Abstract
Fire is a common disturbance in the Brazilian savanna (the cerrado), wherein high-frequency fires drive the vegetation structure, composition, function, and dynamics of savanna ecosystems. Under climate change pressure, further understanding of fire–vegetation relationships and interactions can provide new approaches for establishing integrated fire management strategies and can promote post-fire savanna vegetation recovery. To understand how 15 years of annually manipulated burning has affected vertical and horizontal structures of the vegetation, species composition and diversity metrics (species richness, Shannon’s diversity, and Pielou’s evenness), and aboveground carbon stocks, we surveyed all woody plant species with a diameter greater than three centimeters, in 15 plots of a typical Brazilian savanna (cerrado stricto sensu) at an experimental research station in central Brazil (the cerrado biome). The 15 plots (five plots per treatment) had been differently affected by fire events over a decade, and comprised three treatments: (i) annual fire, (ii) legacy fire (>15 years since the last fire event), and (iii) control (not burned in the past 30 years). A non-metric multidimensional scaling (NMDS) analysis indicated that fire had a significant effect on the species composition among the treatments; some species benefited from fire, such as Erythroxylum suberosum, whereas other species propagated better without fire, such as Roupala montana and Dalbergia miscolobium. Over a decade of annual fire events have led to decreases in Shannon’s diversity, species richness, and stem density, which were significantly lower in the annual fire treatment than in the control and legacy fire treatments. Stem density by diameter and height size classes (except for the 1–2 m class and above 8 m class) was higher in the control than that in the annual fire treatment, but the number of dead trees did not differ between the control and the annual fire treatment. Our results also showed that fire was a factor in changes in the evaluated parameters, such as in the annual fire treatment, where fire reduced the amount of biomass, and therefore the carbon stocks. This study suggests that, if burned yearly, typical Brazilian savannas can become less biodiverse in terms of woody plant species which negatively affects their fire resilience. Therefore, fire management practices should focus on determining the frequency of fire disturbances from which these ecosystems may benefit the most.
1. Introduction
Understanding the natural and anthropic alterations that occur in savanna vegetation and biomass pools is essential for conservation science, especially as it can reflect on the elaboration of protocols for vegetation recovery, and on evaluations of the human-fire influence on the environment. Studies on fire disturbance effects on savanna vegetation have provided evidence and insights on the resilience and dynamics of these ecosystems, in addition to monitoring the factors that govern changes in vegetation structure, composition, and productivity [1]. Tropical savanna biomes are particularly relevant, as there is a predominance of trees without a continuous canopy, a low density in the woody stratum, and a grass dominance [2]. They are among the most widespread terrestrial biomes [3,4], characterized by a biologically diverse flora [5], and potentially resistant and resilient to extreme drought conditions [6].
The Cerrado (Brazilian savanna biome), the second largest biome in Brazil, covers approximately 2.5 million square kilometers, or approximately 22% of the national territory [7,8], and it is one of the world’s biodiversity hotspots [9]. This biome is highly threatened because it is not entirely legally protected and has high rates of anthropic conversion to alternative land uses [4,10]. It is globally recognized for its exuberance and biodiversity, and it provides numerous ecosystem services [11], which stands out in the elaboration of ecosystem restoration protocols and potential for climate change mitigation [12]. Savanna ecosystems in the cerrado are particularly influenced by the availability of water and nutrients, with fire as an agent for maintaining the biomass gradient in this morphoclimatic domain [13,14], altering the vegetation composition and structure. More recently, these savanna ecosystems have been investigated to estimate aboveground biomass because of the complexity of the expansion of anthropogenic activities that tend to affect their different formations [15,16] and to predict information about their nutrient and carbon cycles [15,16].
In addition to intrinsic regional characteristics, such as acidic soils, poor nutrient content, and a superficial water table [17,18], fire is an agent that modifies the structure and composition of vegetation in savannas [19], thereby contributing to the establishment of different habitats. Although fire is a prevailing disturbance in cerrado savanna ecosystems, it is challenging for ecosystem managers and scholars to use preventive fire as a method to avoid highly impactful fire events, which, according to the current legislation, does not cover the specificities of each ecosystem type across the biome, and does not consider the ecological importance of biodiversity maintenance. Therefore, information regarding the need and usefulness of fire management is essential for the development and perpetuation of some plant species in the cerrado [20]. Considering the importance of savanna fire management, there are records of the presence of fires for at least 32 thousand years [21], with fire being responsible for the antagonism of the physiognomies found in the cerrado [22]. Fire management legislation needs to be adapted to deal with fire issues, with the establishment of goals anchored in sustainability as well as effective and attainable long-term commitments to reduce negative fire effects in these environments.
Considering this pressing challenge, one method to minimize immeasurable damage caused by ecosystem fires is the use of integrated fire management, which has excellent applicability and occurs through prescribed, planned, and authorized burnings that consider each ecosystem type. This approach results in different proposals for decision making, including fire as a modeling agent [23], and considers the demands of local populations in the area where it is applied, their cultural traditions, and local socioeconomic conditions [24]. Therefore, there is an improvement in the understanding of fire–human–vegetation relationships in different Brazilian ecosystems, especially those in the cerrado, considering their ecology [25] and acting as agents of the dynamic balance of savannas, forests, and the regulation of the advance and retreat of vegetation [26].
The overall objective of this study was to quantify the effects of fire on the structure, composition, diversity, and aboveground carbon stocks of the shrub-tree component of savanna ecosystems differently exposed to fire in central Brazil. This study was carried out in three savanna treatments, two of which were subjected to constant fire regimes (with and without planned annual fire over 15 years). Specifically, we quantified the effects of fire on (i) the vertical and horizontal structures of the vegetation, (ii) diversity metrics (richness, diversity, and evenness), and (iii) the aboveground biomass carbon stocks of shrub-tree savanna vegetation. We aimed to answer the following questions: (i) Are 15 years of annual fire sufficient to change the floristic composition and diversity of savanna ecosystems? (ii) What is the effect of annual fires on the vertical and horizontal structures of savannah vegetation? (iii) Do aboveground biomass and carbon stocks change after 15 years of annual fire relative to the control areas? We tested the following hypotheses:
Hyptheses 1 (H1).
Annual burning in the cerrado alters the composition and diversity of the vegetation.
Hyptheses 2 (H2).
The incidence of fire diversifies the structure and modifies the distribution of species among size classes.
Hyptheses 3 (H3).
Phytosociology, horizontal structure, and vegetation loss are parameters for evaluating fire effects on a savanna plant community.
Hyptheses 4 (H4).
Burning alters vertical and horizontal parameters, compared to communities where there is no regular fire.
Hyptheses 5 (H5).
Fire influences species richness, diversity, and evenness, and negatively alters richness in regularly burned areas.
2. Materials and Methods
2.1. Study Site
This study was conducted in savanna ecosystems of the Brazilian cerrado in the Federal District. The Federal District has a District Law (Number 742, 28 July 1994) which deals with the protection of its natural vegetation which has a history of unchanged native vegetation until the 1960s. With the onset of the construction of Brasilia, the vegetation in the Federal District was converted into agriculture and urbanization through fires. From that period until now, high rates of deforestation and degradation have been witnessed, especially through the use of fire. This raises questions about the effects of fire on the composition, richness of plant species, and structure of vegetation, motivating this and other studies [20] to answer questions about the recurrence and importance of these anthropic disturbances.
Farm Água Limpa (FAL) is the experimental farm of the University of Brasília (UnB), where cerrado stricto sensu (15°56′–15°59′ S and 47°55′–47°58′ W, between 1048 m and 1150 m elevation) is the predominant phytophysiognomy that covers approximately 1480 ha [27,28]. With about 4340 hectares of total area, the farm is part of the Cerrado Biosphere Reserve [29], which includes the Environmental Protection Areas (APA) Gama and Cabeça-de-Veado, the Botanical Garden of Brasília (JBB), and the Ecological Reserve of the Brazilian Institute of Geography and Statistics (IBGE). At FAL, there are preservation areas, such as the Relevant Area of Ecological Interest (Capetinga-Taquara stream), which covers 2100 hectares and has a history that exceeds 30 years of teaching, research dedicated to protection and conservation, and environmental sustainability [30]. The study area has an Aw-type climate [31], with dry seasons between May and October, rainy seasons from November to April, an average annual rainfall of 1600 mm, and monthly averages between 9 mm and 249 mm [32]. Air temperatures range from 12 to 28.5 °C and the relative humidity is from 15 to 70%. The soils are largely characterized as dystrophic red-yellow latosols, with high acidity and low nutrient availability [33,34], which influence the type of local vegetation. The area is composed of approximately 45% cerrado stricto sensu [35], distributed in flat terrain. For this research, the areas (treatments) selected at FAL were an area with a history of annual burning (Area 1, or annual fire), an area 15 years after the last fire event (Area 2, or legacy fire); and an area with no record of fire occurrence for 30 years (Area 3, or control) (Figure 1).
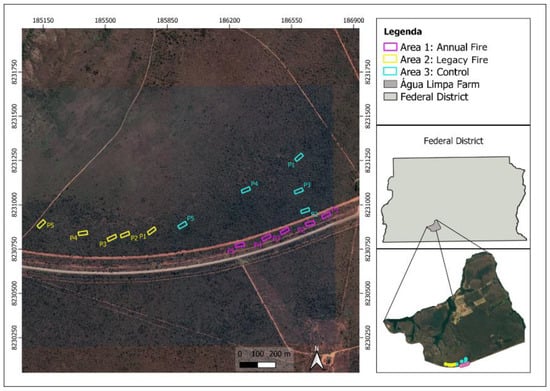
Figure 1.
Study area location: Farm Água Limpa (FAL), Federal District, Brazil. The studied treatments (plots) are delimited in the google earth images (2021), as following: Area 1 (in pink), annual fire; Area 2 (in yellow), legacy fire; Area 3 (in blue), control (fire-free area for the past 30 years).
2.2. Sampling Design
The three cerrado treatments (plots), in this study, were chosen for their high species richness and biodiversity, because the observed environments are easily accessible by the population since they border a highway, and because they have a history of manipulated fire interventions. The three savanna treatments (plots), in this study, were chosen for their high species richness and biodiversity, because the observed environments are easily accessible by the population since they border a highway, and because they have a history of manipulated fire interventions, that is, integrated fire management for over 15 years, which is based on minimizing the availability of dry fuel material. Because of local seasonality, it becomes easily incandescent either due to natural or anthropic specificities, and the mediations arising from this practice do not apply randomly, but contain different cultural, ecological, and technical aspects, and promote the mitigation and prevention of forest fires in Brazilian vegetation. In addition, approximately 38% of the FAL area is composed of savanna ecosystems [36].
Vegetation characterization studies regarding cerrado stricto sensu [37] have identified the main species compositions and their characteristics, analyzed the existing diversity, measured the individuals, and distinguished the vertical and horizontal distributions. In general, in this biome, there are several types of vegetation, typified as grasslands (with Campo Rupestre, clean and dirty), forestry (being Mata Ciliar, Cerradão, Mata Seca and de Galeria), and savannas (having Vereda, Parque de Cerrado, Palmeiral, and the cerrado stricto sensu), comprising 25 phytophysionomies [2]. The immeasurable richness of the cerrado, as well as the physiognomy components, are also described in detail in an analysis by [20], under the action of fire in these formations.
2.3. Fire Experiment
The sample plots were allocated within an area of cerrado stricto sensu covering approximately 7.50 km. Fifteen plots (20 m × 50 m) were randomly allocated, three plots for each treatment, according to a well-established protocol [20], totaling 0.5 ha in each sampled treatment and 1.5 ha in the entire experimental area. Treatment 1 (Figure 1) is characterized by an annual fire over 15 years, defined as black firebreak. The annual burn is conducted in the months of June or July and aimed at reducing the load of combustible material to prevent larger fires. The black firebreak of the annual fire treatment has the support of brigade members from the farm, a team from the Federal District military fire department, the Brazilian army, the Institute of the Environment and Renewable Natural Resources (IBAMA), the Brasília Environmental Institute (IBRAM), and volunteers, and it is created in the vegetation that borders the highway, between the months of June and July, which is the beginning of the fire risk season in the region. It is an activity commonly used to reduce deposited plant material, thus avoiding possible outbreaks of fire or even large fires, and it falls within the regulations established by integrated fire management legislation.
2.4. Vegetation Data Collection in the Field
To estimate aboveground carbon stocks, we collected species identification and diameter and height data of the vegetation through a forest inventory of the tree-shrub component during the dry season. The inventory started at the annual fire treatment, before the black firebreak, between April and December 2021, when each plot was delimited by iron stakes, subdivided into 10 subplots of 100 m2, and all woody individuals of all plots in the three treatments were measured.
We sampled all 0.1 ha plots in each treatment, for a total of 0.5 ha per treatment, and a total of 1.5 ha (3 treatments × 0.5 ha) for all three treatments. All individuals with a base diameter (Db) of 0.30 m above the ground and a height equal to or greater than 5 m [38] were measured and their data recorded. Diametric measurements were performed using a caliper and total height was measured using a hypsometric ruler [39]. Bifurcated individuals were measured separately to obtain basal area and stem biomass. All trees falling within the established criteria were botanically identified at the family, genus, and species levels using the APG IV botanical classification system [40] and updated according to the nomenclature of the Brazilian Flora list [41]. This procedure aimed to quantify Shannon’s Diversity (H’) and Pielou’s equability (J) parameters according to [42,43], as well as stem density (number of woody individuals per unit of area) and richness (number of species per unit of area) [44,45,46].
2.5. Aboveground Carbon Stock Estimation
With the tree data obtained from the forest inventory, specifically stem diameter and total height, we estimated the aboveground carbon stocks using ecosystem-specific adjusted models [27,28]. We multiplied biomass values by a factor of 0.47 [47] to obtain the carbon stock values.
2.6. Statistical Analysis
2.6.1. Floristic Composition and Structure
An analysis of the floristic composition and structure of the vegetation was conducted using the inventory data and the quantitative parameters established by [27,45]. To investigate the floristic composition patterns, a non-metric multidimensional scaling (NMDS) analysis was carried out using the abundances of woody species per plot (i.e., number of individuals per species in each plot) using the R software (R version 4.2.3, metaMDS function in the vegan package; R Foundation for Statistical Computing, Vienna, Austria); ref. [48] based on Bray–Curtis dissimilarity (vegdist function in the vegan package) and standardized data [49] were used to compare the treatments.
The species vectors that were significant at the 99% significance level were included in the NMDS plot. After checking the normality assumption using the Shapiro–Wilk test, ANOVA was performed to detect differences among treatments in the following evaluated diversity and structure parameters: Shannon’s diversity and Pielou’s evenness indices, stem density (woody individuals per plot), and species richness (number of species per plot).
2.6.2. Carbon Stocks
In this study, aboveground biomass was estimated using models adjusted by [28,45]. Carbon stocks were estimated by multiplying the biomass values by a factor of 0.47 [46]. Tree and shrub aboveground carbon stocks across treatments were estimated as: EC = 0.24564 + 0.01456 ∗ Db2 ∗ Ht (R2 = 98.29% and Syx = 25.79%), where EC is the carbon stock per tree, in kg; Db is the diameter taken at the base (30 cm above ground level) in cm; Ht is the total height of the tree or shrub, in m; R2, is the coefficient of determination; Syx is the standard error of the estimate [28].
The results of this evaluation of the analyzed parameters were summarized using boxplots, and other analyses were performed in the R software (version 3.6.2) [50].
3. Results
3.1. Species Composition across Treatments
The NMDS analysis indicated that the species composition differed significantly among treatments based on the species abundance matrix, suggesting that fire influenced the floristic composition of the studied cerrado ecosystems (Figure 2). The NMDS showed that the floristic composition of the control treatment was more similar to that of the legacy fire treatment than that of the annual fire treatment, reflecting the effect of fire on the composition of species in the studied savanna ecosystems. The ordination demonstrated the separation between the species in the treatments, with the control and the legacy fire (record of fires for 15 years) treatment differing from the annual fire treatment. In this case, there is evidence of the existence of a considerable number of species sensitive to repeated fires.
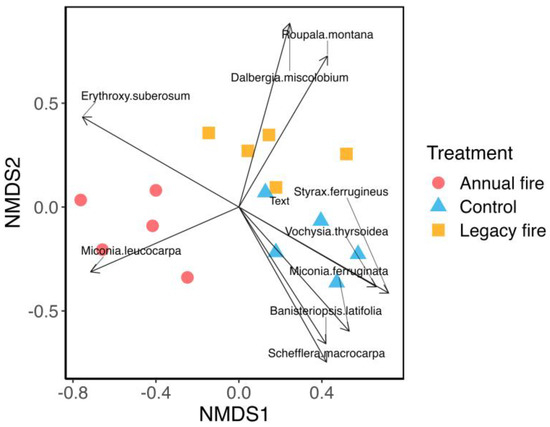
Figure 2.
Ordination of significant species vectors by plot (five plots per treatment). Distribution of species by treatment based on the non–metric multidimensional scaling (NMDS) analysis for the three treatments, including species with vectors significant at the 99% significance level (Appendix A, Table A1). Circles represent the annual fire treatment, triangles represent the control (30 years without fire), and squares represent the legacy fire treatment (i.e., where the fire occurred 15 years prior to the survey).
Among the three treatments, 2734 individuals were identified; the control presented 38.3% of the identified species, followed by the legacy fire treatment with 37.6% of the species, and in the annual fire treatment, the percentage of taxa was 24.1%. Some species, such as Miconia leucocarpa (Figure 2), were strongly associated with the occurrence of fire; however, species such as Roupala montana showed a greater association in the legacy fire treatment, where greater intervals of fire incidence occurred, and species such as Miconia ferruginata had a higher occurrence in the fire extinguishing treatment. These different behaviors may be associated with the strategies of each species, the architecture of individuals [51].
3.2. Diversity Metrics across Treatments
Shannon’s diversity varied significantly among the three treatments, wherein diversity was highest in the control treatment, with values ranging between 2.9 and 3.1, and lowest in the annual fire treatment (Figure 3a). The control treatment did not differ significantly from the legacy fire treatment. Species richness also varied among the treatments, with values in the annual fire treatment lower than those of the other two treatments (Figure 3b).
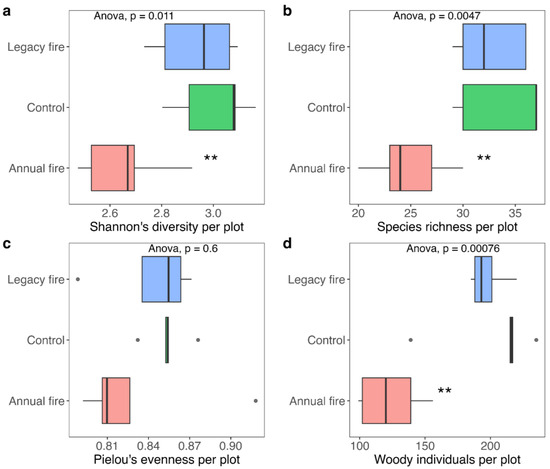
Figure 3.
Structure (woody individuals per plot), richness, diversity (Shannon–Wiener), and evenness of the shrub-tree community in cerrado stricto sensu at Fazenda Água Limpa, DF: (a) Shannon diversity; (b) species richness; (c) Pielou’s evenness; (d) number of woody individuals per plot across the three treatments in the Brazilian cerrado. The boxplots show the median bands representing the median values of the variables, the upper and lower indicate the first and third quartiles. The bars indicate the minimum and maximum values. Dots denote outliers. ** p < 0.01.
Pielou’s evenness values did not differ among treatments, with values ranging from 0.80 to 0.86. The value found for the legacy fire treatment, i.e., 0.85, was the highest average among the three treatments, suggesting that such treatment had the greatest evenness (Figure 3c). The number of woody individuals per treatment was significantly different. Figure 3d shows the effects of fire on the number of woody individuals in each treatment, with the greatest number in the control plots, followed by the legacy fire and annual fire treatments.
3.3. Structural Differences across Treatments
Figure 4a shows the number of dead trees in each treatment. In the control, we identified 19 dead individuals and a similar number was identified in the annual fire treatment as compared with the legacy fire treatment, with 10 dead individuals. Among the diameter distributions obtained in each treatment (Figure 4b), in the 1–5 cm diameter class, the legacy fire treatment had the highest number of individuals; the control treatment stood out with a greater number of individuals in the 5–10 cm diameter class and 165 individuals in the 10–15 cm diameter class. However, in the 15–20 cm diameter class, the annual fire treatment stood out with 42 individuals. Evaluations of the number of individuals among the established diameters demonstrated their uniformity for the established treatments. Among the height classes (Figure 4c), there was variation from a few centimeters (0.4 m) to more than 9 m. In this analysis, the legacy fire treatment had the highest number of individuals, i.e., 606, with heights of up to 3 m. For the height classes from 3–4 m to 7 m, the control treatment group comprised more individuals, i.e., 435 individuals. However, in the 7–8 m height class, the legacy fire treatment stood out with 11 and 10 individuals in the 7–8 and 8–9 m height classes, respectively, compared to 3 individuals in the control treatment and 4 individuals in the annual fire treatment. In the above 9 m height class, two treatments (control and legacy fire) had four individuals each, with no record of this height class for the annual fire treatment.
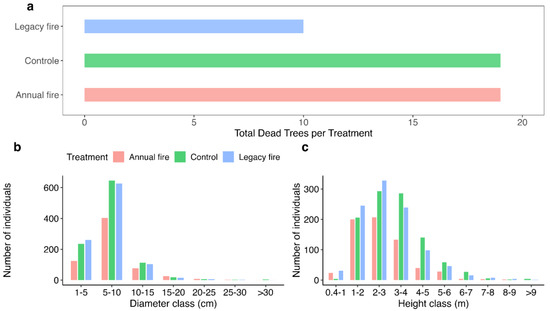
Figure 4.
Structural differences in vegetation among the treatments under the effects of fire, including: (a) The total number of dead individuals per treatment; (b) the total number of individuals per treatment in each diameter class; (c) the total number of individuals per treatment in each height class.
3.4. Aboveground Carbon Stocks across Treatments
Figure 5 shows the variations in live shrub-tree aboveground carbon stocks among the three treatments, indicating substantial variation among the treatments. Our data indicate that aboveground carbon was highest in the control treatment (11.3 Mg C/ha), which was followed by the legacy fire and annual fire treatments (7.2 Mg C/ha). This analysis highlights the main consequences of fire on the vegetation of the Brazilian savanna in terms of carbon stocks.
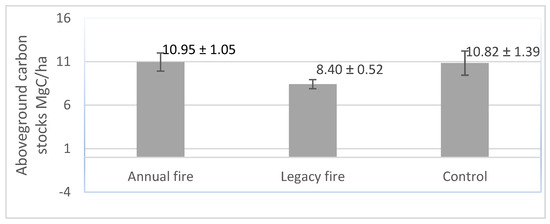
Figure 5.
Estimated live shrub-tree aboveground carbon stocks (in Mg C/ha) per treatment.
4. Discussion
4.1. Relationship between Fire and Cerrado Species Composition
Our findings support the hypothesis regarding the influence of annual burning on changing the composition and diversity of species in the evaluated savanna ecosystem, indicating that the absence of fire for relatively longer periods contributed to increases species density. In general, we expected that the fire differences would negatively affect the composition and diversity of the vegetation among the three treatments in the Brazilian cerrado. Fire is an intrinsic factor that can alter vegetation in this environment [49], conferring the evolution, resistance, and resilience of some species [52,53]. Several studies have shown that, in Brazil, the use of uncontrolled fire for the management of areas for agricultural and livestock purposes has caused high rates of fires every year, modifying human interactions with the vegetation, changing the climate, and changing the soil microorganisms, as well as being the main agent causing environmental problems and negatively affecting and threatening biodiversity [54]. Due to the incidence of fire exposure, we observed that there had been losses in composition and diversity, which have threated the vegetation and contributed to the Brazilian cerrado’s inclusion on the list of global biodiversity hotspots [9].
We demonstrated that species are affected by fire recurrence, such as Miconia leucocarpa from the Melastomaceae family, with the highest representation in the Brazilian cerrado [55] and Davilla elliptica, from the family Dilleniaceae; annual burning changes their ability for regrowth and development [56]. Fire frequency matters for vegetation because of the time needed for the recovery of nutrients needed for regrowth of the aboveground component, which in constant regimes becomes infeasible, and therefore plants do not reach the minimum size for resistance and resilience to fire [20]. However, our research showed that annual, biennial, or four-year burnings did not occur, but with annual fires, the ability to overcome, regrow, and the development of species with medium heights and diameters was significant, such as Dalbergia miscolobium and Roupala montana, developing well with the inclusion of fire in its growth cycle. The findings of previous studies with prescribed fires corroborate ours, showing that these individuals have a greater height after a prescribed fire [19,20].
Fire has detrimental consequences on the radial growth of some plant species and can negatively affect sexual reproduction [57]. The lack of structures in the diaspores of monocotyledonous plants, such as hard integuments or more heat-resistant coverings, also contributes to seed loss during fire exposure. All layers of cerrado vegetation are affected by fire, although in different ways, depending on the phenological characteristics of the species. Fire can negatively affect the sexual reproduction of some species, while favoring others. Some species that inhabit fire-prone environments have developed mechanisms that allow them to survive. According to [21], bark is considered to be the most important mechanism by which trees protect themselves against forest fires because of its excellent thermal insulation capacity. The presence of fire can influence several aspects of plants, such as flowering [58,59], fruit and seed production, clonal reproduction [60], and plant architecture [51]. Ref. [61] concluded that increasing the thickness of the shell promoted a higher level of exchange rate protection. Another survival mechanism in this phytophysiognomy is the promotion of regrowth with the application of fire dating back to the 18th century [62]. In general, the dynamics of cerrado vegetation are favored by biennial fires, in which regrowth ability is favored within this range of fire incidence; however, topkill (death of the aerial part) and regrowth of the basal part in the forest may occur, and if there is an increase in fires, trunk and underground organs are reduced, causing severe damage or death [19,20]. For this vegetation, regrowth is an indispensable mechanism in regeneration, with fire as the maintaining agent [20].
We tested the second hypothesis regarding the effects of fire on vegetation composition and diversity by looking at the differences in the distribution and abundance of species across treatments. Our findings corroborated previous studies that have described fire in the cerrado as a conditioner of some species, where higher density and frequency occurred for individuals in the Araliaceae (Scheflera macrocarpa) and Styracaceae (Styrax ferrugineus) families. In the control and legacy fire treatments, other families followed this trend. This can be explained by the sensitivity of some individuals to environmental changes [2], and it has been reported that in a savanna ecosystem, some species are more tolerant to fire than in other environments [57]. However, it can also depend on the peculiarities of each family. Fire studies in Brazilian savannas suggest that changes are promoted by fire, but to understand which species and families are most fire resistant, several data are needed over relatively longer periods of time [63], unlike the objective of this work.
To this end, the NMDS analysis confirmed the distinction between the species compositions across treatments. Although the results reinforce the interference of recurrent burnings on the cerrado ecosystems, it is still not possible and desirable to completely suppress fire because it is a natural disturbance that is historically needed and the vegetation has adapted [13,64,65,66]. Furthermore, completely suppressing fires is not a viable alternative [67], as it produces an accumulation of organic matter and influences the intensity of fires, contributing to catastrophic events such as increased carbon emissions and loss of biodiversity [68,69,70,71]. Therefore, fires are required to eliminate excess shrubs and grassy materials [72]. These results support decision making for operations using fire, such as the firebreak or black line that the Fazenda Água Limpa conducts annually.
4.2. Fire Effect on Vegetation Diversity
We observed differences in Shannon’s diversity and Pielou’s evenness across treatments. The difference in floristic diversity was strongly correlated with the number of fires in the last 15 years, suggesting that fire is a determining factor in the structure of communities in this savanna and indicating the species are resistant to this fire gradient. Our study demonstrated that the plots excluding fire resulted in a more closed phytophysiognomic formation, dominated by trees such as Tachigali subvelutina and Caryocar brasiliense and shrubs (Erythroxylum suberosum and Erythroxylum tortuosum) sensitive to fire. In general, in places where fire is excluded, there is a possibility of forest invasion onto the savanna, reducing the diversity of shrub and herbaceous vegetation, which we call the process of “woody encroachment” [73]. Our results make sense, since changes in the herbaceous layer can demonstrate a change in the structure of the community, which is influenced by fire, changing the number of species between areas with regular fires and those not burned [61], indicating that periodic fire events gradually reduce species richness and diversity [74].
In this study, it was evident that the average diversity of the Shannon index and species richness in all plots differed significantly among treatments, being larger for the control and legacy fire treatments. Our findings suggest that constant fire changes the richness and diversity in the communities where it occurs. Ratifying studies by [75,76] showed that fire altered the structure, composition, and density of the vegetation, affecting nutrient fluxes and input. Different studies have shown that the absence of fire allows the establishment of sensitive species and the possibility of conversion to different phytophysiognomies [77,78,79], being positive for resistant species and negative for species sensitive to high temperatures [80,81,82,83].
The differences in Pielou’s evenness for all plots across treatments outlined in this study emphasized the interference of fire on the post-fire dynamics of the savanna vegetation. We found differences in the evenness of distributions between the control and legacy fire treatments and that in the annual fire treatment, which can be explained by the fact that areas with high rates of burning have more open tree cover due to a high mortality of adult trees [84]. These results confirm our hypothesis because regular burning simplified the vertical and horizontal parameters of the plant community. Certainly, plant species that are sensitive to fires are lost in areas with intense fires [85]. However, previous studies on the effects of the fire regime in cerrado communities [20] have suggested that fire is essential for the cerrado, acting as a natural element for the biome and contributing to the evolution and diversity of species in this region. physiognomy.
As expected, the plots treated with annual fire had fewer woody individuals, with a total of 147 woody individuals, in contrast with the legacy fire treatment with 200 species and the control with the greatest number of woody species, which, in turn, affected the development of vegetation. A study in the same type of savanna biome [20] evaluated the incidence of fire for a few years and observed a reduction in the shrub-tree vegetation, emphasizing that recurrent burning causes mortality of woody plants [86,87], increases the dominance of grasses [20], alters the landscape, changes the floristic composition [59], impoverishes the complex cerrado system, and modifies the environmental structure [27].
4.3. Fire Effects on Vegetation Structure
A total of 473 live individuals were registered in all treatments in this study, with 110 live individuals in the annual fire treatment, 170 live individuals in the legacy fire treatment, and 193 live individuals in the control. We found a lower tree mortality in the legacy fire treatment, with 10 dead trees, in contrast to the 18 dead trees found in the two other treatments (Figure 4a). This perishing of plant species in Brazilian savannas is remarkable because of the flammability of individuals, favoring selective processes of plants with greater and lesser adaptation [88]. In places with constant burning events, the number of dead trees and species is derived from those with thinner bark and less developed roots, making them more susceptible [89,90,91]. Another factor that contributes to high mortality rates, in this case for an area with an annual burning record, is the amount of fine fuel, grasses, leaves, trunks, and fine branches deposited in the soil [92]. However, we also found that non-occurrence of fire does not mean a reduction in mortality, explaining the need for fire for the growth of species in the Brazilian cerrado. Therefore, mortality does not show major differences between forests where sporadic and interspersed fires occur [90].
Diametric values (Figure 4b) were strongly influenced by the minimum adopted criterion of 5 cm, according to a study that underestimated shrub and subshrub species individuals [93]. Legacy fire treatment stands out for this initial diameter class, indicating that burning, as well as poor soils and rainfall seasonality, are determinants of the vegetation composition of the cerrado [21]. These results indicate non-generalized diametric patterns in the Brazilian cerrado, supporting research where a zero-fire policy does not conserve, much less protect, the existing biodiversity [94]. Different fire regimes determined by frequency and severity and associated with global change factors and fire vectors, influence and drive the diverse characteristics of the biome [95]. As shown in the legacy fire treatment, certain periods of fire benefited species growth, and although fire positively influenced the treatment, for the evaluated metric, its effects differed strongly in each treatment [70]. According to [19], the sporadic nature of fire events can be a renewing factor for plants, with evidence that some species need fire to develop, which may have driven individuals of smaller diameter classes to stand out because of the inclusion of fire in their life cycle.
However, in the evaluation of the diametric classes, for the plants that measured between 5 and 10 cm, the burnings were not favorable, pointing to greater diametric growth; in the control treatment, a greater number of plants were within the mentioned class and regular burning reduced the number of individuals. For the 10–15 cm diameter class, there was a considerable difference in the number of individuals of the three treatments, indicating the main characteristics of the effects of fire on the savanna vegetation, reinforcing that it is an environment with smaller diameter trees and, consequently, thinner bark, and shrub species, which suffer more injuries during burning [70]. The following diameter class values corroborate the suggestion provided by [19] that species with diameters greater than 5 cm survive frequent fires, especially because of the complex dynamics of the savanna ecosystems, reflecting species adaptations to different climate variations, temperature fluctuations, edaphic particularities, anthropic interventions, and post-burn damage, and the interaction among all these variables is reflected in the basal area of savanna ecosystems.
Tree-shrub height ranged from 0.4 m to over 9 m across treatments (Figure 4c), with differences in the number of individuals within each size class among treatments. We noted that the legacy fire treatment stood out with the highest number of individuals (i.e., 606), with heights of up to 3 m. In the 0.4–1 m height class, the treatment with the occurrence of fire presented the most specimens, indicating that for plant growth, fire is essential for development. In the height classes from 3–4 m to 7 m, the control treatment grouped the most individuals (435). However, in the 7–8 m height class, the legacy fire treatment stood out with 11 specimens, and with 10 specimens in the 8–9 m class. In the above 9 m height class, the two treatments (control and legacy fire) had four individuals each, with no record of this height class for the annual fire treatment. In terms of vertical structure of the studied ecosystem, the treatments showed significant differences for the smallest sizes, where the treatments with fire occurrence had the most individuals, supporting the role of fire in shaping the structure of savanna ecosystems [96,97,98].
The legacy fire treatment had the highest stem density within the 1–3 m height class. In this height class, Dalbergia miscolobium, Pouteria ramiflora, Roupala montana, and Miconia burchellii occurred with maximum heights, benefiting from an episode of fire to maximize their development, and thus stood out compared with other species. However, for heights from 3 m to 7 m, as the trees reached larger sizes, in addition to a reduction in the number of species, the treatments also differed. Between 8 m and 9 m, we identified two species in the legacy fire treatment and one species for the three treatments up to 9 m in height. We also identified the species Bowdichia virgilioides and Caryocar brasiliense in the above 9 m height class in the control treatment. Our findings indicated that the control treatment had more individuals in the higher size classes, and there was no occurrence of individuals with great heights. Also, the studied species may have benefited from the indirect effects of fire on the composition and functioning of the ecosystem, increasing the availability of nutrients, light, and water [99]. The direct and indirect effects of fire on the development of tropical vegetation are the result of competition; rapid release of nutrients into the soil; seasonality; and the characteristics of fire, intensity, frequency, and duration [100].
4.4. Fire Effects on Aboveground Carbon Stocks across Treatments
Fire affects ecosystem carbon fluxes [101,102] because trees and shrubs store carbon above and below ground [103,104]. Thus, for fire management in ecosystems with great richness and phytosociological diversity of species, such as in the cerrado stricto sensu [27,58], a factor of great importance in indirect measurements is the accuracy of productivity estimates. This accurate estimation depends on the application of specific allometric equations generated for these environments [27,28], which involve tree attribute data such as diameter, wood density, and height, collected from individuals in the studied plant community [27].
Carbon stock values are good predictors of the structural conditions of a forest [105], such that forests in natural succession with smaller trees have less dispersed distributions of aboveground carbon [106]. In addition to areas with less interference, the intrinsic characteristics of the site influence carbon stocks, composition, and diversity [107]. Our aboveground carbon data support discussions on the changes imposed by fire frequency in the Brazilian cerrado. Less frequent fires (e.g., control treatment) contribute to differentiation of the levels of carbon stocks in a region, influence the height of trees and the type of existing tree cover, and maximize aboveground woody biomass [108,109]. However, savannas affected by fire sporadically, as in the legacy fire treatment, have increased structural complexity, changes in habitat, increases in and diversity of biomass, and consequently, greater availability of nutrients in the soil. Thus, in this study, the high rates of burning were not favorable for carbon stocks, as the seasonality of fire events considerably reduced the input of grasses, branches, leaves, and small shrubs, and therefore the carbon stocks.
Areas with different values of carbon estimates in their natural habitat may be related to peculiarities, such as variations in height and diameter, age, type of anthropic interference, number of individuals [110,111], soil type and fertility, topographies [112], and individual density [113]. In our study, aboveground carbon stocks varied as a function of the direct effects of fire on biomass. The disturbance regime induced the treatment with annual fire and the reduction in the main constituents of the vegetation, influencing the dynamics of the carbon stocks. We suggest that the reduction in tree components where fire occurred regularly increased the availability of light and modified the microclimate causing carbon stocks to change, also due to exposure of the vegetation to high temperatures during fire events. The carbon stock estimates obtained in this research were 10.95 Mg/ha in the annual fire treatment, 8.41 Mg/ha in the legacy fire treatment, and 10.90 Mg/ha in the control. These results were lower than those found by [105], who quantified carbon stockss at the Fazenda Água Limpa and estimated carbon stocks at 8.60 Mg/ha, and [27] who found values close to 3.3 Mg/ha. These differences may have been caused because of the occurrences of fire in the treatments, but in general, we argue that the suppression of fires for this evaluation contributed to the increase in carbon stocks.
Even if the values in this analysis were not relatively high for treatments with fire, because the collections were carried out in a shorter period than most previous studies, measuring the impact of fire on carbon balance was extremely important because of its complexity and performance in controlling species composition, influencing ecosystem development, and impacts on ecosystem services. Thus, for our study site, the frequency of fire occurrence in this vegetation influences aboveground carbon stocks. Furthermore, vegetation responses to fire vary according to the duration, frequency, and intensity [113]. Therefore, the long-term effects of fires can modify the patterns, fluxes, and carbon stocks of Brazilian savannas. Thus, distinguishing the consequences of short- and long-term fire, on the distribution, species composition, and carbon stocks in these places is a challenge, due to the diverse characteristics of each phytophysiognomy, in addition to the fact that the dissemination of harmful effects overlaps the current debates, where fire management in tropical savannas should be considered in decision-making processes. The loss of biomass by fire events contributes to exposure of the tree stratum and loss of woody individuals, altering the climate and plant communities [54]. Our results substantiate claims that fire should not be completely excluded from this ecosystem because of the accumulation of dry biomass, which intensifies the possibility of large and intense fires [65,66,69,70]. Overall, by understanding how the aboveground biomass varies in the cerrado, the carbon stock will bring environmental responses to this parameter, providing information on estimates of carbon stocks and assisting in conservation policies [113].
5. Conclusions
This study explored the effects of fire on the vegetation of a Brazilian neotropical savanna, and suggested that annual fire interferences affect the diversity, composition, richness, and vertical and horizontal distributions of individuals. Our hypotheses related to the composition and structure of the vegetation were supported, since annual fires altered the evaluated vegetation parameters, diversifying the distribution and imposing divergent patterns compared to treatments without regular fire. Contrary to our expectations, tree mortality was determined by both fire and its absence. We deduced that the absence of fire did not characterize the exemption of tree deaths. In general, aboveground carbon stocks are reduced with annual fire events. In the future, the assessment of biomass over a longer period will be able to predict these estimates to consider seasonality and to determine the levels of anthropogenic and natural interference, as well as to establish fire disturbance parameters for restoration and conservation of carbon stocks in these savanna ecosystems.
There are many challenges to overcome when estimating carbon stocks in cerrado ecosystems due to environmental and structural aspects, different types of vegetation, and the application of tools and methodologies that consider the variability and specificity of each location. Additional studies, which take longer to evaluate in the field, are essential to deepen the understanding of the main effects of fire on the entire composition and structure of savanna ecosystems. Future studies should include evaluations of behavioral patterns in the mediation of every component of the biomass, the consequences on nutrient cycling, the development and mortality of woody individuals, and the increment that fire events impose and influence on carbon stocks and on the ecosystem dynamics and post-fire regeneration.
Author Contributions
Conceptualization, S.C.M.N., R.S.P. and P.V.d.S.; methodology, S.C.M.N., R.S.P. and P.V.d.S.; formal analysis, R.S.P.; data curation, S.C.M.N. and R.S.P.; writing—original draft preparation, S.C.M.N., B.B. and R.S.P.; writing—review and editing, S.C.M.N., B.B., R.S.P., P.V.d.S. and A.F.T.; supervision, B.B. and R.S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Graduate Program at the University of Brasilia—UNB and Coordination for the Improvement of Higher Education Personnel—CAPES.
Data Availability Statement
The data can be made available upon request to the first author.
Acknowledgments
The authors thank the Fazenda Água Limpa research station and its employees Sebastião, Geraldo, Rodrigo, Augusto, Alcides, Augustinho, Luciano, Mauro, Miron, Zico, Queen, Alexandre Palermo, Lícia, and teacher Ana Maria for all the support to develop this study, Pâmela Vírgilio for field and laboratory work support; André for his support in carrying out the forest inventory; to the employees of Fazenda Água Limpa, the Forest Sciences graduate program at the University of Brasilia, and CAPES for the doctoral scholarship and financial incentives for survival and carrying out the analyses.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Results of the non-metric multidimensional scaling (NMDS) analysis. R2 is the coefficient of determination and the asterisks refer to the significance level, where those with three asterisks are the vectors included in the NMDS Figure 2.
Table A1.
Results of the non-metric multidimensional scaling (NMDS) analysis. R2 is the coefficient of determination and the asterisks refer to the significance level, where those with three asterisks are the vectors included in the NMDS Figure 2.
| Species | Family | NMDS1 | NMDS2 | R2 | p-Value |
|---|---|---|---|---|---|
| Agonandra brasiliensis Miers ex Benth. & Hook.f. | Opiliaceae | 0.54628 | 0.8376 | 0.2012 | 0.321 |
| Aspidosperm tomentosum Mart. | Apocynaceae | 0.90325 | 0.42912 | 0.1018 | 0.511 |
| Banisteriopsis latifolia (A.Juss.) B. Gates | Malpighiaceae | 0.53772 | −0.84312 | 0.6093 | 0.001 *** |
| Blepharocalyx salicifolius (Kunth) O. Berg. | Myrtaceae | −0.15560 | 0.98782 | 0.0870 | 0.598 |
| Byrsonima pachyphylla A. Juss. | Malpighiaceae | −0.86522 | −0.50139 | 0.1873 | 0.295 |
| Byrsonima verbascifolia (L.) DC. | Malpighiaceae | −0.91416 | −0.40534 | 0.0769 | 0.654 |
| Bowdichia virgilioides Kunth | Fabaceae | 0.29841 | 0.95444 | 0.1670 | 0.328 |
| Casearia sylvestris Sw | Salicaceae | −0.91062 | −0.41324 | 0.3848 | 0.052 |
| Caryocar brasiliense Cambess. | Caryocaraceae | 0.88254 | −0.47024 | 0.4325 | 0.014 * |
| Connarus suberosus Planch. | Connaraceae | −0.93377 | 0.35786 | 0.0402 | 0.779 |
| Dalbergia miscolobium Benth. | Mimosoideae | 0.26686 | 0.96374 | 0.8432 | 0.001 *** |
| Dalbergia elliptica | Fabaceae | 0.63236 | −0.77468 | 0.2087 | 0.275 |
| Davilla elliptica A.St.-Hil. | Dilleniaceae | −0.80026 | −0.59965 | 0.3734 | 0.035 * |
| Diospyros burchellii Hiern | Ebenaceae | −0.57408 | 0.8188 | 0.0911 | 0.676 |
| Diospyros lasiocalyx (Mart.) B.Walln. | Ebenaceae | 0.20319 | 0.97914 | 0.5188 | 0.014 * |
| Dimorphandra mollis Benth. | Fabaceae | 0.38512 | −0.92287 | 0.2701 | 0.056 |
| Enterolobium gummiferum (Mart.) J.F.Macr. | Fabaceae | 0.81475 | −0.57982 | 0.0684 | 0.691 |
| Eremanthus glomerulatus Less | Asteraceae | −0.43466 | −0.90059 | 0.2094 | 0.241 |
| Eriotheca pubescens (Mart.& Zucc.) Schott & Endl. | Malvaceae | 0.99989 | 0.01483 | 0.0304 | 0.823 |
| Erythroxylum deciduum A.St.-Hil | Erythroxylaceae | −0.22797 | −0.97367 | 0.0002 | 0.999 |
| Erythroxy suberosum A.St.-Hil | Erythroxylaceae | −0.86718 | 0.498 | 0.7576 | 0.001 *** |
| Erythroxylum tortuosum A.St.-Hil | Erythroxylaceae | 0.76754 | −0.641 | 0.2761 | 0.125 |
| Guapira noxia (Netto) Lundell | Nyctaginaceae | 0.77366 | −0.6336 | 0.4021 | 0.047 * |
| Handroanthus ochraceus (Chm.) Mattos | Bignoniaceae | 0.84702 | 0.53156 | 0.1538 | 0.370 |
| Hancornia speciosa Gomes | Apocynaceae | −0.22933 | −0.97335 | 0.1786 | 0.412 |
| Hymenaea stigonocarpa Mart. Ex Hayne | Fabaceae | 0.32592 | −0.9454 | 0.4944 | 0.026 * |
| Heteropterys byrsonimiifolia A.Juss | Malpighiaceae | 0.45763 | −0.88914 | 0.0534 | 0.768 |
| Kielmeyera coriacea Mart. & Zucc | Callophyllaceae | 0.05631 | 0.99841 | 0.1677 | 0.411 |
| Kielmeyera speciosa A.St.-Hil. | Callophyllaceae | 0.25950 | 0.96574 | 0.4671 | 0.022 * |
| Lafoensia pacari A.St.-Hil. | Loganiaeceae | 0.88586 | −0.46396 | 0.0725 | 0.861 |
| Leptolobium dasycarpum Vogel | Fabaceae | −0.49854 | −0.86686 | 0.1652 | 0.359 |
| Machaerium opacum Vogel | Fabaceae | 0.73150 | −0.68184 | 0.4537 | 0.013 * |
| Miconia albicans (Sw.) Triana | Melastomataceae | 0.60432 | 0.79674 | 0.2459 | 0.162 |
| Miconia leucocarpa DC | Melastomataceae | −0.91636 | −0.40035 | 0.6099 | 0.002 ** |
| Miconia burchelli Triana | Melastomataceae | 0.16307 | 0.98661 | 0.3891 | 0.046 * |
| Miconia speciosa (A.St.-Hil. & Naudin) Naudin | Melastomataceae | 0.13303 | 0.99111 | 0.1687 | 0.519 |
| Miconia ferruginata DC. | Melastomataceae | 0.66501 | −0.74684 | 0.6406 | 0.004 ** |
| Mimosa claussenii Benth | Fabaceae | 0.76611 | −0.64271 | 0.1956 | 0.265 |
| Myrsine guianensis (Aubl.) Kuntze | Primulaceae | −0.04177 | 0.99913 | 0.1592 | 0.425 |
| Neea theifera Oerst | Nyctaginaceae | −0.9057 | −0.42392 | 0.0731 | 0.640 |
| Ouratea hexasperma (A.St.-Hil.) Baill | Ochnaceae. | 0.10205 | 0.99478 | 0.0456 | 0.739 |
| Palicourea rigida Kunth | Rubiaceae | 0.21823 | 0.9759 | 0.3388 | 0.061 |
| Pouteria ramiflora (Mart.) Radlk | Sapotaceae | 0.60027 | −0.79979 | 0.4059 | 0.027 * |
| Piptocarpha rotundifolia (Less.) Baker | Asteraceae | −0.84519 | −0.53447 | 0.2268 | 0.209 |
| Psidium laruotteanum Cambess | Myrtaceae | 0.19464 | 0.98087 | 0.0218 | 0.913 |
| Plenckia populnea Reissek | Celastraceae | −0.71841 | −0.69562 | 0.2406 | 0.224 |
| Pterodon emarginatus Vogel | Fabaceae | 0.79357 | −0.60848 | 0.3462 | 0.072 |
| Qualea grandiflora Mart. | Vochysiaceae | 0.42728 | −0.90412 | 0.3066 | 0.129 |
| Qualea multiflora Mart. | Vochysiaceae | −0.03812 | 0.99927 | 0.1074 | 0.524 |
| Qualea parviflora Mart. | Vochysiaceae | 0.86802 | −0.49653 | 0.2768 | 0.123 |
| Roupala montana Aubl. | Proteaceae | 0.50624 | 0.86239 | 0.7116 | 0.001 *** |
| Rourea induta Planch. | Connaraceae | 0.98941 | 0.14514 | 0.0767 | 0.674 |
| Salacia crassifolia (Mart. ex Schult.) G.Don | Celastraceae | 0.63236 | −0.77468 | 0.2087 | 0.275 |
| Schefflera macrocarpa (Cham. & Schltdl.) Frodin | Araliaceae | 0.49271 | −0.87019 | 0.7362 | 0.001 *** |
| Symplocos rhamnifolia A.DC. | Symplocaceae. | 0.53729 | 0.8434 | 0.2863 | 0.147 |
| Strychnos pseudoquina A. St.-Hil. | Loganiaceae | 0.83442 | −0.55114 | 0.1168 | 0.541 |
| Syagrus comosa Mart. | Arecaceae | 0.05081 | 0.99871 | 0.0976 | 0.609 |
| Styrax ferrugineus Nees. & Mart | Styracaceae | 0.86754 | −0.49737 | 0.6936 | 0.001 *** |
| Stryphnodendron adstringens (Mart.) Coville | Fabaceae | 0.77755 | −0.62883 | 0.1788 | 0.291 |
| Solanum lycocarpum A.St.-Hill | Solanaceae | 0.00000 | 0 | 0.0000 | 1.000 |
| Tachigali subvelutina (Benth.) Oliveira-Filho | Fabaceae | −0.49209 | −0.87054 | 0.1239 | 0.422 |
| Vatairea macrocarpa (Benth.) Ducke | Leguminoseae | 0.35803 | −0.93371 | 0.0222 | 0.900 |
| Vochysia thyrsoidea Pohl | Vochysiaceae | 0.86539 | −0.50109 | 0.5867 | 0.002 ** |
| Vochysia elliptica Mart. | Vochysiaceae | 0.67429 | 0.73847 | 0.4986 | 0.012 * |
Note: Significance codes *** p < 0.001; ** p < 0.01; * p < 0.05.
References
- Zimbres, B.; Shimbo, J.; Bustamante, M.; Levick, S.; Miranda, S.; Roitman, I.; Silvério, D.; Gomes, L.; Fagg, C.; Alencar, A. A estrutura da vegetação de savana no Cerrado brasileiro permite a estimativa precisa da biomassa acima do solo usando varredura a laser terrestre. For. Ecol. Manag. 2020, 458, 117798. [Google Scholar] [CrossRef]
- Ribeiro, J.F.; Walter, B.M.T. As principais fitofisionomias do bioma Cerrado. In Cerrado: Ecologia e Fóruns; Sano, S.M., Almeida, S.P., Ribeiro, J.F., Eds.; Embrapa Informação Tecnológica: Brasília, Brazil, 2008; pp. 151–212. Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/570911 (accessed on 20 March 2023).
- Terra, M.d.C.N.S.; Prado-Júnior, J.A.D.; de Souza, C.R.; Pinto, L.O.R.; Silveira, E.M.d.O.; Cordeiro, N.G.; Cirne-Silva, T.M.; Mantovani, V.A.; Scolforo, J.R.S.; de Mello, J. MDominância de espécies arbóreas na biomassa e produtividade aérea da savana neotropical. For. Ecol. Manag. 2021, 496, 119430. [Google Scholar] [CrossRef]
- Sano, E.E.; Rodrigues, A.A.; Martins, E.S.; Bettiol, G.M.; Bustamante, M.M.; Bezerra, A.S.; Couto, A.F., Jr.; Vasconcelos, V.; Schüler, J.; Bolfe, E.L. Ecorregiões do Cerrado: Uma estrutura espacial para avaliar e priorizar a diversidade ambiental da savana brasileira para conservação. Rev. Gestão Ambient. 2019, 232, 818–828. Available online: https://pubmed.ncbi.nlm.nih.gov/30529869 (accessed on 15 February 2023).
- Rocha, G.F.; Ferreira, L.G.; Ferreira, N.C.; Ferreira, M.E. Detecção de desmatamento no bioma Cerrado entre 2002 e 2009: Padrões, Tendências e Impactos. Rev. Bras. Cartogr. 2011, 63, 341–349. [Google Scholar] [CrossRef]
- Swann, A.L.S. Plantas e seca num clima em mudança. Curr. Clim. Chang. Rep. 2018, 4, 192–201. [Google Scholar] [CrossRef]
- Strassburg, B.B.; Brooks, T.; Feltran-Barbieri, R.; Iribarrem, A.; Crouzeilles, R.; Loyola, R.; Latawiec, A.E.; Oliveira Filho, F.J.; Scaramuzza, C.A.; Scarano, F.R.; et al. Momento da Verdade Para o Hotspot do Cerrado. Ecol. Evol. Nat. 2017, 1, 99. Available online: https://www.nature.com/articles/s41559-017-0099 (accessed on 27 February 2023). [CrossRef] [PubMed]
- Brasil Ministério do Meio Ambiente. Cadastro Nacional de Unidades de Conservação; MMA: Brasília, Brazil, 2016. Available online: http://www.mma.gov.br/images/arquivo/80112/CNUC_Agosto%20-%20Biomas%201.pdf (accessed on 20 July 2021).
- Myers, N.; Mittermeler, R.A.; Mittermeler, C.G.; Da Fonseca, G.A.B.; Kent, J. Hotspots de biodiversidade para prioridades de conservação. Natureza 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Silveira, E.M.O.; Silva, S.H.G.; Acerbi-Júnior, F.W.; Carvalho, M.C.; Carvalho, L.M.T.; Scolforo, J.R.S.; Wulder, M.A. A modelagem florestal aleatória baseada em objetos de biomassa florestal acima do solo supera uma abordagem baseada em pixels em um ambiente tropical montanhoso heterogêneo. Int. J. Appl. Earth Obs. Geoinf. 2019, 78, 175–188. [Google Scholar] [CrossRef]
- Gomes, L.; Miranda, H.S.; Maria, M. Ecologia e Manejo Florestal Como podemos avançar no conhecimento sobre o comportamento e os efeitos do fogo no bioma Cerrado? Ecol. e Manejo Florest. 2018, 417, 281–290. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0378112717321850 (accessed on 15 April 2023). [CrossRef]
- Strassburgo, B.B.N.; Iribarrem, A.; Beyer, H.L.; Cordeiro, C.L.; Crouzeilles, R.; Jakovac, C.C.; Braga Junqueira, A.; Lacerda, E.; Latawiec, A.E.; Balmford, A.; et al. Áreas prioritárias globais para a restauração de ecossistemas. Natureza 2020, 586, 724–729. [Google Scholar] [CrossRef]
- Coutinho, L.M. O fogo na ecologia do Cerrado brasileiro. In Fogo na Biota Tropical: Processos Ecossistêmicos e Desafios Globais; Goldammer, J.G., Ed.; Springer: Berlin, Germany, 1990; pp. 82–105. Available online: https://link.springer.com/chapter/10.1007/978-3-642-75395-4_6 (accessed on 11 March 2023).
- Mistry, J. Savanas Mundiais: Ecologia e Uso Humano; Pearson Education Limited, Prentice Hall: Grã-Bretanha, UK, 2000. [Google Scholar] [CrossRef]
- Pereira, K.M.G.; Cordeiro, N.G.; Terra, M.d.C.N.S.; Pyles, M.V.; Cabacinha, C.D.; de Mello, J.M.; Berg, E.v.D. Status de proteção como determinante dos drivers de estoque de carbono no Cerrado sensu stricto. J. Ecol. Veg. 2020, 13, 361–368. [Google Scholar] [CrossRef]
- Cianciaruso, M.V.; Silva, I.A.; Batalha, M.A. Biomassa aérea de grupos funcionais na camada subterrânea de savanas sob diferentes frequências de fogo. J. Aust. Bot. 2010, 58, 169–174. [Google Scholar] [CrossRef]
- Haridasan, M. Nutrição mineral de plantas nativas do cerrado. Rev. Bras. Fisiol. Veg. 2000, 12, 54–64. [Google Scholar]
- Eiten, G. Cerrado: Caracterização, Ocupação e Perspectivas; UnB/SEMATEC: Brasília, Brazil, 1994. [Google Scholar]
- Medeiros, M.B.; Miranda, H.S. Mortalidade pós-fogo em espécies lenhosas de campo sujas submetidas a três queimadas prescritas anualmente. Acta Bot. Bras. 2005, 19, 493–500. [Google Scholar] [CrossRef]
- Miranda, H.S. Efeitos do Regime de Fogo Sobre a Estrutura de Comunidades do Cerrado: Projeto Fogo; IBAMA: Brasília, Brazil, 2010; p. 144. [Google Scholar]
- Ferraz-Vicentini, K.R.C. História do Fogo no Cerrado. Ph.D. Thesis, Departamento de Ecologia, Universidade de Brasília, Brasília, Brazil, 1999. [Google Scholar]
- Solbrig, O.T.; Young, M.D. Forças motrizes económicas e ecológicas que afectam as savanas tropicais. In As Savanas do Mundo: Forças Motrizes Económicas, Restrições Ecológicas e Opções Políticas para o uso Sustentável da Terra; Young, M.D., Solbrig, O.T., Eds.; Série Homem e Biosfera; The Parthenon Publishing Group: Unesco, Paris, 1993; Volume 12, pp. 3–18. [Google Scholar]
- Pivello, V.R.; Coutinho, L.M. Firetool: Um sistema especialista para uso de fogos prescritos em áreas de conservação do cerrado. For. Ecol. Manag. 1996, 33, 348–356. [Google Scholar] [CrossRef]
- Eloy, L.; Schmidt, I.B.; Borges, S.L.; Ferreira, M.C.; Dos Santos, T.A. O manejo sazonal do fogo por pecuaristas tradicionais evita a propagação de incêndios florestais no Cerrado brasileiro. Ambio 2019, 48, 890–899. [Google Scholar] [CrossRef]
- Bilbau, B.; Mistério, J.; Millán, A.; Berardi, A. Compartilhando múltiplas perspectivas sobre queimadas: Rumo a uma política participativa e intercultural de manejo do fogo na Venezuela, Brasil e Guiana. Fogo 2019, 2, 39. [Google Scholar] [CrossRef]
- Hopkins, B. Processos ecológicos na fronteira floresta-savana. In Natureza e Dinâmica dos Limites Floresta-Savana; Furley, P.A., Proctor, J., Ratter, J.A., Eds.; Chapman & Hall: London, UK, 1992; pp. 21–33. [Google Scholar]
- Rezende, A.V.; Vale, A.T.; Sanquetta, C.R.; Figueiredo Filho, A.; Felfili, J.M. Comparação de modelos matemáticos para estimativa de volume, biomassa e estoque de carbono do tecido lenhoso de um cerrado sensu stricto em Brasília, DF. Sci. For. 2006, 71, 65–76. [Google Scholar]
- Sanquetta, C.R. Métodos de determinação de biomassa florestal. In As Florestas e o Carbono; Sanquetta, C.R., Ed.; Embrapa Florestas: Curitiba, Brazil, 2002; pp. 119–140. [Google Scholar]
- Felfili, J.M.; Carvalho, F.A.; Haidar, R.F. Manual Para o Monitoramento de Parcelas Permanentes nos Biomas Cerrado e Pantanal; Universidade de Brasília: Brasília, Brazil, 2005. [Google Scholar]
- Granado, L.M.A. Estimativa de Biomassa e Combustível em Diferentes Fitofisionomias do Cerrado. Master’s Thesis, Faculdade de Tecnologia, Universidade de Brasília, Brasília, Brazil, 2019; 71p. [Google Scholar]
- Koppen, W. Climatologia: Com um Estudo dos Climas da Terra; Fundo de Cultura Económica: Mexico City, Mexico, 1948; 479p. [Google Scholar]
- Nimer, E. Climatologia do Brasil; IBGE: Rio de Janeiro, Brazil, 1989; 422p. [Google Scholar]
- Haridasan, M. Adaptações nutricionais de plantas nativas do bioma cerrado em solos ácidos. Braz. J. Fisiol Vegetal. 2008, 20, 183–195. [Google Scholar] [CrossRef]
- Santos, G.L.; Pereira, M.G.; Delgado, R.C.; Torres, J.L.R. Regeneração Natural em Ambientes Antropogênicos Devido ao Uso Agrícola no Cerrado, Uberaba, Mg, Brasil. Rev. Biociênc. 2017, 1, 169–263. [Google Scholar] [CrossRef]
- Felfili, J.M.; Rezende, A.V.; Júnior, M.C.D.S.; Silva, M.A. Mudanças na composição florística do cerrado sensu stricto no Brasil ao longo de um período de nove anos. J. Trop. Ecol. 2000, 16, 579–590. [Google Scholar] [CrossRef]
- Azevedo, G.B. Amostragem e Modelagem da Biomassa de Raízes em um Cerrado Sentido Restrito no Distrito Federal. Master’s Thesis, Universidade de Brasília, Brasília, Brazil, 2014. [Google Scholar]
- Felfili, J.; Nogueira, P.E.; Silva Júnior, M.C.; Marimon, B.S.; Delitti, W.B.C. Composição florística e fitossociologia do cerrado sentido restrito no município de Água Boa-MT. Acta Bot. Bras. 2002, 16, 103–112. [Google Scholar] [CrossRef]
- Felfili, J.M.; Rezende, R.P. Conceitos e Métodos em Fitossociologia; Comunicações Técnicas Florestais; Universidade de Brasília, Departamento de Engenharia Florestal: Brasília, Brazil, 2003; Volume 5, pp. 1–68. [Google Scholar]
- Durigan, G. Estrutura e diversidade de florestas tropicais. In Ecologia de Florestas Tropicais do Brasil; Martins, S.V., Ed.; Editora UF: Viçosa, Brazil, 2009. [Google Scholar]
- Grupo de Filogenia de Angiospermas (APG IV). Uma atualização da classificação do Grupo de Filogenia de Angiospermas para as ordens e famílias de plantas com flores: APG IV. Rev. Bot. Soc. Linneana 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Forzza, R.C.; Baumgratz, J.F.A.; Bicudo, C.E.M.; Canhos, D.A.L.; Carvalho, A.A.; Coelho, M.A.N.; Costa, A.F.; Costa, D.P.; Hopkins, M.G.; Leitman, P.M.; et al. Nova lista florística brasileira destaca desafios de conservação. BioCiência 2012, 62, 39–45. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. A Teoria Matemática da Comunicação; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Pielou, C.E. Diversidade Ecológica; Wiley Interscience: New York, NY, USA, 1975. [Google Scholar]
- Lamprecht, H. Silvicultura nos Trópicos: Ecossistemas Florestais e Espécies Arbóreas Específicas—Possibilidades e Métodos de Aproveitamento Sustentado; República Federal da Alemanha: Rossdorf, Germany, 1990. [Google Scholar]
- Mueller-Dombois, D.; Ellenberg, H. Objetivos e Métodos de Ecologia do Vegetação; The Blackburn Press: Caldwell, NJ, USA, 2002; 547p. [Google Scholar]
- Kent, M.; Coker, P. Descrição da Vegetação: Uma Abordagem Prática; Imprensa de Belhaven: London, UK, 1992; 363p. [Google Scholar]
- IPCC. Painel Intergovernamental sobre Mudanças Climáticas (IPCC), Programa Nacional de Inventários de Gases de Efeito Estufa; Instituto de Estratégias Ambientais Globais: Hayama, Japan, 2006; Available online: http://www.ipcc-nggip.iges.or.jp/public/2006gl/index.html (accessed on 15 May 2023).
- Dixon, P. Vegan, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Legendre, P. Studying beta diversity: Ecological variation partitioning by multiple regression and canonical analysis. J. Plant Ecol. 2008, 1, 3–8. [Google Scholar] [CrossRef]
- Equipe Principal, R. R: Uma Linguagem e Ambiente Para Computação Estatística (Versão v3.6.2). Fundação R para Computação Estatística. 2019. Available online: https://www.R-project.org/ (accessed on 7 May 2023).
- Klink, C.A.; Machado, R.B. Conservação do Cerrado Brasileiro. Conservar. Biol. 2005, 19, 707–713. [Google Scholar] [CrossRef]
- Dantas, J.S. Relação Solo-Paisagem e Predição da Erodibilidade de Solos Coesos dos Tabuleiros Costeiros no Estado do Maranhão. Tese (Programa de Pós Graduação em Agronomia—Ciência do Solo)—Faculdade de Ciências Agrárias e Veterinárias; Universidade Estadual Paulista “Júlio de Mesquita Filho”: Jaboticabal, Brazil, 2013. [Google Scholar]
- Souchie, F.F.; Pinto, J.R.R.; Lenza, E.; Gomes, L.; Maracahipes-Santos, L.; Silvério, D.V. Estratégias de rebrota pós-fogo da vegetação lenhosa no cerrado brasileiro. Acta Bot. Bras. 2017, 31, 260–266. [Google Scholar] [CrossRef]
- Lopes, E.R.N.; Silva, A.P.P.S.; Peruchi, J.F. Zoneamento de Risco de Incêndio e Queimadas no Município de Sorocaba—São Paulo; Revista do Departamento de Geografia; Universidade de São Paulo: São Paulo, Brazil, 2018; Volume 36. [Google Scholar]
- Goldenberg, R.; Baumgratz, J.F.A.; Souza, M.L.D.E.R. Taxonomia de Melastomataceae no Brasil: Retrospectiva, perspectivas e chave de identificação para os gêneros. Rodriguésia 2012, 63, 145–161. [Google Scholar] [CrossRef]
- Medeiros, M.B. Efeitos do Fogo Nos Padrões de Rebrotamento em Plantas Lenhosas, em Campo sujo, após Queimadas Prescritas; Universidade de Brasília: Brasília, Brazil, 2002. [Google Scholar]
- Hoffmann, W.A.; Moreira, A. O papel do fogo na dinâmica populacional de plantas lenhosas. In Ecologia e História Natural de uma Savana Neotropical: Os cerrados do Brasil. A Imprensa da Universidade de Columbia; Oliveira, P.S., Marquês, R.S., Eds.; Columbia University Press: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Felfili, J.M. Dinâmica da regeneração natural na mata de galeria do Gama no Brasil central. For. Ecol. Manag. 1997, 91, 235–245. [Google Scholar] [CrossRef]
- Oliveira, P.T.S.; Leite, M.B.; Mattos, T.; Aproximando-se, M.A.; Scott, R.L.; Oliveira Xavier, R.; Silva Matos, D.M.; Wendland, E. A recarga das águas subterrâneas diminui com o aumento da densidade da vegetação no cerrado brasileiro. Ecohidrologia 2017, 10, 1759. [Google Scholar] [CrossRef]
- Hoffmann, W.A. Incêndio e dinâmica populacional de plantas lenhosas em uma savana neotropical: Projeções de modelos matriciais. Ecologia 1999, 80, 1354–1369. [Google Scholar] [CrossRef]
- do Vale, A.T.; Elias, P.S. Nível de proteção térmica da cascata de quatro espécies lenhosas e a relação da arquitetura da cascata com a transferência de calor. Ciênc. Florest. 2014, 24, 979–987. [Google Scholar] [CrossRef]
- Salgado-Labouriau, M.L.; Ferraz-Vicentini, K.R. Incêndio no Cerrado há 32.000 anos. Pesqui. Atual No Pleistoceno 1994, 11, 85–87. [Google Scholar]
- Lima, J.M.; Castro, A.B.; Lima, A.P.; Magnusson, W.E.; Landeiro, V.L.; Fadini, R.F. Influência do regime de queimadas sobre a riqueza e composição florística de uma savana isolada na Amazônia—PELD Oeste do Pará. Oecologia Aust. 2020, 24, 301–316. [Google Scholar] [CrossRef]
- Miranda, H.S.; Sato, M.; Andrade, S.M.; Haridasan, M.; Moraes, H.C. Queimadas de Cerrado: Caracterização e impactos. In Cerrado: Ecologia e Caracterização; Aguiar, L.M.S., Camargo, A.J.A., Eds.; Embrapa Cerrados: Brasília, Brazil, 2004; pp. 69–123. [Google Scholar]
- Pivello, V.R. Manejo do fogo para conservação biológica no cerrado brasileiro. In Savanas e Florestas Secas; Mistry, J., Berardi, A., Eds.; Routledge: Abingdon-on-Thames, UK, 2017; pp. 141–166. [Google Scholar] [CrossRef]
- Pivello, V.R. O Uso do Fogo no Cerrado e nas Florestas Amazônicas do Brasil: Passado e Presente. Ecol. Do Fogo 2011, 7, 24–39. [Google Scholar] [CrossRef]
- Bowman, D.M.; Murphy, B.P.; Boer, M.M.; A Bradstock, R.; Cary, G.J.; A Cochrane, M.; Fensham, R.J.; A Krawchuk, M.; Price, O.F.; Williams, R.J. Gestão de incêndios florestais, mudanças climáticas e o risco de perdas catastróficas de carbono. Front. Ecol. Environ. 2013, 11, 66–68. [Google Scholar] [CrossRef]
- Bond, W.J.; Archibald, S. Enfrentando a complexidade: Escolhas de políticas de incêndio em parques de savana sul-africanos. Int. J. Wildland Fire 2003, 12, 381–389. [Google Scholar] [CrossRef]
- Batista, E.K.L.; Russell-smith, J.; França, H.; Figueira, J.E.C. Uma avaliação dos regimes contemporâneos de incêndios na savana no Parque Nacional da Canastra, Brasil: Resultados das políticas de supressão de incêndios. J. Meio Ambiente Gerenciar. 2018, 205, 40–49. [Google Scholar]
- Fidelis, A.; Alvarado, S.T.; Barradas, A.C.S.; Pivello, V.R. O ano de 2017: Megaincêndios e gestão no Cerrado. Fire 2018, 1, 49. [Google Scholar] [CrossRef]
- Ramos, P.C.M. Sistema Nacional de Prevenção e Combate aos Incêndios Florestais; IPEF: Piracicaba, Brazil, 1995; pp. 29–58. [Google Scholar]
- Durigan, G. Fogo zero: Não é possível nem desejável no Cerrado do Brasil. Flora 2020, 268, 15161. [Google Scholar] [CrossRef]
- Abreu, R.C.R.; Hoffmann, W.A.; Vasconcelos, H.L.; Pilon, N.A.; Rossatto, D.R.; Durigan, G. O custo da biodiversidade do sequestro de carbono na savana tropical. Sci. Adv. 2017, 3, 1701284. [Google Scholar] [CrossRef] [PubMed]
- Durigan, G.; Leitão Filho, H.F.; Rodrigues, R.R. Fitossociologia e estenose de uma vegetação de cerrado frequentemente queimada no Sudeste do Brasil. Flora 1994, 189, 153–160. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Cummings, D.L.; Ward, D.E. Relações entre fogo, biomassa e dinâmica de nutrientes ao longo de um gradiente de vegetação no Cerrado brasileiro. J. Ecol. 1994, 82, 519–531. [Google Scholar] [CrossRef]
- Koch, A.; Brierley, C.; Maslin, M.M.; Lewis, S.L. Impactos no sistema terrestre de chegada europeia e da Grande Morte nas Américas após 1492. Quat. Ciênc. 2019, 207, 13–36. [Google Scholar]
- Almeida, M.A. Modelagem da Propagação do Fogo Como Ferramenta de Auxílio à Tomada de Decisão no Combate e Prevenção de Incêndios no Parque Nacional das Emas, GO; Instituto Nacional de Pesquisas Espaciais-INPE: São José dos Campos, Brazil, 2012. [Google Scholar]
- Lopes, J.F.; de Andrade, E.M.; de Oliveira Lobato, F.A.; de Queiroz Palácio, H.A.; Arraes, F.D. Deposição e acomodação de serapilheira em área da Caatinga. Rev. Agro@ Mbiente-Line 2009, 2, 72–79. [Google Scholar]
- Moreira, A.G. Efeitos da proteção contra fogo na estrutura das savanas do Brasil Central. Rev. Biogeogr. 2000, 27, 1021–1029. [Google Scholar] [CrossRef]
- Fiedler, N.C.; Azevedo, I.N.; Rezende, A.V.; Medeiros, M.B.; Venturoli, F. Efeito de incêndios florestais na estrutura e composição florística de uma área de cerrado sentido estrito na Fazenda Água Limpa-DF. Rev. Árvore 2004, 28, 129–138. [Google Scholar] [CrossRef]
- Líbano, A.M.; Felfili, M.J. Mudanças temporais na composição florística e na diversidade de um cerrado sensu stricto do Brasil Central em um período de 18 anos. Acta Bot. Bras. 2006, 20, 927–936. [Google Scholar] [CrossRef]
- Roitman, I.; Bustamante, M.M.C.; Haidar, R.F.; Shimbo, J.Z.; Abdala, G.C.; Eiten, G.; Fagg, C.W.; Felfili, M.C.; Felfili, J.M.; Jacobson, T.K.B.; et al. Otimizando estimativas de biomassa de florestas de savana em diferentes escalas espaciais no Cerrado brasileiro: Reavaliando equações alométricas e influências ambientais. PLoS ONE 2018, 13, e0196742. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Metzger, J.P.; Martensen, A.C.; Ponzoni, F.J.; Hirota, M.M. A Mata Atlântica Brasileira: Quanto resta e como está distribuída a floresta remanescente? Implicações para a conservação. Conserv. Biol. 2009, 142, 1141–1153. [Google Scholar] [CrossRef]
- Bernhardt-Römermann, M.; Baeten, L.; Craven, D.; De Frenne, P.; Hédl, R.; Lenoir, J.; Bert, D.; Brunet, J.; Chudomelová, M.; Decocq, G.; et al. Os impulsionadores das mudanças temporais na diversidade de plantas das florestas temperadas variação entre as escalas espaciais. Glob. Chang. Biol. 2015, 21, 3726–3737. [Google Scholar] [CrossRef]
- Altomare, M.; Vasconcelos, H.L.; Raymundo, D.; Lopes, S.; Vale, V.; Prado-Junior, J. Avaliação da resiliência ao fogo do componente arbóreo da savana através de uma abordagem funcional. Acta Oecol. 2021, 111, 103728. [Google Scholar] [CrossRef]
- Sato, N.M. Mortalidade de Plantas do Cerrado Submetidas a Diferentes Regimes de Queima. Ph.D. Thesis, Universidade de Brasília, Brasília, Brazil, 1996. [Google Scholar]
- Ramos-Neto, M.B.; Pivello, V.R. Incêndios de raios em um Parque Nacional do Cerrado Brasileiro: Repensando estratégias de manejo. Gestão Ambient. 2000, 26, 675–684. [Google Scholar] [CrossRef]
- Bond, W.J.; Keeley, J.E. O fogo como um ‘herbívoro’ global: A ecologia e a evolução dos ecossistemas inflamáveis. Tendências Em Ecol. E Evolução 2005, 20, 387–394. [Google Scholar] [CrossRef]
- Pausas, J.G.; Poorter, L. Espessura da casca e regime de fogo. Funct. Ecol. 2015, 29, 315–327. [Google Scholar] [CrossRef]
- Araújo, F.D.; Tng, D.Y.; Apgaua, D.M.; Coelho, P.A.; Pereira, D.G.; Santos, R.M. Regeneração vegetal pós-incêndio em transição florestal-savana fechada. For. Ecol. Manag. 2017, 400, 77–84. [Google Scholar] [CrossRef]
- Souza, C.R.; Coelho De Souza, F.; Maia, V.A.; Aguiar-Campos, N.; Coelho, P.A.; Farrapo, C.L.; Santos, A.B.M.; Araújo, F.C.; Gianasi, F.M.; Paula, G.G.P.; et al. Estrutura e diversidade das florestas tropicais: Uma comparação de escolhas metodológicas. Methods Ecol. Evol. 2021, 12, 2017–2027. [Google Scholar] [CrossRef]
- Lucas, R.; MacArthur, A. Incêndio florestal na Austrália; Serviço de Publicação do Governo Australiano: Canberra, Australia, 1978. [Google Scholar]
- Maracahipes, L.; Marimon, B.S.; Lenza, E.; Marimon-Júnior, B.H.; De Oliveira, E.A.; Mews, H.A.; Gomes, L.; Feldpausch, T.R. Dinâmica pós-fogo da vegetação lenhosa em florestas sazonalmente inundadas (impucas) na zona de transição Cerrado-Floresta Amazônica. Flora 2014, 209, 260–270. [Google Scholar] [CrossRef]
- Silveira, F.A.O.; Ordóñez-Parra, C.A.; Moura, L.C.; Schmidt, I.B.; Andersen, A.N.; Bond, W.; Buisson, E.; Durigan, G.; Fidelis, A.; Oliveira, R.S.; et al. A disparidade de conscientização do bioma é RUIM para a conservação e restauração de ecossistemas tropicais. J. Appl. Ecol. 2021, 59, 1967–1975. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J. Incêndios florestais e mudanças globais. Front. Em Ecol. E Meio Ambiente 2021, 19, 387–395. [Google Scholar] [CrossRef]
- Hagmann, R.; Hessburg, P.; Salter, R.; Merschel, A.; Reilly, M. Os incêndios florestais contemporâneos degradam ainda mais a resistência e a resiliência das florestas excluídas do fogo Ecologia e Gestão Florestal. For. Ecol. Manag. 2022, 506, 119975. [Google Scholar] [CrossRef]
- Hessburgo, P.F.; Miller, C.L.; Povak, N.A.; Taylor, A.H.; Higuera, P.E.; Prichard, S.J.; Norte, D.; Collins, B.M.; Hurteau, M.D.; Larson, A.J.; et al. O clima, o meio ambiente e a história de perturbações governam a resiliência das florestas do oeste da América do Norte. Front. Ecol. Evol. 2019, 7, 239. [Google Scholar] [CrossRef]
- Hessburgo, P.F.; Espiões, T.A.; Perry, D.A.; Skinner, C.N.; Taylor, A.H.; Marrom, P.M.; Stephens, S.L.; Larson, A.J.; Churchill, D.J.; Povak, N.A.; et al. Tamm Review: Gestão de florestas em regime de incêndio de gravidade mista em Oregon, Washington e norte da Califórnia. For. Ecol. Manag. 2016, 366, 221–250. [Google Scholar] [CrossRef]
- Flores, C.; Limites, D.L.; Rubi, D.E. O fogo prescrito beneficia a vegetação das zonas húmidas? Zonas Úmidas 2011, 31, 35–44. [Google Scholar] [CrossRef]
- Elogne, A.G.; Piponiot, C.; Zo-Bi, I.C.; Amani, B.H.; Van der Meersch, V.; Hérault, B. Life after fire—Long-term responses of 20 timber species in semi-deciduous forests of West Africa. For. Ecol. Manag. 2023, 538, 120977. [Google Scholar] [CrossRef]
- Lal, R. Sequestro de carbono. Transações Filosóficas Da R. Soc. B 2008, 363, 815–830. [Google Scholar] [CrossRef]
- Grace, J.; Jose, J.S.; Meir, P.; Miranda, H.S.; Montes, R.A. Produtividade e fluxos de carbono de savanas tropicais. Rev. Biogeogr. 2006, 33, 387–400. [Google Scholar] [CrossRef]
- Bustamante, M.M.C.; Oliveira, E.L. Impacto das atividades agrícolas, florestais e pecuárias nos recursos naturais. In Savanas: Desafios e Estratégias Para o Equilíbrio Entre Sociedade, Agronegócio e Recursos Naturais; Faleiro, F.G., Farias Neto, A.L., Eds.; Embrapa Cerrados: Planaltina, Brazil, 2008. [Google Scholar]
- Houghton, R.A.; Hall, F.; Goetz, S.J. Importância da biomassa no ciclo global do carbono. Biogeosciences 2009, 114, 935. [Google Scholar] [CrossRef]
- Paiva, A.O.; Rezende, A.V.; Pereira, R.S. Estoque de carbono no cerrado sensu stricto no Distrito Federal. Rev. Árvore 2011, 3, 527–538. [Google Scholar] [CrossRef]
- Bastin, J.-F.; Berrahmouni, N.; Grainger, A.; Maniatis, D.; Mollicone, D.; Moura, R.; Patriarca, C.; Picard, N.; Pardal, B.; Abraão, E.M.; et al. A extensão da floresta em biomas de sequeiro. Ciência 2017, 356, 635–638. [Google Scholar]
- Zhou, Y.; Singh, J.; Butnor, J.R.; Coetsee, C.; Boucher, P.B.; Caso, M.F.; Hockridge, E.G.; Davies, A.B.; Staver, A.C. Aumentos limitados nos estoques de carbono da savana ao longo de décadas de supressão de incêndios. Natureza 2022, 603, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L.; Van Der Sande, M.T.; Thompson, J.; Arets, E.J.M.M.; Alarcón, A.; Álvarez-Sánchez, J.; Ascarrunz, N.; Balvanera, P.; Barajas-guzmán, G.; Boit, A.; et al. Diversity enhances carbon storage in tropical forests. Glob. Ecol. Biogeogr. 2015, 24, 1314–1328. [Google Scholar] [CrossRef]
- Piponiot, C.; Derroire, L.G.; Descroix, L.; Mazzei, E.; Rutishauser, P.; Sist, B. Hérault. Avaliando a recuperação do volume de madeira após perturbação em florestas tropicais—Uma nova estrutura de modelagem. Ecol. Model. 2018, 384, 353–369. [Google Scholar] [CrossRef]
- Chaves, J.; Andalo, C.; Marrom, S.; Cairns, M.A.; Câmaras, J.Q.; Éamus, D.; Folster, H.; Fromard, F.; Higuchi, N.; Kira, T.; et al. Alometria de árvores e estimativa aprimorada de estoques e equilíbrio de carbono em florestas tropicais. Ecologia 2005, 145, 87–99. [Google Scholar] [CrossRef]
- Nogueira, E.M.; Nelson, B.W.; Fearnside, P.M.; França, M.B.; Oliveira, A.C.A. Altura das árvores no “arco do desmatamento” do Brasil: árvores mais baixas no sul e sudoeste da Amazônia implicam menor biomassa. For. Ecol. Manag. 2008, 255, 2963–2972. [Google Scholar] [CrossRef]
- Alves, R.J.V.; Kolbek, J. A vegetação do campo rupestre pode ser delimitada floristicamente com base em gêneros de plantas vasculares? Planta Ecol. 2010, 207, 67–79. [Google Scholar] [CrossRef]
- Morandi, P.S.; Marimon, B.S.; Marimon-Junior, B.H.; Ratter, J.A.; Feldpausch, T.R.; Colli, G.R.; Munhoz, C.B.R.; Júnior, M.C.d.S.; Lima, E.d.S.; Haidar, R.F.; et al. Diversidade de árvores e biomassa acima do solo no bioma Cerrado da América do Sul e suas implicações para a conservação. Biodivers. Conserv. 2018, 29, 1519–1536. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).