Introgression as an Important Driver of Geographic Genetic Differentiation within European White Oaks
Abstract
1. Introduction
2. Materials and Methods
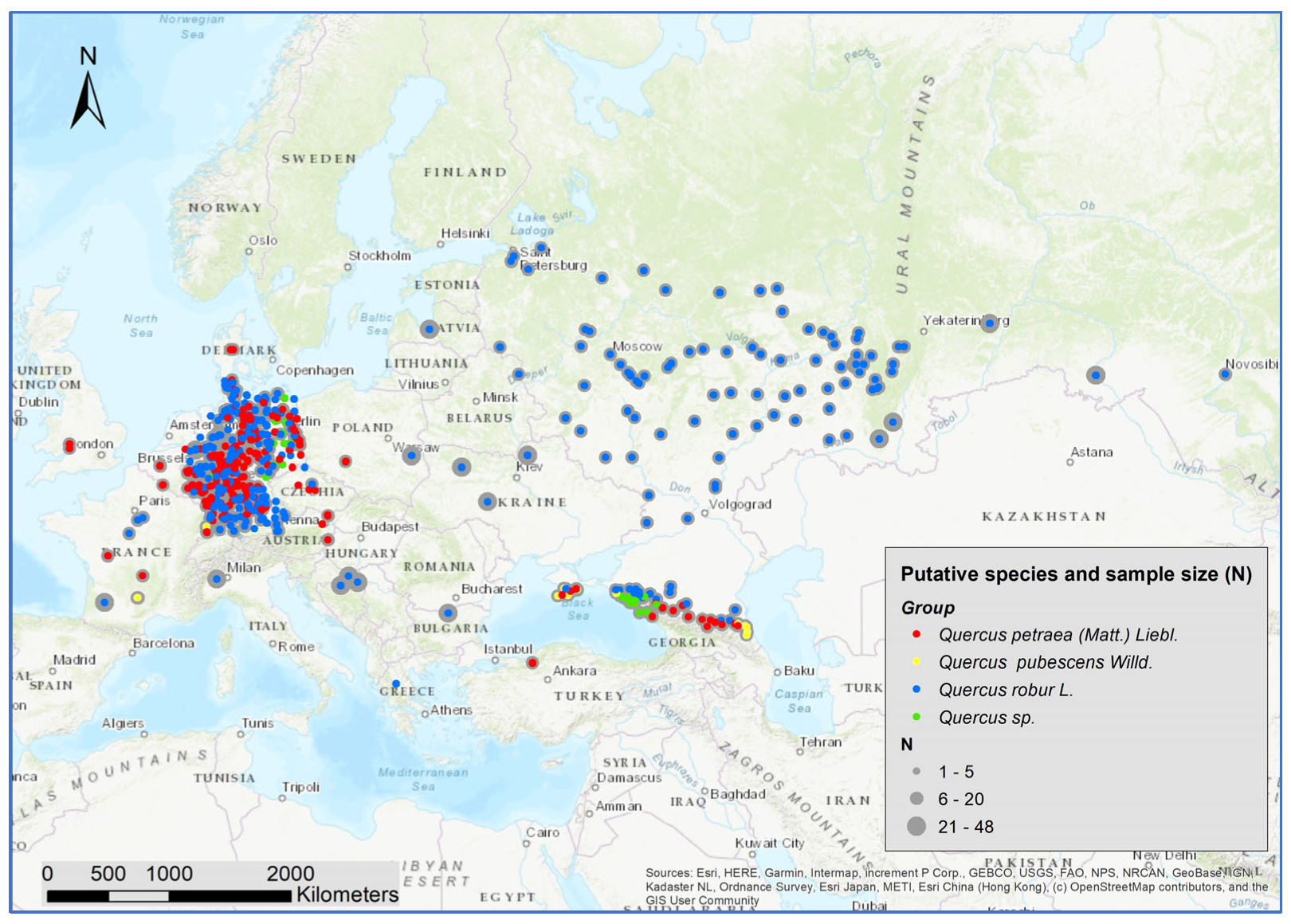
2.1. Sampling
2.2. DNA Extraction and Genotyping
2.3. Data Filtering
2.4. Sparse Non-Negative Matrix Factorization
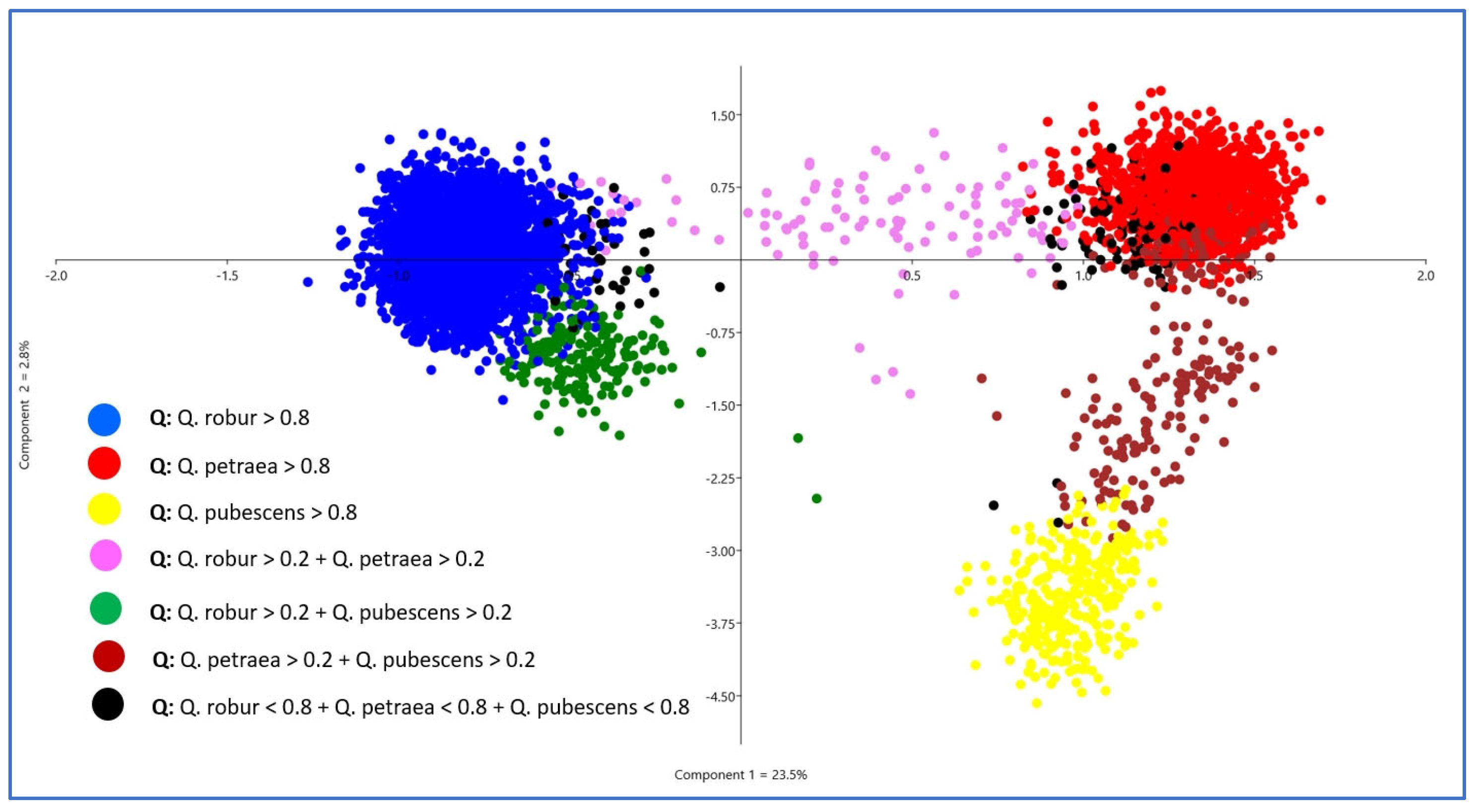
2.5. Principal Component Analysis (PCA)
2.6. Haplotypes
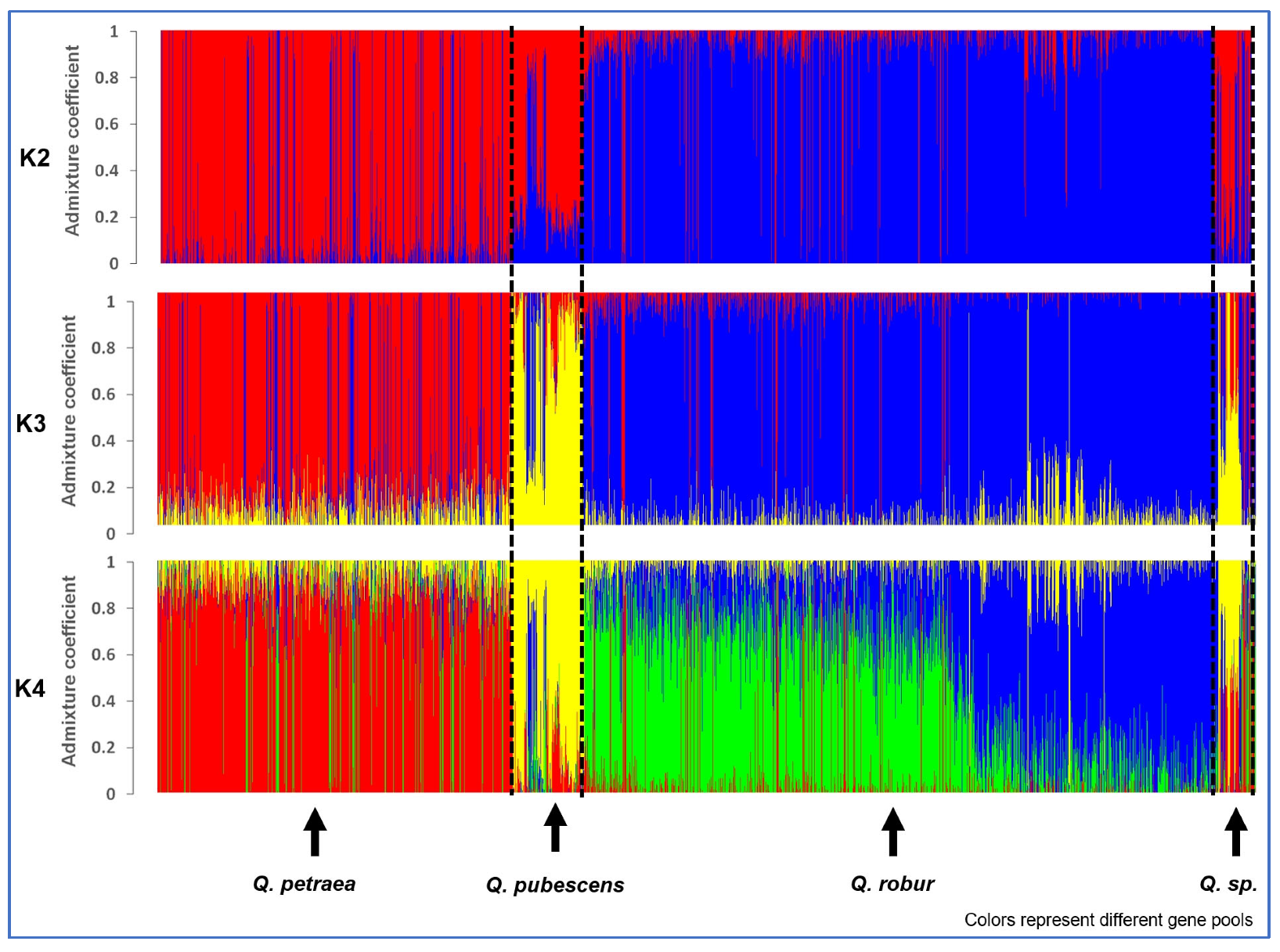
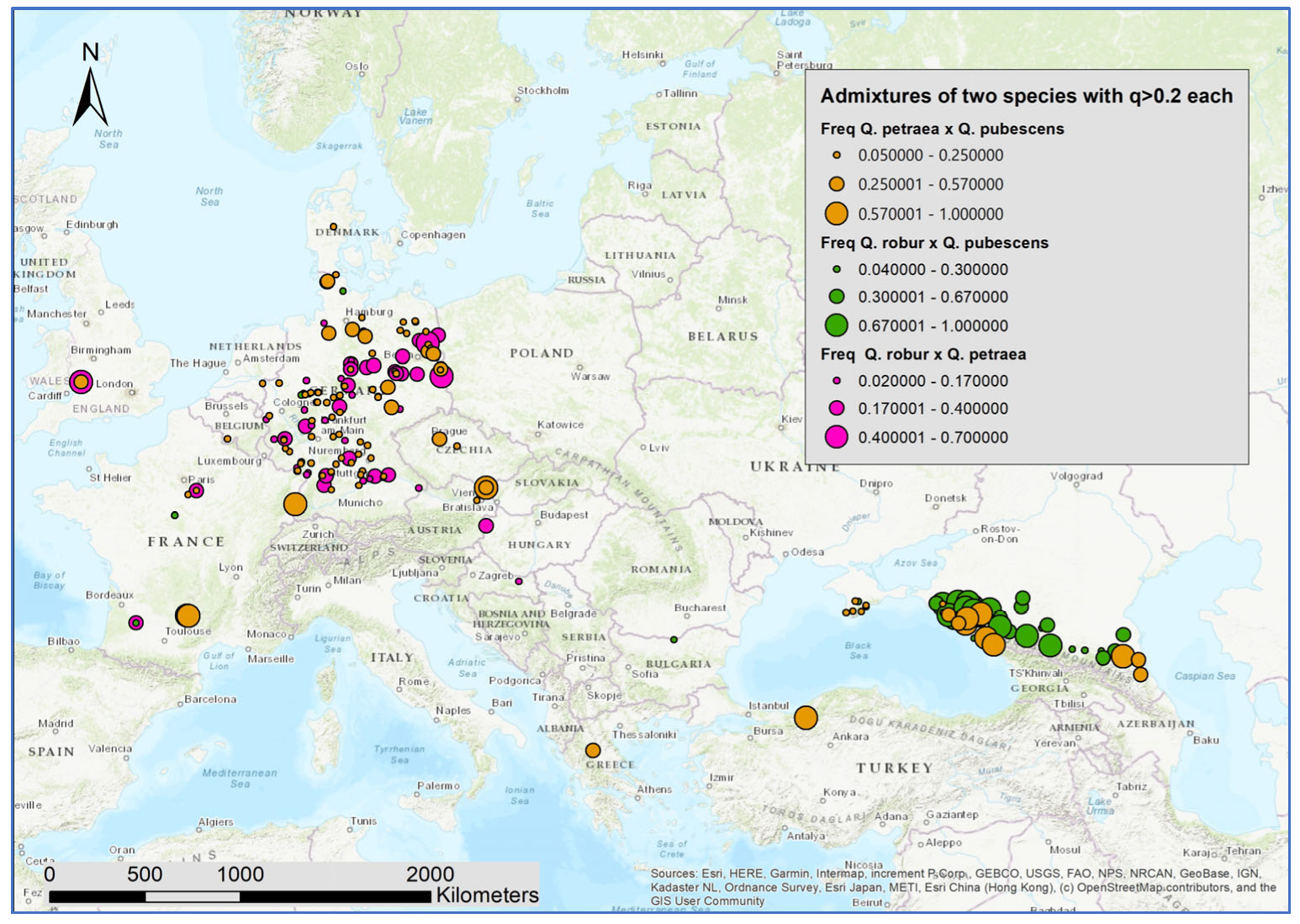
3. Results
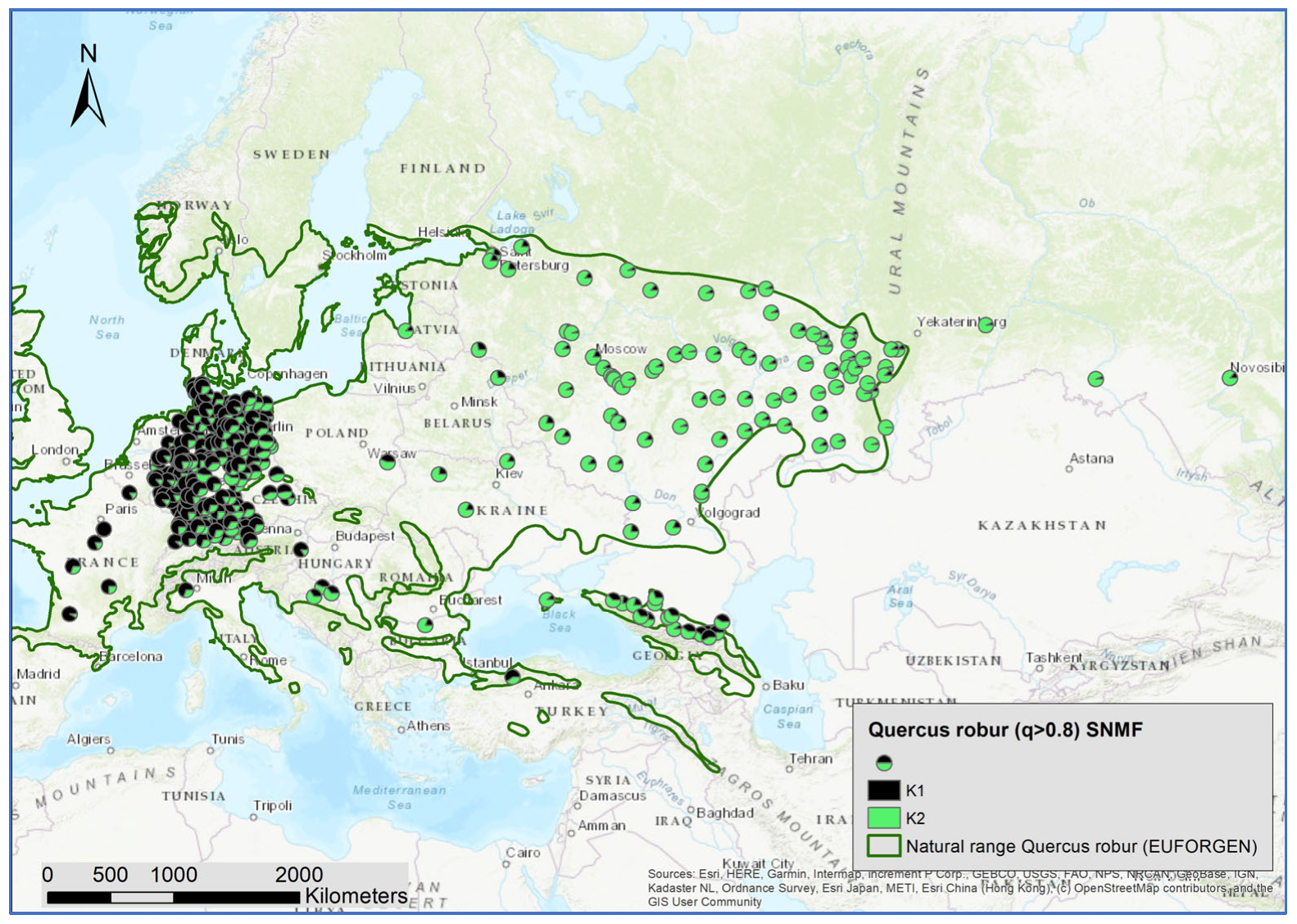
3.1. Number of Genetic Clusters Using All Individuals
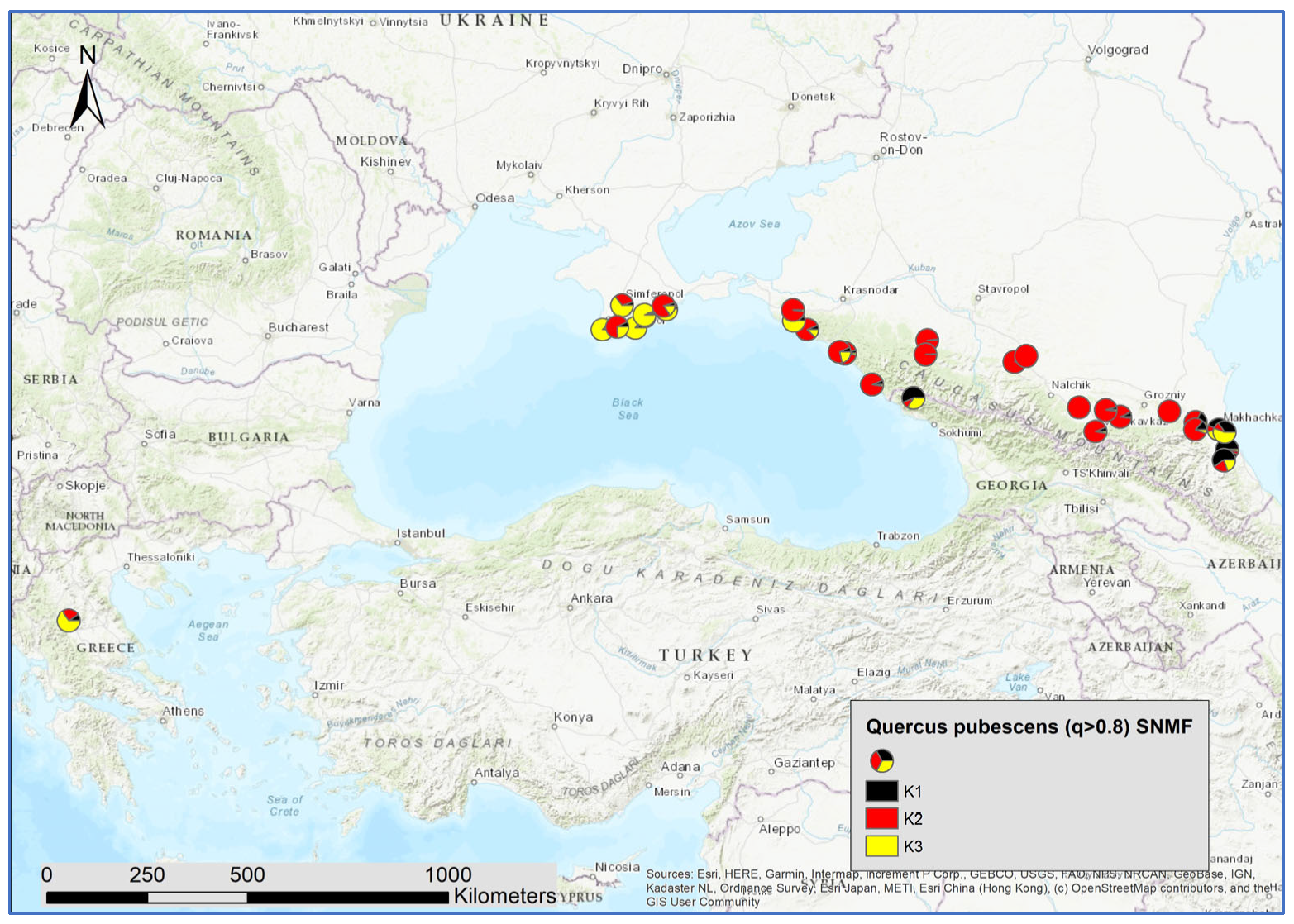
3.2. Genetic Structure within Species
3.3. Distribution of Haplotypes
4. Discussion
4.1. Species Integrity and Introgression
4.2. Introgression as a Source of Within-Species Geographic Genetic Differentiation
4.3. White Oak Species Hotspots in Crimea and the Northern Caucasus
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dyderski, M.K.; Paz, S.; Frelich, L.E.; Jagodzinski, A.M. How much does climate change threaten European forest tree species distributions? Glob. Chang. Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef]
- Reutimann, O.; Dauphin, B.; Baltensweiler, A.; Gugerli, F.; Kremer, A.; Rellstab, C. Abiotic factors predict taxonomic composition and genetic admixture in populations of hybridizing white oak species (Quercus sect. Quercus) on regional scale. Tree Genet. Genomes 2023, 19, 22. [Google Scholar] [CrossRef]
- Diekmann, M. Ecological behaviour of deciduous hardwood trees in Boreo-nemoral Sweden in relation to light and soil conditions. For. Ecol. Manag. 1996, 86, 1–14. [Google Scholar] [CrossRef]
- Bertrand, R.; Perez, V.; Gégout, J.C. Disregarding the edaphic dimension in species distribution models leads to the omission of crucial spatial information under climate change: The case of Quercus pubescens in France. Glob. Chang. Biol. 2012, 18, 2648–2660. [Google Scholar] [CrossRef]
- Kremer, A.; Dupouey, J.L.; Deans, J.D.; Cottrell, J.; Csaikl, U.; Finkeldey, R.; Espinel, S.; Jensen, J.; Kleinschmit, J.; Van Dam, B.; et al. Leaf morphological differentiation between Quercus robur and Quercus petraea is stable across western European mixed oak stands. Ann. For. Sci. 2002, 59, 777–787. [Google Scholar] [CrossRef]
- Musarella, C.M.; Cano-Ortiz, A.; Fuentes, J.C.P.; Navas-Ureña, J.; Gomes, C.J.P.; Uinto-Canas, R.; Cano, E.; Spampinato, G. Similarity analysis between species of the genus Quercus L. (Fagaceae) in southern Italy based on the fractal dimension. PhytoKeys 2018, 113, 79–95. [Google Scholar] [CrossRef]
- Viscosi, V.; Fortini, P.; D’Imperio, M. A statistical approach to species identification on morphological traits of European white oaks: Evidence of morphological structure in Italian populations of Quercus pubescens sensu lato. Acta Bot. Gall. 2011, 158, 175–188. [Google Scholar] [CrossRef]
- Viscosi, V.; Lepais, O.; Gerber, S.; Fortini, P. Leaf morphological analyses in four European oak species (Quercus) and their hybrids: A comparison of traditional and geometric morphometric methods. Plant Biosyst. 2009, 143, 564–574. [Google Scholar] [CrossRef]
- Jensen, J.; Larsen, A.; Nielsen, L.R.; Cottrell, J. Hybridization between Quercus robur and Q. petraea in a mixed oak stand in Denmark. Ann. For. Sci. 2009, 66, 12. [Google Scholar] [CrossRef]
- Kleinschmit, J.R.G.; Bacilieri, R.; Kremer, A.; Roloff, A. Comparison of morphological and genetic traits of pedunculate oak (Q. robur L.) and sessile oak (Q. petraea (MATT) LIEBL). Silvae Genet. 1995, 44, 256–269. [Google Scholar]
- Marcysiak, K.; Lewandowska, A.; Mazur, M.; Meyza, K.; Gawlak, M.; Kaluski, T. Ambiguous leaf morphology of Central European white oaks and their hybrids in an atypical mixed stand. Plant Biosyst. 2022, 156, 635–648. [Google Scholar] [CrossRef]
- Fortini, P.; Di Marzio, P.; Di Pietro, R. Differentiation and hybridization of Quercus frainetto, Q. petraea, and Q. pubescens (Fagaceae): Insights from macro-morphological leaf traits and molecular data. Plant Syst. Evol. 2015, 301, 375–385. [Google Scholar] [CrossRef]
- Rellstab, C.; Bühler, A.; Graf, R.; Folly, C.; Gugerli, F. Using joint multivariate analyses of leaf morphology and molecular-genetic markers for taxon identification in three hybridizing European white oak species (Quercus spp.). Ann. For. Sci. 2016, 73, 669–679. [Google Scholar] [CrossRef]
- Bussotti, F.; Grossoni, P. European and Mediterranean oaks: Taxonomic problems. Ital. For. E Mont. 1997, 52, 240–260. [Google Scholar]
- Enescu, C.M.; Curtu, A.L.; Sofletea, N. Is Quercus virgiliana a distinct morphological and genetic entity among European white oaks? Turk. J. Agric. For. 2013, 37, 632–641. [Google Scholar] [CrossRef]
- Di Pietro, R.; Conte, A.L.; Di Marzio, P.; Gianguzzi, L.; Spampinato, G.; Caldarella, O.; Fortini, P. A multivariate morphometric analysis of diagnostic traits in southern Italy and Sicily pubescent oaks. Folia Geobot. 2020, 55, 163–183. [Google Scholar] [CrossRef]
- Gerber, S.; Chadoeuf, J.; Gugerli, F.; Lascoux, M.; Buiteveld, J.; Cottrell, J.; Dounavi, A.; Fineschi, S.; Forrest, L.L.; Fogelqvist, J.; et al. High rates of gene flow by pollen and seed in oak populations across Europe. PLoS ONE 2014, 9, e85130. [Google Scholar] [CrossRef]
- Truffaut, L.; Chancerel, E.; Ducousso, A.; Dupouey, J.L.; Badeau, V.; Ehrenmann, F.; Kremer, A. Fine-scale species distribution changes in a mixed oak stand over two successive generations. New Phytol. 2017, 215, 126–139. [Google Scholar] [CrossRef]
- Abadie, P.; Roussel, G.; Dencausse, B.; Bonnet, C.; Bertocchi, E.; Louvet, J.M.; Kremer, A.; Garnier-Gere, P. Strength, diversity and plasticity of postmating reproductive barriers between two hybridizing oak species (Quercus robur L. and Quercus petraea (Matt) Liebl.). J. Evol. Biol. 2012, 25, 157–173. [Google Scholar] [CrossRef]
- Petit, R.J.; Brewer, S.; Bordacs, S.; Burg, K.; Cheddadi, R.; Coart, E.; Cottrell, J.; Csaikl, U.M.; van Dam, B.; Deans, J.D.; et al. Identification of refugia and post-glacial colonisation routes of European white oaks based on chloroplast DNA and fossil pollen evidence. For. Ecol. Manag. 2002, 156, 49–74. [Google Scholar] [CrossRef]
- Leroy, T.; Louvet, J.M.; Lalanne, C.; Le Provost, G.; Labadie, K.; Aury, J.M.; Delzon, S.; Plomion, C.; Kremer, A. Adaptive introgression as a driver of local adaptation to climate in European white oaks. New Phytol. 2020, 226, 1171–1182. [Google Scholar] [CrossRef]
- Leroy, T.; Rougemont, Q.; Dupouey, J.L.; Bodenes, C.; Lalanne, C.; Belser, C.; Labadie, K.; Le Provost, G.; Aury, J.M.; Kremer, A.; et al. Massive postglacial gene flow between European white oaks uncovered genes underlying species barriers. New Phytol. 2020, 226, 1183–1197. [Google Scholar] [CrossRef]
- Degen, B.; Yanbaev, Y.; Mader, M.; Ianbaev, R.; Bakhtina, S.; Schroeder, H.; Blanc-Jolivet, C. Impact of gene flow and introgression on the range wide genetic structure of Quercus robur (L.) in Europe. Forests 2021, 12, 1425. [Google Scholar] [CrossRef]
- Curtu, A.L.; Gailing, O.; Finkeldey, R. Evidence for hybridization and introgression within a species-rich oak (Quercus spp.) community. BMC Evol. Biol. 2007, 7, 15. [Google Scholar] [CrossRef]
- Leroy, S.A.G.; Arpe, K. Glacial refugia for summer-green trees in Europe and south-west Asia as proposed by ECHAM3 time-slice atmospheric model simulations. J. Biogeogr. 2007, 34, 2115–2128. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Tarkhnishvili, D. Historical Biogeography of the Caucasus; NOVA Science Publishers: Hauppauge, NY, USA, 2014. [Google Scholar]
- Ekhvaia, J.; Simeone, M.C.; Silakadze, N.; Abdaladze, O. Morphological diversity and phylogeography of the Georgian durmast oak (Q. petraea subsp. iberica) and related Caucasian oak species in Georgia (South Caucasus). Tree Genet. Genomes 2018, 14, 15. [Google Scholar] [CrossRef]
- Lepais, O.; Petit, R.J.; Guichoux, E.; Lavabre, J.E.; Alberto, F.; Kremer, A.; Gerber, S. Species relative abundance and direction of introgression in oaks. Mol. Ecol. 2009, 18, 2228–2242. [Google Scholar] [CrossRef]
- Rushton, B. Natural hybridization within the genus Quercus L. [introgression]. Ann. Sci. For. 1993, 50, 73–90. [Google Scholar] [CrossRef]
- Dumolin, S.; Demesure, B.; Petit, R.J. Inheritance of chloroplast and mitochondrial genomes in pedunculate oak investigated with an efficient PCR method. Theor. Appl. Genet. 1995, 91, 1253–1256. [Google Scholar] [CrossRef]
- Degen, B.; Blanc-Jolivet, C.; Bakhtina, S.; Ianbaev, R.; Yanbaev, Y.; Mader, M.; Nürnberg, S.; Schröder, H. Applying targeted genotyping by sequencing with a new set of nuclear and plastid SNP and indel loci for Quercus robur and Quercus petraea. Conserv. Genet. Resour. 2021, 13, 345–347. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef]
- Degen, B. GDA-NT 2021-a computer program for population genetic data analysis and assignment. Conserv. Genet. Resour. 2022, 14, 347–350. [Google Scholar] [CrossRef]
- Frichot, E.; Francois, O. LEA: An R package for landscape and ecological association studies. Methods Ecol. Evol. 2015, 6, 925–929. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar]
- Puechmaille, S.J. The program structure does not reliably recover the correct population structure when sampling is uneven: Subsampling and new estimators alleviate the problem. Mol. Ecol. Resour. 2016, 16, 608–627. [Google Scholar] [CrossRef]
- Frichot, E.; Mathieu, F.; Trouillon, T.; Bouchard, G.; Francois, O. Fast and efficient estimation of individual ancestry coefficients. Genetics 2014, 196, 973–983. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P. Paleontological statistics software package for education and data analysis. Paleontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Kruskal, J.B. On the shortest spanning subtree of a graph and the traveling salesman problem. Proc. Am. Math. Soc. 1956, 7, 48–50. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. POPART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Olrik, D.C.; Kjaer, E.D. The reproductive success of a Quercus petraea × Q. robur F1-hybrid in back-crossing situations. Ann. For. Sci. 2007, 64, 37–45. [Google Scholar] [CrossRef]
- Curtu, A.L.; Gailing, O.; Finkeldey, R. Patterns of contemporary hybridization inferred from paternity analysis in a four-oak-species forest. BMC Evol. Biol. 2009, 9, 9. [Google Scholar] [CrossRef]
- Salvini, D.; Bruschi, P.; Fineschi, S.; Grossoni, P.; Kjaer, E.D.; Vendramin, G.G. Natural hybridisation between Quercus petraea (Matt.) Liebl. and Quercus pubescens Willd. within an Italian stand as revealed by microsatellite fingerprinting. Plant Biol. 2009, 11, 758–765. [Google Scholar] [CrossRef]
- Reutimann, O.; Gugerli, F.; Rellstab, C. A species-discriminatory single-nucleotide polymorphism set reveals maintenance of species integrity in hybridizing European white oaks (Quercus spp.) despite high levels of admixture. Ann. Bot. 2020, 125, 663–676. [Google Scholar] [CrossRef]
- Timbal, J.; Aussenac, G. An overview of ecology and silviculture of indigenous oaks in France. Ann. Sci. For. 1996, 53, 649–661. [Google Scholar] [CrossRef]
- Lazic, D.; Hipp, A.L.; Carlson, J.E.; Gailing, O. Use of genomic resources to assess adaptive divergence and introgression in oaks. Forests 2021, 12, 690. [Google Scholar] [CrossRef]
- De Dios, R.S.; Benito-Garzón, M.; Sainz-Ollero, H. Hybrid zones between two european oaks: A plant community approach. Plant Ecol. 2006, 187, 109–125. [Google Scholar] [CrossRef]
- Franjic, J.; Liber, Z.; Skvorc, Z.; Idzojtic, M.; Sostaric, R.; Stancic, Z. Morphological and molecular differentiation of the Croatian populations of Quercus pubescens Willd. (Fagaceae). Acta Soc. Bot. Pol. 2006, 75, 123–130. [Google Scholar] [CrossRef]
- Kalinowski, S.T. The computer program STRUCTURE does not reliably identify the main genetic clusters within species: Simulations and implications for human population structure. Heredity 2011, 106, 625–632. [Google Scholar] [CrossRef]
- Chybicki, I.J.; Oleksa, A.; Kowalkowska, K.; Burczyk, J. Genetic evidence of reproductive isolation in a remote enclave of Quercus pubescens in the presence of cross-fertile species. Plant Syst. Evol. 2012, 298, 1045–1056. [Google Scholar] [CrossRef][Green Version]
- Kätzel, R.; Kamp, T.; Höltken, A.M.; Becker, F.; Riederer, H.J.; Schröder, J. Populations of Pubescent Oak (Quercus pubescens Willd.) and its hybrids north of the Alps. Landbauforschung 2014, 64, 73–84. [Google Scholar] [CrossRef]
- Bruschi, P.; Vendramin, G.G.; Bussotti, F.; Grossoni, P. Morphological and molecular differentiation between Quercus petraea (Matt.) Liebl. and Quercus pubescens Willd. (Fagaceae) in Northern and Central Italy. Ann. Bot. 2000, 85, 325–333. [Google Scholar] [CrossRef][Green Version]
- Smid, J.; Douda, J.; Krak, K.; Mandak, B. Analyses of hybrid viability across a hybrid zone between two Alnus species using microsatellites and cpDNA-Markers. Genes 2020, 11, 770. [Google Scholar] [CrossRef]
- An, M.; Deng, M.; Zheng, S.S.; Jiang, X.L.; Song, Y.G. Introgression threatens the genetic diversity of Quercus austrocochinchinensis (Fagaceae), an endangered Oak: A case inferred by molecular markers. Front. Plant Sci. 2017, 8, 15. [Google Scholar] [CrossRef]
- Semerikova, S.A.; Isakov, I.Y.; Semerikov, V.L. Chloroplast DNA variation and phylogeography of Pedunculate Oak (Quercus robur L.) in the eastern part of the range. Russ. J. Genet. 2021, 57, 47–60. [Google Scholar] [CrossRef]
- Semerikova, S.; Podergina, S.; Tashev, A.; Semerikov, V. Phylogeography of oaks in the Crimea reveals Pleistocene refugia and migration routes. Russ. J. Ecol. 2023, 54, 197–212. [Google Scholar] [CrossRef]
- Konig, A.O.; Ziegenhagen, B.; van Dam, B.C.; Csaikl, U.M.; Coart, E.; Degen, B.; Burg, K.; de Vries, S.M.G.; Petit, R.J. Chloroplast DNA variation of oaks in western Central Europe and genetic consequences of human influences. For. Ecol. Manag. 2002, 156, 147–166. [Google Scholar] [CrossRef]

| Pre-Classification | N | Q. robur K2 > 0.8 (%) | Q. petraea K1 > 0.8 (%) | Q. pubescens K3 > 0.8 (%) | Admixtures >0.2 (%) |
|---|---|---|---|---|---|
| Q. robur | 3342 | 91 | 3 | 0 | 6 |
| Q. petraea | 2090 | 11 | 67 | 6 | 16 |
| Q. pubescens | 170 | 0 | 0 | 69 | 31 |
| Q. sp. | 195 | 19 | 12 | 17 | 52 |
| Within-Species Structure | K | Q. petraea (K1) | Q. robur (K3) | Q. pubescens (K2) |
|---|---|---|---|---|
| Q. robur (N = 3287) | 1 | +0.381 *** | −0.162 *** | −0.098 *** |
| 2 | −0.381 *** | +0.162 *** | +0.098 *** | |
| Q. petraea (N = 1525) | 1 | −0.013 ns | +0.023 ns | −0.001 ns |
| 2 | +0.013 ns | −0.023 ns | +0.001 ns | |
| Q. pubescens (N = 298) | 1 | +0.229 *** | +0.232 *** | −0.262 *** |
| 2 | −0.641 *** | −0.114 ns | +0.501 *** | |
| 3 | +0.523 *** | −0.052 ns | −0.350 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degen, B.; Blanc-Jolivet, C.; Mader, M.; Yanbaeva, V.; Yanbaev, Y. Introgression as an Important Driver of Geographic Genetic Differentiation within European White Oaks. Forests 2023, 14, 2279. https://doi.org/10.3390/f14122279
Degen B, Blanc-Jolivet C, Mader M, Yanbaeva V, Yanbaev Y. Introgression as an Important Driver of Geographic Genetic Differentiation within European White Oaks. Forests. 2023; 14(12):2279. https://doi.org/10.3390/f14122279
Chicago/Turabian StyleDegen, Bernd, Celine Blanc-Jolivet, Malte Mader, Vasilina Yanbaeva, and Yulai Yanbaev. 2023. "Introgression as an Important Driver of Geographic Genetic Differentiation within European White Oaks" Forests 14, no. 12: 2279. https://doi.org/10.3390/f14122279
APA StyleDegen, B., Blanc-Jolivet, C., Mader, M., Yanbaeva, V., & Yanbaev, Y. (2023). Introgression as an Important Driver of Geographic Genetic Differentiation within European White Oaks. Forests, 14(12), 2279. https://doi.org/10.3390/f14122279






