Abstract
This study aimed to verify the effects of prescribed personalized forest walking by considering individuals’ characteristics. To prescribe individualized exercise programs, we developed an algorithm to calculate exercise intensity based on each participant’s age, regular exercise, fatigue level, and chronic disease type, if any. To investigate the effects of forest walking on physical and mental health based on exercise prescription, we recruited 59 participants (average age: 39.1 ± 19.0 years old) aged 18 years or older. Physiological and psychological responses were compared before and after walking in the forest. Systolic blood pressure, diastolic blood pressure, percent body fat, negative affect, and emotional exhaustion significantly decreased, while the pulse rate significantly increased following the forest walking. Additionally, we investigated the effects of exercise relative to successfully maintaining one’s target heart rate and found that these effects were even greater when success score of maintaining the target heart rate while walking improved. Comparison of the groups relative to successfully achieving the target heart rate indicated that the high-achievement group had significant reductions in systolic and diastolic blood pressure, body fat mass, percent body fat, negative affect, and emotional exhaustion, and a significant increase in pulse rate. However, the low-achievement group only showed a significant reduction in emotional exhaustion. This study showed that prescribed forest walking has a positive impact on human health and is expected to have a positive effect on the motivation to start and continue exercising.
1. Introduction
Considering the recent expansion of sedentary lifestyles, with the risk of metabolic syndrome and obesity on the rise, the practice of walking is particularly important. Studies have shown that forest walking can not only promote physical health but also assist in stress relief. Ochiai et al. [1] reported that it increases positive mood and decreases negative mood. Park et al. [2] found that it reduces the level of cortisol, the stress hormone, and blood pressure while enhancing parasympathetic nervous system activity. Li et al. [3] indicated that forest walking decreases the levels of norepinephrine and dopamine, as well as blood pressure, while increasing anti-stress hormones. Song et al. [4] reported that a short 15 min forest walk increases parasympathetic nervous system activity compared to walking in urban areas, reducing sympathetic nervous system activity and heart rate. Horiuchi et al. [5] highlighted that, in the elderly, it contributes to blood pressure reduction and mood state improvement.
As the number of people visiting forests increases, the incidence of hiking accidents is also on the rise [6,7,8]. These accidents are mainly caused by human factors such as carelessness, poor judgment, and noncompliance with safety rules rather than external environmental factors such as avalanches and falling rocks [9,10,11].
Unlike external environmental factors, accidents caused by human factors can be reduced by raising individual safety awareness; therefore, various institutions present safety rules and provide related education. The Union Internationale des Associations d’Alpinisme (UIAA) has issued guidelines for safe hiking and uploaded videos related to climbing technique and skills [12]. In South Korea, hiking routes are classified into five levels based on their gradient and difficulty, allowing individuals to choose trails suitable for their capabilities [13]. In addition, educational programs and practical training for safe forest walking have been conducted [14]. However, despite studies showing that accident rates during hiking depend on characteristics such as age [7,15], sex [16], the presence of chronic illnesses [17,18], and regular exercise habits [19], information regarding forest walking tailored to individuals’ health conditions and characteristics is insufficient.
In the field of sports medicine, research is being conducted to determine the intensity of exercise to maximize its effect while minimizing the risks and injuries that may occur during exercise. Previous studies have mainly been conducted to improve the skills of sports players [20] or to restore function during rehabilitation from injury or disease [21,22,23]. However, recently, research has also been conducted on exercise prescriptions for improving physical strength and health, as health problems have emerged due to individuals’ sedentary lifestyles [24,25,26].
Applying exercise prescriptions that consider individuals’ health status and characteristics to forest walking could reduce accident rates and enhance the effectiveness of exercise, ultimately benefiting hikers. In addition, forest walking based on exercise prescription can elicit interest, motivating individuals to initiate and sustain physical activity [27]. Therefore, this study aims to derive a safe and efficient method of forest walking based on the principles of exercise prescriptions. The objectives of this study are as follows: (1) To develop optimal personalized forest walking exercise prescriptions based on information such as the individual’s age, regular exercise, and fatigue levels, as well as the presence and type of any chronic illnesses. (2) To verify the effects of participating in forest walking exercises three times a week as per the developed exercise prescriptions. (3) To compare the differences in these effects between the group that closely followed the exercise prescription and the group that did not.
2. Materials and Methods
2.1. Participants
This study was approved by the Institutional Review Board of Kongju National University (approval no. KNU_IRB_2022-085). The study participants were adults aged 18 years and older who understood the research objectives. All participants provided written, informed consent. The exclusion criteria, as specified on the participant recruitment poster, were the following: being unable to communicate, receiving treatment at a hospital, having a history of heart or cerebrovascular disease, and being unable to walk due to joint pain. Participants were recruited via promotional articles related to participation in the experiment that were posted on the bulletin board of an online local community website. The experiment was conducted on 59 participants (Table 1). All study participants were residents of Yesan-gun. They all lived within an approximately 20–30 min driving distance from the experimental site.

Table 1.
Participant information.
2.2. Experimental Site
The experiment was conducted from 5 October to 20 November 2022, and during this period, the daily average temperature was 12.1 ± 2.7 °C and the humidity was 75.0 ± 9.8%.
This study was conducted on a forest trail at a forest welfare facility located in Yesan-gun, South Korea. Forest welfare refers to economic, social, and emotional assistance designed to enhance people’s well-being through government-provided, forest-based welfare services [28], which are provided by the Korean government through forest welfare facilities. The total area of the experimental site was 134 ha, and the dominant tree species were Pinus densiflora (53 ha, 40%) and oak (42 ha, 31%), including Quercus variabilis and Quercus acutissima. Detailed information on the forest trail, including distance, height, and slope, is provided in Table 2.

Table 2.
Geographical information of the forest trail.
2.3. Exercise Prescription
We aimed to calculate the appropriate exercise intensity for each individual to maximize the benefits of forest walking while minimizing the potential risks and injuries. Referring to the American College of Sports Medicine (ACSM) FITT-VP guidelines for health promotion and cardiovascular fitness [29], we developed an algorithm to calculate exercise intensity ranging from 30% to 60% based on individuals’ health information, age, regular exercise, and perceived fatigue levels (Figure 1) [30]. Approximately 30%–60% represents a relative range of the maximum exercise intensity, which is associated with an individual’s maximum heart rate [31].
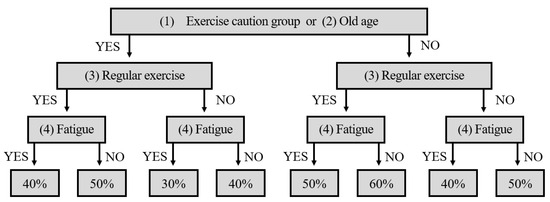
Figure 1.
The algorithm was used to determine forest exercise prescriptions.
Those who answered “yes” to question (1) in Table 3 participated in the experiment after receiving confirmation from the doctor that they could proceed with the walking exercise. The experiment was conducted safely by setting the exercise intensity according to the steps listed in Figure 1.

Table 3.
The algorithmic questions for exercise prescription.
2.4. Experimental Design
The experiment comprised pre-measurement, exercise prescription, forest walking, and post-measurement stages (Figure 2). Participants were restricted from consuming caffeine, meals, or cigarettes, all of which could affect the body’s physiological responses, 2 h prior to the start of the experiment.
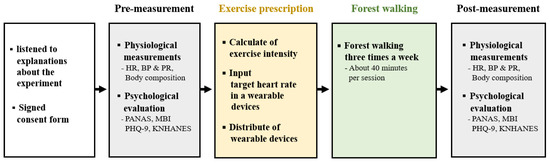
Figure 2.
Experiment design. HR (heart rate), BP (blood pressure), PR (pulse rate), PANAS (positive and negative affect scale), MBI (maslach burnout inventory), PHQ-9 (patient health questionnaire-9), and KNHANES (korean national health and nutrition examination survey).
The pre-measurement procedure was as follows: The participants visited the laboratory, sat in a chair, listened to sufficient explanations about the background, purpose, and methods of this study, and signed a consent form for participation in the experiment. After resting for approximately 5 min while sitting in the chair, their resting heart rate, blood pressure, and body composition were measured. They then completed a pre-experiment questionnaire containing information necessary for the exercise prescription, such as age, regular exercise, and psychological evaluation.
The exercises were conducted using the following methods and sequences: The individual’s maximum heart rate, indicating the heart rate at maximum exercise intensity for an individual, was calculated using the formula developed by Fox et al. [32]: “220 − (age)”. Their target heart rate was calculated using Karvonen’s [33] formula: “(maximum heart rate − resting heart rate) × exercise intensity + resting heart rate”. Exercise intensity was assigned to participants using the procedure shown in Figure 1, based on the information provided in the pre-experiment questionnaire. The target heart rate range, which was entered into a wearable wristband device, was set at ±10 bpm around the target heart rate. Subsequently, the participants were given the wearable device and instructed on how to wear and use it. Participants were instructed to walk within their designated target heart rate range during the forest walking exercise.
The participants visited the experimental site three times a week when they wanted to walk through the designated forest trail. When walking in the forest, if their heart rate was outside the preset target range, the wearable device vibrated so that the exercise could be performed smoothly.
After the last forest walk, participants returned to the laboratory for post-measurements within 24 h, at the same time as for the pre-measurements. The precautions (no eating, smoking, or consuming caffeine for 2 h prior to measurements) and time, indicators, and post-measurement procedure were the same as those in the pre-measurement stage.
2.5. Physiological Measurements
2.5.1. Heart Rate
Heart rate refers to the number of heartbeats in one minute [34]. In this study, a heart rate sensor (H10, Polar, Kempele, Finland) and smartwatch (Pacer Pro, Polar, Finland) were used to measure and calculate participants’ heart rate by recording electrocardiogram (ECG) data. The resting heart rate was measured while seated with eyes closed for five minutes, and the average value was used.
2.5.2. Blood Pressure
Systolic blood pressure (SBP) represents the pressure exerted on the blood vessels when the heart contracts, while diastolic blood pressure (DBP) represents the pressure exerted on the blood vessels when the heart is relaxed. In this study, an oscillometric method (BPBIO330n; Inbody, Seoul, Korea) was used to measure blood pressure. Two measurements were obtained from the right upper arm of each participant. If the difference in systolic blood pressure between the first and second measurements was equal to or exceeded 10 mmHg or the difference in diastolic blood pressure was equal to or exceeded 6 mmHg, an additional measurement was taken, and the average of the second and third measurements was used.
The pulse rate, which is the number of times the arterial wall vibrates per minute owing to contractions of the heart, was also measured [35].
2.5.3. Body Composition
In this study, body composition was measured using a bioelectrical impedance analysis (BIA) device (Inbody270, Inbody, Seoul, Korea), which assesses differences in electrical conductivity based on tissue biological characteristics. BIA can be measured easily and conveniently by estimating the body composition ratio [36], and it also shows high reliability and validity as an evaluation index of objective body components [37]; therefore, it is actively used for diagnosing obesity. In this study, weight, skeletal muscle mass, body fat mass, body mass index (BMI), and percent body fat were used as variables.
2.6. Psychological Measurements
2.6.1. Positive and Negative Affect Scale (PANAS)
In this study, we used the Positive and Negative Affect Scale (PANAS) questionnaire developed by Watson et al. [38] and translated into Korean by Lee et al. [39]. The questionnaire has a two-subscale design consisting of 10 items each for positive affect and 10 items for negative affect, which were rated using a 5-point Likert scale. The maximum and minimum scores for both positive and negative affect are 40 and, respectively, 0 points. In the study by Park and Lee [40], the Cronbach’s α values for the subfactors of positive and negative affect were reported as 0.80 and 0.86, respectively. In our study, these values were found to be 0.69 and, correspondingly, 0.61.
2.6.2. Maslach Burnout Inventory (MBI)
This study used the Maslach Burnout Inventory (MBI) questionnaire developed by Maslach and Jackson [41] and translated into Korean by Shin et al. [42]. The questionnaire has a three-subscale design consisting of personal accomplishment, emotional exhaustion, and depersonalization. In this study, only one subscale—emotional exhaustion—was used. Items were rated on a 5-point Likert scale, with higher scores indicating more severe emotional exhaustion. The maximum and minimum scores for emotional exhaustion are 20 and, respectively, 0 points. In the study by Shin et al. [42], the Cronbach’s α value was reported as 0.9. In our study, this value was calculated as 0.58.
2.6.3. Patient Health Questionnaire-9 (PHQ-9)
The Patient Health Questionnaire-9 (PHQ-9) questionnaire developed by Spitzer et al. [43] and translated into Korean by Park et al. [44] was used in this study. This is a self-reported questionnaire designed to diagnose major depressive disorder. It consists of nine items rated on a 4-point Likert scale. A total score of 5 or higher suggests the possibility of depression; if the score is 10 or higher, professional counseling is recommended. The maximum and minimum scores for emotional exhaustion are 27 and, respectively, 0 points. In the study by Park et al. [44], the Cronbach’s α values were reported as 0.81. In our study, these values were found to be 0.59.
2.6.4. The Korean National Health and Nutrition Examination Survey (KNHANES)
The Korean National Health and Nutrition Examination Survey (KNHANES) questionnaire, developed by Lee et al. [45], was used in this study. It consists of 20 items structured into three subscales: fatigue, depression, and anger. Higher scores indicate higher stress levels. The maximum and minimum scores for emotional exhaustion are 45 and, respectively, 0 points. In the study by Choi et al. [46], the Cronbach’s α value was reported as 0.79. In our study, this value was found to be 0.57.
2.7. Data Analysis
The Statistical Package for the Social Sciences (SPSS) (version 27.0) was used for statistical analysis, and the significance level was set at p < 0.05. Regarding the physiological measurements, one individual was excluded from the body composition analysis due to missing data. In the psychological measurement, two people were excluded from PHQ-9, one from MBI, and one from the subscale of negative affect in PANAS as the participants had missed questions.
The following three analyses were conducted: (1) To investigate the effect of participating in prescribed forest walking three times a week, participants’ pre- and post-measurement values were compared; (2) to confirm the difference in effect according to the degree of achievement of remaining within the target heart rate range, the pre- and post-measurement values were compared by dividing the scores of the high- and low-achievement groups based on the median value of the target heart rate range achievement rate; (3) changes (post-measurement − pre-measurement) were calculated, and the scores were compared between the groups.
For analyses (1) and (2), as the data were paired, the physiological responses from populations exhibiting normal distribution were analyzed using the paired t-test, while psychological responses from populations not exhibiting normal distribution were analyzed using the Wilcoxon signed-rank test. For analysis (3), as the data were independent, the physiological responses from populations exhibiting normal distribution were analyzed using the independent samples t-test, while psychological responses from populations not exhibiting normal distribution were analyzed using the Mann–Whitney U test.
3. Results
3.1. Comparison of Pre- and Post-Measurements for All Participants
When comparing the pre- and post-measurements for all participants, systolic blood pressure, diastolic blood pressure, and percent body fat significantly decreased, and the pulse rate significantly increased (Table 4). In addition, negative affect and emotional exhaustion scores significantly decreased (Table 5). No significant differences were found in the other indicators.

Table 4.
Comparison of physiological responses between pre- and post-measurements for all participants.

Table 5.
Comparison of psychological responses between pre- and post-measurements for all participants.
3.2. Comparison between High-Achievement and Low-Achievement Groups
The achievement rate of walking within the target HR range was calculated to compare the difference in the effect according to the degree of achievement. Among the 59 participants, the lowest and highest achievement rates were 12.94% and 95.98%, respectively, confirming that the achievement rate differed by individual. Based on the median value of 59.66%, those with an achievement rate of 59.66% or more were classified as high-achievement (n = 30), while those with an achievement rate less than 59.66% were classified as low-achievement (n = 29). A group comparison was then conducted.
The homogeneity of the demographic variables (gender, chronic disease, drinking, and smoking) between the high- and low-achievement groups was confirmed (Table 6). No differences were found for any of the indicators, indicating homogeneity among the groups.

Table 6.
The results of an intergroup homogeneity test.
The physiological and psychological measurement results of the two groups are presented in Table 7 and Table 8, respectively. When comparing the pre-test measurements between the two groups, no significant differences were found in any of the indicators, confirming the homogeneity of the two groups.

Table 7.
Comparison of physiological responses between the pre- and post-measurements of the two groups.

Table 8.
Comparison of psychological responses between the pre- and post-measurements of the two groups.
When comparing the pre- and post-measurement results within each group, the high-achievement group showed significant decreases in systolic blood pressure, diastolic blood pressure, body fat mass, percent body fat, and negative affect and emotional exhaustion scores, while pulse rate significantly increased. In contrast, in the low-achievement group, only the emotional exhaustion score significantly decreased. When comparing the changes (post − pre) between the groups, we found that the reduction in negative affect score and diastolic blood pressure and increase in positive affect score and pulse rate were significantly higher in the high-achievement group than in the low-achievement group.
4. Discussion
This study was conducted to verify the effects of personalized forest walking prescriptions that consider individual characteristics. Physiological responses were measured using blood pressure, pulse rate, and body composition analyses, and psychological responses were evaluated using PANAS, PHQ-9, KNHANES, and MBI.
Blood pressure analysis revealed that systolic and diastolic blood pressure were significantly decreased following the forest walking exercise. This is consistent with previous studies that indicate that forest walking reduces blood pressure [1,2,3,5,47]. Furthermore, research has shown that walking in forests not only lowers the blood pressure of individuals with high blood pressure but also increases the blood pressure of individuals with low blood pressure to normal levels [48]. Walking in forests may be beneficial for those seeking to efficiently enhance and maintain their cardiovascular health. The results of the pulse rate analysis showed that the post-measurement pulse rate increased significantly compared to the pre-measurement pulse rate. A number of studies [49,50,51,52] have reported that exercise lowers blood pressure and heart rate simultaneously by reducing sympathetic nerve activity; however, these results contrast with the findings of our study. However, some studies have shown that exercise reduces blood pressure and increases heart and pulse rates. Jeon et al. [53] examined the effects of exercise on blood pressure and heart rate by measuring these parameters at rest and 20 min post-exercise periods. The results showed that blood pressure post-exercise significantly decreased compared to resting blood pressure, but heart rate significantly increased. They reported that the drop in blood pressure after exercise was caused by relaxation of resistance vessels, such as arterioles, rather than a decrease in cardiac output. Kim et al. [54] performed a step test for 8 min in a forest and compared blood pressure and pulse rate at rest, immediately after exercise, 5 min after exercise, and 10 min after exercise. Compared to the resting period, blood pressure had decreased 10 min after exercise, while the pulse rate had increased. However, there are few studies related to blood pressure reduction and pulse rate elevation, which makes it difficult to generalize the results. Furthermore, studies that simultaneously investigate blood pressure and pulse rate are lacking. Future research should accumulate sufficient data to investigate this issue in further detail.
Body composition analysis showed that the percent body fat decreased after the intervention. This is similar to the results of previous studies showing that steady exercise reduces body fat [55,56,57,58]. Previous studies have also reported that continuous aerobic exercise not only reduces body fat but also increases muscle strength [59,60]. However, in this study, a significant difference was observed only in percent body fat. In previous studies, an exercise period of at least a month was set, and in this study, forest walking was conducted three times a week. It is possible that the inconsistencies between these results may be caused by differences in the duration of exercise.
Following the psychological response analysis, we found that negative affect and emotional exhaustion scores significantly decreased after the intervention. This is consistent with previous studies showing that walking in a forest aids in psychological relaxation [4,61,62,63,64,65]. Forest walking not only reduces depression [63] and negative effects such as confusion, anger, depression, and anxiety [4,65], but it also helps to improve mood [64]. This intervention is believed to be helpful for people who want to reduce their stress.
In this study, the participants were divided into high- and low-achievement groups based on their achievement of maintaining their target heart rate during the prescribed exercise. Regarding physiological responses, systolic and diastolic blood pressure significantly decreased, and pulse rate significantly increased after the intervention in the high-achievement group, but no significant differences were found in the low-achievement group. Psychological response measurements revealed that negative affect and emotional exhaustion scores significantly decreased in the high-achievement group. However, in the low-achievement group, only the emotional exhaustion score significantly decreased. When comparing the changes between the groups, we found that the reduction in negative affect score and diastolic blood pressure and increase in positive affect score and pulse rate were significantly higher in the high-achievement group than in the low-achievement group. This suggests that forest walking aids in physiological and psychological relaxation, but effectiveness is dependent on the achievement of the exercise prescription. Therefore, exercise prescriptions should be administered to individuals.
Forest walking based on exercise prescription can promote individuals’ intrinsic motivation to engage in exercise. An increase in intrinsic motivation aids in maintaining the willingness to sustain exercise and has a positive impact on goal-setting and achievement [66]. These intrinsic motivations can increase further if they support an individual’s “autonomy”, “competence”, and “relevance” [67]. Therefore, future research should contemplate methods of forest walking that can further enhance intrinsic motivation.
Recently, as the amount of time individuals spend being sedentary has increased worldwide, the time spent on physical activity has decreased [68,69], the risk of various diseases has increased, and the importance of exercise is increasing. Insufficient physical activity is associated with a 6%–10% increase in the risk of major non-communicable diseases such as coronary artery disease, heart disease, type 2 diabetes, breast cancer, and colorectal cancer [70]. For adults who spend more than 7 h a day sitting, each additional hour of sedentary time increases the probability of disease-related mortality by 5% [71]. It is estimated that 9.4% of the 57 million deaths worldwide are due to physical inactivity, emphasizing the risk of diseases caused by reduced physical activity [72]. This study is significant in that it presents a safe and efficient forest walking method that considers individual health conditions and is expected to be used as a guideline for forest walking exercises in the future.
Our study has several limitations. First, owing to difficulties in participant recruitment caused by the COVID-19 pandemic, this study was unable to establish a control group in which no exercise prescription was applied. In future studies, it will be necessary to compare the experimental and control groups. Second, although we observed differences in the effects of exercise based on the target heart rate range and achievement rate, the specific factors leading to decreased achievement rates were not identified. Future research should focus on analyzing the factors that contribute to low achievement rates and exploring methods to improve these rates. Third, this study was conducted only for a week. The more continuous and repetitive the exercise, the higher its efficiency [73]. Future studies should focus on the effects of forest walking with a long-term exercise prescription of more than one month. Fourth, prior research has reported that human responses may vary depending on the concentration of monoterpenes in the forest atmosphere during walking [74,75]. In our study, participants engaged in forest walking on different days, which may result in varying monoterpene concentrations. These fluctuations may influence the physiological responses of the participants differently. Therefore, future studies should incorporate measurements of air quality and monoterpene concentration to analyze this aspect more comprehensively.
5. Conclusions
In this study, prescribed forest walking physiologically reduced systolic and diastolic blood pressure, percent body fat, increased pulse rate, and psychologically reduced negative affect and emotional exhaustion scores. These effects were even greater when the success scores for walking and maintaining the target heart rate while walking improved. In conclusion, forest walking based on exercise prescriptions has a positive impact on human health.
Author Contributions
Conceptualization, Y.Y., B.-J.P. and C.S.; methodology, C.K., Y.Y., B.-J.P. and C.S.; formal analysis, Y.Y., B.-J.P. and C.S.; investigation, C.K., J.K., I.S. and C.S.; data curation, C.K., J.K., I.S. and C.S.; writing—original draft preparation, C.K.; writing—review and editing, C.S.; visualization, C.K. and C.S.; supervision, Y.Y., B.-J.P. and C.S.; funding acquisition, C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Korea Forestry Promotion Institute (grant number: 2021385C10-2323-0101).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This study was performed with permission from the National Center for Forest Therapy, Yesan. We appreciate their cooperation. We are also grateful to Sujeong Lee for her valuable contributions in the data collection phase of this study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of this study, in the collection, analysis, or interpretation of data, in the writing of this manuscript, or in the decision to publish the results.
References
- Ochiai, H.; Ikei, H.; Song, C.; Kobayashi, M.; Takamatsu, A.; Miura, T.; Kagawa, T.; Li, Q.; Kumeda, S.; Imai, M.; et al. Physiological and Psychological Effects of Forest Therapy on Middle-Aged Males with High-Normal Blood Pressure. Int. J. Environ. Res. Public. Health 2015, 12, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Kagawa, T.; Miyazaki, Y. The Physiological Effects of Shinrin-Yoku (Taking in the Forest Atmosphere or Forest Bathing): Evidence from Field Experiments in 24 Forests across Japan. Environ. Health Prev. Med. 2010, 15, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Otsuka, T.; Kobayashi, M.; Wakayama, Y.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Li, Y.; Hirata, K.; Shimizu, T.; et al. Acute Effects of Walking in Forest Environments on Cardiovascular and Metabolic Parameters. Eur. J. Appl. Physiol. 2011, 111, 2845–2853. [Google Scholar] [CrossRef]
- Song, C.; Ikei, H.; Kagawa, T.; Miyazaki, Y. Effects of Walking in a Forest on Young Women. Int. J. Environ. Res. Public. Health 2019, 16, 229. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, M.; Endo, J.; Akatsuka, S.; HasEgawa, T.; YaMaMoto, E.; Uno, T.; Kikuchi, S. An Effective Strategy to Reduce Blood Pressure after Forest Walking in Middle-Aged and Aged People. J. Phys. Ther. Sci. 2015, 27, 3711–3716. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lischke, V.; Byhahn, C.; Westphal, K.; Kessler, P. Mountaineering Accidents in the European Alps: Have the Numbers Increased in Recent Years? Wilderness Environ. Med. 2001, 12, 74–80. [Google Scholar] [CrossRef]
- Faulhaber, M.; Pocecco, E.; Niedermeier, M.; Ruedl, G.; Walter, D.; Sterr, R.; Ebner, H.; Schobersberger, W.; Burtscher, M. Fall-Related Accidents among Hikers in the Austrian Alps: A 9-Year Retrospective Study. BMJ Open Sport. Exerc. Med. 2017, 3, e000304. [Google Scholar] [CrossRef]
- Ministry of the Interior and Safety. “Disaster Yearbook”. Available online: https://www.safekorea.go.kr/idsiSFK/neo/sfk/cs/csc/bbs_conf.jsp?bbs_no=27&emgPage=Y&menuSeq=736&viewtype=read&bbs_ordr=2143 (accessed on 16 August 2023).
- Knott, J. Causes of Injuries in the Mountains: A Review of Worldwide Reports into Accidents in Mountaineering. BMJ Mil. Health 2011, 151, 92–99. [Google Scholar] [CrossRef]
- Wang, S.W.; Heo, J.H.; Lee, J.C. A Case Study of Climbing Safety Accidents and Its Prevention. J. Korean Soc. Disaster Secur. 2013, 9, 73–87. [Google Scholar]
- Chamarro, A.; Fernández-Castro, J. The Perception of Causes of Accidents in Mountain Sports: A Study Based on the Experiences of Victims. Accid. Anal. Prev. 2009, 41, 197–201. [Google Scholar] [CrossRef]
- Union Internationale des Associations d’Alpinisme (UIAA, lit. International Union of Alpine Clubs). Available online: https://theuiaa.org/safety/ (accessed on 21 August 2023).
- Kim, T.G.; Cho, Y.H. Evaluation of Trail Difficulty in Jirisan National Park Using GIS. J. Natl. Park Res. 2011, 2, 129–136. [Google Scholar]
- National Mountaineering School. “Curriculum Announcement”. Available online: https://www.nationalmschool.kr/ (accessed on 16 August 2023).
- Gasser, B. Half of Emergency Calls in Hikers Are Injuries from Falls in 50–70 Year-Olds. Dtsch. Z. Sportmed. 2019, 70, 209–214. [Google Scholar] [CrossRef]
- Chu, W.Y.C.; Chong, Y.C.; Mok, W.Y. Hiking-Related Orthopaedic Injuries: Another Epidemic during the COVID-19 Pandemic. J. Orthop. Trauma Rehabil. 2021, 28, 22104917211059543. [Google Scholar] [CrossRef]
- Dehnert, C.; Bärtsch, P. Can Patients with Coronary Heart Disease Go to High Altitude? High Alt. Med. Biol. 2010, 11, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, M.; Ponchia, A. The Risk of Cardiovascular Events during Leisure Time Activities at Altitude. Prog. Cardiovasc. Dis. 2010, 52, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Windsor, J.S.; Firth, P.G.; Grocott, M.P.; Rodway, G.W.; Montgomery, H.E. Mountain Mortality: A Review of Deaths That Occur during Recreational Activities in the Mountains. Postgrad. Med. J. 2009, 85, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Córdova, A.; Sureda, A.; Tur, J.A.; Pons, A. Immune Response to Exercise in Elite Sportsmen during the Competitive Season. J. Physiol. Biochem. 2010, 66, 1–6. [Google Scholar] [CrossRef]
- Garvey, C.; Fullwood, M.D.; Rigler, J. Pulmonary Rehabilitation Exercise Prescription in Chronic Obstructive Lung Disease: US Survey and Review of Guidelines and Clinical Practices. J. Cardiopulm. Rehabil. Prev. 2013, 33, 314–322. [Google Scholar] [CrossRef]
- Hannan, A.L.; Harders, M.P.; Hing, W.; Climstein, M.; Coombes, J.S.; Furness, J. Impact of Wearable Physical Activity Monitoring Devices with Exercise Prescription or Advice in the Maintenance Phase of Cardiac Rehabilitation: Systematic Review and Meta-Analysis. BMC Sports Sci. Med. Rehabil. 2019, 11, 14. [Google Scholar] [CrossRef]
- Sweegers, M.G.; Altenburg, T.M.; Chinapaw, M.J.; Kalter, J.; Verdonck-De Leeuw, I.M.; Courneya, K.S.; Newton, R.U.; Aaronson, N.K.; Jacobsen, P.B.; Brug, J.; et al. Which Exercise Prescriptions Improve Quality of Life and Physical Function in Patients with Cancer during and Following Treatment? A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Br. J. Sports Med. 2018, 52, 505–513. [Google Scholar] [CrossRef]
- Phillips, E.M.; Kennedy, M.A. The Exercise Prescription: A Tool to Improve Physical Activity. Phys. Med. Rehabil. 2012, 4, 818–825. [Google Scholar] [CrossRef]
- O’Donoghue, G.; Blake, C.; Cunningham, C.; Lennon, O.; Perrotta, C. What Exercise Prescription Is Optimal to Improve Body Composition and Cardiorespiratory Fitness in Adults Living with Obesity? A Network Meta-Analysis. Obes. Rev. 2021, 22, e13137. [Google Scholar] [CrossRef] [PubMed]
- Kirton, M.J.; Burnley, M.T.; Ramos, J.S.; Weatherwax, R.; Dalleck, L.C. The Effects of Standardised versus Individualised Aerobic Exercise Prescription on Fitness-Fatness Index in Sedentary Adults: A Randomised Controlled Trial. J. Sports Sci. Med. 2022, 21, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, R.E.; Fiala, B. Building Motivation and Sustainability into the Prescription and Recommendations for Physical Activity and Exercise Therapy: The Evidence. Physiother. Theory Pract. 2009, 25, 424–441. [Google Scholar] [CrossRef] [PubMed]
- Korea Forest Welfare Institute. Available online: https://www.fowi.or.kr/user/eng/forestWelfare.do (accessed on 6 September 2023).
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Kim, C.; Kim, J.; Song, I.; Yi, Y.; Park, B.J.; Song, C. Analysis of Preferred Course for Forest Exercise Prescription Considering Individual Characteristics. Korean Inst. For. Recreat. Welf. 2023, 27, 91–105. [Google Scholar]
- Mann, T.; Lamberts, R.P.; Lambert, M.I. Methods of Prescribing Relative Exercise Intensity: Physiological and Practical Considerations. Sports Med. 2013, 43, 613–625. [Google Scholar] [CrossRef]
- Fox, S.M., 3rd. Physical activity in the prevention of coronary heart disease. Ann. Clin. Res. 1971, 3, 404–432. [Google Scholar] [CrossRef]
- Karvonen, M.J.; Kentala, E.; Mustala, O. The Effects of Training on Heart Rate; a Longitudinal Study. Ann. Med. Exp. Bil. Fenn. 1957, 35, 307–315. [Google Scholar]
- Cacioppo, J.T.; Berntson, G.G.; Binkley, P.F.; Quigley, K.S.; Uchino, B.N.; Fieldstone, A. Autonomic Cardiac Control. II. Noninvasive Indices and Basal Response as Revealed by Autonomic Blockades. Psychophysiology 1994, 31, 586–598. [Google Scholar] [CrossRef]
- Beevers, G.; Lip, G.Y.H.; O’brien, E. ABC of Hypertension Blood Pressure Measurement Part I-Sphygmomanometry: Factors Common to All Techniques Methods of Blood Pressure Measurement. Br. Med. J. 2001, 332, 981–985. [Google Scholar] [CrossRef]
- Wyatt, S.B.; Winters, K.P.; Dubbert, P.M. Overweight and Obesity: Prevalence, Consequences, and Causes of a Growing Public Health Problem. Am. J. Med. Sci. 2006, 331, 166–174. [Google Scholar] [CrossRef]
- Roubenoff, M.D.R.; Gerard, E.; Peter, W.F. Predicting Body Fatness: The Mass Index vs Estimation by Bioelectrical Impedance. Am. J. Public Health 1995, 85, 726–728. [Google Scholar] [CrossRef]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and Validation of Brief Measures of Positive and Negative Affect: The PANAS Scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Lee, H.H.; Kim, E.J.; Lee, M.K. A Validation Study of Korea Positive and Negative Affect Schedule: The PANAS Scales. Korean J. Clin. Psychol. 2003, 22, 935–946. [Google Scholar]
- Park, H.; Lee, J. A Validation Study of Korean Version of PANAS-Revised. Korean J. Psychol. Gen. 2016, 35, 617. [Google Scholar] [CrossRef]
- Maslach, C.; Jackson, S.E. The measurement of experienced burnout. J. Organ. Behav. 1981, 2, 99–113. [Google Scholar] [CrossRef]
- Shin, K. The Maslach Burnout Inventory-General Survey (MBI-GS): An Application in South Korea. Korean J. Ind. Organ. Psychol. 2003, 16, 1–17. [Google Scholar]
- Park, S.J.; Choi, H.R.; Choi, J.H.; Kim, K.; Hong, J.P. Reliability and Validity of the Korean Version of the Patient Health Questionnaire-9 (PHQ-9). Anxiety Mood 2010, 6, 119–124. [Google Scholar]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W. Validation and Utility of a Self-Report Version of PRIME-MD The PHQ Primary Care Study. J. Am. Med. Assoc. 1999, 282, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Shin, H.C.; Yang, Y.J.; Cho, J.J.; Ahn, K.Y.; Kim, S.H. Development of the Stress for KNHANES: Report of Scientific Study Service; Korea Disease Control and Prevention Agency: Cheongju-si, Republic of Korea, 2010. [Google Scholar]
- Choi, Y.G.; Choi, B.J.; Park, T.H.; Uhm, J.Y.; Lee, D.B.; Chang, S.S.; Kim, S.Y. A Study on the Characteristics of Maslach Burnout Inventory-General Survey (MBI-GS) of Workers in One Electronics Company. Ann. Occup. Environ. Med. 2019, 31, e29. [Google Scholar] [CrossRef]
- Sung, D.S.; Park, J.S.; Lim, W.H. Analysis of the Healing Effect of Walking Activities According to the Difference in forest environment. Public Value 2020, 5, 1–16. [Google Scholar]
- Song, C.; Ikei, H.; Miyazaki, Y. Elucidation of a Physiological Adjustment Effect in a Forest Environment: A Pilot Study. Int. J. Environ. Res. Public. Health 2015, 12, 4247–4255. [Google Scholar] [CrossRef] [PubMed]
- Collins, H.L.; Dicarlo, S.E. Daily Exercise Attenuates the Sympathetic Component of the Arterial Baroreflex Control of Heart Rate. Am. J. Physiol.-Heart Circ. Physiol. 2023, 273, H2613–H2619. [Google Scholar] [CrossRef] [PubMed]
- Dicarlo, S.E.; Stahl, L.K.; Bishop, V.S. Daily Exercise Attenuates the Sympathetic Nerve Response to Exercise by Enhancing Cardiac Afferents. Am. J. Physiol.-Heart Circ. Physiol. 1997, 273, H1606–H1610. [Google Scholar] [CrossRef] [PubMed]
- Chandler, M.P.; Rodenbaugh, D.W.; Dicarlo, S.E. Arterial Baroreflex Resetting Mediates Postexercise Reductions in Arterial Pressure and Heart Rate. Am. J. Physiol.-Heart Circ. Physiol. 1998, 275, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Kulics, J.M.; Collins, H.L.; Dicarlo, S.E. Postexercise Hypotension Is Mediated by Reductions in Sympathetic Nerve Activity. Am. J. Physiol.-Heart Circ. Physiol. 1999, 276, 27–32. [Google Scholar] [CrossRef]
- Jeon, J.; Jeon, B.; Kim, S. Studies on the Optimal Exercise Intensity for Post-Exercise Hypotension and Its Mechanism. Exerc. Sci. 2003, 12, 197–222. [Google Scholar]
- Kim, J.; Song, I.; Kim, C.; Gho, H.; An, S.; Song, D.; Joung, D.; Kang, K.; Yi, Y.; Park, B.J.; et al. Thermal Comfort and Human Responses According to Tree Den-2 Sity in Forest Environments during Exercise in the Summer. Forests 2022, 14, 120. [Google Scholar] [CrossRef]
- Thomasa, E.L.; Brynes, A.E.; McCarthy, J.; Goldstone, A.P.; Hajnal, J.V.; Saeed, N.; Frost, G.; Bell, J.D. Preferential Loss of Visceral Fat Following Aerobic Exercise, Measured by Magnetic Resonance Imaging. Lipids 2000, 35, 769–776. [Google Scholar] [CrossRef]
- Johnson, N.A.; Sachinwalla, T.; Walton, D.W.; Smith, K.; Armstrong, A.; Thompson, M.W.; George, J. Aerobic Exercise Training Reduces Hepatic and Visceral Lipids in Obese Individuals without Weight Loss. Hepatology 2009, 50, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, G.J.; Wang, Z.J.; Chu, Z.D.; Sauer, P.J.J.; Haymond, M.W.; Rodriguez, L.M.; Sunehag, A.L. A 12-Week Aerobic Exercise Program Reduces Hepatic Fat Accumulation and Insulin Resistance in Obese, Hispanic Adolescents. Obesity 2010, 18, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Muscella, A.; Stefàno, E.; Lunetti, P.; Capobianco, L.; Marsigliante, S. The Regulation of Fat Metabolism during Aerobic Exercise. Biomolecules 2020, 10, 1699. [Google Scholar] [CrossRef] [PubMed]
- Mosher, P.E.; Underwood, S.A.; Ferguson, M.A.; Arnold, R.O. Effects of 12 Weeks of Aerobic Circuit Training on Aerobic Capacity, Muscular Strength, and Body Composition in College-Age Women. J. Strength. Cond. Res. 1994, 8, 144–148. [Google Scholar]
- Konopka, A.R.; Harber, M.P. Skeletal Muscle Hypertrophy after Aerobic Exercise Training. Exerc. Sport. Sci. Rev. 2014, 42, 53–61. [Google Scholar] [CrossRef]
- Morita, E.; Imai, M.; Okawa, M.; Miyaura, T.; Miyazaki, S. A before and after Comparison of the Effects of Forest Walking on the Sleep of a Community-Based Sample of People with Sleep Complaints. Biopsychosoc. Med. 2011, 5, 13. [Google Scholar] [CrossRef]
- López-Pousa, S.; Bassets Pagès, G.; Monserrat-Vila, S.; De Gracia Blanco, M.; Hidalgo Colomé, J.; Garre-Olmo, J. Sense of Well-Being in Patients with Fibromyalgia: Aerobic Exercise Program in a Mature Forest—A Pilot Study. Evid.-Based Complement. Altern. Med. 2015, 2015, 614783. [Google Scholar] [CrossRef]
- Bang, K.S.; Lee, I.; Kim, S.; Lim, C.S.; Joh, H.K.; Park, B.J.; Song, M.K. The Effects of a Campus Forest-Walking Program on Undergraduate and Graduate Students’ Physical and Psychological Health. Int. J. Environ. Res. Public. Health 2017, 14, 728. [Google Scholar] [CrossRef]
- Pasanen, T.; Johnson, K.; Lee, K.; Korpela, K. Can Nature Walks with Psychological Tasks Improve Mood, Self-Reported Restoration, and Sustained Attention? Results from Two Experimental Field Studies. Front. Psychol. 2018, 9, 2057. [Google Scholar] [CrossRef]
- Song, C.; Ikei, H.; Park, B.J.; Lee, J.; Kagawa, T.; Miyazaki, Y. Psychological Benefits of Walking through Forest Areas. Int. J. Environ. Res. Public. Health 2018, 15, 2804. [Google Scholar] [CrossRef]
- Oman, R.; McAuley, E. Intrinsic motivation and exercise behavior. J. Health Educ. 1993, 24, 232–238. [Google Scholar] [CrossRef]
- Ahmadi, A.; Noetel, M.; Parker, P.; Ryan, R.M.; Ntoumanis, N.; Reeve, J.; Beauchamp, M.; Dicke, T.; Yeung, A.; Ahmadi, M.; et al. A classification system for teachers’ motivational behaviors recommended in self-determination theory interventions. J. Educ. Psychol. 2023, 115, 1158–1176. [Google Scholar] [CrossRef]
- Bauman, A.; Ainsworth, B.E.; Sallis, J.F.; Hagströmer, M.; Craig, C.L.; Bull, F.C.; Pratt, M.; Venugopal, K.; Chau, J.; Sjöström, M. The Descriptive Epidemiology of Sitting: A 20-Country Comparison Using the International Physical Activity Questionnaire (IPAQ). Am. J. Prev. Med. 2011, 41, 228–235. [Google Scholar] [CrossRef]
- Ng, S.W.; Popkin, B.M. Time Use and Physical Activity: A Shift Away from Movement across the Globe. Obes. Rev. 2012, 13, 659–680. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T.; Alkandari, J.R.; Andersen, L.B.; Bauman, A.E.; Brownson, R.C.; et al. Effect of Physical Inactivity on Major Non-Communicable Diseases Worldwide: An Analysis of Burden of Disease and Life Expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Chau, J.Y.; Grunseit, A.C.; Chey, T.; Stamatakis, E.; Brown, W.J.; Matthews, C.E.; Bauman, A.E.; Van Der Ploeg, H.P. Daily Sitting Time and All-Cause Mortality: A Meta-Analysis. PLoS ONE 2013, 8, e80000. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Blair, S.N.; Katzmarzyk, P.T. Less Sitting, More Physical Activity, or Higher Fitness? Mayo Clin. Proc. 2015, 90, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Willis, F.B.; Smith, F.M.; Willis, A.P. Frequency of Exercise for Body Fat Loss: A Controlled, Cohort Study. J. Strength. Cond. Res. 2009, 23, 2377–2380. [Google Scholar] [CrossRef] [PubMed]
- Donelli, D.; Meneguzzo, F.; Antonelli, M.; Ardissino, D.; Niccoli, G.; Gronchi, G.; Baraldi, R.; Neri, L.; Zabini, F. Effects of Plant-Emitted Monoterpenes on Anxiety Symptoms: A Propensity-Matched Observational Cohort Study. Int. J. Environ. Res. Public. Health 2023, 20, 2773. [Google Scholar] [CrossRef] [PubMed]
- Donelli, D.; Antonelli, M.; Baraldi, R.; Corli, A.; Finelli, F.; Gardini, F.; Margheritini, G.; Meneguzzo, F.; Neri, L.; Lazzeroni, D.; et al. Exposure to Forest Air Monoterpenes with Pulmonary Function Tests in Adolescents with Asthma: A Cohort Study. Forests 2023, 14, 2012. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).