The Structural, Physical, and Mechanical Properties of Wood from Scots Pine (Pinus sylvestris L.) Affected by Scots Pine Blister Rust
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brzezicka-Szymczyk, K. Grzyby rdzawnikowe (Uredinales)—Pasożyty roślin. Kosmos 1992, 41, 223–233. [Google Scholar]
- Samils, B.; Stenlid, J. A Review of Biology, Epidemiology and Management of Cronartium pini with Emphasis on Northern Europe. Scand. J. For. Res. 2022, 37, 153–171. [Google Scholar] [CrossRef]
- Kirk, P. Species Fungorum (Version Oct 2017). Catalogue of Life—2019 Annual Checklist. In Species 2000 & ITIS Catalogue of Life, 2019 Annual Checklist; Roskov, Y., Ower, G., Orrel, T., Nicolson, D., Kirk, P., Bourgoin, T., DeWalt, R., Decock, W., van Nieukerken, E., Zarucchi, J., et al., Eds.; Species 2000; Naturalis: Leiden, The Netherlands, 2019; ISSN 2405-884X. [Google Scholar]
- DGLP. Raport o Stanie Lasów w Polsce 2010–2021; Centrum Informacyjne Lasów Państwowych: Warszawa, Poland, 2021; ISSN 1641-3229. [Google Scholar]
- Kaitera, J. Susceptibility and Lesion Development in Scots Pine Saplings Infected with Peridermium pini in Northern Finland. For. Pathol. 2003, 33, 353–362. [Google Scholar] [CrossRef]
- Kim, M.-S.; Hantula, J.; Kaitera, J.; Zambino, P.J.; Woodward, S.; Richardson, B.A.; Stewart, J.E.; Spaine, P.; Shaw, D.C.; Takeuchi, Y.; et al. Recovery Plan for Scots Pine Blister Rust Caused by Cronartium pini. Plant Health Prog. 2022, 23, 105–130. [Google Scholar] [CrossRef]
- Kaitera, J. Analysis of Cronartium Flaccidum Lesion Development on Pole-Stage Scots Pines. Silva Fenn. 2000, 34, 21–27. [Google Scholar] [CrossRef]
- Geils, B.W.; Jacobi, W.R. Effects of Comandra Blister Rust on Growth and Survival of Lodgepole Pine. Phytopathology 1993, 83, 638–644. [Google Scholar] [CrossRef]
- Kaitera, J.; Aalto, T.; Jalkanen, R. Effect of Resin-top Disease Caused by Peridermium pini on the Volume and Value of Pinus sylvestris Saw Timber and Pulpwood. Scand. J. For. Res. 1994, 9, 376–381. [Google Scholar] [CrossRef]
- Sullivan, M. CPHST Pest Datasheet for Cronartium Flaccidum (Alb. & Schwein) Winter; Revised July 2015 by D. Z. Mackesy; USDA-APHISPPQ-CPHST: Raleigh, NC, USA, 2010; pp. 1–18. [Google Scholar]
- Mirski, R.; Wieruszewski, M.; Malinowski, Z. Zmienność rozkładu wad drewna okrągłego w dojrzałych drzewostanach sosnowych. Sylwan 2019, 163, 913–923. [Google Scholar] [CrossRef]
- Karlman, L.; Mörling, T.; Martinsson, O. Wood Density, Annual Ring Width and Latewood Content in Larch and Scots Pine. EurasianJ. For. Res. 2005, 8, 91–96. [Google Scholar]
- Mańkowski, P.; Krzosek, S.; Burawska-Kupniewska, I.; Grześkiewicz, M.; Mirski, R. Correlation between the Share of Latewood and the Density of Sawn Timber from the Silesian Forestry Region. Ann. Wars. Univ. Life Sci. SGGW For. Wood Technol. 2020, 109, 70–75. [Google Scholar] [CrossRef]
- Bektas, İ.; Tutuş, A.; Eroğlu, H. A Study of the Suitability of Calabrian Pine (Pinus brutia Ten.) for Pulp and Paper Manufacture. Turk. J. Agric. For. 1999, 23, 589–598. [Google Scholar]
- Rikala, J. Spruce and Pine on Drained Peatlands—Wood Quality and Suitability for the Sawmill Industry; Publications 35; University of Helsinki, Department of Forest Resource Management: Helsinki, Finland, 2003; ISBN 951-45-9092-9. [Google Scholar]
- Gryc, V.; Vavrčík, H.; Horn, K. Density of Juvenile and Mature Wood of Selected Coniferous Species. J. For. Sci. 2011, 57, 123–130. [Google Scholar] [CrossRef]
- Farsi, M.; Kiaei, M.; Miar, S.; Mohammadnezhad Kiasari, S. Effect of Seed Source on Physical Properties of Scots Pine (a Case Study in Neka, Iran). Drv. Ind. 2013, 64, 183–191. [Google Scholar] [CrossRef]
- Kask, R. The Influence of Growth Conditions on Physico-Mechanical Properties of Scots Pine (Pinus sylvestris L.) Wood in Estonia. Ph.D. Thesis, Estonian University of Life Sciences, Tartu, Estonia, 2015. [Google Scholar]
- Schönfelder, O.; Zeidler, A.; Borůvka, V.; Bílek, L.; Lexa, M. Shrinkage of Scots Pine Wood as an Effect of Different Tree Growth Rates, a Comparison of Regeneration Methods. J. For. Sci. 2018, 64, 271–278. [Google Scholar] [CrossRef]
- Tomczak, A.; Jelonek, T.; Pazdrowski, W. Basic Density of Scots Pine Wood-Relationships between Values Calculated at Different Heights of the Trun. Ann. Wars. Univ. Life Sci. SGGW For. Wood Technol. 2013, 84, 241–246. [Google Scholar]
- Tomczak, A.; Jelonek, T. Comparison of selected physical properties of the juvenile and mature wood of Scots pine (Pinus sylvestris L.) from mature stands. Sylwan 2010, 154, 809–817. [Google Scholar] [CrossRef]
- Witkowska, J.; Lachowicz, H. Analysis of variation in pure density of Scots pine wood (Pinus sylvestris L.) along a trunk height depending on selected factors. Przegląd Pap. 2012, 68, 573–578. [Google Scholar]
- Witkowska, J.; Lachowicz, H. Variability of conventional wood density of Scots pine (Pinus sylvestris L.) depending on the selected factors. Sylwan 2013, 157, 336–347. [Google Scholar] [CrossRef]
- Mäkinen, H.; Hynynen, J. Wood Density and Tracheid Properties of Scots Pine: Responses to Repeated Fertilization and Timing of the First Commercial Thinning. For. Int. J. For. Res. 2014, 87, 437–447. [Google Scholar] [CrossRef]
- Janusz, S.; Danilov, D. Density of Wood of Pine and Spruce in the Postagrogenic Soil of the Boreal Zone. In Proceedings of the 24th International Scientific Conference Research for Rural Development, Jeglava, Latvia, 16–18 May 2018; pp. 92–96. [Google Scholar]
- Schönfelder, O.; Zeidler, A.; Borůvka, V.; Bílek, L. Impact of Silvicultural Measures on the Quality of Scots Pine Wood: Part II. Effect of Site. Wood Res. 2019, 64, 789–798. [Google Scholar]
- Kantieva, E.; Snegireva, S.; Platonov, A. Formation of Density and Porosity of Pine Wood in a Tree Trunk. IOP Conf. Ser.Earth Environ. Sci. 2021, 875, 012016. [Google Scholar] [CrossRef]
- Pikiński, P.M.; Szaban, J.; Šilingienė, G.; Korzeniewicz, R.; Pazdrowski, W. Selected Physical and Mechanical Properties of Scots Pine (Pinus sylvestris L.) Wood from Stands of Younger Age Classes as Criteria for Rational Utilization of Timber. Balt. For. 2021, 27, 363. [Google Scholar] [CrossRef]
- Pikk, J.; Kask, R. Mechanical Properties of Juvenile Wood of Scots Pine (Pinus sylvestris L.) on Myrtillus Forest Site Type. Balt. For. 2004, 10, 72–78. [Google Scholar]
- Roszyk, E.; Mania, P.; Iwańska, E.; Kusiak, W.; Broda, M. Mechanical Performance of Scots Pine Wood from Northwestern Poland—A Case Study. BioRes 2020, 15, 6781–6794. [Google Scholar] [CrossRef]
- Wąsik, R.; Michalec, K.; Barszcz, A.; Mudryk, K. Variability of Selected Macrostructure Features, Density and Compression Strength along the Grain of “Tabórz” Scots Pine Wood (Pinus sylvestris L.). Wood 2020, 63, 171–182. [Google Scholar] [CrossRef]
- Konofalska, E.; Kozakiewicz, P.; Buraczyk, W.; Szeligowski, H.; Lachowicz, H. The Technical Quality of the Wood of Scots Pine (Pinus sylvestris L.) of Diverse Genetic Origin. Forests 2021, 12, 619. [Google Scholar] [CrossRef]
- Kask, R.; Pikk, J.; Kangur, A. Effect of Growth Conditions on Wood Properties of Scots Pine (L.). For. Stud. 2021, 75, 176–187. [Google Scholar] [CrossRef]
- Pazdrowski, W.; Splawa-Neyman, S. Badania wybranych wlasciwosci drewna sosny zwyczajnej [Pinus silvestris L.] na tle klas biologicznych w drzewostanie. Folia For. Pol. Ser. B Drzew. 1994, 24, 133–145. [Google Scholar]
- Raczkowska-Helinska, L.; Raczkowski, J.; Krauss, A.; Ciazynska, I. Tracheids Length Variation in Scots Pine [Pinus silvestris L.] Trees Belonging to Different Tree Growth Classes. Folia For. Pol. Ser. B Drzew. 1994, 24, 123–132. [Google Scholar]
- Kokociński, W. Anatomia Drewna, 2nd ed.; Prodruk: Poznań, Poland, 2005; ISBN 83-88518-42-9. [Google Scholar]
- Przybysz, K. System Kontroli Jakości Masy Włóknistej. Cz. 2. Wymiary i Kształt Włókien. Przegląd Pap. 2005, 61, 212–216. [Google Scholar]
- Jelonek, T.; Gzyl, J.; Arasimowicz-Jelonek, M.; Tomczak, A.; Remlein, A. Wpływ wybranych wskaźników stabilności drzew na grubość ścian cewek u sosny zwyczajnej (Pinus sylvestris L.). Acta Sci. Pol. Silvarum Colendarum Ratio Ind. Lignaria 2016, 15, 13–21. [Google Scholar] [CrossRef]
- Area, M.C.; Popa, V. Characteristics of Cells and Properties of Pulps. In Wood Fibers for Papermaking, Smithers Pira; Smithers Information Ltd.: Shawbury, UK, 2014; Chapter 4; pp. 65–88. ISBN 978-1-909030-86-2. [Google Scholar]
- Sadiku, A.; Oluyege, A.; Ajayi, B. Fibre Dimension and Chemical Characterisation of Naturally Grown Bambusa Vulgaris for Pulp and Paper Production. J. Bamboo Ratt. 2016, 15, 33–43. [Google Scholar]
- ISO 3129:2019; Wood—Sampling Methods and General Requirements for Physical and Mechanical Testing of Small Clear Wood Specimens. International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 13061-2:2014; Physical and Mechanical Properties of Wood—Test Methods for Small Clear Wood Specimens—Part 2: Determination of Density for Physical and Mechanical Tests. International Organization for Standardization: Geneva, Switzerland, 2014.
- ISO 13061-17:2017; Physical and Mechanical Properties of Wood—Test Methods for Small Clear Wood Specimens—Part 17: Determination of Ultimate Stress in Compression Parallel to Grain. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 13061-1:2014; Physical and Mechanical Properties of Wood—Test Methods for Small Clear Wood Specimens—Part 1: Determination of Moisture Content for Physical and Mechanical Tests. International Organization for Standardization: Geneva, Switzerland, 2014.
- Cybis Elektronik & Data. CooRecorder—Cybis Coordinate Recorder, 64bit; Version 7.6; Cybis Elektronik & Data: Saltsjöbaden, Sweden, 2012. [Google Scholar]
- Cybis Elektronik & Data. CDendro—Cybis Dendro Dating Program, 64bit; Version 7.6; Cybis Elektronik & Data: Saltsjöbaden, Sweden, 2012. [Google Scholar]
- TIBCO. Statistica, an Advanced Analytics Software Package; Version 13.3; TIBCO: Palo Alto, CA, USA, 2017. [Google Scholar]
- Grekin, M.; Surini, T. Shear Strength and Perpendicular-to-Grain Tensile Strength of Defect-Free Scots Pine Wood from Mature Stands in Finland and Sweden. Wood Sci. Technol. 2008, 42, 75–91. [Google Scholar] [CrossRef]
- Bektas, I.; Alma, H.; As, N. The Effect of 120 Years of Service on Various Physical and Mechanical Properties of Scots Pine Wood Used as Roof Beam. Wood Res. 2005, 50, 27–32. [Google Scholar]
- Awoyemi, L. Reversibility of Dimensional Changes in Birch (Betula Pubescens) and Scots Pine (Pinus sylvestris L.) Wood. Taiwan J. For. Sci. 2004, 19, 97–101. [Google Scholar]
| Structural, Physical and Mechanical Properties | Health Status | M | Me | Min | Max | SD | M–W |
|---|---|---|---|---|---|---|---|
| Annual ring width, mm | Healthy | 2.44 | 2.44 | 0.90 | 6.03 | 1.01 | 0.019 |
| Infected | 2.75 | 2.58 | 1.07 | 5.61 | 1.12 | ||
| Latewood proportion, % | Healthy | 30.14 | 30.16 | 16.84 | 42.76 | 5.41 | 0.747 |
| Infected | 29.90 | 29.96 | 13.37 | 51.99 | 6.77 | ||
| Air-dry density (), kg m−3 | Healthy | 461.56 | 458.95 | 378.52 | 645.87 | 45.61 | <0.001 |
| Infected | 632.51 | 637.31 | 405.49 | 974.76 | 106.70 | ||
| Oven-dry density (), kg m−3 | Healthy | 420.58 | 420.05 | 339.17 | 518.43 | 45.64 | <0.001 |
| Infected | 585.23 | 587.98 | 378.55 | 796.35 | 87.74 | ||
| Basic density (,), kg m−3 | Healthy | 371.38 | 372.25 | 300.39 | 457.55 | 36.69 | <0.001 |
| Infected | 527.92 | 529.91 | 329.74 | 730.33 | 81.61 | ||
| Proportion of wood substance (D), % | Healthy | 28.04 | 28.00 | 22.61 | 34.56 | 3.04 | <0.001 |
| Infected | 39.02 | 39.20 | 25.24 | 53.09 | 5.85 | ||
| Porosity (c), % | Healthy | 71.96 | 72.00 | 65.44 | 77.39 | 3.04 | <0.001 |
| Infected | 60.98 | 60.80 | 46.91 | 74.76 | 5.85 | ||
| Total longitudinal shrinkage (% | Healthy | 0.15 | 0.07 | 0.0324 | 1.62 | 0.203 | 0.302 |
| Infected | 0.19 | 0.03 | 0.0323 | 1.65 | 0.312 | ||
| Total radial shrinkage (), % | Healthy | 4.10 | 4.19 | 1.84 | 6.25 | 0.91 | <0.001 |
| Infected | 3.48 | 3.51 | 1.22 | 6.49 | 1.10 | ||
| Total tangential shrinkage (),% | Healthy | 7.69 | 7.75 | 4.55 | 10.35 | 1.20 | <0.001 |
| Infected | 6.41 | 6.29 | 3.08 | 9.36 | 1.248 | ||
| Total volumetric shrinkage (), % | Healthy | 11.60 | 11.72 | 7.28 | 14.67 | 1.64 | <0.001 |
| Infected | 9.84 | 9.83 | 5.69 | 13.13 | 1.74 | ||
| Total longitudinal shrinkage coefficient () | Healthy | 0.005 | 0.002 | 0.0011 | 0.054 | 0.007 | 0.302 |
| Infected | 0.006 | 0.001 | 0.0011 | 0.055 | 0.001 | ||
| Total radial shrinkage coefficient () | Healthy | 0.14 | 0.14 | 0.06 | 0.21 | 0.031 | <0.001 |
| Infected | 0.12 | 0.12 | 0.04 | 0.22 | 0.037 | ||
| Total tangential shrinkage coefficient () | Healthy | 0.26 | 0.26 | 0.15 | 0.35 | 0.04 | <0.001 |
| Infected | 0.21 | 0.21 | 0.10 | 0.31 | 0.10 | ||
| Total volumetric shrinkage coefficient () | Healthy | 0.39 | 0.39 | 0.24 | 0.49 | 0.05 | <0.001 |
| Infected | 0.33 | 0.33 | 0.19 | 0.44 | 0.06 | ||
| Shrinkage anisotropy index () | Healthy | 1.95 | 1.86 | 1.11 | 3.56 | 0.45 | 0.371 |
| Infected | 2.02 | 1.88 | 0.70 | 4.57 | 0.69 | ||
| Compression strength parallel to grain (), MPa | Healthy | 42.55 | 41.49 | 31.9 | 59.8 | 6.27 | 0.027 |
| Infected | 44.24 | 43.49 | 22.1 | 57.8 | 6.01 | ||
| Coefficient of compression strength parallel to grain (), km | Healthy | 9.19 | 9.16 | 7.71 | 10.93 | 0.72 | <0.001 |
| Infected | 7.16 | 6.98 | 3.39 | 9.86 | 1.37 |
| Parameters and Indicators of Wood Fiber Structure | Health Status | Type of Wood | M | Me | Min | Max | SD | p (M–W) | p (K–W) |
|---|---|---|---|---|---|---|---|---|---|
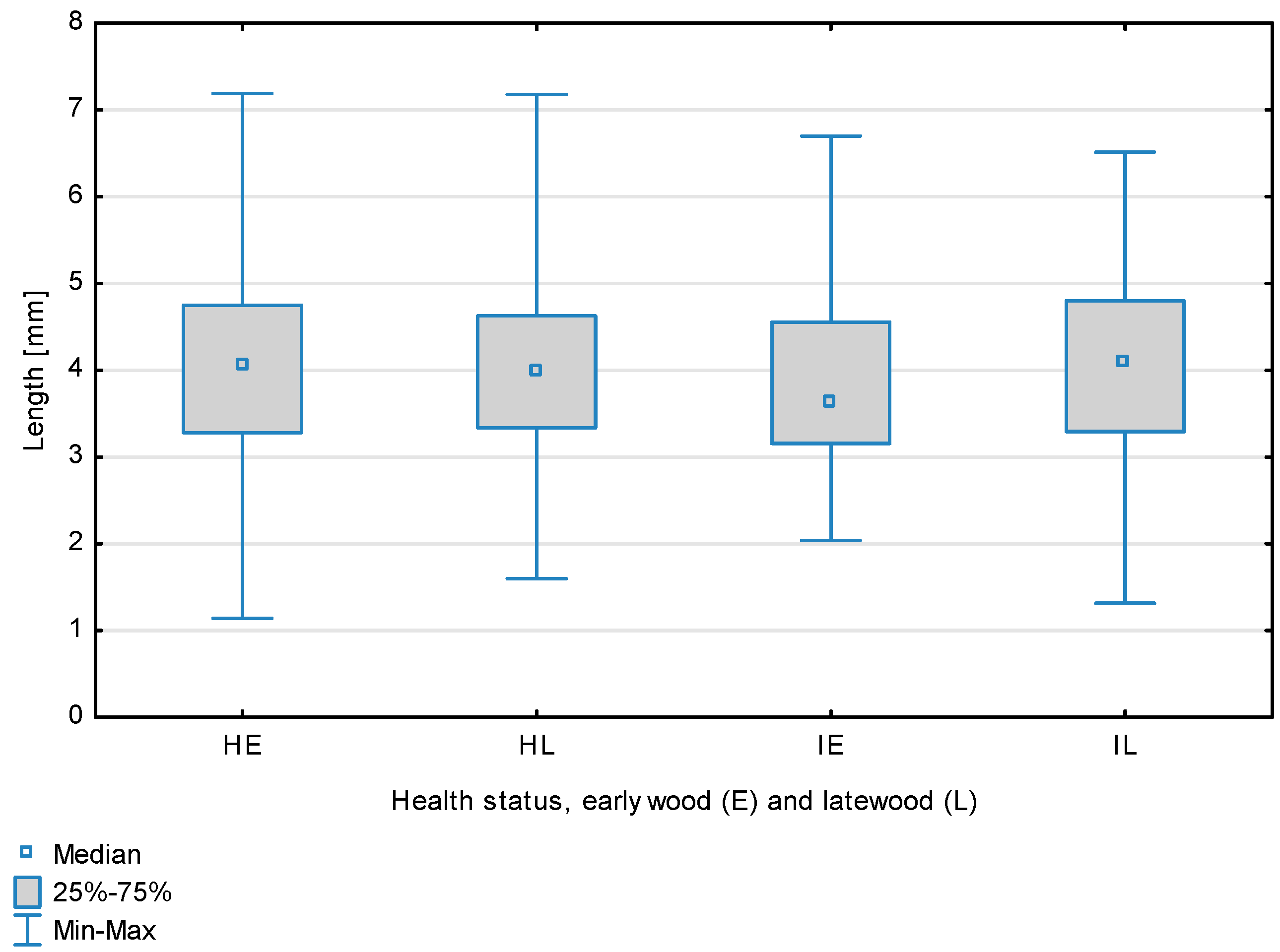
| Length, mm | Healthy | Earlywood | 3.99 | 4.06 | 1.14 | 7.19 | 1.12 | 0.514 | 0.387 |
| Latewood | 3.99 | 3.97 | 1.60 | 7.18 | 1.04 | ||||
| Overall | 3.99 | 4.02 | 1.08 | 7.19 | 1.14 | ||||
| Infected | Earlywood | 3.93 | 3.63 | 2.04 | 6.70 | 1.09 | |||
| Latewood | 4.03 | 4.11 | 1.32 | 6.51 | 1.05 | ||||
| Overall | 3.98 | 3.88 | 1.07 | 6.70 | 1.32 | ||||
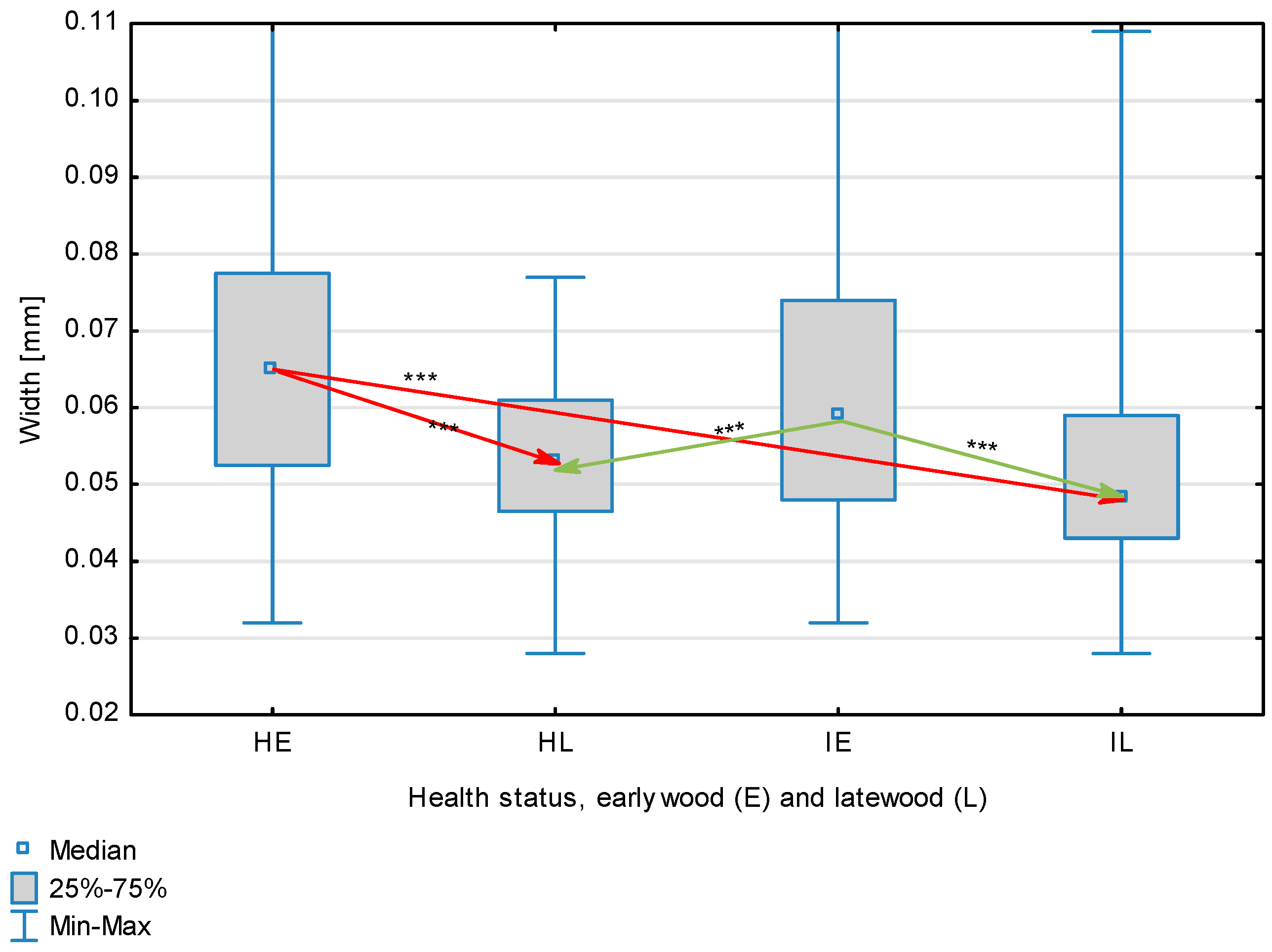
| Diameter, mm | Healthy | Earlywood | 0.0670 | 0.0650 | 0.0320 | 0.1190 | 0.0191 | 0.001 | <0.001 |
| Latewood | 0.0535 | 0.0530 | 0.0280 | 0.0770 | 0.0102 | ||||
| Overall | 0.0603 | 0.0575 | 0.022 | 0.119 | 0.0167 | ||||
| Infected | Earlywood | 0.0629 | 0.0590 | 0.0320 | 0.1190 | 0.0188 | |||
| Latewood | 0.0513 | 0.0480 | 0.0280 | 0.1090 | 0.0130 | ||||
| Overall | 0.0571 | 0.0540 | 0.028 | 0.119 | 0.0171 | ||||
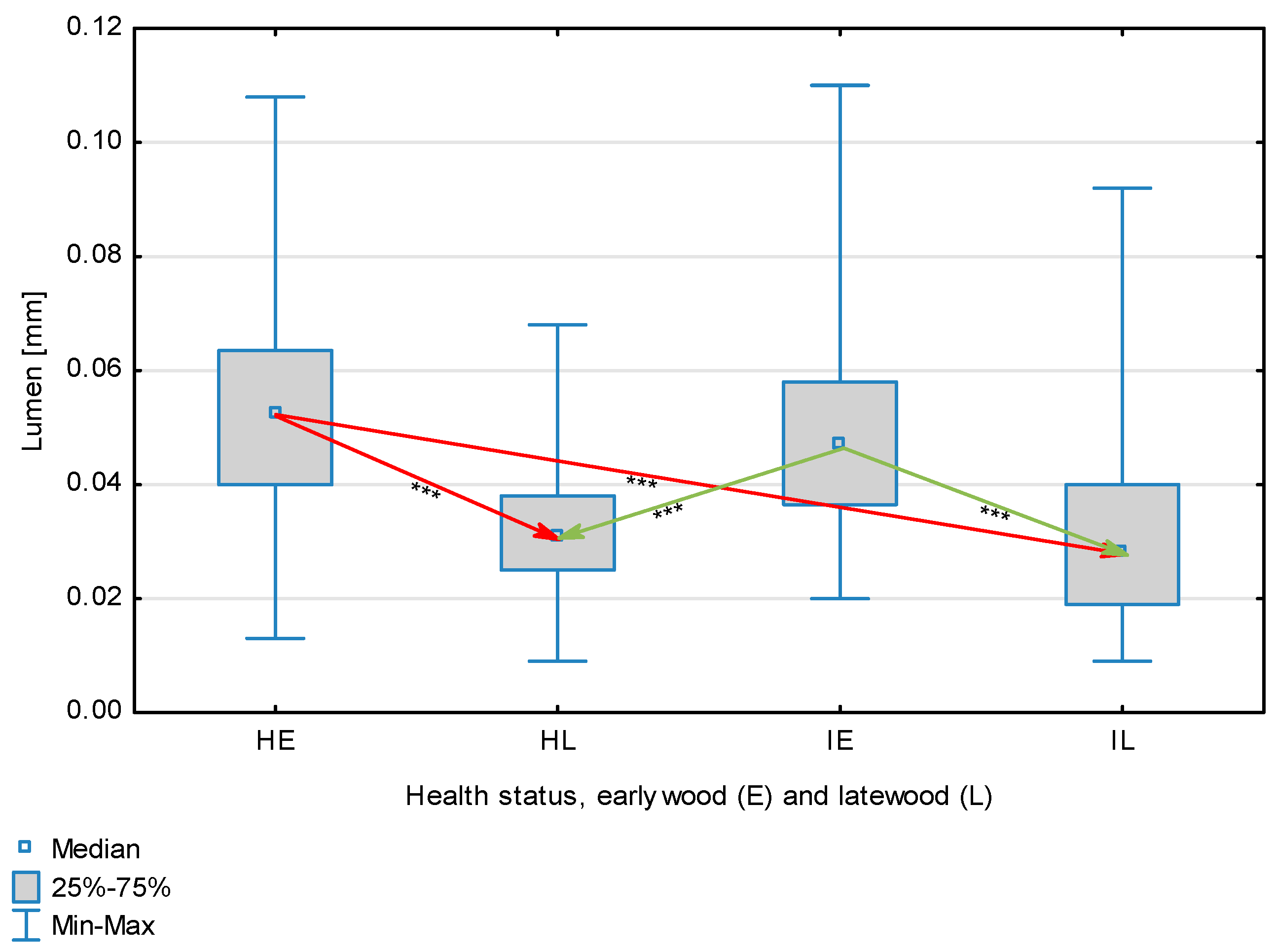
| Lumen, mm | Healthy | Earlywood | 0.0523 | 0.0525 | 0.0130 | 0.1080 | 0.0196 | 0.040 | <0.001 |
| Latewood | 0.0330 | 0.0310 | 0.0090 | 0.0680 | 0.0116 | ||||
| Overall | 0.0426 | 0.0390 | 0.0090 | 0.1080 | 0.0188 | ||||
| Infected | Earlywood | 0.0491 | 0.0470 | 0.0200 | 0.1100 | 0.0168 | |||
| Latewood | 0.0302 | 0.0280 | 0.0090 | 0.0920 | 0.0133 | ||||
| Overall | 0.0397 | 0.0380 | 0.0090 | 0.1100 | 0.0178 | ||||
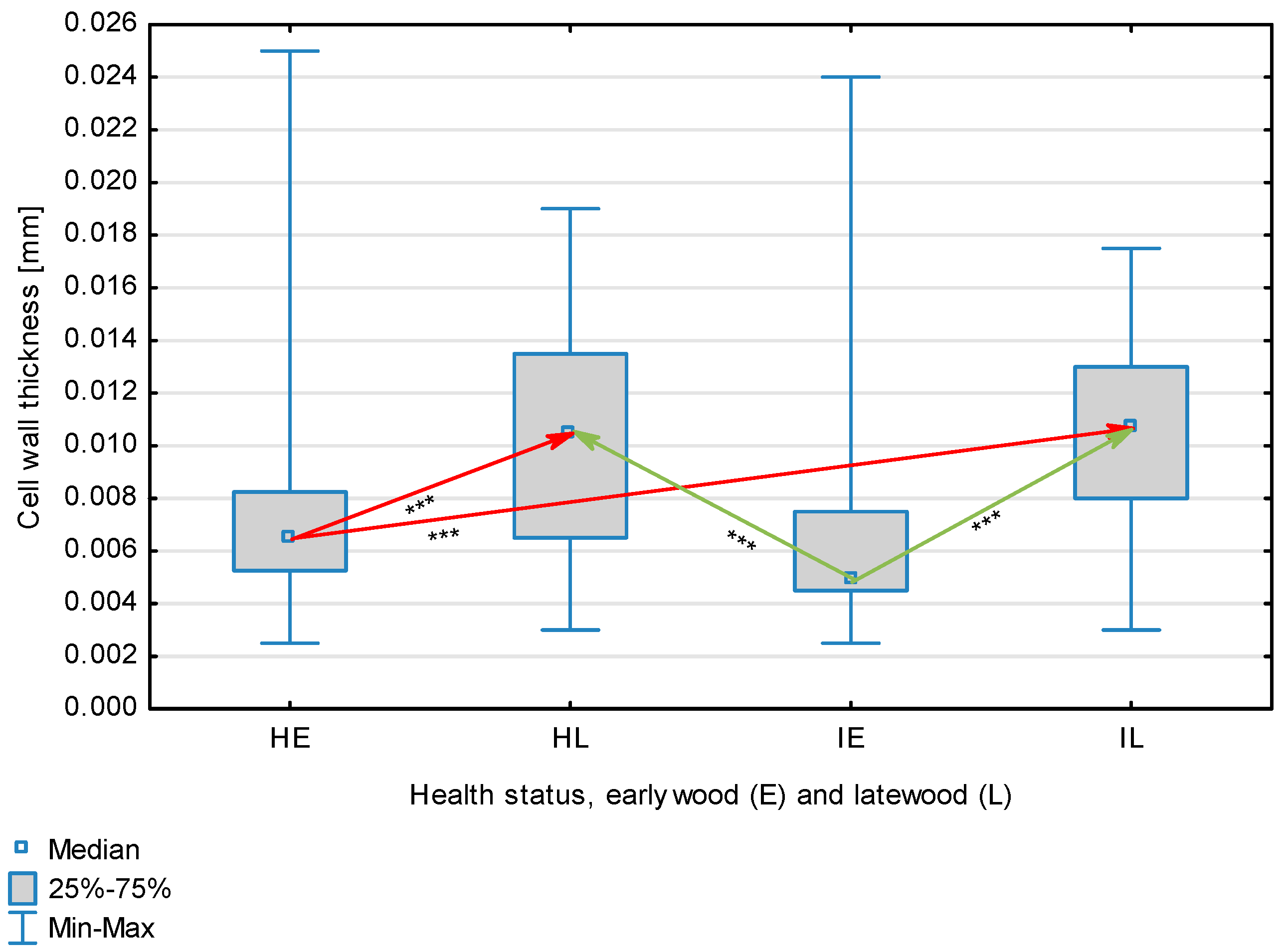
| Wall Thickness, mm | Healthy | Earlywood | 0.0074 | 0.0650 | 0.0025 | 0.0250 | 0.0036 | 0.601 | <0.001 |
| Latewood | 0.0103 | 0.0105 | 0.0030 | 0.0190 | 0.0042 | ||||
| Overall | 0.0088 | 0.0750 | 0.0025 | 0.0250 | 0.0042 | ||||
| Infected | Earlywood | 0.0069 | 0.0050 | 0.0025 | 0.0240 | 0.0042 | |||
| Latewood | 0.0105 | 0.0108 | 0.0030 | 0.0175 | 0.0032 | ||||
| Overall | 0.0087 | 0.0080 | 0.0025 | 0.0240 | 0.0042 | ||||
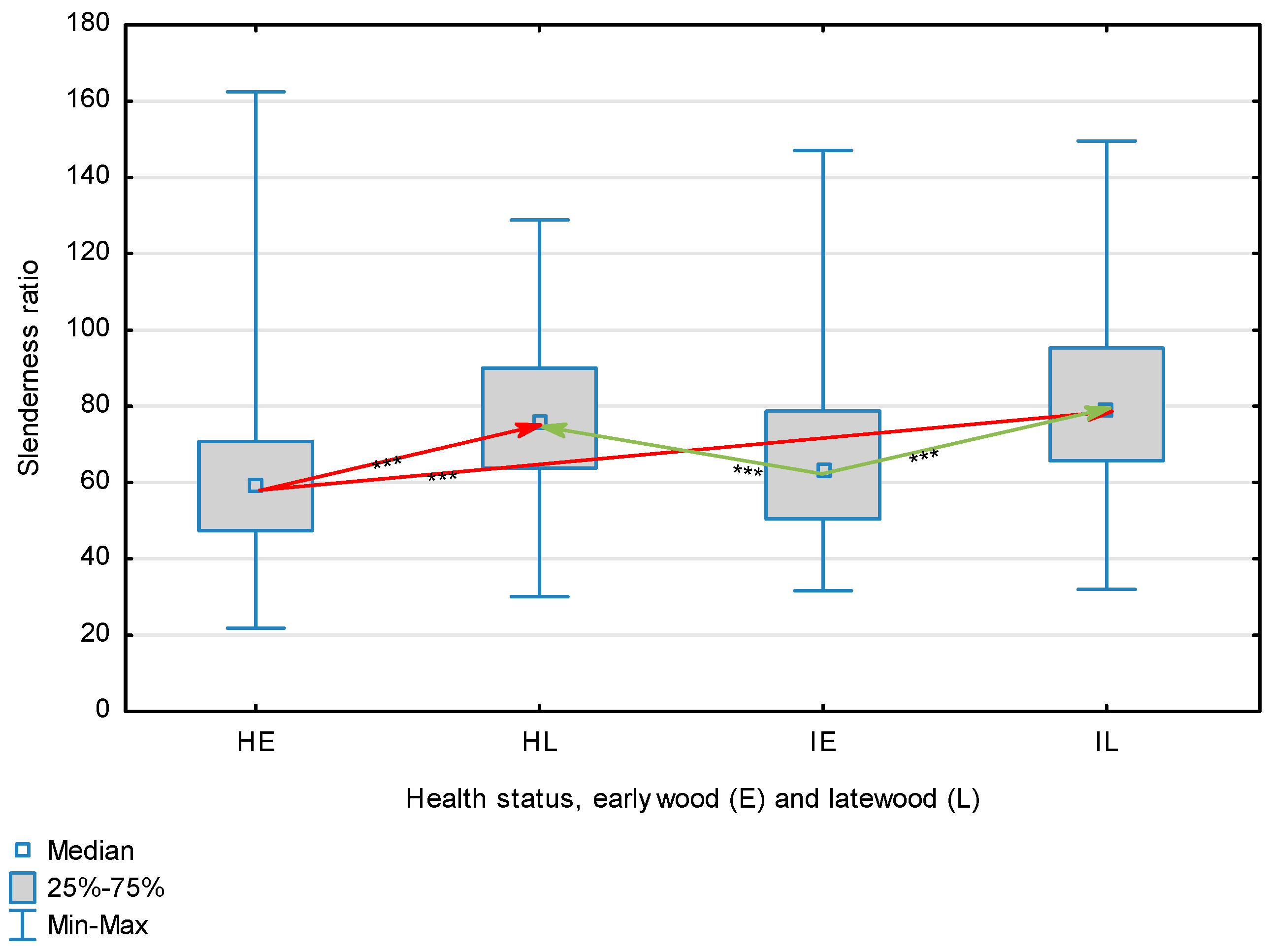
| Slenderness Ratio | Healthy | Earlywood | 63.14 | 59.55 | 21.81 | 162.46 | 24.29 | 0.024 | <0.001 |
| Latewood | 75.72 | 75.55 | 30.15 | 128.81 | 18.48 | ||||
| Overall | 69.52 | 67.52 | 21.81 | 162.46 | 22.63 | ||||
| Infected | Earlywood | 66.00 | 63.07 | 31.64 | 147.03 | 21.14 | |||
| Latewood | 81.06 | 79.36 | 32.02 | 149.55 | 22.62 | ||||
| Overall | 73.53 | 71.57 | 31.64 | 149.55 | 23.13 | ||||
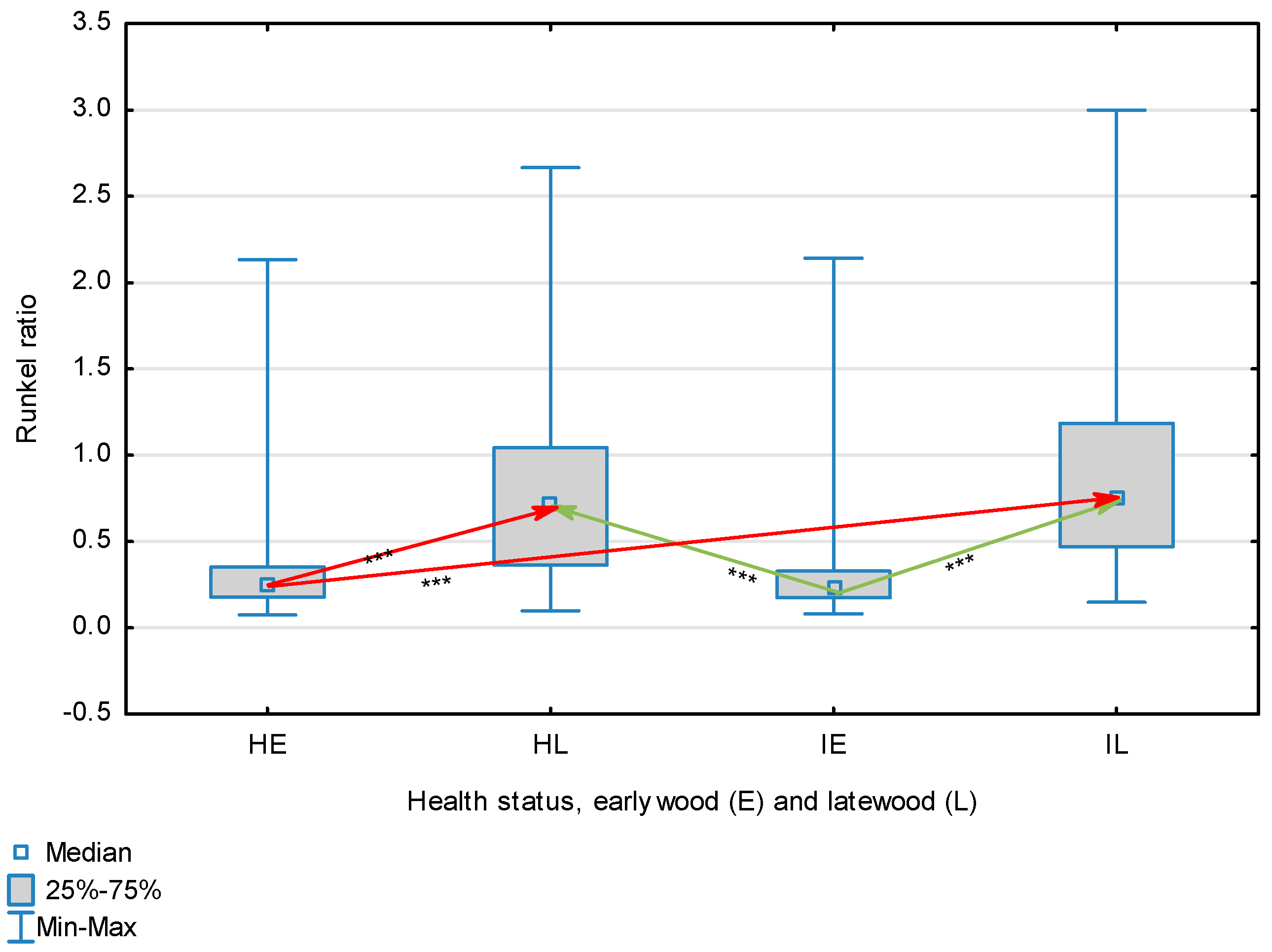
| Runkel Ratio | Healthy | Earlywood | 0.377 | 0.248 | 0.074 | 2.133 | 0.381 | 0.345 | <0.001 |
| Latewood | 0.755 | 0.701 | 0.098 | 2.667 | 0.474 | ||||
| Overall | 0.565 | 0.351 | 0.074 | 2.667 | 0.470 | ||||
| Infected | Earlywood | 0.318 | 0.227 | 0.082 | 2.143 | 0.280 | |||
| Latewood | 0.873 | 0.751 | 0.150 | 3.000 | 0.527 | ||||
| Overall | 0.595 | 0.398 | 0.082 | 3.000 | 0.505 | ||||
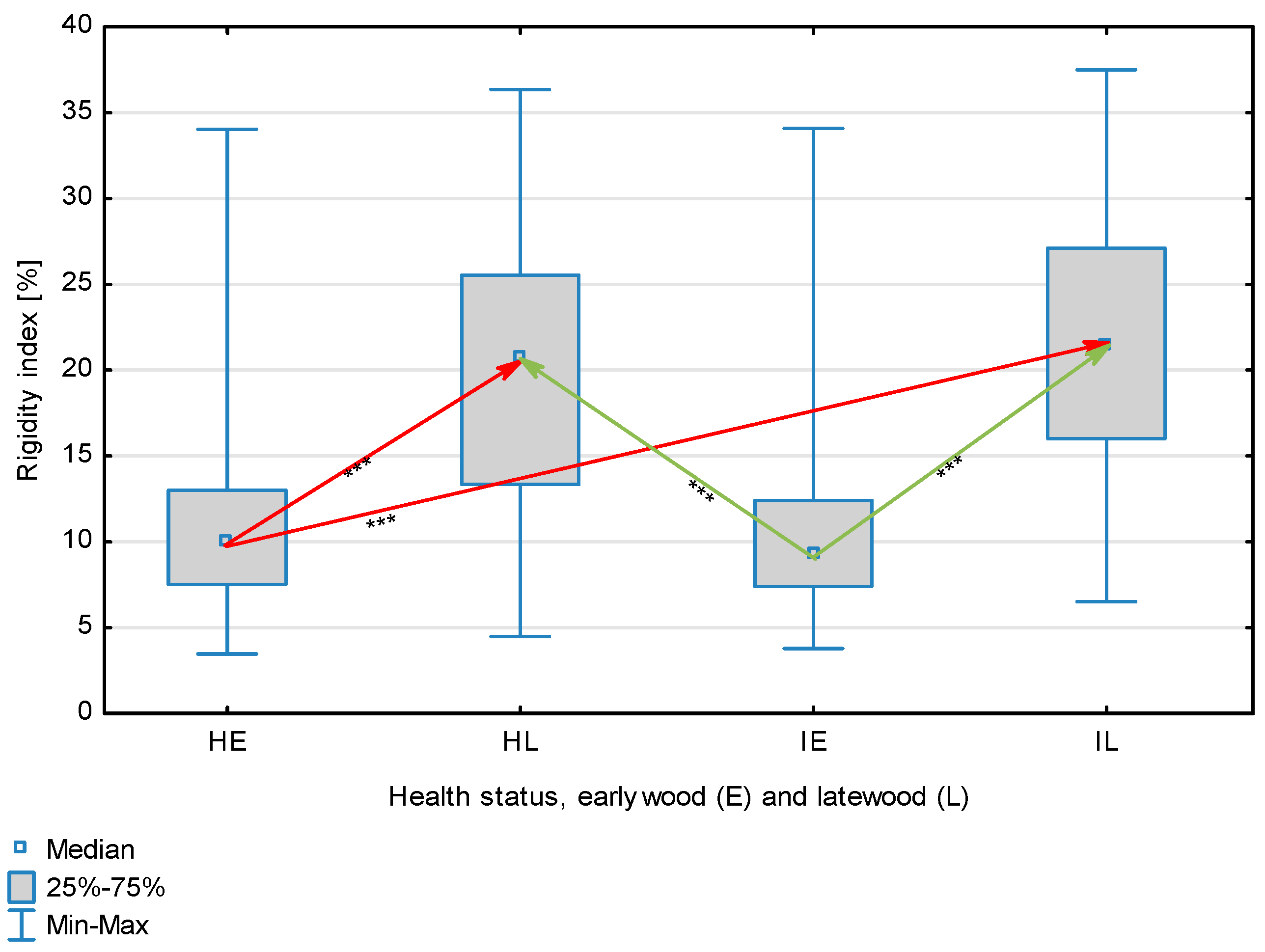
| Rigidity Index, % | Healthy | Earlywood | 11.87 | 9.93 | 3.47 | 34.04 | 6.815 | 0.344 | <0.001 |
| Latewood | 19.54 | 20.61 | 4.48 | 36.36 | 7.726 | ||||
| Overall | 15.68 | 12.99 | 3.47 | 36.36 | 8.22 | ||||
| Infected | Earlywood | 10.95 | 9.24 | 3.78 | 34.09 | 5.468 | |||
| Latewood | 21.38 | 21.44 | 6.52 | 37.50 | 7.202 | ||||
| Overall | 16.17 | 14.23 | 3.78 | 37.50 | 8.25 | ||||
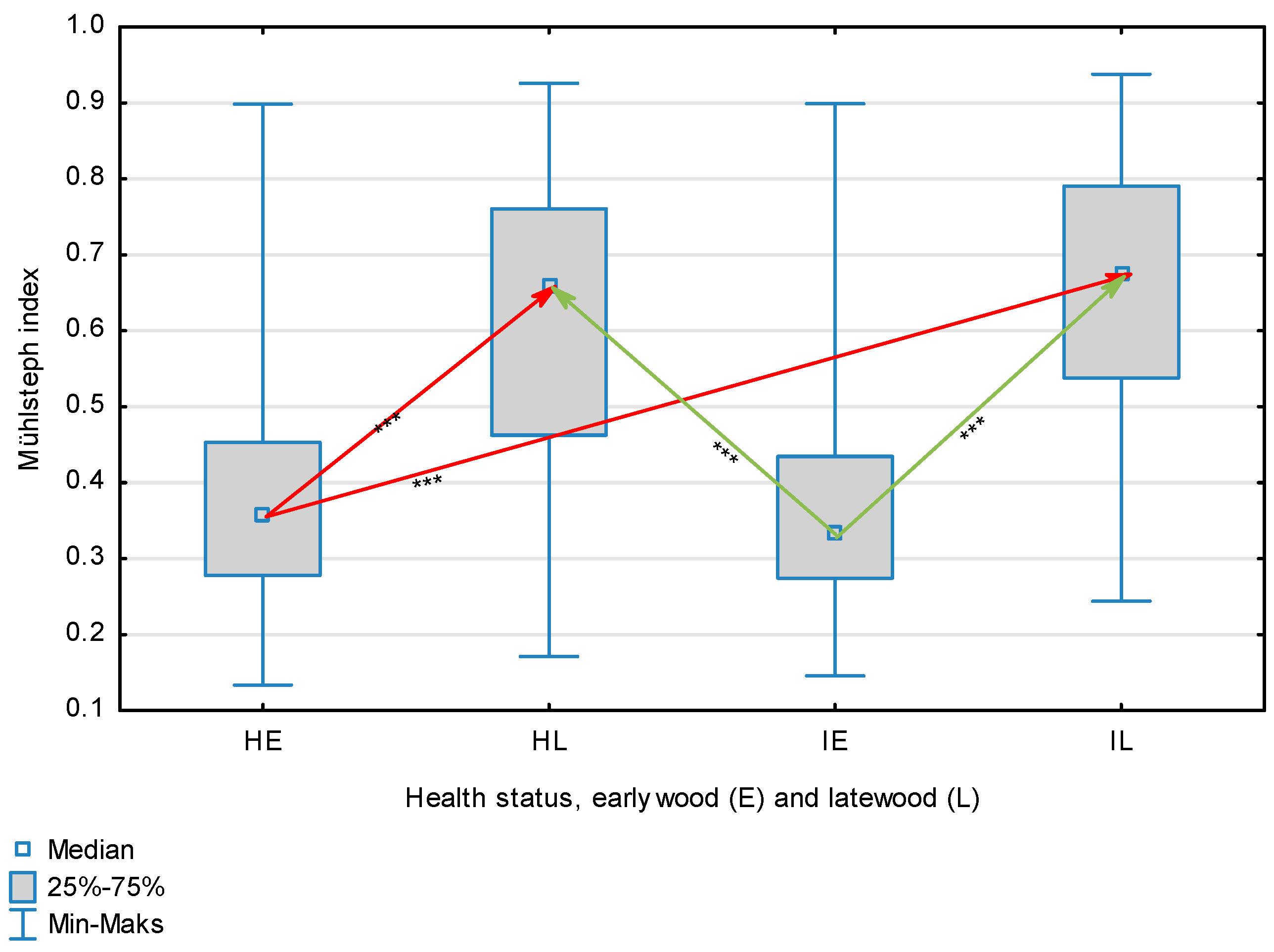
| Mühlsteph Index | Healthy | Earlywood | 0.3999 | 0.358 | 0.1338 | 0.8981 | 0.1793 | 0.345 | <0.001 |
| Latewood | 0.6052 | 0.655 | 0.1711 | 0.9256 | 0.1961 | ||||
| Overall | 0.5018 | 0.452 | 0.1338 | 0.9256 | 0.2138 | ||||
| Infected | Earlywood | 0.3780 | 0.336 | 0.1455 | 0.8988 | 0.1505 | |||
| Latewood | 0.6518 | 0.674 | 0.2439 | 0.9375 | 0.1676 | ||||
| Overall | 0.5149 | 0.488 | 0.1455 | 0.9375 | 0.2100 | ||||
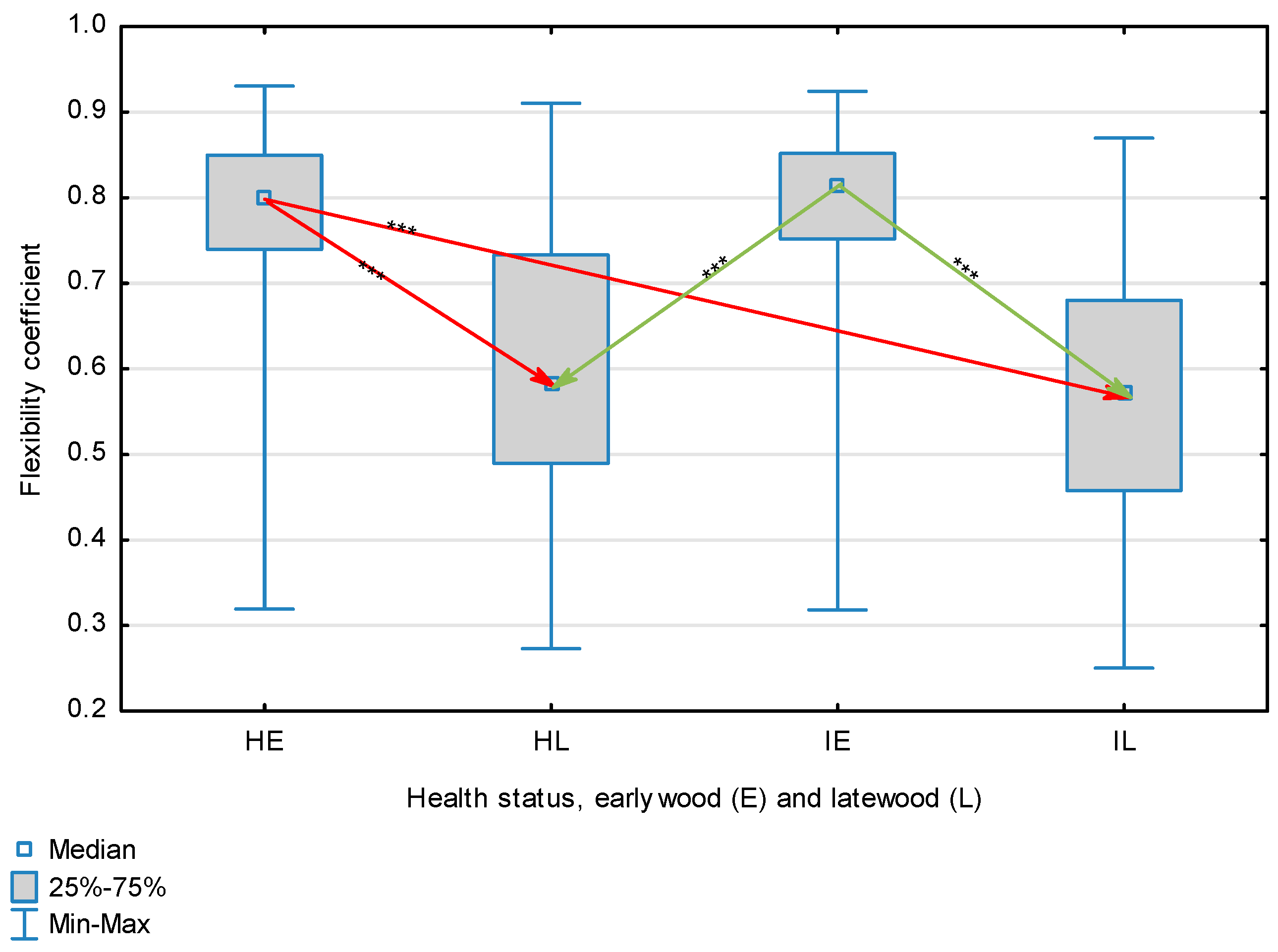
| Flexibility Coefficient | Healthy | Earlywood | 0.801 | 0.801 | 0.319 | 0.931 | 0.136 | 0.345 | <0.001 |
| Latewood | 0.588 | 0.588 | 0.273 | 0.910 | 0.155 | ||||
| Overall | 0.686 | 0.740 | 0.273 | 0.931 | 0.164 | ||||
| Infected | Earlywood | 0.815 | 0.815 | 0.318 | 0.924 | 0.109 | |||
| Latewood | 0.571 | 0.571 | 0.250 | 0.870 | 0.144 | ||||
| Overall | 0.677 | 0.715 | 0.250 | 0.924 | 0.165 | ||||
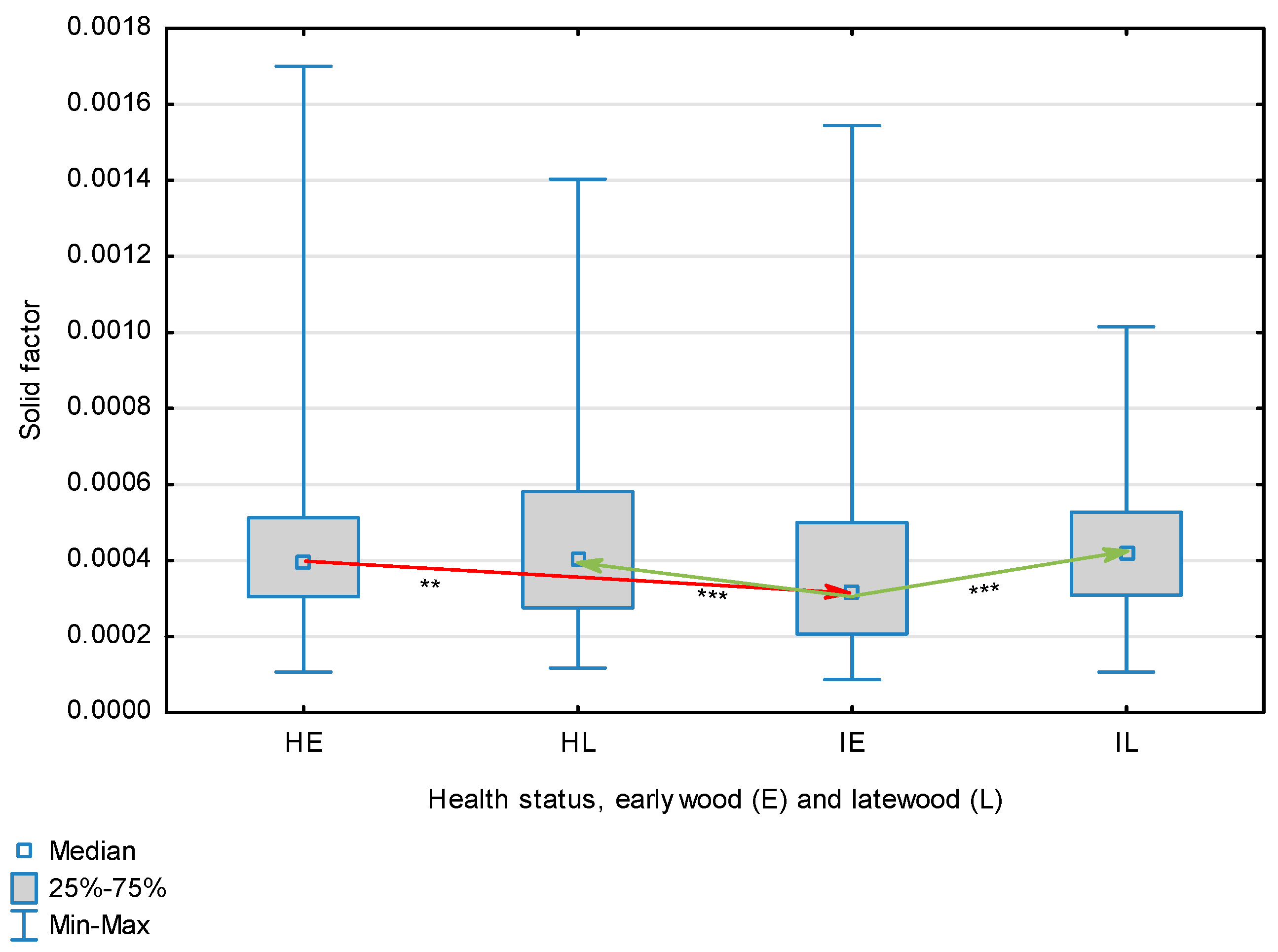
| Solid Factor | Healthy | Earlywood | 0.00045 | 0.00040 | 0.00011 | 0.00170 | 0.00025 | 0.039 | <0.001 |
| Latewood | 0.00046 | 0.00040 | 0.00012 | 0.00140 | 0.00024 | ||||
| Overall | 0.00045 | 0.00040 | 0.00007 | 0.00170 | 0.00024 | ||||
| Infected | Earlywood | 0.00042 | 0.00031 | 0.00009 | 0.00154 | 0.00030 | |||
| Latewood | 0.00043 | 0.00042 | 0.00011 | 0.00101 | 0.00016 | ||||
| Overall | 0.00042 | 0.00038 | 0.00038 | 0.00009 | 0.00024 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulak, P.; Lachowicz, H.; Moskalik, T.; Piętka, J.; Aniszewska, M.; Gendek, A. The Structural, Physical, and Mechanical Properties of Wood from Scots Pine (Pinus sylvestris L.) Affected by Scots Pine Blister Rust. Forests 2023, 14, 2161. https://doi.org/10.3390/f14112161
Kulak P, Lachowicz H, Moskalik T, Piętka J, Aniszewska M, Gendek A. The Structural, Physical, and Mechanical Properties of Wood from Scots Pine (Pinus sylvestris L.) Affected by Scots Pine Blister Rust. Forests. 2023; 14(11):2161. https://doi.org/10.3390/f14112161
Chicago/Turabian StyleKulak, Patrycja, Hubert Lachowicz, Tadeusz Moskalik, Jacek Piętka, Monika Aniszewska, and Arkadiusz Gendek. 2023. "The Structural, Physical, and Mechanical Properties of Wood from Scots Pine (Pinus sylvestris L.) Affected by Scots Pine Blister Rust" Forests 14, no. 11: 2161. https://doi.org/10.3390/f14112161
APA StyleKulak, P., Lachowicz, H., Moskalik, T., Piętka, J., Aniszewska, M., & Gendek, A. (2023). The Structural, Physical, and Mechanical Properties of Wood from Scots Pine (Pinus sylvestris L.) Affected by Scots Pine Blister Rust. Forests, 14(11), 2161. https://doi.org/10.3390/f14112161






