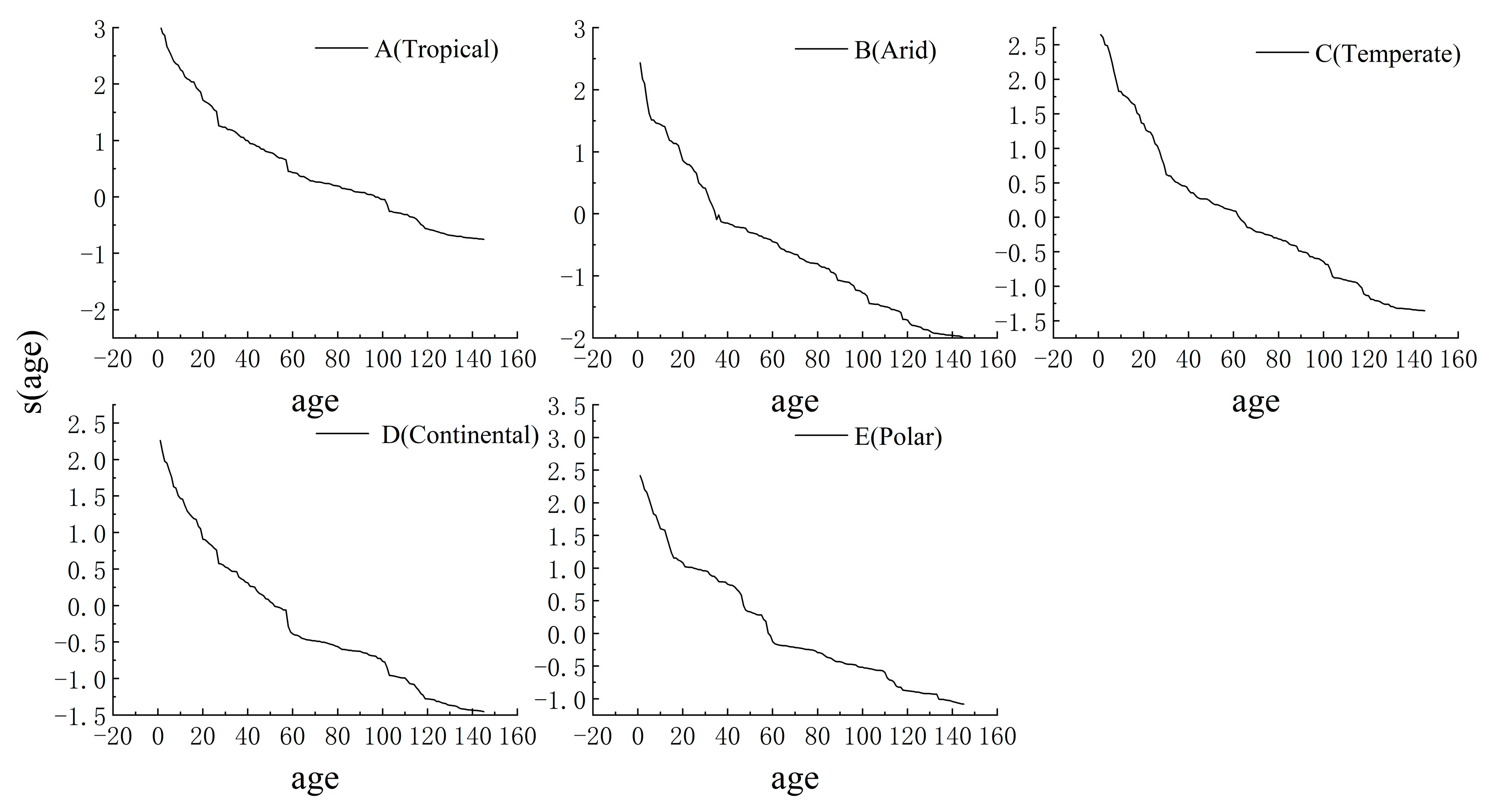
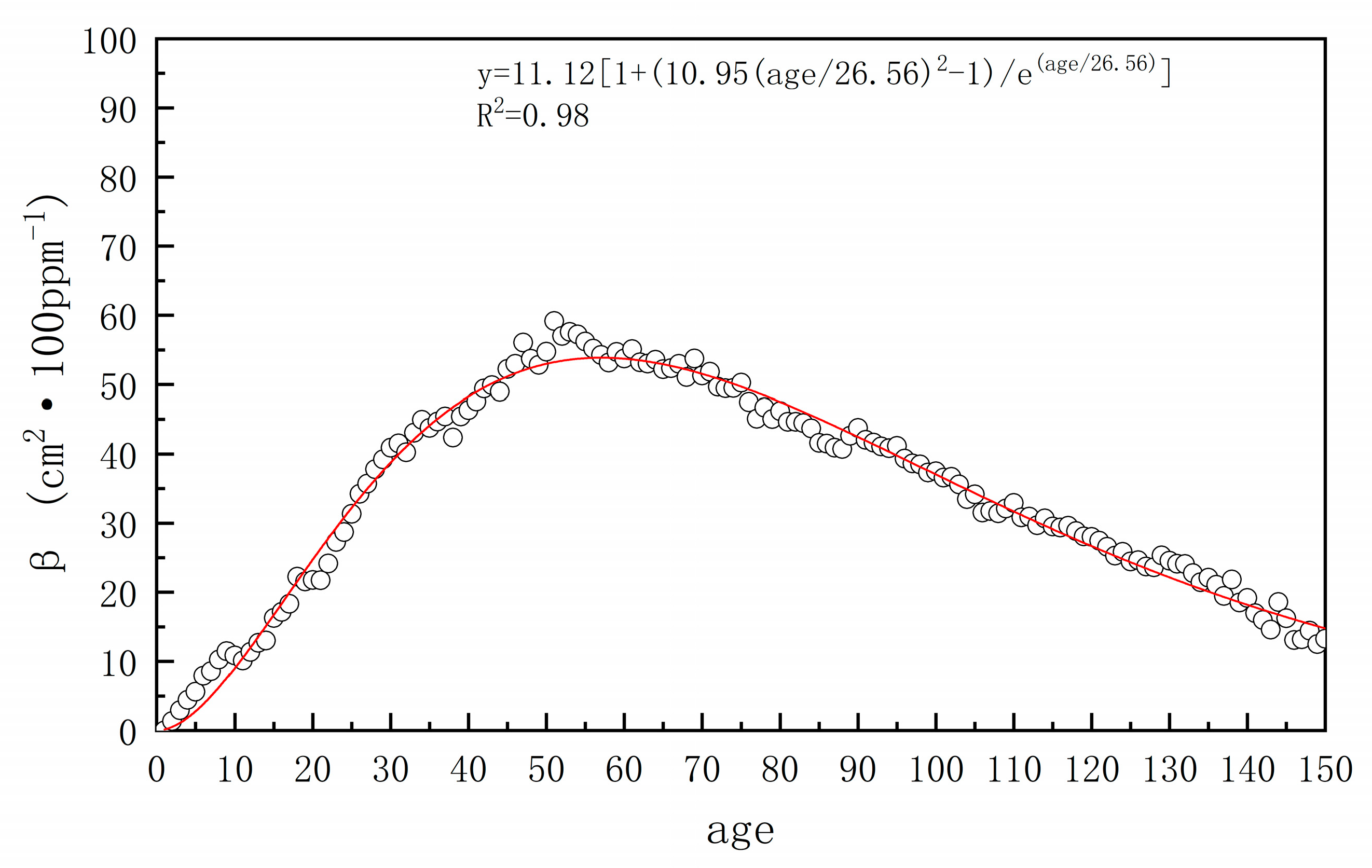

| Climate Zone | No. of Samples | Plant Number |
|---|---|---|
| A (Tropical) | 15 | 241 |
| B (Arid) | 235 | 7744 |
| C (Temperate) | 55 | 1657 |
| D (Continental) | 1085 | 25,033 |
| E (Polar) | 55 | 1657 |
| Parameter Term | Nonparametric Term | |||||||
|---|---|---|---|---|---|---|---|---|
| Climate Zone | Intercept | Standard Error | t-Value | p-Value | Variant | Freedom | p-Value | Radj2 |
| A (Tropical) | 0.89 | 0.33 | 43.82 | <0.01 | DBH | 17.44 | <0.01 | 0.83 |
| Age | 3.21 | <0.01 | ||||||
| B (Arid) | 1.84 | 0.05 | 33.34 | <0.01 | DBH | 16.46 | <0.01 | 0.79 |
| Age | 9.30 | <0.01 | ||||||
| C (Temperate) | 1.98 | 0.04 | 55.04 | <0.01 | DBH | 25.89 | <0.01 | 0.87 |
| Age | 5.25 | <0.01 | ||||||
| D (Continental) | 1.78 | 0.72 | 24.49 | <0.01 | DBH | 15.28 | <0.01 | 0.75 |
| Age | 4.49 | <0.01 | ||||||
| E (Polar) | 0.61 | 0.04 | 15.53 | <0.01 | DBH | 18.30 | <0.01 | 0.81 |
| Age | 7.52 | <0.01 | ||||||
| Climate Zone | Parameters | R2 | SSE | RMSE | RF | |||
|---|---|---|---|---|---|---|---|---|
| a | b | c | d | |||||
| A (Tropical) | 26.67 | 13.29 | 29.10 | 1.66 | 0.86 | 49508.65 | 18.41 | 146 |
| B (Arid) | 9.73 | 13.41 | 24.22 | 2.17 | 0.96 | 2784.55 | 4.37 | 146 |
| C (Temperate) | 9.46 | 16.92 | 28.85 | 1.92 | 0.97 | 9828.68 | 8.20 | 146 |
| D (Continental) | 11.25 | 9.14 | 29.25 | 1.95 | 0.98 | 521.96 | 1.89 | 146 |
| E (Polar) | 8.08 | 9.69 | 29.99 | 1.73 | 0.63 | 2626.73 | 4.08 | 146 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, B.; Li, M.; Kong, X.; Bian, S. Trends in Atmospheric CO2 Fertilization Effects with Stand Age Based on Tree Rings. Forests 2023, 14, 2441. https://doi.org/10.3390/f14122441
Chen Y, Wang B, Li M, Kong X, Bian S. Trends in Atmospheric CO2 Fertilization Effects with Stand Age Based on Tree Rings. Forests. 2023; 14(12):2441. https://doi.org/10.3390/f14122441
Chicago/Turabian StyleChen, Yanxi, Bin Wang, Mingze Li, Xiangqi Kong, and Shaojie Bian. 2023. "Trends in Atmospheric CO2 Fertilization Effects with Stand Age Based on Tree Rings" Forests 14, no. 12: 2441. https://doi.org/10.3390/f14122441
APA StyleChen, Y., Wang, B., Li, M., Kong, X., & Bian, S. (2023). Trends in Atmospheric CO2 Fertilization Effects with Stand Age Based on Tree Rings. Forests, 14(12), 2441. https://doi.org/10.3390/f14122441





