Spatial Distribution of the Anecic Species of Earthworms Dendrobaena nassonovi nassonovi (Oligochaeta: Lumbricidae) in the Forest Belt of the Northwestern Caucasus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Identifying of Earthworms
2.4. Statistical Analysis of Field Data
2.5. Environmental Predictors
2.6. Model Building via Maxent and Model Evaluation
3. Results
3.1. Confinement of D. n. nassonovi to Various Forest Types (Field Data Results)
3.2. Modeling of Current Potential Areas of Earthworms
4. Discussion
Consequences and Limitations of the Maxent Method
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kooch, Y.; Jalilvand, H. Earthworms as ecosystem engineers and the most important detritivors in forest soils. Pak. J. Biol. Sci. 2008, 11, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Le Bayon, R.C.; Bullinger-Weber, G.; Schomburg, A.; Turberg, P.; Schlaepfer, R.; Guenat, C. Earthworms as ecosystem engineers: A review. In Earthworms: Types, Roles and Research; Nova Science Publishers: New York, NY, USA, 2017; pp. 129–178. [Google Scholar]
- Bouche, M.B. Strategies lombriciennes. Ecol. Bull. 1977, 25, 122–132. (In French) [Google Scholar]
- Perel’, T.S. Range and Regularities in the Distribution of Earthworms of the USSR Fauna; Nauka: Moscow, Russia, 1979. (In Russian) [Google Scholar]
- Huang, W.; González, G.; Zou, X. Earthworm abundance and functional group diversity regulate plant litter decay and soil organic carbon level: A global meta-analysis. Appl. Soil Ecol. 2020, 150, e103473. [Google Scholar] [CrossRef]
- Edwards, C.A.; Arancon, N.Q. The role of earthworms in organic matter and nutrient cycles. In Biology and Ecology of Earthworms; Springer: New York, NY, USA, 2022; pp. 233–274. [Google Scholar] [CrossRef]
- Capowiez, Y.; Sammartino, S.; Michel, E. Burrow systems of endogeic earthworms: Effects of earthworm abundance and consequences for soil water infiltration. Pedobiologia 2014, 57, 303–309. [Google Scholar] [CrossRef]
- Lavelle, P. Earthworm activities and the soil system. Biol. Fertil. Soils 1988, 6, 237–251. [Google Scholar] [CrossRef]
- Geraskina, A.P.; Shevchenko, N.E. Biotopic Allocation of Morpho-Ecological Groups of Earthworms (Oligochaeta, Lumbricidae) to the Main Forest Types in the Bol’shaya Laba River Basin (Northwestern Caucasus). Bio. Bull. 2021, 48, 1176–1188. [Google Scholar] [CrossRef]
- Lee, K.E. Earthworms: Their Ecology and Relationships with Soils and Land Use; Academic Press: Sydney, Australia; Orlando, FL, USA, 1985; pp. 1–411. [Google Scholar]
- Potvin, L.R.; Lilleskov, E.A. Introduced earthworm species exhibited unique patterns of seasonal activity and vertical distribution, and Lumbricus terrestris burrows remained usable for at least 7 years in hardwood and pine stands. Biol. Fertil. Soils 2017, 53, 187–198. [Google Scholar] [CrossRef]
- Genise, J.F. Soil neighbors I: Traces of other organisms in paleosols. Crustaceans and earthworms. In Ichnoentomology: Insect Traces in Soils and Paleosols; Springer: Cham, Switzerland, 2017; pp. 383–415. [Google Scholar] [CrossRef]
- Jégou, D.; Cluzeau, D.; Balesdent, J.; Tréhen, P. Effects of four ecological categories of earthworms on carbon transfer in soil. Appl. Soil Ecol. 1998, 9, 249–255. [Google Scholar] [CrossRef]
- Tiunov, A.V.; Kuznetsova, N.A. Environmental activity of anecic earthworms (Lumbricus terrestris L.) and spatial organization of soil communities. Bio. Bull. 2000, 27, 510–518. [Google Scholar]
- Kvavadze, E.S. Earthworms (Lumbricidae) of the Caucasus; Metsniereba: Tbilisi, GA, USA, 1985; pp. 1–238. (In Russian) [Google Scholar]
- Perel, T.S. Dozdevye cervi reliktovych lesov Zapadnogo Zakavkaz’ja i Talysa. Pedobiologia 1967, 7, 93–120. [Google Scholar] [CrossRef]
- Vsevolodova-Perel, T.S. Earthworms of the Fauna of Russia: Cadastre and Determinant; Nauka: Moscow, Russia, 1997; pp. 1–102. (In Russian) [Google Scholar]
- Vsevolodova-Perel, T.S. Addition to the fauna of earthworms (Oligochaeta, Lumbricidae). Zool. Zhurn. 2003, 82, 275–280. [Google Scholar]
- Rapoport, I.B. Fauna, ecology and altitudinal distribution of earthworms in the central part of the North Caucasus. In Candidate’s Dissertation in Biology; Institute of Ecology of the Volga Basin RAS: Tolyatti, Russia, 2011. (In Russian) [Google Scholar]
- Rapoport, I.B. Altitude distribution of earthworms (Oligochaeta, Lumbricidae) in the central part of the North Caucasus. Zool. Zh. 2013, 1, 3–10. (In Russian) [Google Scholar] [CrossRef]
- Rapoport, I.B.; Tsepkova, N.L. Earthworm Populations (Oligochaeta, Lumbricidae) in the Basin of the Middle Reaches of the Bol’shaya Laba River (Northwestern Caucasus, Buffer Zone of Caucasian Nature Reserve). Bio. Bull. 2019, 46, 1012–1029. [Google Scholar] [CrossRef]
- Rapoport, I.B.; Puzachenko, A.Y.; Tsepkova, N.L.; Csuzdi, C. Preliminary Estimation of the Influence of Cydalima perspectalis Invasion on the Species Composition and Structure of Earthworm Population (Oligochaeta: Lumbricidae, Acanthodrilidae) in the Range of Buxus sempervirens (Western Caucasus). Russ. J. Biol. Invasions 2022, 13, 140–157. [Google Scholar] [CrossRef]
- Misirlioǧlu, M.; Reynolds, J.; Stojanović, M.; Trakić, T.; Sekulić, J.; James, S.; Csuzdi, C.; Decaëns, T.; Lapied, E.; Phillips, H.R.P.; et al. Earthworms (Clitellata, Megadrili) of the world: An updated checklist of valid species and families, with notes on their distribution. Zootaxa 2023, 5255, 417–438. [Google Scholar] [CrossRef]
- Salako, G.; Russell, D.J.; Stucke, A.; Eberhardt, E. Assessment of Multiple Model Algorithms to Predict Earthworm Geographic Distribution Range and Biodiversity in Germany: Implications for soil-monitoring and species-conservation needs. Biodivers. Conserv. 2023, 32, 2365–2394. [Google Scholar] [CrossRef]
- Marchan, D.F.; Refoyo, P.; Novo, M.; Fernandez, R.; Trigo, D.; Cosin, D.J.D. Predicting soil micro-variables and the distribution of an endogeic earthworm species through a model based on large-scale variables. Soil Biol. Biochem. 2015, 81, 124–127. [Google Scholar] [CrossRef]
- Hughes, F.M.; Cortes-Figueira, J.E.; Drumond, M.A. Anticipating the response of the Brazilian giant earthworm (Rhinodrilus alatus) to climate change: Implications for its traditional use. Anais da Academia Brasileira de Ciências 2019, 91, e20180308. [Google Scholar] [CrossRef]
- Li, X.; Wu, K.; Hao, S.; Kang, L.; Ma, J.; Zhao, R.; Zhang, Y. Mapping of suitable habitats for earthworms in China. Soil Biol. Biochem. 2023, 84, e109081. [Google Scholar] [CrossRef]
- Sherlock, E.; Coates, M.; Csuzdi, C. Modelling of climatic tolerances of three earthworm species; Satchellius mammalis, Lumbricus friendi and Lumbricus festivus using Maximum Entropy Modeling. Opusc. Zool. Bp. 2022, 53, 51–65. [Google Scholar] [CrossRef]
- Serniak, L.T.; Chan, S.S.; Lajtha, K. Predicting habitat suitability for Amynthas spp. in the United States: A retrospective analysis using citizen science data from iNaturalist. Biol. Invas. 2023, 25, 817–825. [Google Scholar] [CrossRef]
- FAO. IUSS Working Group World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports; FAO: Rome, Italy, 2015. [Google Scholar]
- Omodeo, P.; Rota, E. Earthworms of Turkey. Ital. J. Zool. 1989, 56, 167–198. [Google Scholar] [CrossRef]
- Omodeo, P.; Rota, E. Earthworms of Turkey II. Boll. Di Zool. 1991, 58, 171–181. [Google Scholar] [CrossRef]
- Csuzdi, C.; Zicsi, A.; Misirlioðlu, M. An annotated checklist of the earthworm fauna of Turkey (Oligochaeta: Lumbricidae). Zootaxa 2006, 1175, 1–29. [Google Scholar] [CrossRef]
- Szederjesi, T. The first combined checklist of earthworms of the Northeastern Mediterranean region (Clitellata: Megadrili). Opusc. Zool. 2017, 48, 77–116. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1 km spatial resolution climate surfaces for global land areas. Intern. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudik, M. Modeling of species distributions with MAXENT: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Peterson, A. Predicting the Geography of Species’ Invasions via Ecological Niche Modeling. Q. Rev. Biol. 2004, 78, 419–433. [Google Scholar] [CrossRef]
- Buhl-Mortensen, L.; Burgos, J.M.; Steingrund, P.; Buhl-Mortensen, P.; Olafsdottir, S.H.; Ragnarsson, S.A. Vulnerable Marine Ecosystems (VMEs): Coral and Sponge VMEs in Arctic and sub-Arctic Waters–Distribution and Threats; Nordic Council of Ministers: Copenhagen, Denmark, 2019; pp. 1–144. [Google Scholar]
- Kramer-Schadt, S.; Niedballa, J.; Pilgrim, J.D.; Schröder, B.; Lindenborn, J.; Reinfelder, V.; Stillfried, M.; Heckmann, I.; Scharf, A.K.; Augeri, D.M.; et al. The importance of correcting for sampling bias in Maxent species distribution models. Divers. Distrib. 2013, 19, 1366–1379. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar]
- Sillero, N.; Arenas-Castro, S.; Enriquez-Urzela, U.; Vale, C.G.; Sousa-Guedes, D.; Martínez-Freiría, F.; Real, R.; Barbosa, A.M. Want to model a species niche? A step-by-step guideline on correlative ecological niche modelling. Ecol. Model. 2021, 456, e109671. [Google Scholar] [CrossRef]
- Ligthart, T.N. Development of earthworm burrow systems and the influence of earthworms on soil hydrology. In Candidate’s Dissertation in Biology; University of Wageningen: Wageningen, The Netherlands, 1996; pp. 1–140. [Google Scholar]
- Shevchenko, N.; Geraskina, A. Northwest Caucasus forest spreading evaluation by GIS mogeling and historical and geographic data analysis. Ecol. Ques. 2019, 30, 47–55. [Google Scholar] [CrossRef]
- Prokonova, T.V. Species composition and classification of groups of earthworms (Lumbricidae, Oligochaeta) of the Central Ciscaucasia. Bull. High. Educ. Instit. 2005, 3, 70–74. (In Russian) [Google Scholar]
- Luo, Y.; Wang, L.; Cao, T.; He, W.; Lu, S.; Li, F.; Zhang, Z.; Chang, T.; Tian, X. Legacy effect of plant chemical defence substances on litter decomposition. Plant Soil 2023, 487, 93–108. [Google Scholar] [CrossRef]
- Briones, M.J.I.; Ostle, N.J.; Piearce, T.G. Stable isotopes reveal that the calciferous gland of earthworms is a CO2-fixing organ. Soil Biol. Biochem. 2008, 40, 554–557. [Google Scholar] [CrossRef]
- Baldwin, R.A. Use of maximum entropy modeling in wildlife research. Entropy 2009, 11, 854–866. [Google Scholar] [CrossRef]

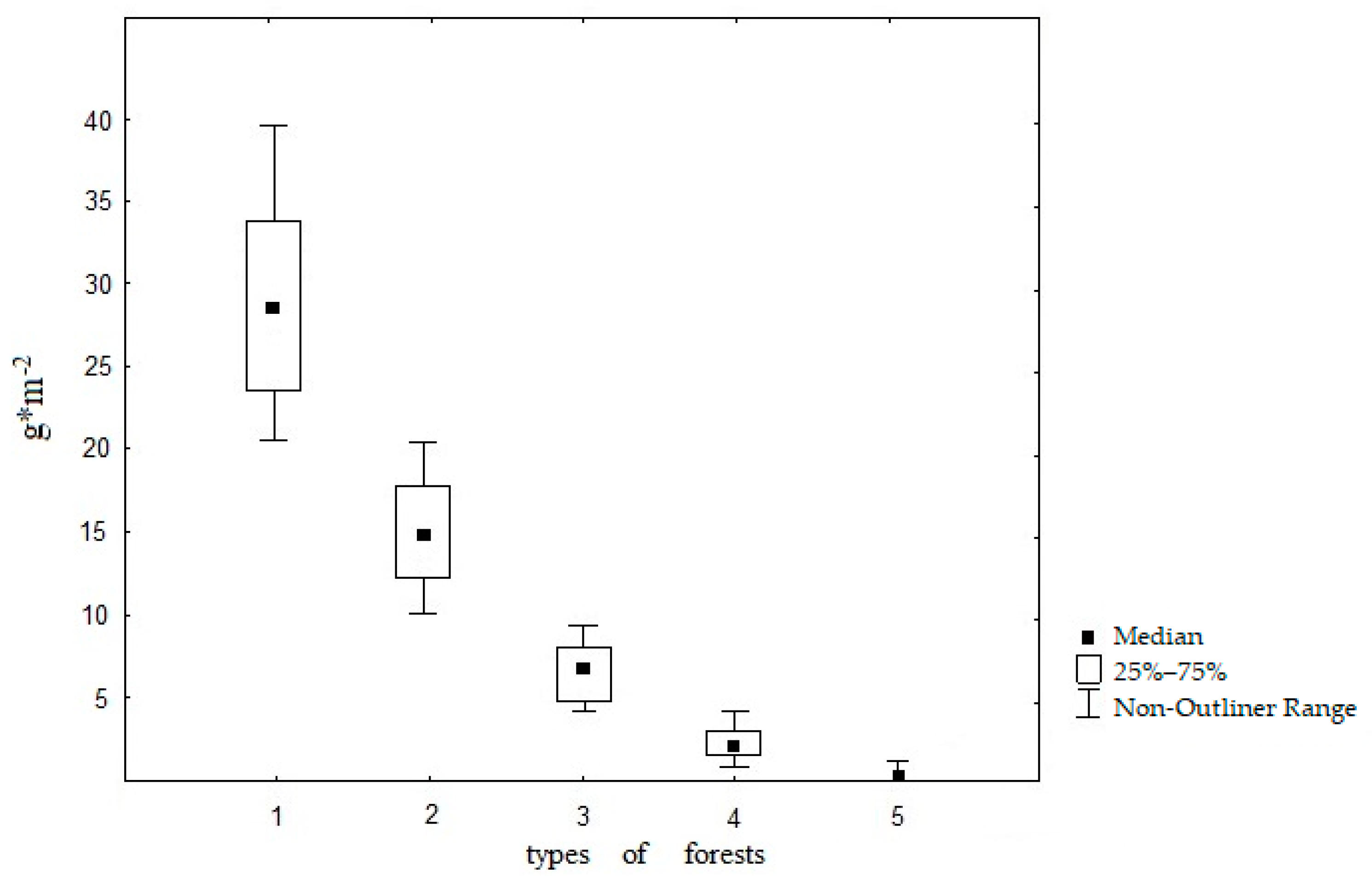

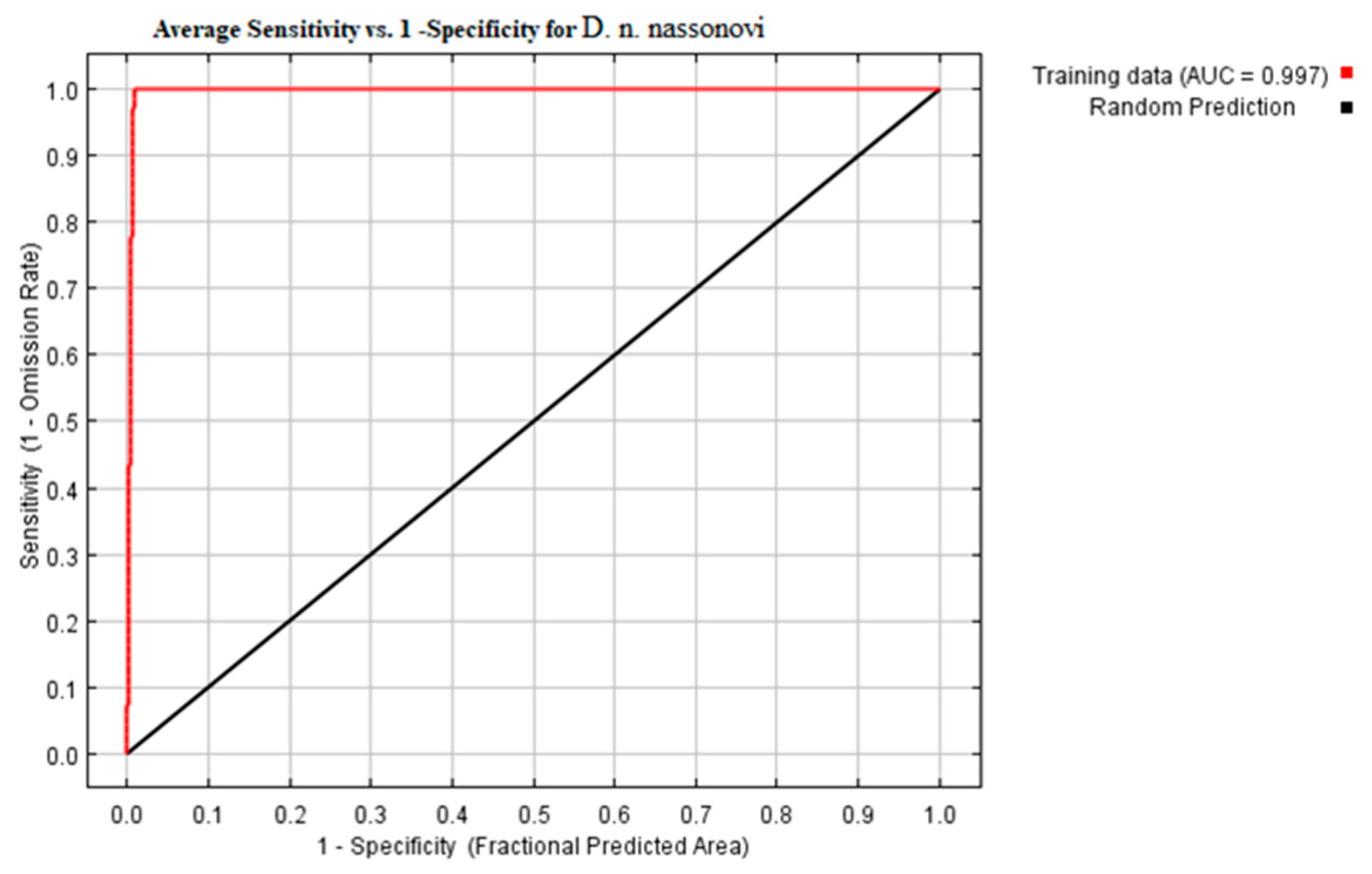
| Forest Types | Number of Geographical Locations | Number of Soil Samples | Plant Litter Thickness, cm | Soil Moisture, % | Soil pH |
|---|---|---|---|---|---|
| Black alder forests (large-fern and tall-grass) | 34 | 102 | 2–8 | 45 ± 3 | 6.0 ± 0.5 |
| Small-leaved forests (small-grass, multi-grass) | 305 | 915 | 1–3 | 35 ± 2 | 5.8 ± 0.6 |
| Hornbeam and beech forests (wood–fern, tall-grass, small-grass, fescue) | 280 | 840 | 1–2 | 30 ± 5 | 5.6 ± 0.2 |
| Mixed coniferous–deciduous forests (small-grass, multi-grass, dead-cover) | 281 | 843 | 2–4 | 25 ± 5 | 5.5 ± 0.1 |
| Coniferous forests (dead-cover, small-grass, green-moss) | 281 | 843 | 2–4 | 25 ± 5 | 5.5 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geraskina, A.; Shevchenko, N. Spatial Distribution of the Anecic Species of Earthworms Dendrobaena nassonovi nassonovi (Oligochaeta: Lumbricidae) in the Forest Belt of the Northwestern Caucasus. Forests 2023, 14, 2367. https://doi.org/10.3390/f14122367
Geraskina A, Shevchenko N. Spatial Distribution of the Anecic Species of Earthworms Dendrobaena nassonovi nassonovi (Oligochaeta: Lumbricidae) in the Forest Belt of the Northwestern Caucasus. Forests. 2023; 14(12):2367. https://doi.org/10.3390/f14122367
Chicago/Turabian StyleGeraskina, Anna, and Nikolay Shevchenko. 2023. "Spatial Distribution of the Anecic Species of Earthworms Dendrobaena nassonovi nassonovi (Oligochaeta: Lumbricidae) in the Forest Belt of the Northwestern Caucasus" Forests 14, no. 12: 2367. https://doi.org/10.3390/f14122367
APA StyleGeraskina, A., & Shevchenko, N. (2023). Spatial Distribution of the Anecic Species of Earthworms Dendrobaena nassonovi nassonovi (Oligochaeta: Lumbricidae) in the Forest Belt of the Northwestern Caucasus. Forests, 14(12), 2367. https://doi.org/10.3390/f14122367





