Abstract
The effect of pruning treatments on growth, photosynthesis characteristics, and metabolites were was studied in Eucommia ulmoides Oliver (E. ulmoides). The experiment was carried out from March–August 2019. Three treatments were used: non-pruned trees (CK), a height of 20 cm above the top edge of the flowerpot (T1), and a height of 10 cm above the top edge of the flowerpot (T2). The results showed that the branches branch number, leaves leaf number, and stem diameter increased significantly (p < 0.05) in pruning treatments compared with CK. Similarly, the net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (Tr), maximum photosynthetic efficiency (Fv/Fm), and non-photochemical quenching coefficient (NPQ) increased significantly in pruning treatments (p < 0.05). Interestingly, the contents of Chl a, Chl b, Chl, Car, and the rate between the Chl a content and the Chl b content increased significantly (p < 0.05) in T2, respectively. These verified that it was a better way to enhance the plants growth of E. ulmoides for pruning treatments. The GC-MS analysis showed that 36 different primary metabolites were identified, including 11 sugars, 13 acids, 5 alcohols, and 7 other compounds, the relative content of their metabolites were was higher in the T2 treatment than that in the T1 treatment, which was mainly concentrated in four main enrichment pathways (Galactose metabolism; Citrate cycle; Glyoxylate and dicarboxylate metabolism; and starch and sucrose metabolism) via KEGG analysis. Meanwhile, correlation analysis showed there were was a positive correlation between the accumulation of D-Galactose, D-Mannose, Succinic acid, and photosynthetic pigment content, and the rate of photosynthesis in T2 treatment (p < 0.05). The pruning height above the top edge of the flowerpot changed the accumulation of primary metabolites and promoted plant regeneration ability in E. ulmoides. Finally, the yield of main secondary metabolites from leaves (Genipin, Geniposide, Geniposidic acid, and Pinoresinol diglucoside) were was increased in pruning treatments by UPLC analysis. It provided a reference for the directional ecological cultivation of E. ulmoides.
1. Introduction
Eucommia ulmoides Oliver (E. ulmoides) is part of the Eucommia genus and is a distinctive relic species in China [1]. It is a rare and endangered protected tree species; the leaves of E ulmoides contain various active compounds and nutritional components, which have successively been included as raw materials in the Chinese Pharmacopoeia [2]. The high-value utilization of resources led to the reduction in wild E. ulmoides. The efficient cultivation of E ulmoides is an important basis for improving raw materials, and so the planting of E. ulmoides has been expanded [3,4].
Pruning is a technique used in plant cultivation; it can enhance the regeneration capacity of plants and change the contents of metabolites of plants [5]. Proper pruning techniques were used to minimize the hazard of pathogens and increase the yield of plants [6]. It has been shown that pruning treatment enhanced the biomass accumulation of roots and leaves, increased leaves area and number, and promoted growth in Manchurian Ash [7]. In Gossypium hirsutum L., pruning treatment affects photosynthesis and photo-assimilate partitioning in relation to yield formation, which increases cotton yields mainly by increasing canopy photosynthesis [8]. Similarly, healthy leaves in pruning treatment are found to be approximately five times more than non-pruned treatment in Diospyros melanoxylon Roxb [9]. Meanwhile, in Vitis vinífera L. cv. ‘Malbec’, the yield of fresh leaves after pruning treatments increased over five folds compared with control, and the contents of carbohydrates also increased significantly (p < 0.05) [10]. The net photosynthetic rate (Pn), transpiration (Tr), and stomatal conductance (Gs) resulted in an increase in oil in Cinosi N.’s [11] study under summer pruning treatment. Pruning, as a vital management factor, removes the apical meristem branches and leaves, which changes plant architecture, plant biomass allocation, and the yield of inflorescences and cannabinoids per plant and area in medicinal Cannabis [12,13]. In addition, the different heights of the pruning treatments may affect plant growth, biomass investment, and distribution [14]. Appropriate pruning treatments can promote growth and improve the biomass of the plant. Therefore, in the study, the different heights of the pruning treatments were set. The characteristics of growth, photosynthetic parameters, primary metabolites, and secondary metabolites in E. ulmoides were analyzed. The study aims to provide a reasonable pruning height of E. ulmoides to enhance the yield of secondary metabolites, which supports novel insights into medicinal plant cultivation.
2. Material and Methods
2.1. Plant Materials
Three-year-old Eucommia ulmoides Oliver (E. ulmoides) potted seedlings were grown in the greenhouse of Key Laboratory of Forest Plant Ecology of Ministry of Education, Northeast Forestry University (45.75° N, 126.63° E), Harbin, China. The cultivated soil was a 1:1 uniform mixture of nutrient soil and common soil. The contents of soil organic carbon, nitrogen, and phosphorus, respectively, are 571.11 ± 38.48, 24.92 ± 0.98, and 2.35 ± 0.25 (g/kg). In our research, 90 mature plants were selected: 30 plants were pruned to a height of 20 cm above the top edge of the flowerpot (T1), 30 plants were pruned to a height of 10 cm above the top edge of the flowerpot (T2), and the others were unpruned as the control (CK) (Figure 1). After 6 months, leaves leaf samples collected were used for measuring leaves leaf number, leaf area, fresh and dry mass, photosynthetic parameters, primary metabolites, and secondary metabolites. Branches samples collected were used for the determination of branch numbers. Stem samples collected were used for the determination of stem diameter. Each treatment includes three biological replicates.
Figure 1.
Different height of pruning treatments from E. ulmoides seedling. CK (unpruned); T1 (pruned at a height 20 cm above the top edge of flowerpot); T2 (pruned at a height 10 cm above the top edge of flowerpot).
2.2. Measurement of Growth and Photosynthetic Characteristics
2.2.1. Growth Indices
The leaves leaf area was calculated according to the tracing numbers grid method. The leaf’s fresh weight was weighted with an analytical balance (MS205DU; Mettler Toledo, Columbus, OH, USA). The leaf number, branch number, and stem diameter were determined.
2.2.2. Photosynthetic Parameters
Photosynthetic parameters were determined using a portable photosynthesis system (LI–6400XT, LI–COR, Lincoln, NE, USA). The net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular carbon dioxide concentration (Ci), and transpiration rate (Tr) were measured. The water use efficiency (Ewu), Leaf carboxylation efficiency (Ec), and stomatal limitation (Ls) were calculated according to Dong’s et al. [15] method formula:
Ewu = Pn/Tr
Ec = Pn/Ci
Ls = 1 − Ci/Ca
Ca was 400 μmol/mol.
2.2.3. Chlorophyll Fluorescence Parameters
According to Cen’s et al. method [16], a portable chlorophyll fluorescence meter (PAM-2500, Shanghai Zequan Technology Company, Shanghai, China) was used to measure chlorophyll fluorescence parameters. The maximum photosynthetic efficiency (Fv/Fm), actual photosynthetic efficiency (ΦPS II), non-photochemical quenching coefficient (NPQ), photochemical quenching coefficient (QP), the quantum yield of PS II regulatory energy dissipation (YNPQ), the quantum yield of PS II non-regulatory energy dissipation (YNO), and the relative electron transfer rate of PS II (ETR) were calculated according to the method of Yao et al. [15].
The Fv/Fm, ΦPS II, NPQ, QP, YNPQ, YNO, and ETR were calculated using the following formula:
Fv/Fm = (Fm − Fo)/Fm
ΦPS II = (Fm′ − F′)/Fm′
NPQ = Fm/Fm′ − 1
QP = (Fm′ − F)/(Fm′ − Fo′)
YNPQ = 1 − ΦPS II − 1/[NPQ + 1 + qP (Fm/Fo − 1)]
YNO = 1/[NPQ + 1 + QP (Fm/Fo − 1)]
ETR = PAR · ΦPS II × 0.84 × 0.5
2.2.4. Photosynthetic Pigments Content
Photosynthetic pigments (chlorophyll a, chlorophyll b, chlorophyll, and carotenoid) were estimated according to Wei’s [17] method. An amount of 0.05 g fresh leaf weight of was taken and homogenized by grinding with 5 mL dimethyl sulfoxide. Samples were analyzed using a spectrophotometer (Shimadzu UV-2550, Kyoto, Japan) at wavelengths of 480, 649, and 665 nm. The Chl a, Chl b, Chl, Car, and Chl a/b content were calculated using the following formula:
Chl a = 12.19 × A665 − 3.45 × A649
Chl b = 21.99 × A649 − 5.32 × A665
Chl = CChl a + CChl b.
Car = (1000 × A480 − 2.14 × Chl a − 70.16 × Chl b)/220
Chl a/b = Chl a/Chl b
2.3. Determination of Primary Metabolites
The leaves were saved at −80 °C, and GC-MS sample extraction was carried out according to Liu’s et al. method [18]. An Agilent (Agilent) 7890A series automatic sampler (Agilent Technologies, Santa Clara, CA, USA) with an Agilent 5975C gas chromatography–mass spectrometer and a non-polar DB-5 capillary chromatography column (30 m × 250 μm ID, J&W Scientific, Folsom, CA, USA) were used for chromatographic extraction. With a flow rate of 1.0 mL/min, the temperature isothermal was set at 8 °C, and then increased from 60 °C to 120 °C at 8 °C/min; held at then increased from 125 °C to 210 °C at 4 °C/min; held at then increased from 210 °C to 270 °C at 5 °C/min; and finally increased from 270 °C to 305 °C at the rate of 10 °C/min. This temperature was maintained at 3 min. The injection port temperature was 260 °C; the electron impact ionization source (EI) temperature was 260 °C; and the voltage was −70 V.
2.4. Determination of Secondary Metabolites
The major secondary metabolites were measured according to Zhang’s et al. method [19]. Ultra-performance liquid chromatography (UPLC) (Agilent 1260, Santa Clara, CA, USA) was equipped with a column (Sigma-Aldrich, St. Louis, MO, USA), 4.6 mm × 250 mm). Mobile phase solvents methanol (Shanghai Yaokan Chemical Limited Liability Company, Shanghai, China) and 0.5% phosphoric acid (Shijiazhuang Haifa Chemical Limited Liability Company, Hebei Province, China) methanol and phosphoric acid (0.5%) were used as used as mobile phase A and B; the flow rate was 1.0 mL/min. From 0 to 15 min, the detection wavelengths were 206 nm, 236 nm from 15 to 55 min, with a rate of 1.0 mL/min. The gradient elution was from 0 to 30 min, 5% → 10% A, 95% → 90% B; from 30 to 70 min, 10% → 25% A, 90% → 75% B.
2.5. Statistical Analysis
Origin 2023 and Excel 2016 software were used for data analysis, Values in the figures are average ± SE.
Orthogonal partial least squares discriminant analysis (PLS-DA) was analyzed using SIMCA 14.1 software. VIP > 1 and p < 0.05 (Student’s t-test) were mainly screened as differential metabolites in this study. The enrichment pathways of different metabolites were analyzed using the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database.
3. Results
3.1. Growth Indices Changes in Eucommia ulmoides Oliver (E. ulmoides) in Different Treatments
Compared with CK, the whole seedlings and leaves showed significant changes in morphology and growth by pruning; the single leaf sizes from pruned plants were increased (Figure 2a). There were no differences in the branch number between T1 and T2 treatments; the branch number reached a maximum of 6.00 ± 0.03 (branches) in the T1 treatment and increased by 50.0% (p < 0.05) compared with CK (Figure 2b). Similarly, the maximum number of stem diameters was recorded at 9.04 ± 0.75 (mm) in the T1 treatment (p < 0.05) (Figure 2b). Furthermore, the total leaf number reached 72.00 ± 1.28 (pieces) in the T1 treatment, which increased by 63.6% compared with the CK (p < 0.05) (Figure 2c). Interestingly, the leaf area, fresh weight, and dry weight had no significant difference between treatments and CK (Figure 2c,d).
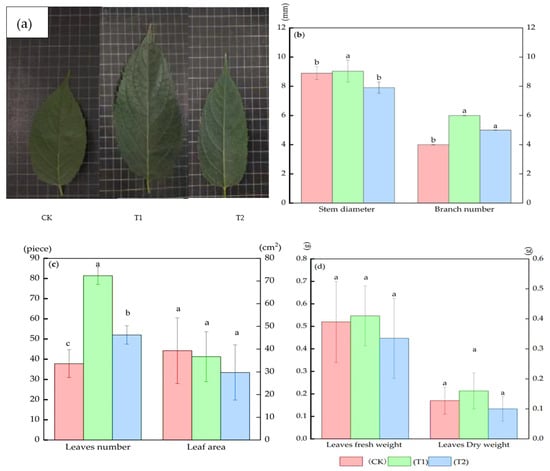
Figure 2.
The changes in growth indexes in Eucommia ulmoides Oliver (E. ulmoides) in different treatments. Leaf morphology (a); branch number, stem diameter (b); leaf number, leaf area (c); leaf fresh weight, dry weight (d). Note: lowercase letters represent significance of differences between different treatments (p < 0.05). CK (unpruned); T1 (pruned at a height 20 cm above the top edge of flowerpot); T2 (pruned at a height 10 cm above the top edge of flowerpot).
3.2. Photosynthetic Parameters Changes in Eucommia ulmoides Oliver (E. ulmoides) in Different Treatments
3.2.1. Photosynthetic Parameters
The Pn, Gs, Tr, Ewu, and EC increased significantly (p < 0.05) with an increase in pruning intensities; however, the opposite trend was observed for Ci values. In the T1 treatment, the Pn, Gs Tr, Ewu, and EC increased markedly by 31.4%, 19.5%, 9.8%, 10.5%, and 66.7% compared with CK, respectively (p < 0.05). Similarly, compared with CK, the Fv/Fm and the NPQ were increased in the pruning treatments (T1, 8.3% and T2, 5.5%) (T1, 53.9% and T2, 98.8%) respectively (p < 0.05). However, the Φ (II) and ERT had no differences between pruning treatments and CK (Table 1).

Table 1.
The changes in photosynthetic indexes in Eucommia ulmoides Oliver (E. ulmoides) in different treatments.
3.2.2. Photosynthetic Pigments Contents
The contents of Chl a, Chl b, and Car, respectively, reached a maximum of 2.09 ± 0.05 (mg/g), 1.03 ± 0.02 (mg/g), and 0.22 ± 0.003 (mg/g) in T2 treatment, increased by 69.9%, 58.4%, and 27.2% compared with CK (p < 0.05), there were no differences between T1 treatment and CK (p > 0.05). In addition, the Chl content and the rate between Chl a content and Chl b content reached 3.14 ± 0.07 (mg/g) and 2.01 ± 0.01 (mg/g) in T2 treatment and increased by 40.0% and 7.49% compared with CK (p < 0.05), respectively (Table 2).

Table 2.
The changes in photosynthetic pigments in Eucommia ulmoides Oliver (E. ulmoides) in different treatments.
3.3. Primary Metabolites Changes in Eucommia ulmoides Oliver (E. ulmoides) in Different Treatments
3.3.1. Primary Metabolites Analysis
The leaves of plants in different treatments were subjected to targeted metabolites analysis. Different primary metabolites were identified according to the criteria of VIP > 1, p-value < 0.05. Principal component analysis (PCA) results demonstrated that the metabolites of different pruning treatments were quite varied (Figure 3). A total of 36 different metabolites were screened from the three pruning treatments, including 11 sugars, 13 acids, 5 alcohols, and 7 other compounds (Figure 4).
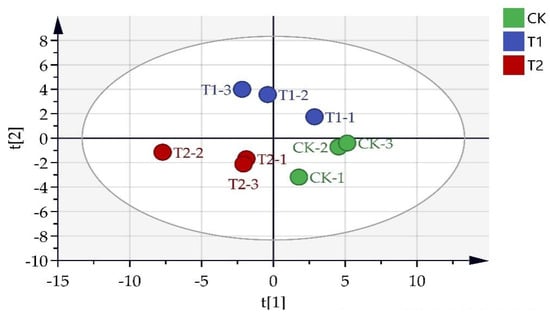
Figure 3.
PCA analysis of primary metabolites in Eucommia ulmoides Oliver (E. ulmoides) in different treatments. Note: CK (unpruned); T1 (pruned at a height 20 cm above the top edge of flowerpot); T2 (pruned at a height 10 cm above the top edge of flowerpot). CK−1, CK−2, and CK−3 represent three CK biological replicates; T1−1, T1−2, and T1−3 represent three T1 biological replicates; T2−1, T2−2, and T2−3 represent three T2 biological replicates.
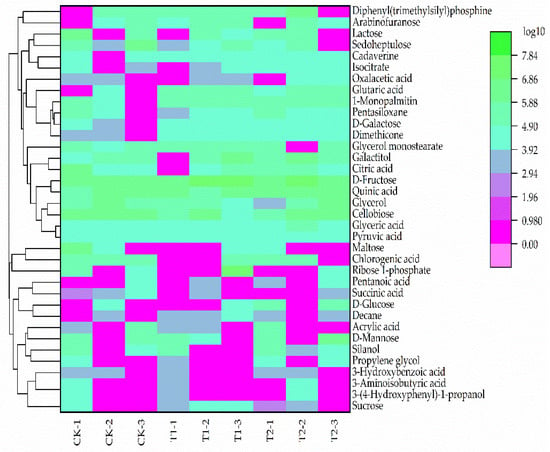
Figure 4.
Primary metabolites heat map in Eucommia ulmoides Oliver (E. ulmoides) in different treatments. Note: Green represents increase while purple represents decrease. CK (unpruned); T1 (pruned at a height 20 cm above the top edge of flowerpot); T2 (pruned at a height 10 cm above the top edge of flowerpot). CK−1, CK−2, and CK−3 represent three CK biological replicates; T1−1, T1−2, and T1−3 represent three T1 biological replicates; T2−1, T2−2, and T2−3 represents three T2 biological replicates.
3.3.2. Primary Metabolites Pathways Analysis
According to the metabolites pathway of Arabidopsis thaliana (L.) Heynh., the KEGG analysis showed that there were five main enrichment pathways in Eucommia ulmoides Oliver (E. ulmoides), including Galactose metabolism, Citrate cycle (TCA cycle), Glyoxylate and dicarboxylate metabolism, Alanine, aspartate and glutamate metabolism, and starch and sucrose metabolism (Figure 5).
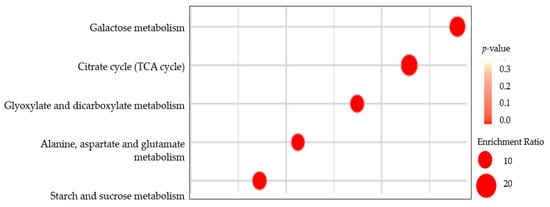
Figure 5.
KEGG enrichment map of primary metabolites in different pruning treatments. Note: The dot represents the number of differential metabolites. From red to orange indicates the p-value level.
Figure 6 showed that in Galactose metabolism, the relative contents of D-Galactose, Galactino, and D-Mannose increased by 14.8%, 16.1%, and 8.19% in T2 treatment compared with CK (p < 0.05). In starch and sucrose metabolism, the relative contents of D-Glucose and D-Fructose increased by 13.6% and 2.6% in T2 treatment compared with CK (p < 0.05). In the Citrate cycle, the relative contents of Oxaloacetate and Isocitric acid increased by 24.7% and 6.03% in T2 treatment compared with CK (p < 0.05). In Glyoxylate and dicarboxylate metabolism, the relative contents of Pyruvic acid and Glyceric acid increased by 9.01% and 9.49% in T2 treatment compared with CK (p < 0.05). The results showed that the relative contents of primary metabolites from five main enrichment pathways were increased in pruning treatments compared with CK (p < 0.05), and the relative contents of primary metabolites in T2 treatment were higher than that in T1 treatment.
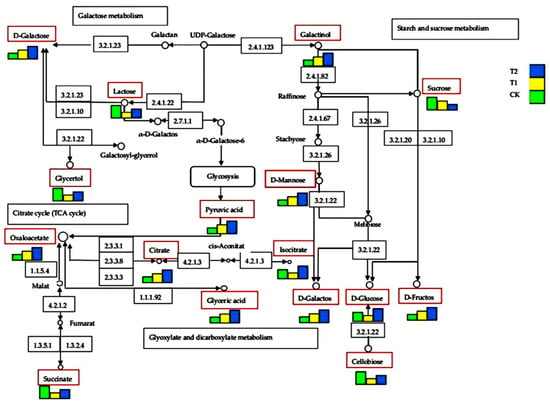
Figure 6.
The main enrichment pathway was based on KEGG from Arabidopsis thaliana (L.) Heynh. Note: The primary metabolites were distributed in the red rectangular boxes; the rectangular box size represents the relative content of primary metabolites.
3.3.3. Correlations Analysis between Primary Metabolites and Growth Indices, Photosynthetic Parameters in T2 Treatment
A correlation analysis identified that there was a positive correlation between the content of Chl a and stem diameters and fresh weight (p < 0.05) in T2 treatment. There was a positive correlation between Pn and the contents of Chl and Chl b (p < 0.05). There was a positive correlation between the D-Galactose relative content and Pn and the contents of Chl b, Chl (p < 0.05). There were was a positive correlation between the D-Mannose relative content and the content of Chl a (p < 0.05), the rate beweeen between Chl a content and Chl b content and leaf area (p < 0.05). There was a positive correlation between the content of Car and the relative contents of Citric acid (p < 0.05) and pyruvic acid (p < 0.05). There were was a positive correlation between the Succinic acid relative content and the content of Chl a, the content of Chl b, and the leaf area (p < 0.05) (Figure 7). The results showed that the accumulation of D-Galactose, D-Mannose, and Succinic acid might promote the photosynthetic pigment contents and the rate of photosynthesis, which can increase biomass.
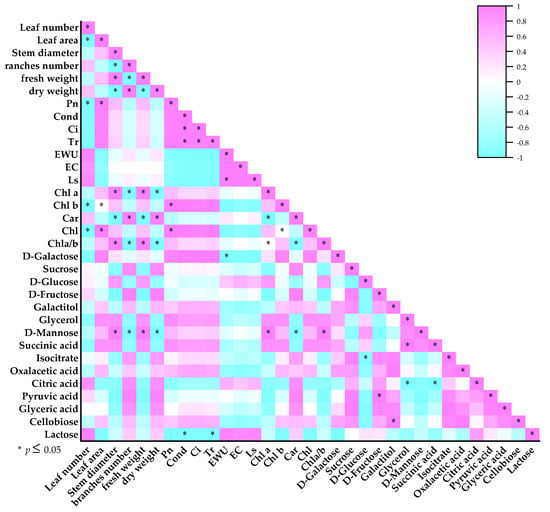
Figure 7.
Correlations analysis between primary metabolites and growth indices and photosynthetic parameters in T2 treatment.
3.4. Secondary Metabolites Yield Changes in Eucommia ulmoides Oliver (E. ulmoides) in Different Treatments
The yields of the main secondary metabolites (Chlorogenic acid, Genipin, Geniposide, Pinoresinol diglucoside, Harpagide, Caffeic acid, Asperuloside, and Geniposidic acid) from leaves were determined and increased in pruning treatments. In the T1 treatment, the yields of Genipin, Geniposide, Pinoresinol diglucoside, and Geniposidic acid were increased by 51.5%, 50%, 66.7%, and 26.1% compared with CK (p < 0.05), respectively. In the T2 treatment, the yields of Genipin, Geniposide, Pinoresinol diglucoside, and Geniposidic acid were increased by 9.84%, 2.56%, 11.1%, and 49.8% compared with CK (p < 0.05), respectively (Figure 8).
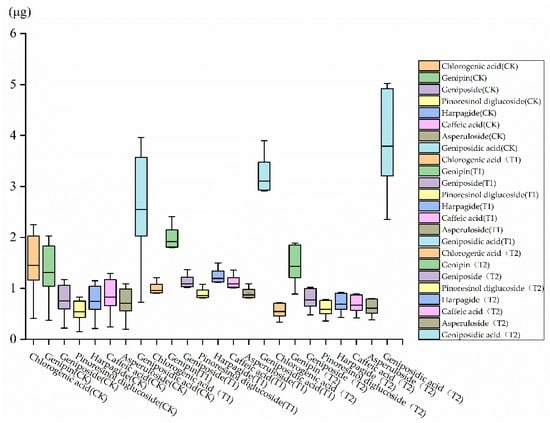
Figure 8.
The changes in main secondary metabolites yields in different pruning treatments.
4. Discussion
In our study, the growth indices, photosynthetic indexes, the relative contents of primary metabolites, and secondary metabolite yields were analyzed in the pruning treatments of 3-year-old Eucommia ulmoides Oliver (E. ulmoides) seedlings. The branch number, leaf number, and stem diameter were increased significantly (p < 0.05) in pruning treatments. Our results were consistent with previous studies in pruning 1-year-old Fraxinus mandshurica seedlings; it can result in an increase in the quantity and leaves area, an increase in the biomass of the leaves, and the development of new branches [7]. The fresh weight and dry weight of plants were increased after pruning in our studies, which was consistent with the results from [20]. Similarly, leaf biomass increased in the Zea mays L. [21]. The main photosynthetic organ is the leaves, when leaf area increased and photosynthetic capacity can be increased [22]. The photosynthetic indexes (Pn, Gs Tr, Ewu, and EC) increased markedly compared with CK (p < 0.05) in our results. Photosynthesis is the primary determinant of plant yield and growth [23]. Furthermore, photosynthetic pigments (chlorophyll a, chlorophyll b, and carotenoids) are essential for the normal course of energy processes for plant growth and development [24], which contributed to improving the accumulation of biomass [25]. In our studies, the chlorophyll content was increased after pruning treatment, which could accelerate the synthesis rate of chlorophyll and increase leaf photosynthesis. In the process of photosynthesis, carbon dioxide is transformed into glucose and other types of sugars within the plant [26]. The glucose is passed throughout the plant to various compartments and utilized to facilitate plant growth [27]. Plant polysaccharide contributes to the metabolic and organ development processes that occur as plants mature [28]. Pruning treatment has a significant impact on saccharide and tricarboxylic acid (TCA) metabolism; the processes of glycolysis and the TCA cycle have the ability to furnish the carbon structure needed to produce different types of amino acids [29,30,31], which are crucial pathways for primary and secondary metabolism.
The suitable pruning treatment can stimulate branch and leaf germination and provide adequate preparations for further secondary metabolite synthesis [32]. Pruning treatment in E. ulmoides seedings improved the plant’s metabolite pathways and promoted the absorption and transportation ability of N and P, which maintained the rapid recovery and growth of the plant [33,34]. Similarly, during the compensatory recovery and growth after pruning, the resource use efficiency and nutrient retention ability were gradually strengthened, which promoted the branch’s ability and helped with the renewal and recovery of plants [35,36]. There were four enrichment metabolite pathways in E. ulmoides in our study; these primary pathways are interlinked with the cycling of carbon and nitrogen [37]. The absorption of the resource enables effectively facilitating plant development, which promotes the increase in leaf biomass in E. ulmoide. In our study, the biomass of E. ulmoides significantly increased (p < 0.05) in pruning treatments, and the yield of the main secondary metabolites (Genipin, Geniposide, Pin oresinol diglucoside, and Geniposidic acid) from leaves was increased in pruning treatments compare with CK (p < 0.05). Therefore, pruning could accelerate plant metabolism and significantly improve the plant vitality.
5. Conclusions
Pruning treatment stimulated the compensatory growth of vegetative branches and changed the within-plant distribution of branches. The plant structure change affected canopy photosynthesis and dry matter partitioning, which affected yield formation in the end. In our study, the growth indices, the contents of photosynthetic pigments, and the rate of photosynthesis were significantly increased (p < 0.05) in pruning treatments. The KEGG analysis showed that there were five main enrichment pathways after pruning treatment in Eucommia ulmoides Oliver (E. ulmoides), and the contents of critic acid and sugar accumulation were significantly (p < 0.05) higher in T2 treatment compared with CK. These results indicated that the height in the T2 treatment was superior to the T1 treatment for plant growth. A suitable pruning treatment promoted the growth of E. ulmoides and the accumulation of secondary metabolites. It provided a new idea for the artificial cultivation of other medicinal plants, which increased the contents of secondary metabolites. Different pruning heights as a pruning treatment and the interactive effect of various pruning cuts should be assessed in follow-up studies to allow for a more complete understanding of pruning responses at the crown level. In addition, light availability can influence branch production and growth; pruning responses should also be investigated in mature Eucommia ulmoides Oliver, while accounting for the vertical light distribution gradients and for different shade levels. Finally, to assess whether manipulations of the crown structure in young Eucommia ulmoides Oliver trees have a positive impact on Eucommia ulmoides Oliver yield, long-term pruning field experiments that extend into are also needed.
Author Contributions
J.Y. performed the statistical analysis, wrote the first draft of the manuscript, and modified the manuscript; S.X. completed the research; D.D. completed the research; W.Z. completed the research; H.W. completed the research; Y.Z. completed the research; D.L. contributed to the conception and design of the study and modified the manuscript; Y.L. and Z.T. modified the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Heilongjiang province Natural Science Fund project (No. LH2021C014); the National Forestry and Grassland Science and Technology Achievement Promotion Project (No. 2023133125); the Project 111 of Heilongjiang Goose Innovation Team (No. B20088); and the Horizontal project (No. 2021).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Acknowledgments
We can acknowledge any support given that is not covered by the authors’ contribution or funding sections.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yan, J.K.; Shi, X.L.; Donkor, P.O.; Ding, L.Q.; Qiu, F. Four new megastigmane glycosides from the leaves of Eucommia ulmoides Oliver. Phytochem. Lett. 2018, 27, 208–213. [Google Scholar] [CrossRef]
- Meng, Y.D.; Du, Q.X.; Du, H.Y.; Wang, Q.; Wang, L.; Du, L.Y.; Liu, P.F. Analysis of chemotypes and their markers in leaves of core collections of Eucommia ulmoides Oliver using metabolomics. Front. Plant Sci. 2023, 13, 1029907. [Google Scholar] [CrossRef]
- Huang, L.; Lyu, Q.; Zheng, W.; Yang, Q.; Cao, G. Traditional application and modern pharma cological research of Eucommia ulmoides Oliver. Chin. Med. 2021, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Yamane, K.; Mariyama, M.; Hirooka, Y.; Iijima, M. Root pruning is effective in alleviating the inhibition of soybean growth caused by anaerobic stress for a short period. J. Integr. Agric. 2023, 22, 1035–1044. [Google Scholar] [CrossRef]
- Badrulhisham, N.; Othman, N. Knowledge in tree pruning for sustainable practices in urban setting: Improving our quality of life. Procedia-Soc. Behav. Sci. 2016, 234, 210–217. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Ogawa, Y.; Iwase, A.; Sugimoto, K. Plant regeneration: Cellular origins and molecular mechanisms. Development 2016, 143, 1442–1451. [Google Scholar] [CrossRef]
- Wen, Y.B.; Jiang, H.L.; Long, S.H.; Chen, S.X.; Peng, Z. Effect of Mounding and Top-Pruning on Survival and Growth of Manchurian Ash Seedlings Planted under the Secondary Forest of the Species. Sci. Silvae Sin. 2015, 51, 104–113. [Google Scholar]
- Nie, J.; Li, Z.; Zhang, Y.; Zhang, D.; Xu, S.; He, N.; Zhan, Z.; Dai, J.; Li, C.; Li, W.; et al. Plant pruning affects photosynthesis and photo assimilate partitioning in relation to the yield formation of field-grown cotton. Ind. Crops Prod. 2021, 173, 114087. [Google Scholar] [CrossRef]
- Mehtan, N.; Jain, A.; Rajkumar, M. Impact of pruning of Diospyros melanoxylon Roxb. (Tendu) bushes on yield and quality of leaves in Maharashtra. J. Pharmacogn. Phytochem. 2020, 9, 1360–1365. [Google Scholar] [CrossRef]
- Morgani, M.B.; Fanzone, M.; Peña, J.E.; Sari, S.; Gallo, A.E.; Tournier, M.G.; Prieto, J.A. Late pruning modifies leaf to fruit ratio and shifts maturity period, affecting berry and wine composition in Vitis vinífera L. cv. ‘Malbec’ in Mendoza, Argentina. Sci. Hortic. 2023, 313, 111861. [Google Scholar] [CrossRef]
- Cinosi, N.; Moriconi, F.; Farinelli, D.; Marchionni, D.; Lodolini, E.M.; Rosati, A.; Famiani, F. Effects of summer pruning on the water status and physiology of olive trees and on fruit characteristics and oil quality. Sci. Hortic. 2024, 324, 112612. [Google Scholar] [CrossRef]
- Danziger, N.; Bernstein, N. Shape Matters: Plant Architecture Affects Chemical Uniformity in Large-Size Medical Cannabis Plants. Plants 2021, 10, 1834. [Google Scholar] [CrossRef] [PubMed]
- Danziger, N.; Bernstein, N. Plant Architecture Manipulation Increases Cannabinoid Standardization in ‘drug-Type’ MedicalCannabis. Ind. Crops Prod. 2021, 167, 113528. [Google Scholar] [CrossRef]
- Jia, X.Y.; Zhou, J.J.; Su, T.T.; Zhou, Y.; Chen, J.B.; Ma, H.B.; Ma, J.L.; Wang, X.F. Effects of different cropping densities on the habitat of artificial Caragana intermedia in desert steppe. Acta Ecol. Sin. 2020, 40, 4126–4136. [Google Scholar]
- Dong, X.Y.; Li, P.H.; Wang, Y.Z.; Liu, C.L.; Wang, S.Q.; Lin, Q. Effects of water stress on Water Utilization Efficiency and Carboxylation Efficiency in the peach leaves of different growth type. J. Irrig. Drain. 2005, 24, 67–69. [Google Scholar]
- Cen, H.Y.; Weng, H.Y.; Yao, J.N.; He, M.B.; Lv, J.W.; Hua, S.J.; Li, H.Y.; He, Y. Chlorophyll fluorescence imaging uncov ers photosynthetic fingerprint of Citrus Huanglongbing. Front. Plant Sci. 2017, 29, 1509. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Sun, D.; Cen, H.; Xu, H.; Weng, H.; Yuan, F.; He, Y. Phenotyping of Arabidopsis drought stress re sponse using kinetic chlorophyll fluorescence and multicolor fluorescence imaging. Front. Plant Sci. 2018, 9, 603. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.X. Effects of Exogenous NO on Physiological Characteristics of Pinus Koreiansis under UV-B Stress. Master’s Thesis, Northeast Fortheast University, Harbin, China, 2011. [Google Scholar]
- Liu, Y.; Liu, J.; Abozeid, A.; Wu, K.X.; Guo, X.R.; Mu, L.Q.; Tang, Z.H. UV-B Radiation Largely Promoted the Transformation of Primary Metabolites to Phenols in Astragalus mongholicus seedlings. Biomolecules 2020, 10, 504. [Google Scholar] [CrossRef]
- Zhang, Q.C. Study on extraction technology of chemical active components from Eucommia ulmoides Oliver leaves. Liaoning Chem. Ind. 2022, 51, 1379–1381. [Google Scholar]
- Zou, K.; Liu, X.; Zhang, D.; Yang, Q.; Fu, S.; Meng, D.; Chang, W.; Li, R.; Yin, H.; Liang, Y. Flavonoid Biosynthesis Is Likely More Susceptible to Elevation and Tree Age Than Other Branch Pathways Involved in Phenylpropanoid Biosynthesis in Ginkgo Leaves. Front. Plant Sci. 2019, 10, 983. [Google Scholar] [CrossRef]
- Yang, H.W.; Chai, Q.; Yin, W.; Hu, F.L.; Qin, A.Z.; Fan, Z.L.; Yu, A.Z.; Zhao, C.; Fan, H. Yield photosynthesis and leaf anatomy of maize in inter and mono-cropping systems at varying plant densities. Crop J. 2022, 10, 893–903. [Google Scholar] [CrossRef]
- Wu, Y.S.; Gong, W.Z.; Wang, Y.M.; Yong, T.W.; Yang, F.; Liu, W.G.; Wu, X.L.; Du, J.B.; Shu, K.; Liu, J.; et al. Leaf area and photosynthesis of newly emerged trifoliolate leaves are regulated by mature leaves in soybean. J. Plant Res. 2018, 131, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Kamble, P.N.; Giri, S.P.; Mane, R.S.; Tiwana, A. Estimation of chlorophyll content in young and adult leaves of some selected plants. Univers. J. Environ. Res. Technol. 2015, 5, 306–310. [Google Scholar]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Wilfried, S.; Rachel, D.R.; Efraim, L. Biosynthesis of plant-derived flavor com pounds. Plant J. 2008, 54, 712–732. [Google Scholar]
- Chen, Y.; Zhu, C.; Zhao, Y.; Zhang, S.; Wang, W. Transcriptomics Integrated with Changes in Cell Wall Material of Chestnut (Castanea mollissima Blume) during Storage Provides a New Insight into the “Calcification” Process. Foods 2022, 11, 1136. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.X.; Chen, M.H.; Shen, J.G.; Zhang, Y.Z.; Yuan, J.P. Study on components of haematochrome in Wild Zingiber strioatum from Mount Fanjing. Med. Plant 2012, 5, 62–64. [Google Scholar]
- Zhang, H.Q.; Liu, X.W.; Song, B.T.; Nie, B.H.; Zhang, W.; Zhao, Z.Q. Effect of excessive nitrogen on levels of amino acids and sugars, and differential response to postharvest cold storage in potato (Solanum tuberosum L.) tubers. Plant Physiol. Biochem. 2020, 157, 38–46. [Google Scholar] [CrossRef]
- Rivero Meza, S.L.; de Castro Tobaruela, E.; Benedetti Pascoal, G.; Louro Massaretto, I.; Purgatto, E. Post-harvest treatment with methyl jasmonate impacts lipid metabolism in tomato pericarp (Solanum lycopersicum L. cv. Grape) at different ripening stages. Foods 2021, 10, 877. [Google Scholar] [CrossRef]
- Gokavi, N.; Mote, K.; Jayakumar, M.; Raghuramulu, Y.; Surendran, U. The effect of modified pruning and planting systems on growth, yield, labour use efficiency and economics of Arabica coffee. Sci. Hortic. 2021, 276, 109764. [Google Scholar] [CrossRef]
- Beidler, K.V.; Taylor, B.N.; Strand, A.E.; Cooper, E.R.; Schönholz, M.; Pritchard, S.G. Changes in root architecture under elevated concentrations of CO2 and nitrogen reflect alternate soil exploration strategies. New Phytol. 2015, 205, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Baoyin, T.; Sun, J.; Minggagud, H.; Li, X. Plant sizes mediate mowing induced changes in nutrient stoichiometry and allocation of a perennial grass insemiarid grassland. Ecol. Evol. 2018, 8, 3109–3118. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Minggagud, H.; Baoyin, T.; Li, F.Y. Plant production decreases whereas nutrients concentration increases in response to the decrease of mowing stubble height. J. Environ. Manag. 2020, 253, 109745. [Google Scholar] [CrossRef]
- Yuan, J.H.; Li, H.Y.; Yang, Y.F. The compensatory tillering in the forage grass hordeum brevisubulatum after simulated grazing of different severity. Front. Plant Sci. 2020, 11, 792. [Google Scholar] [CrossRef]
- Naliwajski, M.R.; Sklodowska, M. The relationship between carbon and nitrogen metabolism in cucumber leaves acclimated to salt stress. Peer J. 2018, 6, 6043. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).