Abstract
In Fraxinus mandshurica Rupr. (F. mandshurica), the mature seeds exhibit a deep dormancy trait, and the seedlings are vulnerable to external environmental factors, such as low temperature and drought, leading to ecological dormancy. In order to investigate the role of FmDELLA in growth and development, the variation in FmDELLA transcriptional level, the endogenous hormone content in seed germination and bud dormancy release, and the effects of the month, organs, and exogenous hormones on FmDELLA were determined. The results showed that FmDELLA genes had a synergistic impact with the XERICO, PP2C, and DOG genes on regulating hypocotyl elongation during seed germination. Unlike growing buds, the dormant buds had much higher levels of FmDELLA transcripts. Still, these transcript levels were lowered by using 100 mg/L exogenous gibberellin acid (GA), which could promote bud dormancy release. Exogenous hormones regulated the transcription of FmDELLA, which primarily occurred in the stems, leaves, buds, and flowers and reached its lowest level in September. The transition from dormancy to germination for buds and seeds was related to increased GA, auxin, and cytokinin and decreased abscisic acid. In conclusion, our study revealed the role of FmDELLA in the seed germination and release of bud dormancy and provided a solid basis for F. mandshurica tissue culture and micropropagation.
1. Introduction
Dormancy is a defense mechanism plants develop in seasonal climate environments to defend themselves from adverse environments like long-term cold or drought stress [1]. Fraxinus mandshurica Rupr. (F. mandshurica) is a perennial deciduous tree that uses dormant buds as resting organs. In order to avoid water loss and low-temperature damage [2], the dormant buds are wrapped with multiple layers of scales. After entering autumn, F. mandshurica responds to seasonal changes in bud elongation cessation, resident bud formation, and dormancy establishment. According to the inducing factors, bud dormancy is generally divided into paradormancy, endodormancy, and ecodormancy [3]. The structure of the plant itself induces paradormancy, and neighboring tissues also have an impact. For example, apical dominance in the plant inhibits lateral bud growth. An adequate low-temperature accumulation can break the endodormancy of buds. Endodormancy is a form of dormancy regulated by internal factors. Low temperature and drought cause growth stagnation, an external environmental factor, resulting in ecodormancy [3,4].
Several studies have found that sufficient gibberellin acid (GA3) threshold levels are necessary for the release of bud dormancy and the occurrence of sprouting [5]. GAs can promote plant growth by inducing the degradation of DELLA proteins (DELLAs), which are characterized by a DELLA motif (Asp-Glu-Leu-Leu-Ala) [6]. DELLAs play a key role in the signal transduction of gibberellin [7]. Five members of the DELLA protein family are found in the model plant Arabidopsis thaliana genome, and each responds uniquely and redundantly to gibberellin signals [8]. The DELLA protein is involved in various biological processes due to its interaction with many factors. DELLA regulates the response to pathogens due to its direct protein interaction with JAZ1 [9]. When light is a limiting factor for plant growth, plants will alter the distribution of substances in their bodies by promoting the degradation of DELLA protein, such as increasing the allocation for defense while decreasing the allocation for growth [10]. In Oryza sativa L., the DELLA protein SLR1 can integrate with and enhance innate immunity dependent on salicylic acid and jasmonic acid [6]. When interacting physically with CONSTANS and FLC in Arabidopsis, DELLA proteins regulate flowering [11,12]. Additionally, Arabidopsis DELLA interacts with phytochrome interacting factors 3 and 4 (PIF3 and PIF4) and blocks their DNA-binding abilities to their target gene promoters, resulting in short hypocotyls in light-grown plants [13]. The transcriptional level of the PsRGL1 gene was significantly downregulated in tree peonies throughout the process of inducing bud dormancy release by GA3, and the low-temperature stratification and silencing of PsRGL1 could accelerate the release of bud dormancy [7]. The overexpression of PmRGL2 in poplar caused a delay in the onset of bud dormancy and dwarf plants relative to wild-type trees [3]. Gibberellin signaling, which is DELLA-mediated, regulates nod factor signaling and rhizobial infection in Medicago truncatula [14]. The PlDELLA gene in herbaceous peony negatively regulates dormancy release and plant growth [15]. The GA content in pear buds determines the timing of endodormancy release, and GA4 may be the critical gibberellin to promote peach leaf bud endodormancy release [3].
Environmental signals and plant growth and development signals can usually alter the expressions of GA 20-oxidase gene (GA20ox), GA 3-oxidase gene (GA3ox), and GA 2-oxidase gene (GA2ox), which regulate GA signaling by altering GA content [16]. The essential genes synthesizing gibberellin are induced during the transition from dormancy to release. Plants have identified key GA signaling components, such as the GA receptors gibberellin-insensitive dwarf1 (GID1), DELLA, GID2, or Sleepy1 (SLY1). It is well known that an active GA binds to GID1, which subsequently binds to DELLA to form a GA-GID1-DELLA complex [17]. Plants then exhibit GA responses due to DELLA being recruited to the SCF SLY1/GID2 E3 ubiquitin ligase for polyubiquitination and subsequent degradation by the 26S proteasome. PIF3 and PIF4 or Alcatraz are only a few of the transcription factors or transcriptional regulators that DELLAs directly inactivate or block when GA levels are low [8,18]. Additionally, DELLA regulates the expression of the putative E3 ligase gene, XERICO, a GA-repressed gene, promoting ABA accumulation and mediating the interaction between the GA and ABA pathways [19].
F. mandshurica Rupr., an Oleaceae deciduous tree of the Fraxinus genus, is a precious broad-leaved tree species in northern China, and abiotic stress will cause ecological dormancy of its buds. After being transplanted for 45–60 days, the tissue-cultured seedlings of F. mandshurica will successively form apical buds for dormancy. After transplantation, the question arises of how to break dormancy and maintain the growth of F. mandshurica seedlings. Furthermore, F. mandshurica mature seeds exhibit a deep dormancy trait. It was found that the DELLA gene only had two members in F. mandshurica: GA insensitive (GAI) and a repressor of gal-1 (RGA1) through the analysis of the genome and multiple transcriptomes of F. mandshurica in our research group. Therefore, the following questions were primarily investigated in this study: (1) What variations exist at the transcriptional level of genes related to dormancy during seed germination? (2) What variations exist at the transcriptional level of FmDELLA during the occurrence and release of bud dormancy? (3) What are the response patterns of FmDELLA to exogenous hormones? (4) What relationship exists between endogenous hormones and dormancy?
2. Materials and Methods
2.1. Plant Materials and Culture Conditions
2.1.1. Seed Germination
To observe the process of seed germination and determine the variation of genes transcriptional levels at different stages of germination, 1800–2000 F. mandshurica seeds were inoculated into WPM medium (Table 1) according to the method of Yu et al. [20]. WPM (product number: HBZ0609) is a plant culture medium purchased from the HOPEBIO company (Qingdao, China). A total of 400–500 sterile seedlings were obtained.

Table 1.
Medium used in the cultivation and processing of the sterile seedlings.
2.1.2. Culture Methods and Conditions
The bud-free stem segments between two adjacent buds of the sterile seedlings of F. mandshurica from 28 days of seed germination were cut and inoculated on callus induction medium and adventitious bud induction medium (Table 1). Subculturing was conducted every 30 days. Adventitious buds were transferred to a secondary proliferation medium when the adventitious buds elongated, and basal callus began to brown after 60 days. Some seedlings stopped growing as terminal buds appeared in tissue culture bottles after 80 days. To restore growth, sterile seedlings were transferred to secondary proliferation medium containing 1 mg/L GA when aseptic seedlings exhibited ecological dormancy after 90 days.
2.1.3. Transplanting Process
Sterile seedlings were transplanted to a nonwoven bag (diameter 9.5 cm, height 11 cm) containing peat soil: vermiculite 3:1 mixed matrix. The seedling box was placed in culture conditions at a temperature of 25 °C, 90% humidity, and a light intensity of 80 μmol *· m−2 · s−1, with light-dark alternate culturing in a greenhouse with a light time of 16 h [20]. After transplantation, their nutritional mode undergoes a transition from heterotrophic to autotrophic. During this transition, seedlings are prone to ecological dormancy. To relieve the dormancy of the top buds, 0 mg/L, 30 mg/L, 50 mg/L, 100 mg/L, and 300 mg/L GA were sprayed on the seedlings, which were transplanted for 45–60 days. Each group had 20 seedlings, with three replicates.
2.2. Extraction of Endogenous Hormones
Endogenous hormones, such as GA3, cytokinins, abscisic acid (ABA), and indole acetic acid (IAA), were extracted from the dormant seeds, germinating seeds, dormant buds, and germinating buds. For the determination, three biological replicates were set for each sampling point, and three technical replicates were set.
Germinating seeds had undergone variable temperature stratification (first 20–25 °C for 60 days, then 0–4 °C for 60 days), whereas the dormant seeds had not undergone variable temperature stratification. Germinating buds were the growth buds of the seedlings from which the seeds originated. Dormant buds were the apical buds formed after 45–60 days of sterile seedling transplantation. The extraction and analysis method of endogenous hormones was performed by following Yu et al. [20].
2.3. Sample Preparation for Transcriptional Profiling
2.3.1. Sample for Dormancy-Related Genes
The transcriptional levels of the genes related to dormancy during seed germination were determined. The method of disinfection and inoculation for the F. mandshurica seeds was performed by following Yu et al. [20]. Samples (three biological replicates) were obtained at 12 different time points during the embryonic development of F. mandshurica, including the 0th, 1st, 3rd, 4th, 5th, 7th, 8th, 10th, 14th, 18th, 21st, and 28th days.
2.3.2. Sample for FmDELLA Genes for Different Months, Tissues, and Developmental Stages
The samples (leaves; three biological replicates) were collected at 10 a.m. on the 15th of each month from the F. mandshurica seedlings cultivated in greenhouses in May, June, July, August, September, and October.
Mature seedlings with uniform growth in the forest farm of Northeast Forestry University (five trees in total; repeated three times) were harvested for their male flowers, female flowers, buds, and seeds. The roots, stems, and leaves were harvested from seedlings cultivated in a greenhouse for 3 years.
Sterile F. mandshurica seedlings were cut into segments with no buds and inoculated on an adventitious medium after 28 days of seed germination. They were subcultured every 30 days and cultured alternately in dark/light conditions. The terms “induction medium” and “subculture medium” were used to refer to the findings of our previous research [20]. The growth status of inoculated sterile buds was observed at different stages, and the samples (three biological replicates) of different growth stages were obtained promptly.
The samples mentioned above were frozen in liquid nitrogen and stored at −80 °C prior to RNA extraction.
2.3.3. Effects of Different Exogenous Hormone Signals on FmDELLA Genes
The 45-day-old, evenly grown, and well-shaped F. mandshurica seedlings were treated with 100 μM of ABA, GA3, IAA, Methyl jasmonate (MeJA), salicylic acid (SA), and brassinolide (BR), with untreated seedlings as controls. The samples (three biological replicates) were collected at 0 h, 1 h, 3 h, 6 h, 12 h, 24 h, and 48 h, frozen in liquid nitrogen, and stored at −80 °C to extract the RNA.
2.3.4. Effects of Exogenous GA on Top Bud Dormancy Release in Transplanted Seedlings
The apical buds, grown after 45–60 days of sterile seedling transplantation, were sprayed with 0 mg/L, 30 mg/L, 50 mg/L, 100 mg/L, and 300 mg/L of GA. Each group had 20 seedlings with three replicates. The growth conditions were 25 °C, 90% humidity, 80 μmol/m2s of light intensity, and 16 h illumination. After 15 d of treatment, the dormant bud state and germination rate were re-observed and measured. Simultaneously, the samples (three biological replicates) were collected, frozen in liquid nitrogen, and stored at −80 °C prior to RNA extraction.
2.4. RNA Extraction and cDNA Library Preparation
The Tris-CTAB method for RNA extraction was carried out as follows:
- (1)
- A total of 650 μL Tris CTAB and 50 μL β-Mercaptoethanol were added to a 1.5 mL sterile centrifuge tube, and the extraction buffer was preheated at 65 °C (aqueous solution).
- (2)
- The mortar, rod, and spoon were thoroughly cooled with liquid nitrogen three–four times, and 0.1–0.5 g of blades (−40 °C) were put into liquid nitrogen and ground to powder.
- (3)
- The prepared powder was added to the preheated Tris-CTAB extraction buffer, subjected to intense vortex oscillation for 30 s, and a water bath at 65 °C for 15 min.
- (4)
- A total of 700 μL of chloroform was added to a centrifuge tube, which was centrifuged for 10 min at 4 °C.
- (5)
- Extract the supernatant and repeat step 4 twice.
- (6)
- Extract the supernatant and add 560 μL lithium chloride (5 M), 350 μL anhydrous ethanol, and 60 μL sodium acetate and stand on ice for 60–90 min.
- (7)
- Centrifuge (12,000 rmp) at a low temperature (4 °C) for 10 min; extract the supernatant and discard it.
- (8)
- The sediment was rinsed twice with 70% ethanol; the centrifuge tube was opened in the fume hood to air dry the sediment, and 20 μL ddH2O was added to the centrifuge tube.
In this study, cDNA was synthesized with TransGen Biotech’s (Beijing, China) reverse transcription kit (AT341). According to the manufacturer’s instructions, the genomic DNA was first digested by gDNA Rmover, and then 0.5 µg total RNA was reverse transcribed into single-stranded cDNA. To verify the success of cDNA synthesis, a polymerase chain reaction (PCR) of microtubule protein genes was performed using single-stranded cDNA as the template.
2.5. Reverse Transcriptional Quantitative PCR(RT-qPCR)
The transcriptional levels of genes (GA2ox, GA 20-oxidase; GAI, GA insensitive; RGA1, repressor of gal-1; PP2C, protein phosphatase 2C; GID1, gibberellin-insensitive dwarf1; XERICO, a putative E3 ligase; and DOG1 and DOG2, delay of germination 1 and 2) were determined, and the method of RT-qPCR was taken from Yu et al. [20]. Three technical replicates were set for each sample. The primers were designed using Primer 5.0, and the list is provided in Table S1.
2.6. Statistical Analysis
The data were analyzed using the Statistical Package for Social Sciences 19.0 statistical software of IBM company (Almond, NY, USA). Duncan’s multiple comparison tests showed a significant difference between treatments (p = 0.05). The column charts used in this study were drawn using Origin 8.0 and GraphPad Prism 8.0.2. Heat maps of gene transcriptional levels were drawn using R: pheatmap (a coding program for creating heat maps in R Project for Statistical Computing).
3. Results
In F. mandshurica, the mature seeds exhibit a deep dormancy. Variable temperature stratification is frequently used in the seedling production process to break the deep dormancy of seeds and ensure normal germination and the neat emergence of the seeds. First, the growth and development processes of embryos at 12 time points, including 0 day, 1 day, 3 day, 4 day, 5 day, 7 day, 8 day, 10 day, 14 day, 18 day, 21 day, and 28 day, was analyzed to investigate the roles of GA signaling pathway genes (GA2ox, GID1, GA1, and RGA1), ABA signaling pathway genes (PP2C and XERICO), and seed dormancy genes (DOG1 and DOG2) in the process of the dormancy release and germination of F. mandshurica seeds. Then, the transcription levels of the genes mentioned above were measured during seed germination.
3.1. Germination Characteristics of the F. mandshurica Embryo
According to morphology, the progression from seed to seedling is divided into three stages (Figure 1). The cotyledons of the sterile embryos began to open somewhat after 3 days of inoculation (DI) and turned green after 4 DI in the first stage (Figure 1A). In the second stage, the cotyledons turned green, and the embryonic roots started to bend after 5 DI. The hypocotyl expanded and started to produce villous roots after 7 DI, the cotyledons stretched and opened after 8 DI, and the hypocotyl significantly extended after 10 DI, leading to overall plant enlargement (Figure 1B). In the third stage, the hypocotyl of the plant continued to elongate, the cotyledons grew from 14 DI to 18 DI, new leaves grew after 21 DI, and both pairs of leaves stretched and developed roots, stems, and leaves after 28 DI (Figure 1C). Additionally, the morphology of the embryo (with a significant difference after seeds were inoculated on 0 DI, 4 DI, 8 DI, and 28 DI) is shown in Figure 1D.

Figure 1.
Phenotypic variation in F. mandshurica seed germination at different stages. (A) The initial morphologies of seed embryo germination at 0 days, 1 day, 3 days, and 4 days; (B) the morphologies during the transition from seeds to seedlings at 5 days, 7 days, 8 days, and 10 days; (C) the morphologies of seedlings at 14 days, 18 days, 21 days, and 28 days; (D) the morphologies during the transition from seeds to seedlings at 0 days, 4 days, 8 days, and 28 days.
3.2. Variations in the Transcription Levels of Genes Related to GA and ABA Signaling Pathways and DOG Genes during Seed Development
The transcription levels of germination-inhibitory factors (DOG1, DOG2, GAI, RGA1, and XERICO) and germination-promoting factors (GA2ox, PP2C, and GID1) were measured at different stages of embryonic development using RT-qPCR. The findings revealed that germination was started by the inoculation of the F. mandshurica embryos into the sterile culture medium on day 0 of embryo inoculation, as evidenced by the transcriptional levels of the germination-promoting factor GA2ox gene significantly increasing on that day. The germination-inhibiting factor DOG1, DOG2, GAI, RGA1, and XERICO genes significantly decreased (Figure 2).
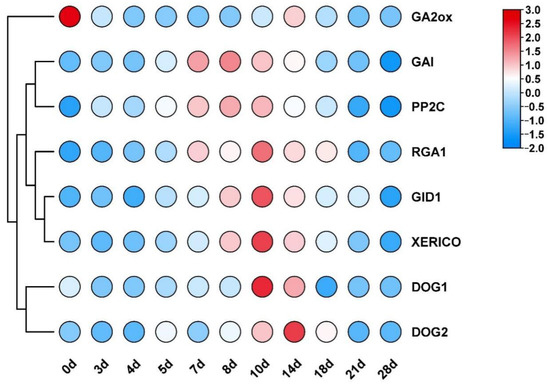
Figure 2.
Transcriptional analysis of genes related to the gibberellin acid and abscisic acid signaling pathways and DOG genes during seed germination. Note: GA2ox, GA 20oxidase gene; GAI, GA insensitive; RGA1, repressor of gal1; PP2C, protein phosphatase 2C; GID1, gibberellininsensitive dwarf1; XERICO, a putative E3 ligase gene; DOG1 and DOG2, delay of germination 1 and 2.
After seed germination, the transcriptional levels of GA2ox and FmDELLA genes (RGA1 and GAI) varied; the former was significantly inhibited, whereas the latter displayed a trend of first increasing and then decreasing. The transcriptional level of the RGA1 gene began to increase at 5 days, peaked at 10 days, was 59.43-fold higher than the control, and remained high until 18 days. Developing embryonic roots and hypocotyls was crucial during the sterile seed cultivation period between 5 days and 18 days. Hypocotyl elongation was thought to be regulated by the RGA1 gene, facilitating the transition of embryos into seedlings. The transcriptional level of the GAI gene was relatively high from 7 days to 14 days, peaking at 8 days when it was 12.86-fold higher than the control. The cotyledons and hypocotyls developed during this crucial period, and it was hypothesized that RGA1 and GAI had functional redundancy. However, there was no difference in the transcriptional level of the FmDELLA genes in the early stages of embryonic development (0–4 days) and the late stages of seedling formation (21–28 days) compared to the control group. In conclusion, the regulation of hypocotyl elongation was the primary function of the FmDELLA genes. The transcriptional level of genes such as XERICO, PP2C, and DOG1/DOG2 had a similar trend as the FmDELLA genes.
3.3. Spatiotemporal Transcriptional Patterns of FmDELLA Genes
The spatiotemporal transcriptional patterns of FmDELLA genes in seven parts and six growth time points in the F. mandshurica seedlings were determined to further investigate the role of FmDELLA genes in growth and development. The findings demonstrated that FmDELLA genes were transcribed in the leaves, buds, stems, and male flowers, with tissue-specific transcriptional levels (Figure 3A). The RGA1 gene was highly transcribed in the stems, leaves, and buds, with the maximum transcriptional level in the leaves being 5.88-fold higher than the internal reference gene. The GAI gene was highly transcribed in the stems, leaves, buds, male flowers, and female flowers, with a maximum transcriptional level 8.87-fold higher than the internal reference gene in buds. The GAI gene had the highest transcriptional level in male flowers, 12.45-fold higher than the internal reference gene. In summary, the FmDELLA genes were mainly transcribed in the stems, leaves, buds, and flowers.
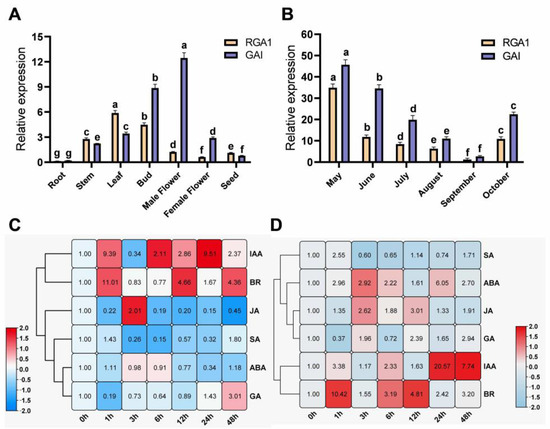
Figure 3.
The transcriptional levels of FmDELLA genes. (A,B) The spatiotemporal transcriptional patterns of FmDELLA genes; (C,D) the effect of hormone signaling on the transcriptional level of FmDELLA genes. C: RGA1; D: GAI. Different letters above bars within statistically significant differences between different tissues (A) and months (B) at the p < 0.05 level according to Duncan’s multiple range test for genes of RGA1 and GAI.
Samples of the leaves of the F. mandshurica seedlings cultured in a greenhouse from May to October were collected to determine the transcriptional variation of FmDELLA genes during various months of the growth season (Figure 3B). The results showed that the transcriptional level of FmDELLA genes started to drop in May, reached its lowest level in September, and then increased in October. The RGA1 gene transcription levels were 34.91, 1.21, and 10.85 in May, September, and October, respectively. The levels in May were 28.85-fold higher than those in September. The transcriptional levels of the GAI gene were 45.72, 2.73, and 22.46 in May, September, and October, respectively. The levels in May were 16.75-fold higher than those in September. In October, they were 8.23-fold higher than those in September. It was found that the FmDELLA genes were transcribed in specific parts of F. mandshurica and participated in growth regulation based on the analysis of their spatiotemporal transcriptional patterns.
3.4. Effect of Exogenous Hormone Signals on the Transcriptional Levels of FmDELLA Genes
The transcriptional levels of the FmDELLA genes were measured at 0, 1, 3, 6, 12, 24, and 48 h after treatment to determine whether the FmDELLA genes also responded to hormone signals due to the direct or indirect regulatory role of the DELLA gene in multiple signaling pathways across various species. The leaves of 45-day-old F. mandshurica seedlings were sprayed with six plant hormones: ABA, GA3, IAA, MeJA, SA, and BR. The findings demonstrated that the FmDELLA genes responded to six hormone signals (Figure 3C,D), but the response patterns to each hormone signal differed. The ABA treatment downregulated the expression of the RGA1 gene (Figure 3C), which had the lowest transcriptional level at 24 h and was 0.34-fold higher than the control. The transcriptional level of the RGA1 gene fluctuated under the influence of IAA, showing an increase, decrease, and increase pattern, with higher transcriptional levels at 1 h and 24 h, reaching 9.39-fold higher than the control. BR significantly increased the transcriptional levels of the RGA1 gene, which was 11.01-fold higher than the control. The RGA1 gene was downregulated overall and did not respond significantly to MeJA (Figure 3C). The transcriptional level was at its lowest when it was 0.15-fold higher than the control. The RGA1 gene was upregulated after SA treatment at 1 h. This was followed by a significant downward trend, with the lowest transcriptional level occurring at 6 h, which was 0.15-fold higher than the control. The gene transcriptional level of the RGA1 gene was significantly inhibited by GA3, and it displayed a trend of first decreasing and then increasing. The transcriptional level of the RGA1 gene was 0.19-fold higher than that of the control at 1 h. It was upregulated after 24 h, and the transcriptional level was 3.0-fold higher than that of the control at 48 h. Figure 3D shows that ABA, IAA, and BR all increased the transcription of the GAI gene. At 24 h, ABA and IAA induced the highest transcriptional levels, 6.05- and 20.57-fold higher than the control, respectively. BR significantly increased the transcriptional level of the GAI gene, 10.42-fold higher than the control within 1 h. MeJA significantly upregulated the GAI gene, with the highest transcriptional level occurring at 12 h, which was 3.01-fold higher than the control (Figure 3D). The transcriptional level of the GAI gene under SA treatment fluctuated, initially increasing, then decreasing, and then increasing. The highest transcriptional level was at 1 h, 2.55-fold higher than the control, and the lowest was at 3 h, 0.6-fold higher than the control. The GAI gene under GA3 treatment showed a trend of first decreasing and then increasing, with the lowest transcriptional level at 1 h, which was 0.37-fold higher than the control. However, the highest transcriptional level was at 48 h, 2.94-fold higher than the control. Nevertheless, it was upregulated after 3 h.
3.5. Variation Analysis of FmDELLA Genes’ Transcriptional Levels at Different Developmental Stages of Tissue Culture Seedlings
F. mandshurica constantly undergoes dormancy and aging during tissue culture propagation, leading to the cessation of growth or death. The transcriptional levels of the FmDELLA genes at various stages of tissue culture seedlings were analyzed by RT-qPCR to further analyze the relationship between FmDELLA genes and dormancy regulation. The samples were obtained at significant time points during the tissue culture process.
First, the growth stages of the tissue-cultivated seedlings of F. mandshurica were separated and described. Stem segments from sterile seedlings were cut and injected into callus induction medium and adventitious bud induction medium. Subcultures were carried out every 30 days, with the stem segments used at this time as a control (0 days). After 20 days, a delicate, green, semi-dense callus tissue (Figure 4A) was produced. After 30 days, when the adventitious buds started to grow in the semi-dense gap of the callus tissue block (Figure 4B), the adventitious buds were transferred to a new adventitious bud culture medium. After 40 days, the adventitious buds formed clusters (Figure 4C). When the adventitious buds expanded and the basal callus started to brown (Figure 4D) after 60 days, the adventitious buds were moved to a robust seedling culture medium. After 80 days, some sterile seedlings developed as terminal buds in tissue culture bottles and stopped growing (Figure 4E). The stem of these seedlings started to age, and some gray tissues started sticking to it. After 90 days, the aseptic seedlings began demonstrating ecological dormancy, including deeper stem aging and certain branches and leaves aging and breaking off (Figure 4F). The sterile seedlings were moved to a robust seedling culture medium containing 1 mg/L GA. After 100 days, the aged tissues adhering to the stems started to fall off, and the dormant top buds of the sterile seedlings started to sprout (Figure 4G). After 110 days, the top buds of the sterile seedling continued to grow, the new leaves turned delicate and green, and the old stem tissue shrank further (Figure 4H). After 120 days, the seedlings were strong, the color of the leaves darkened, and the gray aging tissues linked to the stems essentially vanished (Figure 4I).
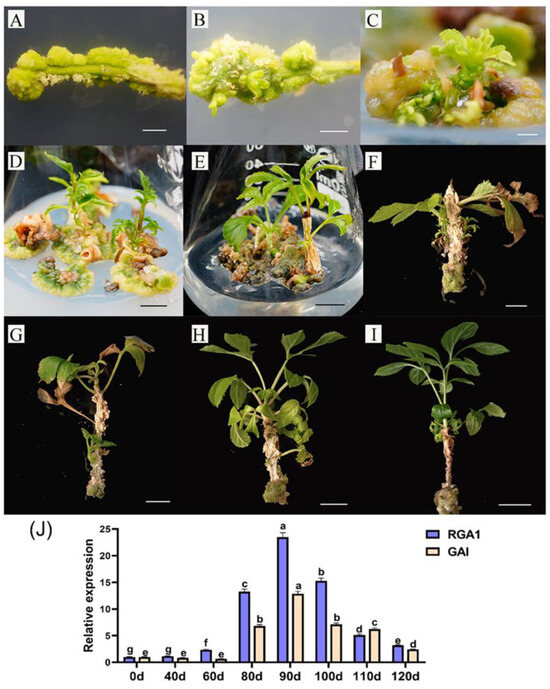
Figure 4.
Variation analysis of FmDELLA gene morphology and transcriptional levels at different developmental stages of tissue culture seedlings. Note: (A): morphology at 20 days of induction culture (scale: 3 mm); (B): morphology at 30 days of induction culture (scale: 3 mm); (C): morphology at 40 days of induction culture (scale: 5 mm); (D): morphology at 60 days of induction culture (scale: 1 cm); (E): morphology of the first subculture for 20 days (scale: 1 cm); (F): morphology of the first subculture for 30 days (scale: 1 cm); (G): morphology of the second subculture for 10 days (scale: 1 cm); (H): morphology of the second subculture for 20 days (scale: 2 cm), (I): morphology of the second subculture for 30 days (scale: 2 cm), and (J) the transcriptional levels of FmDELLA gene at different developmental stages of tissue culture seedlings. Different letters above bars within statistically significant differences between different developmental stages of tissue culture seedlings at the p < 0.05 level according to Duncan’s multiple range test for genes of RGA1 and GAI.
The transcriptional levels of FmDELLA genes at various developmental stages of tissue culture seedlings were analyzed by RT-qPCR based on the phenomenon of dormancy during the propagation of F. mandshurica tissue culture. The results showed that the transcriptional levels of the FmDELLA genes were significantly higher during the dormancy stage than during the growth stage for the tissue culture seedlings. RGA1 and GAI were 23.51- and 12.86-fold higher than the control at 90 days when the transcriptional level of the FmDELLA genes peaked. Simultaneously, the sterile seedlings exhibited passive dormancy and an obvious aging phenomenon (Figure 4F).
3.6. Variation in the Transcriptional Level of the FmDELLA Gene in the Dormant Top Buds of Transplanted F. mandshurica Seedlings and Top Bud Dormancy Release
The tissue culture seedlings grew at a constant temperature for a long time. Their nutritional mode transitions from heterotrophic to autotrophic after transplantation. Ecological dormancy is a risk for F. mandshurica seedlings during this transition. As shown in Figure 5A–D, the tissue-cultured seedlings displayed apical buds and dormancy after 45 days of transplantation. Different gibberellin concentrations (0–300 mg/L) were sprayed on those seedlings that had been transplanted for 45–60 days to release top bud dormancy. The results showed (Table 2) that the top buds were released from dormancy (Figure 5E–H), and after 15 days of spraying 100 mg/L of exogenous GA, 90% of the new buds had germinated (Table 2). The new leaves were tender and green, and the plants were robust (Figure 5I–K). However, the germination rate of untreated top buds was only 11.67% (Table 2). This technology improved the growth rate and germination rate of top buds by 78.33%.
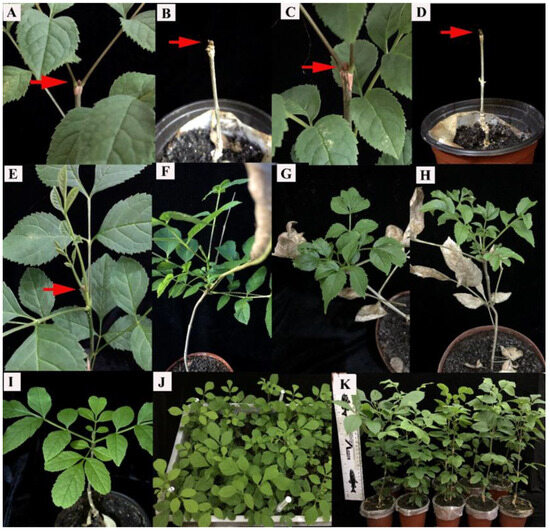
Figure 5.
Dormancy release of F. mandshurica seedlings treated with gibberellin acid (GA). Note: (A–D): the phenotype of the passive dormant bud of the F. mandshurica transplants (red arrow points to the terminal bud); (E–H): the initiation of dormant buds after 15 days of GA treatment; (I–K): the growth state of F. mandshurica transplants after GA3 treatment.

Table 2.
Effect of gibberellin acid on disrupting the dormancy of F. mandshurica seedlings (15 days).
RT-qPCR was used to assess the transcriptional levels of FmDELLA genes in the dormant topbuds treated with and without GA (Figure 6A,B). According to the results, the transcriptional levels of the RGA1 and GAI genes were 19.29- and 10.08-fold higher in the dormant bud than in the control, respectively (Figure 6C,D). The transcriptional levels of the FmDELLA genes were significantly decreased by GA, with the transcriptional levels of the RGA1 and GAI genes being 2.34- and 3.19-fold higher than that of the control, and these decreased by 87.87% and 68.35%, respectively.
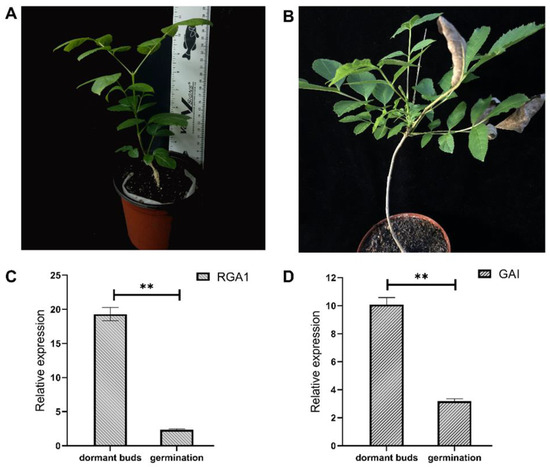
Figure 6.
Transcriptional levels of FmDELLA genes during the dormancy/release period of F. mandshurica transplantation. Note: (A): dormant buds first appeared 45 days after transplanting; (B): morphology of seedlings after treatment with gibberellin acid for 15 days; (C,D): transcriptional levels of FmDELLA genes in dormant buds/germinating buds. The ** on the bars indicate significant differences by t-test (p < 0.01).
3.7. Relationship between Endogenous Hormones and Dormancy
Gibberellin, ABA, auxin, and cytokinin content were determined in the four critical stages of F. mandshurica (dormant seeds, germinating seeds, dormant buds, and germinating buds) to investigate the relationship between endogenous hormones and the dormancy of the seeds and buds in F. mandshurica. When the seeds transitioned from dormant to germination, the contents of gibberellin, auxin, and cytokinin significantly increased, but the content of ABA significantly decreased (Figure 7). Additionally, there was a significant increase in the ratio of cytokinin and gibberellin to ABA. Similar to seeds, the transformation of buds from dormancy to germination was related to the increase in gibberellin, auxin, and cytokinin content and the decrease in ABA in F. mandshurica (Figure 7).
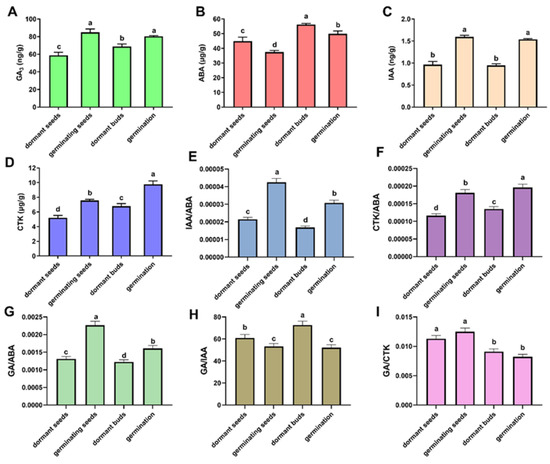
Figure 7.
Determination of the hormone content in the seeds and buds of F. mandshurica from dormancy to germination. (A): The gibberellin (GA3) content; (B) the abscisic acid (ABA) content; (C) the auxin (IAA) content; (D) the cytokinin (CTK) content; (E–I) the ratio between different hormones. Different letters above bars within statistically significant differences among 4 different materials at the p < 0.05 level according to Duncan’s multiple range test.
4. Discussion
4.1. Variations in the Transcriptional Level of Genes Related to GA and ABA Signaling Pathways and DOG Genes during Seed Development
DOG1 (delay of germination 1) and ABA are positive regulatory factors of seed dormancy [21,22]. The seed or embryo will be dormant and unable to germinate when the transcriptional level of the DOG1 gene and ABA content increase simultaneously [23,24]. An increase in PP2C (protein phosphatase 2C) content will promote seed germination since PP2C is a negative regulator of ABA signaling [25]. The gibberellin oxidase gene GA2ox is a crucial enzyme in GA biosynthesis, and GA can promote seed germination [13]. The increased transcriptional level of GA2ox increases seed or embryo germination. The DELLA gene negatively regulates the GA signaling pathway [26], and an increase in DELLA protein content can inhibit seed germination. DELLA upregulates the transcriptional level of XERICO, an E3 ubiquitin ligase. XERICO is a negative regulatory factor for seed germination because it counteracts the effects of GA by promoting ABA accumulation [19]. The transcriptional level of the germination-promoting factor GA2ox gene significantly increased in this study on day 0 of embryo inoculation. In contrast, the transcriptional levels of the germination-inhibiting factor DOG1, DOG2, GAI, RGA1, and XERICO genes significantly decreased (Figure 2). This finding suggests that the germination of F. mandshurica embryos began during medium insertion. The primary role of FmDELLA genes during the development process after seed germination was in regulating hypocotyl elongation. The transcriptional level of genes, such as XERICO, PP2C, and DOG1/DOG2, showed the same trend as FmDELLA genes, indicating that FmDELLA had synergistic effects with XERICO, PP2C, and DOG1/DOG2 genes throughout the embryonic development of F. mandshurica.
4.2. The Function of FmDELLA in the Passive Dormancy of F. mandshurica
According to the literature, the DELLA protein, an essential node and negative regulatory factor, induces dormancy in the gibberellin regulatory pathway [27,28,29]. F. mandshurica frequently experiences passive dormancy during tissue culture and micropropagation due to environmental changes or discomfort, which is particularly prominent during transplantation. This study explains the role of FmDELLA genes in regulating passive dormancy in the tissue culture of F. mandshurica. It focuses on the phenomenon of passive dormancy. The transcriptional level of FmDELLA genes was significantly higher during the dormancy stage than during the growth stage for the tissue culture seedlings based on the phenomenon of dormancy during the propagation of F. mandshurica tissue culture. Simultaneously, sterile seedlings exhibited passive dormancy and an evident aging phenomenon (Figure 4F). This suggested that the senescence and dormancy of F. mandshurica might be regulated by FmDELLA genes. However, GA treatment could promote bud dormancy release in F. mandshurica by reducing the transcriptional level of FmDELLA genes.
4.3. The Spatiotemporal Transcriptional Expression Patterns of DELLA
In dormant buds, the transcription level of the FmDELLA genes was high. The DELLA gene was highly expressed in the study of dormancy in pear flower buds during three critical times: the critical dormancy release period, the release period, and the germination period. Inhibiting the expression of the AG gene during flower bud germination is possible when the AtRGA gene is overexpressed [30]. AtRGA, AtRGL2, and AtRGL1 exert inhibitory effects on the development of petals, stamens, and anthers [31]. However, effective pollen development in Arabidopsis depends on DELLA activity [32]. RGA1 and GAI are the only two DELLA family members found in F. mandshurica. The peak expression of these two genes was significantly different from that of other tissues occurring in the bud. Therefore, it was hypothesized that the signal transduction pathway regulating flower bud dormancy would involve the FmDELLA genes. The DELLA protein had different roles and redundancy in other species, according to the high expression of GAI in male and female flowers. The expression of the DELLA gene varies between kinds, which require various cooling temperatures to release dormancy, according to a study of dormancy regulation in plum and pear flowers. In GA-releasing flower bud dormancy, DELLA protein and low-temperatures play crucial roles [33]. Further research is required to determine how the DELLA gene participates in the mechanism of dormancy regulation and release in F. mandshurica. According to the soil environments, DELLA proteins, which function as integrators of GA action, are emerging as important regulators of root system architecture and harmonize root development [34,35]. However, the vigorous growth of root tip cells and an increase in GA expression levels, which significantly inhibited the expression of FmDELLA genes, may have contributed to the very low transcriptional levels of FmDELLA genes in roots.
F. mandshurica grows across the forest from late May to July. Beginning in early August, the fast growth of F. mandshurica slows down as dormant buds progressively form to resist the environment better and avoid being frostbitten by low temperatures. The growth cycle of F. mandshurica can be prolonged under the constant greenhouse environmental factors of temperature, humidity, and stable light intensity, combined with specific fertilization treatments. Therefore, it is possible to analyze the gene expression patterns in different months by collecting leaves from May to October. The transcriptional levels of the GAI gene were 16.75-fold higher in May than in September and 8.23-fold higher in October than in September, indicating that the transcriptional levels of FmDELLA genes were higher in dormancy than in growth. It was discovered that the FmDELLA genes were expressed in specific parts of F. mandshurica and participated in growth regulation based on the analysis of the spatiotemporal transcriptional expression pattern of the FmDELLA genes. The transcriptional levels of FmDELLA genes were significantly higher during the dormancy stage than during the growth stage for the tissue culture seedlings, indicating that the FmDELLA genes could be involved in dormancy. Moreover, the FmDELLA genes responded to ABA, GA3, IAA, MeJA, SA, and BR, but the response patterns to each hormone signal differed.
4.4. Spraying Gibberellin Can Break the Dormancy of Apical Buds
The DELLA protein inhibits plant growth and works with other hormones to respond to stress, increasing plant adaptability [36,37]. Ecological dormancy occurs while tissue-cultured seedlings are cultured, and the DELLA gene regulates the dormancy. Therefore, DELLA can be inhibited, dormancy can be broken, and plant growth can be maintained by adding gibberellin to the culture medium and spraying gibberellin externally during transplantation. The seeds of F. mandshurica exhibit the characteristic of deep dormancy, with a dormancy period of up to several months. The prolonged vernalization treatment slows down the reproduction rate of F. mandshurica. Therefore, members of our research group and others were able to obtain a significant number of sterile tissue culture seedlings of F. mandshurica using methods including rejuvenation, callus induction from explants, and the establishment of adventitious bud regeneration systems [38]. It is necessary to root and transplant the early-stage tissue culture seedlings of F. mandshurica to achieve the large-scale propagation of seedlings. However, the F. mandshurica seedlings stopped growing and entered a dormant state after 45 days of transplantation because they began to develop terminal buds. After transplantation, the growth of tissue-cultured F. mandshurica seedlings can be significantly impacted by spraying exogenous GA3, which can disrupt the dormancy state of seedlings and restore their growth by lowering the transcriptional levels of the FmDELLA genes. Studies have shown that multiple plant hormones and miRNAs are involved in plant dormancy [23,39,40]. Additionally, the increase in gibberellin, auxin, and cytokinin content and the decrease in ABA in F. mandshurica were related to the transition of seeds and buds from dormancy to germination.
5. Conclusions
Plants respond to stressful environments by inhibiting the growth of specific organs or entire plants and passively entering into a dormant state to resist adversity [41]. Dormancy frequently occurs during tissue culture and the micropropagation of F. mandshurica due to environmental changes or discomfort. This is particularly prominent during the transplantation process. In addition to enabling the seedling form, GA is an essential hormone for regulating seed germination and dormancy [42]. The DELLA protein can inhibit plant growth and development because it is a negative regulatory factor in the GA signal transduction pathway. It is unclear whether FmDELLA is involved in the regulation of dormancy during this process. The relationship between stress-induced dormancy and the DELLA genes served as the foundation for this article. A solution to the dormancy phenomena after transplanting F. mandshurica tissue culture seedlings was proposed by examining the changes in endogenous hormones at critical dormancy/release periods and the expression patterns of FmDELLA genes in F. mandshurica. A technique for breaking dormancy is developed using the FmDELLA regulatory mechanism, and the role of DELLA protein in the dormancy and growth of F. mandshurica is explained. In conclusion, this study used DELLA as the core to study the regulation of forest dormancy, laying the foundation for a more comprehensive understanding of the genetic mechanism regulating dormancy release and providing new perspectives on plant breeding efforts in the context of global climate change.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14112128/s1, Table S1: The primer nucleotide sequence of genes for RT-qPCR.
Author Contributions
Methodology, L.Y., C.L. and N.L.; Formal Analysis, X.Z., L.Y., C.L. and N.L.; Data Curation, X.Z., L.Y. and N.L.; Writing—original draft, X.Z. and L.Y.; Writing—review & editing, X.Z., L.Y., F.Z. and Y. Z; Visualization, L.Y.; Supervision, F.Z. and Y.Z; Funding acquisition, Y.Z., X.Z. and L.Y. contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China, grant number: 2021YFD2200303, and the APC was funded by the National Key R&D Program of China, grant number: 2021YFD2200303.
Data Availability Statement
This article contains all the data generated or analyzed during this study. The seeds used in the experiment were obtained from our laboratory, and the complete plants were cultured in the greenhouse of the Northeast Forestry University. The relevant regulations were followed.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bassel, G.W. To Grow or not to Grow? Trends Plant Sci. 2016, 21, 498–505. [Google Scholar] [CrossRef] [PubMed]
- El Yaacoubi, A.; Malagi, G.; Oukabli, A.; Citadin, I.; Hafidi, M.; Bonhomme, M.; Legave, J.M. Differentiated dynamics of bud dormancy and growth in temperate fruit trees relating to bud phenology adaptation, the case of apple and almond trees. Int. J. Biometeorol. 2016, 60, 1695–1710. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Q.; Wen, B.; Zhang, R.; Jing, X.; Xiao, W.; Chen, X.; Tan, Q.; Li, L. Endodormancy Release Can Be Modulated by the GA(4)-GID1c-DELLA2 Module in Peach Leaf Buds. Front. Plant Sci. 2021, 12, 713514. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.T.; Horvath, D.P.; Dharmawardhana, P.; Priest, H.D.; Mockler, T.C.; Strauss, S.H. Extensive Transcriptome Changes During Natural Onset and Release of Vegetative Bud Dormancy in Populus. Front. Plant Sci. 2015, 6, 989. [Google Scholar] [CrossRef] [PubMed]
- Horvath, D. Common mechanisms regulate flowering and dormancy. Plant Sci. 2009, 177, 523–531. [Google Scholar] [CrossRef]
- De Vleesschauwer, D.; Seifi, H.S.; Filipe, O.; Haeck, A.; Huu, S.N.; Demeestere, K.; Hofte, M. The DELLA Protein SLR1 Integrates and Amplifies Salicylic Acid- and Jasmonic Acid-Dependent Innate Immunity in Rice. Plant Physiol. 2016, 170, 1831–1847. [Google Scholar] [CrossRef]
- Gao, L.; Niu, D.; Chi, T.; Yuan, Y.; Liu, C.; Gai, S.; Zhang, Y. PsRGL1 negatively regulates chilling and gibberellin induced dormancy release by PsF box1 mediated targeting for proteolytic degradation in tree peony. Hortic. Res. 2023, 10, uhad044. [Google Scholar] [CrossRef]
- Daviere, J.M.; Wild, M.; Regnault, T.; Baumberger, N.; Eisler, H.; Genschik, P.; Achard, P. Class I TCP-DELLA interactions in inflorescence shoot apex determine plant height. Curr. Biol. 2014, 24, 1923–1928. [Google Scholar] [CrossRef]
- Hauvermale, A.L.; Ariizumi, T.; Steber, C.M. Gibberellin signaling: A theme and variations on DELLA repression. Plant Physiol. 2012, 160, 83–92. [Google Scholar] [CrossRef]
- Leone, M.; Keller, M.M.; Cerrudo, I.; Ballare, C.L. To grow or defend? Low red: Far-red ratios reduce jasmonate sensitivity in Arabidopsis seedlings by promoting DELLA degradation and increasing JAZ10 stability. N. Phytol. 2014, 204, 355–367. [Google Scholar] [CrossRef]
- Xu, F.; Li, T.; Xu, P.B.; Li, L.; Du, S.S.; Lian, H.L.; Yang, H.Q. DELLA proteins physically interact with CONSTANS to regulate flowering under long days in Arabidopsis. FEBS Lett. 2016, 590, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; An, F.; Li, W.; Ma, M.; Feng, Y.; Zhang, X.; Guo, H. DELLA proteins interact with FLC to repress flowering transition. J. Integr. Plant Biol. 2016, 58, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Hirano, K.; Sato, T.; Mitsuda, N.; Nomoto, M.; Maeo, K.; Koketsu, E.; Mitani, R.; Kawamura, M.; Ishiguro, S.; et al. DELLA protein functions as a transcriptional activator through the DNA binding of the indeterminate domain family proteins. Proc. Natl. Acad. Sci. USA 2014, 111, 7861–7866. [Google Scholar] [CrossRef] [PubMed]
- Fonouni-Farde, C.; Tan, S.; Baudin, M.; Brault, M.; Wen, J.; Mysore, K.S.; Niebel, A.; Frugier, F.; Diet, A. DELLA-mediated gibberellin signalling regulates Nod factor signalling and rhizobial infection. Nat. Commun. 2016, 7, 12636. [Google Scholar] [CrossRef] [PubMed]
- Bian, T.T.; Ma, Y.; Guo, J.; Wu, Y.; Shi, D.M.; Guo, X.F. Herbaceous peony (Paeonia lactiflora Pall.) PlDELLA gene negatively regulates dormancy release and plant growth. Plant Sci. 2020, 297, 110539. [Google Scholar] [CrossRef] [PubMed]
- Veerabagu, M.; van der Schoot, C.; Tureckova, V.; Tarkowska, D.; Strnad, M.; Rinne, P.L.H. Light on perenniality: Para-dormancy is based on ABA-GA antagonism and endo-dormancy on the shutdown of GA biosynthesis. Plant Cell Environ. 2023, 46, 1785–1804. [Google Scholar] [CrossRef]
- Sun, T.P. Gibberellin-GID1-DELLA: A pivotal regulatory module for plant growth and development. Plant Physiol. 2010, 154, 567–570. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, Z.; Zhu, Z. DELLA-PIF Modules: Old Dogs Learn New Tricks. Trends Plant Sci. 2016, 21, 813–815. [Google Scholar] [CrossRef]
- Zentella, R.; Zhang, Z.L.; Park, M.; Thomas, S.G.; Endo, A.; Murase, K.; Fleet, C.M.; Jikumaru, Y.; Nambara, E.; Kamiya, Y.; et al. Global analysis of della direct targets in early gibberellin signaling in Arabidopsis. Plant Cell 2007, 19, 3037–3057. [Google Scholar] [CrossRef]
- Yu, L.; Li, X.; Tian, H.; Liu, H.; Xiao, Y.; Liang, N.; Zhao, X.; Zhan, Y. Effects of Hormones and Epigenetic Regulation on the Callus and Adventitious Bud Induction of Fraxinus mandshurica Rupr. Forests 2020, 11, 590. [Google Scholar] [CrossRef]
- Graeber, K.; Linkies, A.; Steinbrecher, T.; Mummenhoff, K.; Tarkowská, D.; Turečková, V.; Ignatz, M.; Sperber, K.; Voegele, A.; de Jong, H.; et al. DELAY OF GERMINATION 1 mediates a conserved coat-dormancy mechanism for the temperature- and gibberellin-dependent control of seed germination. Proc. Natl. Acad. Sci. USA 2014, 111, E3571–E3580. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, J.; Palusinska, M.; Wroblewska-Swiniarska, A.; Pietras, Z.; Szewc, L.; Dolata, J.; Jarmolowski, A.; Swiezewski, S. Alternative Polyadenylation of the Sense Transcript Controls Antisense Transcription of DELAY OF GERMINATION 1 in Arabidopsis. Mol. Plant 2017, 10, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Obroucheva, N.V. Distinct regulatory patterns of seed dormancy release and germination commencement. Seed Sci. Technol. 2010, 38, 265–279. [Google Scholar] [CrossRef]
- Obroucheva, N.V. Transition from hormonal to nonhormonal regulation as exemplified by seed dormancy release and germination triggering. Russ. J. Plant Physiol. 2012, 59, 546–555. [Google Scholar] [CrossRef]
- Nishimura, N.; Tsuchiya, W.; Moresco, J.J.; Hayashi, Y.; Satoh, K.; Kaiwa, N.; Irisa, T.; Kinoshita, T.; Schroeder, J.I.; Yates, J.R., 3rd; et al. Control of seed dormancy and germination by DOG1-AHG1 PP2C phosphatase complex via binding to heme. Nat. Commun. 2018, 9, 2132. [Google Scholar] [CrossRef]
- Hou, X.; Lee, L.Y.; Xia, K.; Yan, Y.; Yu, H. DELLAs modulate jasmonate signaling via competitive binding to JAZs. Dev. Cell 2010, 19, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Josse, E.M.; Gan, Y.B.; Bou-Torrent, J.; Stewart, K.L.; Gilday, A.D.; Jeffree, C.E.; Vaistij, F.E.; Martinez-Garcia, J.F.; Nagy, F.; Graham, I.A.; et al. A DELLA in Disguise: SPATULA Restrains the Growth of the Developing Arabidopsis Seedling. Plant Cell 2011, 23, 1337–1351. [Google Scholar] [CrossRef] [PubMed]
- Ariizumi, T.; Hauvermale, A.L.; Nelson, S.K.; Hanada, A.; Yamaguchi, S.; Steber, C.M. Lifting della repression of Arabidopsis seed germination by nonproteolytic gibberellin signaling. Plant Physiol. 2013, 162, 2125–2139. [Google Scholar] [CrossRef]
- Penfield, S.; Gilday, A.D.; Halliday, K.J.; Graham, I.A. DELLA-mediated cotyledon expansion breaks coat-imposed seed dormancy. Curr. Biol. 2006, 16, 2366–2370. [Google Scholar] [CrossRef]
- Yu, H.; Ito, T.; Zhao, Y.; Peng, J.; Kumar, P.; Meyerowitz, E.M. Floral homeotic genes are targets of gibberellin signaling in flower development. Pans 2004, 101, 7827–7832. [Google Scholar] [CrossRef]
- Cheng, H.; Qin, L.; Lee, S.; Fu, X.; Richards, D.E.; Cao, D.; Luo, D.; Harberd, N.P.; Peng, J. Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development 2004, 131, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Plackett, A.R.G.; Ferguson, A.C.; Powers, S.J.; Wanchoo-Kohli, A.; Phillips, A.L.; Wilson, Z.A.; Hedden, P.; Thomas, S.G. DELLA activity is required for successful pollen development in the Columbia ecotype of Arabidopsis. N. Phytol. 2014, 201, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, R.; Zhu, K.M.; Jiang, T.; Ding, P.; Gao, Y.; Tan, X.L. DELLAs directed gibberellins responses orchestrate crop development: A brief review. Crop Sci. 2022, 63, 1–28. [Google Scholar] [CrossRef]
- Fonouni-Farde, C.; Diet, A.; Frugier, F. Root Development and Endosymbioses: DELLAs Lead the Orchestra. Trends Plant Sci. 2016, 21, 898–900. [Google Scholar] [CrossRef] [PubMed]
- Ubeda-Tomas, S.; Swarup, R.; Coates, J.; Swarup, K.; Laplaze, L.; Beemster, G.T.; Hedden, P.; Bhalerao, R.; Bennett, M.J. Root growth in Arabidopsis requires gibberellin/DELLA signalling in the endodermis. Nat. Cell Biol. 2008, 10, 625–628. [Google Scholar] [CrossRef]
- Daviere, J.M.; Achard, P. A Pivotal Role of DELLAs in Regulating Multiple Hormone Signals. Mol. Plant 2016, 9, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R.R.; Lynch, T.J. Overexpression of ABI5 Binding Proteins Suppresses Inhibition of Germination Due to Overaccumulation of DELLA Proteins. Int. J. Mol. Sci. 2022, 23, 5537. [Google Scholar] [CrossRef]
- He, L.; Zhang, J.; Guo, D.; Tian, H.; Cao, Y.; Ji, X.; Zhan, Y. Establishment of the technology of cambial meristematic cells (CMCs) culture from shoots and high expression of FmPHV (PHAVOLUTA) functions in identification and differentiation of CMCs and promoting the shoot regeneration by hypocotyl in Fraxinus mandshurica. Plant Physiol. Biochem. 2021, 160, 352–364. [Google Scholar] [CrossRef]
- Cui, J.W.; Zhao, J.G.; Zhao, J.Y.; Xu, H.M.; Wang, L.; Jin, B. Cytological and miRNA expression changes during the vascular cambial transition from the dormant stage to the active stage in Ginkgo biloba L. Trees-Struct. Funct. 2016, 30, 2177–2188. [Google Scholar] [CrossRef]
- Gao, J.; Ni, X.P.; Li, H.T.; Hayat, F.; Shi, T.; Gao, Z.H. miR169 and PmRGL2 synergistically regulate the NF-Y complex to activate dormancy release in Japanese apricot (Prunus mume Sieb. et Zucc.). Plant Mol. Biol. 2021, 105, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Artlip, T.; McDermaid, A.; Ma, Q.; Wisniewski, M. Differential gene expression in non-transgenic and transgenic “M.26” apple overexpressing a peach CBF gene during the transition from eco-dormancy to bud break. Hortic. Res. 2019, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Chu, C. Gibberellin Metabolism and Signaling: Targets for Improving Agronomic Performance of Crops. Plant Cell Physiol. 2020, 61, 1902–1911. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).