Abstract
Environmental stresses can disrupt protein structure, resulting in unfolded or misfolded proteins, thereby triggering endoplasmic reticulum (ER) stress. The unfolded protein response (UPR), particularly as activated by Arabidopsis AtbZIP60 gene, is pivotal for counteracting ER stress and ensuring cell survival. The medicinal plant, Cyclocarya paliurus, known for its wealth of beneficial compounds, is threatened by environmental stresses, limiting the exploration of its therapeutic potential. In order to better exploit and utilize its value, it is necessary to understand the signal pathway of environmental stresses. Here, we identify a homolog of AtbZIP60 in C. paliurus, termed CpbZIP11, which can be upregulated by tunicamycin. The conserved double stem-loop structure in its mRNA is spliced under environmental stresses. This splicing event results in a novel CpbZIP11 mRNA variant, leading to the production of a nuclear-localized CpbZIP11 protein with transcriptional activation activity in yeast. We further delve into the study of evolutionary lineage and motif conservation of CpbZIP11 homologs across various plant groups. This research illuminates the stress adaptation mechanisms in C. paliurus and deepens our understanding of the bZIP evolution, which endows versatility for the understanding of this transcription factor.
1. Introduction
The sweet tea tree, Cyclocarya paliurus (Batal.) Iljinskaja, is a deciduous tree native to China [1,2,3]. It belongs to the Juglandaceae family, which also includes walnuts and pecans [4]. This tree is predominantly found in the central and southern regions of China, particularly in the provinces of Hubei, Hunan, and Guizhou [5]. Cyclocarya paliurus (C. paliurus) is associated with putative therapeutic properties and is reported to contains significant bioactive compounds such as triterpenoids, polysaccharides, and flavonoids [6,7]. In traditional Chinese medicine, the leaves of C. paliurus are extensively used to treat various ailments, and the tea made from these leaves is well known for its health benefits [5,6]. C. paliurus primarily exists in natural forests, with a small population that is mostly scattered in remote and inaccessible areas, including certain nature reserves [8].
However, due to environmental stresses such as drought, cold, and heavy metal contamination and the deep dormancy characteristics of its seeds, the natural regeneration ability of C. paliurus is relatively weak, resulting in limited population expansion [9]. Furthermore, in recent years, excessive human exploitation has led to a significant reduction in natural forest resources, greatly impacting the conservation and sustainable development of this species [5,10]. In order to improve the survival ability of C. paliurus under environmental stress, it is important to understand the signal pathway of environmental stress in C. paliurus. Transcription factors are a class of important regulatory proteins that influence various biological processes, such as development, differentiation, and response to environmental stimuli, by controlling gene expression [11,12,13]. For example, the NAC family transcription factors have been found to be upregulated under drought stress [14]. Similarly, under salt stress, WRKY family transcription factors are upregulated in response to salt stress and other biological effectors such as jasmonates, hydrogen sulfide, and nitric oxide [15]. While the extent of environmental stresses such as drought, cold, and heavy metal contamination may vary, it is crucial to recognize their potential impact on the natural regeneration and growth of C. paliurus in specific regions or changing conditions. Understanding how this tree adapts to these stresses has broader implications for plant adaptation to challenging environments. Furthermore, C. paliurus holds economic and ecological significance, making research on this species valuable for its conservation and cultivation.
The endoplasmic reticulum (ER) is responsible for the maturation and folding of most secreted and transmembrane proteins. Endoplasmic reticulum stress can be caused by disturbances in the ER’s internal or external environment, which can accumulate misfolded or unfolded proteins [16]. Environmental stress including cold and heat can lead to protein misfolding or nonfolding in plants, causing ER stress, which can be detrimental to plant health [17]. In response to ER stress, cells activate the unfolded protein response (UPR), which comprises a series of intracellular signaling pathways [18]. The UPR aims to restore ER homeostasis by enhancing protein folding capacity, reducing protein synthesis, promoting proteolysis, and modifying cellular metabolic and signaling pathways [19]. In eukaryotes, the UPR is mediated by specific ER membrane-localized transcription factors, including ATF4, ATF6, and PERK in metazoans [20,21]. These pathways are conserved in animals and plants [22]. In Arabidopsis, two important ER stress-related transcription factors have been identified, including bZIP28 and bZIP60 [23,24,25]. Unconventional splicing of bZIP60 by IRE1 has been observed in various plant species, including Arabidopsis, rice, maize, tomato, and Nicotiana. In Arabidopsis, there are three IRE1 homologs, namely IRE1A, IRE1B, and IRE1C [26]. IRE1A and IRE1B redundantly function in mRNA splicing, while IRE1C is involved in gametogenesis. In the presence of ER stress inducers such as tunicamycin (tm) and dithiothreitol (DTT), AtIRE1A and AtIRE1B splice AtbZIP60 mRNA and remove 23 ribonucleotides, resulting in an activated form of AtbZIP60. This activated form enters the nucleus and may activate downstream UPR genes through specific cis-acting elements [25,27,28]. Unlike Arabidopsis, the splicing of HAC1 and XBP1 mRNA targets, respectively, is made possible by the dimerization/oligomerization and autophosphorylation of yeast IRE1 or mammalian IRE1a [29]. In addition, the S2P-bZIP28 pathway results in the proteolytic cleavage of the ER membrane-associated transcription factor bZIP28 [30]. These factors are normally inactive, but when an excessive amount of unfolded or improperly folded proteins accumulate, they are translocated to the Golgi, where they are then activated by cleavage [31]. The active form then controls downstream UPR gene expression in the nucleus to aid in cell survival.
AtbZIP60, a significant transcription factor in Arabidopsis thaliana, orchestrates a variety of biological responses. It governs gene expression related to endoplasmic reticulum stress, heat stress, pathogen infection, defense hormone responses, and other crucial processes [25,32]. In maize, ZmbZIP60 contributes to a myriad of plant adaptations. It assists in adapting to elevated temperatures, preserving endoplasmic reticulum homeostasis, bolstering resistance against pathogens, and aiding in drought conditions [17]. Similarly, in tomato plants, SlbZIP60 becomes activated during environmental stress. This activation modulates the expression of specific genes, enhancing the tomato plant’s resilience against environmental stressors, including high temperatures and viral infections [33]. Lastly, in rice, OsbZIP74 plays a pivotal role in the regulation of genes associated with endoplasmic reticulum stress, influencing rice growth and development [34].
In our transcriptome data, we analyzed the expression of members of the CpbZIP family upregulated by endoplasmic reticulum stress. We found that CpbZIP11, which is a homologous gene of Arabidopsis AtbZIP60, was induced when treated with an ER stress inducer TM. Furthermore, we also investigated CpbZIP11 unconventional splicing during development, with a particular focus on its induction by ER stressors and environmental stresses. The identification of the CpbZIP11 transcription factor in C. paliurus provides insights into the specific mechanisms and regulatory pathways that this plant species employs to cope with ER stress. Understanding the role of CpbZIP11 in the UPR can shed light on how C. paliurus adapts and survives under adverse conditions. The genetic information obtained from our research holds value for future studies and can be used to investigate desired traits such as disease resistance or improved growth. These traits have the potential to be applied in breeding programs or genetic engineering efforts.
2. Materials and Methods
2.1. Plant Materials and Stress Treatment
A controlled growing environment with a temperature of 28 °C and a photoperiod of 16 h of light and 8 h of darkness was employed to grow the seedlings for the experiment. When they were about two months old, they went through various stress treatments and had their RNA extracted. For stress treatment, the leaves of the seedlings were plucked and placed in a solution containing a specific concentration of reagent ½ MS liquid culture medium. These concentrations were as follows: 2 mM of DTT, 5 μg/mL of tunicamycin (TM), and 1 mM of salicylic acid (SA). For different stress treatments, each treatment was subjected to three biological replicates and materials were collected at four different time points, namely 0 h, 1 h, 4 h, and 16 h. In the case of tunicamycin treatment, a concentration of 5 μg/mL of tunicamycin was added to the treated samples. In the control samples, an equal amount of dimethyl sulphoxide (DMSO) was added to rule out any effects it may have on the seedlings, as DMSO was the solvent used for tunicamycin. To serve as control samples, the seedlings were transplanted into a 1/2 MS liquid medium without the addition of any reagents. This control group allowed for comparison and evaluation of the effects of the specific stress treatments on gene expression and other molecular responses.
2.2. Differential Expression Analysis
In this experiment, we employed the DESeq R package (version 1.10.1) to determine the differential expressions between the two groups/conditions. The DESeq package utilizes statistical procedures and a model based on negative binomial distributions to analyze differential expression in digital gene expression [35]. To minimize false-positive results, we further adjusted the p value [36]. Genes were considered differentially expressed when the adjusted p value determined by DESeq was <0.05, following the approach proposed by Huang et al. [37].
2.3. Classification and Sequence Analysis of bZIP Members
The outgroup in this study refers to a taxon that is closely related to, but not part of, the group of interest (ingroup). Its purpose is to serve as a reference point for rooting the phylogenetic tree and elucidating evolutionary relationships within the ingroup. By comparing the ingroup with the outgroup, we can infer the common ancestor and trace the evolutionary trajectory. In our study, we selected Molluginaceae as the outgroup based on its established taxonomic relationship and previous research. The phylogenetic tree was constructed using the Poisson model and the neighbor-joining (NJ) method implemented in MEGA 7.0. However, in order to better distinguish and understand the bZIP evolutionary map, we used the JTT model with the maximum likelihood (ML) method to construct a phylogenetic tree in MEGA 7.0.
These sequences for monocotyledonous and dicotyledonous plants were obtained from the Phytozome database, using the information provided in the relevant literature. We used a phylogram with equal edge lengths to the analysis evolutionary relationship in monocots and dicots. Furthermore, the MEME online program was employed to identify conserved motifs in the WRKY proteins we discovered [38]. The generated file was then imported into TBtools for visualization. Motif annotation information was obtained from the Conserved Domain Database (CDD) and Pfam databases.
2.4. CpbZIP11 mRNA Secondary Structure Prediction
To identify the splice site, we utilized CentroidFold tools, a secondary structure prediction tool accessible at http://www.ncrna.org/ (accessed on 25 April 2023) [39]. By utilizing the CpbZIP11 mRNA and AtbZIP60 mRNA sequences, we predicted the secondary structures using energy minimization parameters.
2.5. mRNA Splicing Assay and RT–PCR
Leaf tissues were used for the extraction of total RNA, and one microgram of the used RNA was employed for cDNA synthesis. The cDNA synthesis was carried out using Moloney murine leukemia virus (M-MLV) reverse transcriptase from Invitrogen, following the instructions provided by the manufacturer. To ensure precise quantification, the 18S RNA was utilized as a normalization control for the quantity of cDNA [40]. RT-PCR analysis was performed to investigate the splicing of CpbZIP11. Flanking primers were used for measurement: forward primer: 5′-CGCATTT-GGTGCTTCCAT-3′; reverse primer: 3′-GGGCAGAGTGAATAGGCACA-5′. For the splicing-specific primer assays, two different primer pairs were used, one for the unspliced form of CpbZIP11 and the other for the spliced form of CpbZIP11: unspliced form primer pair: forward primer: 5′-CTGCTGTGCTCTTGTTGGAA-3′, reverse primer: 3′-CTTTTTGACCCTCCTAGAGC-5′; spliced form primer pair: forward primer: 5′-AGGAG-TCTGCTGTTGGGTTCC-3′, reverse primer: 3′-CTTTTTGACC0CTCCTAGAGC-5′.
2.6. Subcellular Localization Analysis
The vector of pSKY36 was digested using AscI and SpeI enzymes. As the vector pSKY36 contained a green fluorescence-labeled protein GFP, the cDNA of CpbZIP11(D) and CpbZIP11(P) were inserted between the AscI and SpeI sites of the vector. The resulting construct was then transformed into Agrobacterium tumefaciens using electric shock and subsequently injected into tobacco plants for immediate expression. After three days, the fluorescence signal of GFP was visualized and detected using confocal microscopy. The forward primer used for amplification was 5′-TTGGCGCGCCATGAAAGACGATGACGGT-3′ for both amplicons, while the reverse primers were 3′-GGACTAGTGCTTGGTTATGGAAGCACCA-5′ for CpbZIP11(D) and 3′-GGACTAGAAGAACAAGGTAATTCGGTT-5′ for CpbZIP11(P) cDNA. To confirm the nuclear localization of the protein, tobacco leaves were placed on PBS containing 0.1% (v/v) Triton X-100 and cultured in 1 mg mL−1 DAPI (Sigma-Aldrich, purchased at Invitrogene in Shanghai, China in 2021) solution for 30 min using DAPI staining.
2.7. Transcriptional Activation Activity Assay in Yeast
For the transcriptional activation activity assay in yeast, the cDNA of CpbZIP11 (D) was inserted into the pGBKT7 vector (Purchased at Invitrogene in Shanghai, China in 2020) between the EcoRI and BamHI restriction sites. The forward primer was 5′-CGGAATTCATGAAAGACGATGACGGTAT-3′ for two amplicon, and the reverse primer was 3′-CCGGATCCGCTTGGTTATGGAAGCACCA-5′ for CpbZIP11(D). Plasmids were transferred to yeast cells using the LiAc–PEG transformation method. Transcriptional activation was assessed by evaluating the activation of two reporter genes, HIS3 and LacZ. The HIS3 reporter measured growth on selective media without histidine, indicating successful activation. The LacZ reporter measured β-galactosidase activity driven by the transcription factor. This determined the transcriptional activation activity of CpbZIP11(D).
3. Results
3.1. The Expression Profile of CpbZIP Genes under ER Stress
In the Arabidopsis bZIP family, two members, AtbZIP28 and AtbZIP60, have been identified as being upregulated in response to endoplasmic reticulum (ER) stress. These proteins play a crucial role in promoting the unfolded protein response (UPR) and enhancing cell survival in the ER stress pathway. To explore bZIP genes related to ER stress in Cyclocarya paliurus (C. paliurus), we analyzed the expression of the bZIP family under treatment with tunicamycin (TM) via transcriptomic data (GSE133027) [2]. We performed a cluster analysis of expression patterns in the WRKY family genes of C. paliurus (Figure 1). There were 58 CpbZIP genes identified in C. paliurus [41]. Out of the 58 CpbZIP genes analyzed, the expression of 12 genes were not detected in this transcriptomic data, while the remaining 46 genes exhibited differential expression. After 6 h of TM treatment, 17 CpbZIP genes were upregulated and 29 genes were downregulated. Similarly, after 14 h of TM treatment, 18 genes were upregulated and 28 genes were downregulated. Thirteen CpbZIP genes showed consistent upregulation at both 6 and 14 h after TM treatment. This observation suggests that these genes may play a crucial role in the response to ER stress induced by TM and could potentially be important regulators in the cellular stress response pathway.
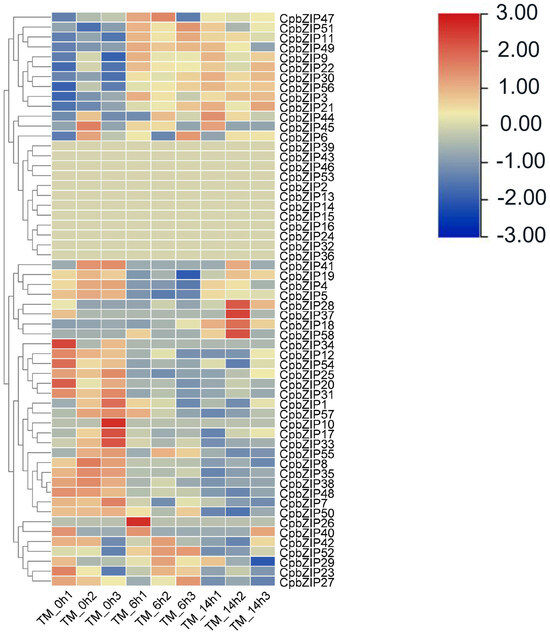
Figure 1.
Cluster analysis of expression patterns in WRKY family genes of C. paliurus under TM stress. The TM concentration used was 5 μg/mL. The time points TM_0h (control), TM_6h, and TM-14h denote the different durations of treatment. Transcript abundance levels were normalized and categorized by comparing the log2 (FPKM + 1) values among the various treated genes. Expression values are illustrated using a color scale: red indicates high expression, while blue signifies low expression.
3.2. CpbZIP11 Sequence Homology with AtbZIP60 and Splicing Site Prediction
In a recently study conducted by Deng et al. [42], the significance of AtbZIP60 in signal transduction and gene regulation within the unfolded protein response (UPR) was discovered. This discovery reinforces the goal of this study to identify a similar pathway during the development of the high-value medicinal plant C. paliurus. To achieve this, we conducted a homologous comparison search between the protein sequence of AtbZIP60 and the gene annotation data for C. paliurus, which is accessible at https://ngdc.cncb.ac.cn. Our analysis identified the C. paliurus gene GWHPBEHY011792 as having the highest similarity score (E value = 1 × 10−41) for a conserved protein sequence compared to AtbZIP60. This gene encodes the CpbZIP transcription factor CpbZIP11, which can be upregulated under ER stress (Figure 1) and shares 45% similarity with the inactivated AtbZIP60 protein in Arabidopsis. This finding is consistent with the previous phylogenetic results that placed CpbZIP11 and AtbZIP60 in the same clade [39]. In Arabidopsis, AtbZIP60 is a membrane-associated transcription factor with a transmembrane domain that is removed through conserved mRNA unconventional splicing. The truncated form, AtbZIP60D, lacking the transmembrane domain, can enter the nucleus and upregulate UPR genes. To further confirm if CpbZIP11 is a homolog of AtbZIP60, we analyzed the structure of CpbZIP11. Our analysis revealed that CpbZIP11 is also predicted to be a type II membrane protein, with a DNA-binding domain at the N-terminus facing the cytoplasm and a C-terminal transmembrane domain (Figure 2A).
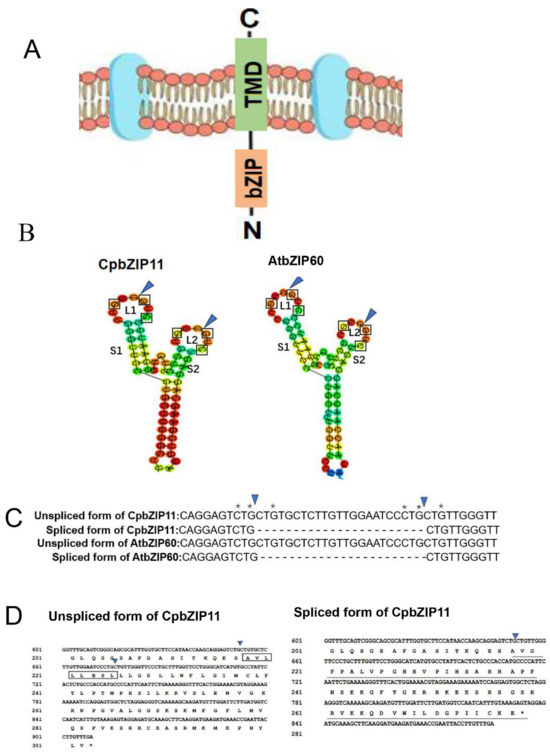
Figure 2.
Predicted splicing site of CpbZIP11 mRNA. (A) Domain structure of CpbZIP11 protein. TMD indicates the transmembrane domain. (B) Predicted twin stem-loop structures in Arabidopsis bZIP60 mRNA (AtbZIP60) and Cyclocarya paliurus bZIP11 mRNA (CpbZIP11). Each structure has two stems (S1 and S2) and two loops (L1 and L2). Conserved nucleotides within each loop are boxed. The genuine and predicted cleavage sites are denoted with arrows and arrowheads, respectively. (C) The sequencing results of CpbZIP11 were compared with those of the unspliced and spliced versions of AtbZIP60 focusing on the splicing sites. Asterisks and triangles represent conserved nucleotides and splicing sites, respectively. (D) A segment of nucleotides and the associated amino acid sequences of unspliced or spliced variants of CpbZIP11. Arrows indicate splicing sites of CpbZIP11 sequence sequencing results, as shown in (C). An arrowhead highlights the joining site. The transmembrane domain in the unspliced variant of CpbZIP11 is boxed (upper), while the new C-terminus from splicing is underlined and bolded (lower). An asterisk indicates the stop codon.
To predict mRNA unconventional splicing in CpbZIP11, we employed the CentroidFold tools (http://www.ncrna.org/, accessed on 25 April 2023) to analyze the RNA secondary structure and splice sites of the CpbZIP11 coding sequence (Figure 2B). Interestingly, we observed the conservation of two kissing stem-loop structures in both Arabidopsis and C. paliurus sequences. Based on the splicing pattern observed in AtbZIP60, we anticipated the splicing out of a 23-nucleotide intron from the CpbZIP11 mRNA stem-loop structure (Figure 2B, marked by arrowheads). We predicted the unspliced and spliced forms of the mRNA, designated as the unspliced form of CpbZIP11 and the spliced form of CpbZIP11, respectively (Figure 2C). The splicing event was projected to result in a smaller protein for the spliced form of CpbZIP11 (Figure 2D). This is due to the frameshift caused by the splicing of a 23-base oligonucleotide fragment, leading to the emergence of a new termination codon. The transition from the unspliced form of CpbZIP11 to the spliced form of CpbZIP11 causes the CpbZIP11 protein to lose the transmembrane domain (TMD) that anchors the unspliced form of CpbZIP11 to the ER membrane. These findings suggest that CpbZIP11 undergoes a similar unconventional splicing event as AtbZIP60, resulting in the removal of the transmembrane domain.
To validate the predicted splicing event in CpbZIP11, we designed a pair of primers (CpbZIP11-F and CpbZIP11-R) flanking the putative splice sites (Figure 3A). We treated C. paliurus young leaves with different abiotic stresses, including TM and DTT. Upon gel electrophoresis, we observed an additional band with faster migration, indicating that nucleotides were indeed spliced out under these stress conditions. To discriminate between the unspliced and spliced forms of CpbZIP11, we generated additional specific primers (Figure 3B). The SPU forward primer crosses the spliced “intron”, while the SPS primer bridges the “intron”. We confirmed the amplification specificity using plasmid DNA containing either the unspliced or spliced form of CpbZIP11 as the template. When using the SPU primer, only the unspliced form of mRNA was detected, whereas the spliced form of mRNA was detected when amplified with the SPS primer (Figure 3C). Then, we constructed fragments of both unspliced and spliced forms on the T-vector for sequencing validation. The sequencing results showed that the spliced form produced a 23-base oligonucleotide fragment (Supplementary Figure S1), consistent with our predicted results (Figure 2D). Furthermore, we conducted a comparison of nucleotide and amino acid sequences between the bZIP11 truncated fragment and its homolog, bZIP60 in Arabidopsis, to identify conserved elements (Supplementary Figure S2). This analysis provided valuable insights into the potential functional and evolutionary relationships between these two homologous genes across different plant species.
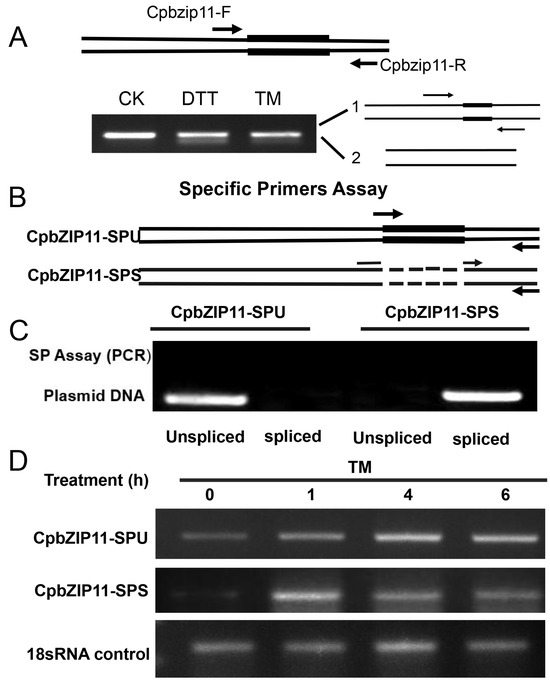
Figure 3.
Analysis of CpbZIP11 mRNA splicing and specific primer assays amid ER stress. (A) CpbZIP11 mRNA splicing upon exposure to ER stress agents, alongside the electrophoresis pattern from RT-PCR of untreated (CK) or treated (DTT, TM) Cyclocarya paliurus leaves. Primers immediately flanking the splicing sites (arrows) were used for the splicing assay; the spliced region is highlighted with bold lines (upper). (B) Details of primers employed for specific primer assays to amplify the unspliced (SPU) and spliced (SPS) versions of CpbZIP11. (C) Validation in the primer specificity shown in (B) via PCR with plasmid DNA carrying both the unspliced and spliced sequences of CpbZIP11. (D) Time-course analysis revealing the splicing dynamics of CpbZIP11 mRNA when subjected to ER stress.
To further investigate the splicing of CpbZIP11 mRNA, we conducted RT-PCR splicing assay using C. paliurus treated with tunicamycin treatment at different times. We used both unspliced and splice-specific primers to detect the splicing event. The results showed that splicing occurred after tunicamycin treatment, and splicing mRNA (CpbZIP11-SPS) can be detected after treatment with C. paliurus at different times (0 h, 1 h, 4 h, and 6 h) (Figure 3D). These experimental findings provide further support for the hypothesis that CpbZIP11 is a homolog of AtbZIP60 and may function in a similar manner in the UPR pathway. The detection of splicing under different stress conditions and the confirmation of splicing after tunicamycin treatment strengthen our understanding of the regulatory mechanisms of CpbZIP11 in response to ER stress.
3.3. CpbZIP11 mRNA Splicing Produces the Active Form of Transcription Factor bZIP11
AtbZIP60 features a transmembrane domain (TMD) from amino acids 224 to 244, following the bZIP domain (amino acids 140–197) [43]. The full-length AtbZIP60 protein is localized in the endoplasmic reticulum (ER) membrane. However, a truncated form of AtbZIP60, known as AtbZIP60D (amino acids 1–216), lacks the transmembrane domain and is able to localize to the nucleus. AtbZIP60D functions as a transcription factor and has the ability to activate the promoters of genes that respond to ER stress. To determine the subcellular localization of CpbZIP11(P) in its full length and truncated transmembrane domain form (CpbZIP11(D)), we fused a monomer GFP (mGFP) tag to the N-terminal of the protein (Figure 4A,B). The resulting fusion construct was transiently expressed in tobacco leaves, and the localization was examined using confocal laser scanning microscopy. Our findings revealed that the full-length form of CpbZIP11 is predominantly localized outside the nucleus, while the truncated form, lacking the transmembrane domain, is primarily present within the nuclei. This localization pattern of the truncated form of CpbZIP11 is consistent with the localization of AtbZIP60. To further confirm the nuclear localization of the truncated form, we performed DAPI staining, a fluorescent stain that binds strongly to adenine–thymine-rich regions in DNA (Supplementary Figure S3). The DAPI staining results provided additional evidence supporting the nuclear localization of the truncated form of CpbZIP11.
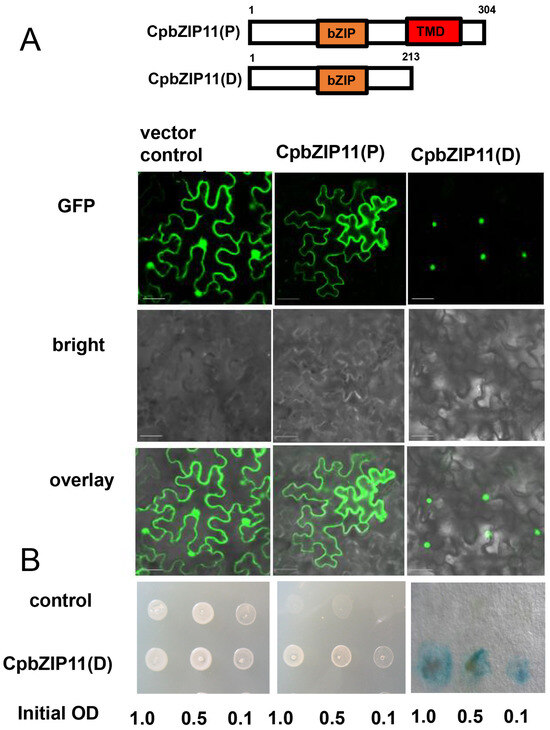
Figure 4.
Examination of subcellular localization and transcriptional activation activity of CpbZIP11. (A) Subcellular localization of the full-length unspliced CpbZIP11(P), the truncated CpbZIP11(D), and empty mGFP serving as a negative control. Scale bar represents 50 um. (B) Assay determining the transcriptional activation activity of CpbZIP11(D) in yeast cells. A series of yeast cell dilutions were employed in the assessment.
To investigate the transcriptional activation activity of CpbZIP11(D), we conducted transcriptional activation assays. Compared to the empty vector control (containing only the GAL4 DNA binding domain), the truncated CpbZIP11(D) fusion proteins were found to activate the HIS3 and LacZ reporter genes (Figure 4B). This result confirms the transcriptional activation activity of CpbZIP11(D) proteins and provides insights into their potential functional roles in gene regulation. In the transcriptional activation assay, the presence of blue coloration in yeast cells containing CpbZIP11(D) in the presence of X-gal further substantiated their ability to activate the LacZ reporter gene (Figure 4B). This colorimetric evidence aligns with our molecular findings, confirming the role of CpbZIP11(D) as transcriptional activators (Figure 4B).
To investigate whether CpbZIP11(D) forms homodimers, we conducted yeast two-hybrid (Y2H) assays. In the absence of histidine and presence of 3-AT, the yeast cells harboring both the bait and prey constructs grew normally on the medium. However, in the presence of histidine and 3-AT, the yeast cells failed to grow normally, which is consistent with the behavior of AtbZIP60 in Arabidopsis (Supplementary Figure S4). These observations suggest that while CpbZIP11(D) functions as a transcriptional activator, it may not form homodimers in plants.
3.4. CpbZIP11 Is Induced by Environmental Stresses
Previous studies have demonstrated the close relationship between the unfolded protein response (UPR) and the response of Arabidopsis to various abiotic and biotic stresses. In this study, we aimed to investigate the splicing of CpbZIP11 mRNA in response to abiotic stress treatments (Figure 3A). To validate the hypothesis that CpbZIP11 mRNA undergoes splicing under abiotic stress, we performed RT-PCR using CpbZIP11 flanking primers. Two different primer sets were used for RT-PCR validation: one for the unspliced form of CpbZIP11-SPU and the other for the spliced form of CpbZIP11-SPS. The experimental results indicate that the non-spliced form of CpbZIP11 mRNA (CpbZIP11-SPU) can be detected under both untreated and treated conditions. However, under conditions of heat stress (45 °C), SA treatment, as well as treatment with ER stress inducers DTT and TM, the expression level of the spliced form of CpbZIP11 (CpbZIP11-SPS) significantly increases. This suggests that heat stress, SA treatment, and DTT and TM treatments can induce the splicing of the CpbZIP11 gene (Figure 5). These findings provide further support for the hypothesis that CpbZIP11 mRNA undergoes splicing in response to different abiotic stresses.
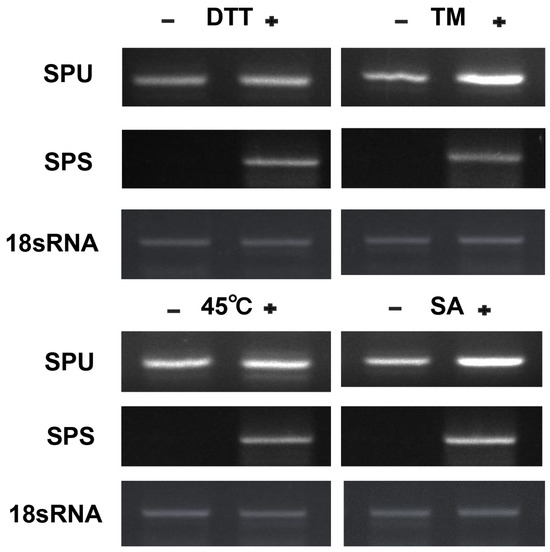
Figure 5.
Splicing analysis of CpbZIP11 under environmental stresses. Leaves of Cyclocarya paliurus were subjected to diverse abiotic stresses, including 2 mM DTT, 5 μg/mL TM, and heat stress at 45 °C. They were also exposed to the biotic stress hormone treatment of 1 mM salicylic acid (SA). Specialized primers were subsequently used to detect and verify the splicing of CpbZIP11 mRNA. SPU is the detection of unspliced form primers and SPS is the detection of spliced form primers. 18sRNA was employed as an internal control.
3.5. Evolutionary Relationship and Motif Analysis of CpbZIP11 Homologous Genes across Monocots and Dicots
To investigate the evolutionary relationship of the CpbZIP11 gene, we constructed a phylogenetic tree (Figure 6) using neighbor-joining (NJ) based on homologous amino acid sequences of CpbZIP11 from common species of monocotyledonous and dicotyledonous plants. In this tree, bZIP family members from monocots plants are represented by a light blue background, while members from dicots plants are represented by a light brown background. We found that CpbZIP11 from C. paliurus is closely related to homologs from Carya illinoinensis and Juglans regia, as they are located on the same branch. This suggests a close evolutionary relationship among these species within the Juglandaceae family, possibly sharing common genetic and functional characteristics. The evolutionary relationships were verified through the application of the maximum likelihood (ML) method (Supplementary Figure S5). By combining the results of both methods, we can gain a better overall understanding of the evolutionary relationship of the CpbZIP11 gene.
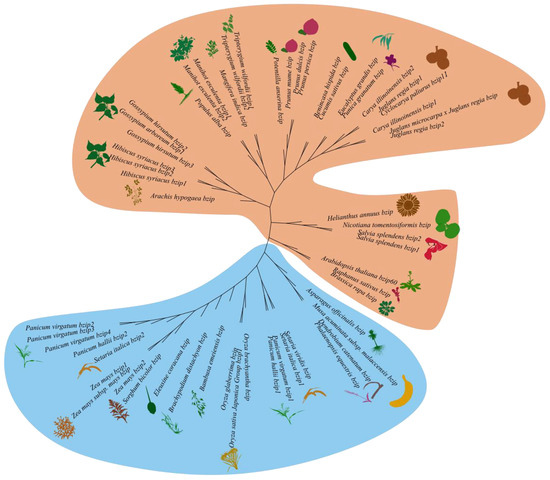
Figure 6.
Tracing the evolutionary lineage of CpbZIP11 homologous genes among monocots and dicots. Utilizing MEGA 7.0 software, a NJ phylogenetic tree was constructed, elucidating the evolutionary connections among CpbZIP11 genes. This tree, founded upon CpbZIP11 homologous amino acid sequences from prevalent species within the monocots and dicots families, features color-coded branches: light blue represents bZIP family members from monocotyledons, whereas light brown denotes those from dicotyledons.
To identify conserved motifs or functional domains present in these proteins, we also conducted a motif analysis to examine the motif composition of CpbZIP11 across different species (Figure 7). Most species, except for Gossypium hirsutum CpbZIP11 homologs and Hibiscus syriacus CpbZIP11 homologs, contained seven motifs (motif 5, motif 8, motif 7, motif 1, motif 3, motif 2, and motif 4). Interestingly, motif 6 was conserved in monocots but absent in dicots. Conversely, motif 10 was not found in monocots but was present in a small number of species, including C. paliurus, Juglans regia bZIP, Carya illinoinensis, Juglans macrocarpa_x_ Juglans regia bZIP, and Juglans regia. These results provide insights into the evolutionary relationship and motif composition of CpbZIP11 proteins in monocots and dicots. They contribute to our understanding of the functional diversity and evolutionary history of this important family of transcription factors. The identification of specific motifs can aid in characterizing functional domains and regulatory elements within these proteins, which is valuable for further research on the roles and mechanisms of bZIP transcription factors in plant biology.
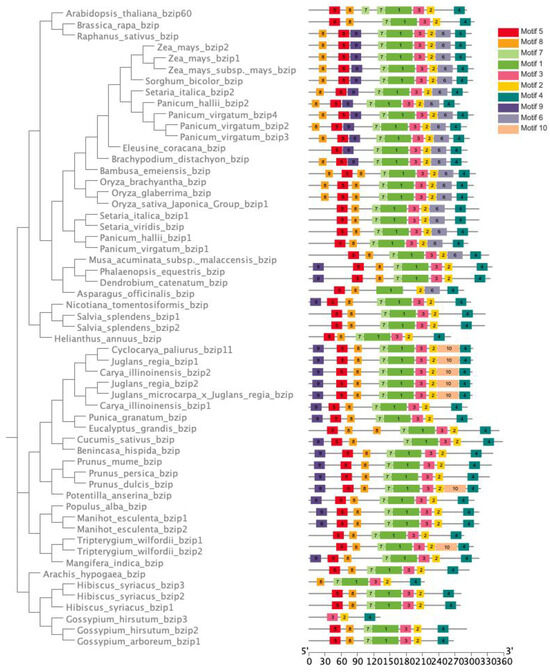
Figure 7.
Deciphering the conserved motifs in CpbZIP11 homologous genes from monocots and dicots. Phylogenetic trees, curated from the full-length proteins from CpbZIP11 homologous genes in monocotyledon and dicotyledon plants, offer a vivid portrayal of motif compositions. Distinctive patterns, labeled from 1 to 10, are represented by differently colored boxes. For a proximate understanding of protein length, refer to the scale at the figure’s base.
4. Discussion
Cyclocarya paliurus (C. paliurus) is a medicinal herb that holds significance in traditional Chinese medicine [5]. This herb is renowned for its abundant presence of beneficial compounds, including flavonoids, polysaccharides, triterpenoid acids, and trace elements [5,6]. The presence of these compounds in C. paliurus imparts a wide range of biological and physiological activities to the herb, thereby establishing it as a valuable herbal resource [7]. Studies have shown that C. paliurus has a variety of medicinal value, such as the treatment of hyperlipidemia, coronary heart disease, and anti-hyperglycemia effect [6]. C. paliurus possesses antioxidant properties that aid in combating oxidative stress in the liver and kidneys [44]. Additionally, research has shown that C. paliurus has potential in supporting and enhancing the immune system. However, it is important to consider that environmental factors, including seasonal changes, can influence the yield and quality of the leaves, particularly in terms of the accumulation of flavonoids and phenolic compounds [45].
In plants, bZIP TFs (transcription factors) have been identified as key players in numerous developmental processes and abiotic stress tolerance [46]. These genes are involved in essential biological processes such as cell elongation, seed and flower development, as well as nitrogen/carbon and energy metabolism [47]. For instance, AtbZIP17, AtbZIP24, and OsbZIP72 have been demonstrated to positively regulate plant responses to salt stress, either through direct or indirect mechanisms [47,48,49,50]. Furthermore, in rice, OsbZIP52 functions as a negative regulator in cold signaling [51].
The endoplasmic reticulum is the mature and folding site of most secreted and transmembrane proteins. Environmental changes can lead to instability or suboptimal modification of protein folding in the endoplasmic reticulum, leading to endoplasmic reticulum stress. Compared to yeast or mammalian cells, plants have much less understanding of the molecular mechanisms underlying endoplasmic reticulum stress response [52]. The UPR pathway is regulated by several transcription factors, with AtbZIP60 playing a crucial role in Arabidopsis [25]. Normally, AtbZIP60 is synthesized as a transmembrane protein and remains anchored to the ER membrane [53]. However, during ER stress, the mRNA of AtbZIP60 undergoes unconventional splicing, leading to the removal of introns and the generation of cytoplasmic forms of the protein. This splicing process is mediated by the IRE1 branch of the UPR pathway [54].
In the current study, through a homology analysis of the bzip family between Cy-clocarya paliurus and Arabidopsis, we identified the homologous gene CpbZIP11 of AtbZIP60. We further found that when dealing with the tender leaves of C. paliurus, such as TM or DTT treatment, as well as heat stress response and SA hormone treatment, it can trigger the splicing of CpbZIP11 mRNA. SA hormone can induce the synthesis of related proteins and improve the plant’s disease resistance after being infected by pathogenic microorganisms. According to a subcellular localization analysis, CpbZIP11 is normally located on the endoplasmic retina. Under environmental stress, the conserved double helix structure of CpbZIP11 mRNA is spliced away, and a new CpbZIP11 protein is localized in the nucleus. Splicing the CpbZIP11 protein showed transcriptional activation activity in yeast cells, indicating its role as a transcription factor in regulating stress-response genes.
The motif analysis revealed the presence of seven conserved motifs (motif 5, motif 8, motif 7, motif 1, motif 3, motif 2, and motif 4) in most species, except for Gossypium hirsutum bZIP2 and Hibiscus syriacus bZIP3. Notably, motif 6 was prevalent in monocots but absent in dicots, while motif 10 was found in only a small number of species, including Cyclocarya paliurus CpbZIP11, Juglans regia bZIP1, Carya illinoinensis, Juglans macrocarpa_x_ Juglans regia bZIP, and Juglans regia. Although the close relationship between Cyclocarya paliurus CpbZIP11, Carya illinoinensis, and Juglans regia was not to suggest surprise, the close relationship between Cyclocarya paliurus CpbZIP11, Carya illinoinensis, and Juglans regia within the Juglandaceae family emphasizes the potential shared evolutionary history and potentially similar functional roles for their respective bZIP genes.
These findings contribute to our understanding of the evolutionary history, functional diversity, and motif composition of bZIP proteins in monocots and dicots. The identification of specific motifs can aid in the characterization of functional domains and regulatory elements within these proteins, providing valuable information for further research on the roles and mechanisms of bZIP transcription factors in plant biology.
The splicing mode of IRE1 is an important mechanism in the endoplasmic reticulum stress response. Recent progress has been made in understanding this process. IRE1 consists of an endonuclease domain and an extracellular domain. The activation of the endonuclease domain is a crucial step in IRE1 cleavage [55]. Activation of IRE1 is regulated by intracellular ER stress signals, such as protein aggregation and phosphorylation [56]. IRE1 primarily cleaves XBP1 mRNA, resulting in the production of the active transcription factor XBP1s [57]. XBP1s enters the nucleus and regulates the transcription of genes related to ER stress response [58]. IRE1 can also cleave other mRNAs, like ATF6 and RIDD, to regulate various aspects of ER stress response [59]. The cleavage mode of IRE1 is influenced by different regulatory mechanisms, including phosphorylation, protein interactions, and substrate binding. Molecular chaperones and cofactors also play a role in IRE1 cleavage regulation [60]. The IRE1 cleavage mode is crucial in regulating processes like protein synthesis, folding, and degradation in ER stress. It is also closely associated with cell survival, adaptability, and disease development. Further research on IRE1 cleavage in C. paliurus will enhance our understanding of its molecular mechanism in ER stress response and the adaptability of C. paliurus under adverse conditions.
Our findings on the role of CpbZIP11 in mitigating ER stress pave the way for enhancing the resilience of C. paliurus against environmental adversities. The upregulation of CpbZIP11, triggered by tunicamycin and environmental stress, suggests a potential molecular target for stress management. Biotechnological tools, such as gene editing technologies, could be developed to modulate the expression of CpbZIP11, potentially boosting the plant’s stress-tolerance mechanisms. Moreover, understanding the splicing mechanism that generates the nuclear-localized CpbZIP11 protein variant provides a foundation for exploring strategies to optimize this process under stress conditions. Furthermore, the evolutionary conservation of CpbZIP11 across plant groups hints at a conserved functional role in stress adaptation, which could be leveraged to transfer stress-resilience traits to other valuable plant species. Additionally, this study can inform breeding programs aiming at selecting stress-tolerant Cyclocarya paliurus varieties, thus ensuring sustained production and exploration of its therapeutic compounds. In conclusion, the characterization of CpbZIP11 provides a basis for developing stress management strategies, crucial for unlocking the full therapeutic potential of this medicinal plant under varying environmental conditions.
5. Conclusions
In our research, we discovered a counterpart of AtbZIP60 called CpbZIP11 in C. paliurus. CpbZIP11 is found in the endoplasmic reticulum membrane, and when exposed to environmental stresses, the conserved double stem-loop structures of CpbZIP11 mRNA are removed through splicing. This splicing generates a new CpbZIP11 mRNA that produces a nucleus-localized form of the CpbZIP11 protein, exhibiting transcriptional activation activity in yeast cells. Furthermore, we examined the evolutionary relationship and conserved motifs of CpbZIP11 homologues among monocots and dicots. These findings offer valuable insights into the stress response pathway in C. paliurus, enhancing our understanding of the functional diversity and evolutionary history of bZIP transcription factors.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/f14102104/s1, Figure S1: Sequencing results comparing unspliced and spliced forms of CpbZIP11; Figure S2: Comparative sequence alignment of nucleotides and amino acids between Arabidopsis AtZIP60 (D) and CpbZIP11 (D). (A) Amino acid sequence alignment. (B) Nucleotide sequence alignment; Figure S3: Nuclear localization of the truncated form of CpbZIP11D confirmed by DAPI staining. To corroborate the nuclear localization of this truncated form, DAPI staining was executed. DAPI, a stain that binds robustly to adenine–thymine-rich regions in DNA, serves as a primary marker for cell nuclei. Scale bar represents 50 μm; Figure S4: Inability of nuclear form CpbZIP11(D) to form homodimers in yeast cells. The cDNA sequence of CpbZIP11(D) was cloned into bait vector pGBKT7 and prey vector pGADT7. Upon co-transfection into yeast cells with empty vectors and in the presence of 3-AT to suppress CpbZIP11(D) self-activation, it was observed that the homodimer CpbZIP11(D) failed to form in yeast; Figure S5: maximum likelihood (ML) phylogenetic analysis of CpbZIP11 homologous genes among monocots and dicots. Light blue shading represents bZIP family members from monocotyledons, while light brown shading denotes those from dicots.
Author Contributions
Z.Y. and K.L. designed the research. Z.Y., Y.A., F.H., Q.Y., M.T., Y.Z. and S.F. performed the study. Z.Y., Y.A., F.H. and K.L. analyzed the data. Y.A., F.H., Z.Y. and K.L. wrote the manuscript, K.L. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by National Natural Science Foundation of China (grant number 32360074 and 31600214), Guizhou Provincial Natural Science Foundation of Department of Education [2022]077, and the Joint Fund of the National Natural Science Foundation of China and the Karst Science Research Center of Guizhou Province (grant number U1812401).
Data Availability Statement
All data are available upon reasonable request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Xie, J.; Wang, W.; Dong, C.; Huang, L.; Wang, H.; Li, C.; Nie, S.; Xie, M. Protective effect of flavonoids from Cyclocarya paliurus leaves against carbon tetrachloride-induced acute liver injury in mice. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 119, 392–399. [Google Scholar] [CrossRef]
- Yang, Z.; Xiong, M.; Yan Jian, S.F.; Luo, S.; An, Y.; Li, K.; Yi, Y. Transcriptome Analysis of ER Stress—Related Genes and Validation of Reference Genes in Gene Expression RT-qPCR for Cyclocarya paliurus (Batal.) Iljinskaja. Int. J. Agric. Biol. 2019, 22, 1588–1598. [Google Scholar]
- Sun, T.; Ablaev, A.G.; Wang, Y.; Li, C. Cyclocarya paliurus (Batal.) Iljinskaja (Juglandaceae) from the Hunchun Formation (Eocene), Jilin Province, China. J. Integr. Plant Biol. 2005, 47, 1281–1287. [Google Scholar] [CrossRef]
- Zheng, X.; Xiao, H.; Chen, J.; Zhu, J.; Fu, Y.; Ouyang, S.; Chen, Y.; Chen, D.; Su, J.; Xue, T. Metabolome and Whole-Transcriptome Analyses Reveal the Molecular Mechanisms Underlying Hypoglycemic Nutrient Metabolites Biosynthesis in Cyclocarya paliurus Leaves During Different Harvest Stages. Front. Nutr. 2022, 9, 851569. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Peng, Y.; Zhu, X.; Li, H.; Zhang, L.; Kong, F.; Wang, J.; Yu, D. The phytochemicals and health benefits of Cyclocarya paliurus (Batalin) Iljinskaja. Front. Nutr. 2023, 10, 1158158. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, C.; Gao, Z.; Huang, Y.; Zhang, B.; Wei, J.; Zhao, L.; Tong, X. Potential Role of Natural Plant Medicine Cyclocarya paliurus in the Treatment of Type 2 Diabetes Mellitus. J. Diabetes Res. 2021, 2021, 1655336. [Google Scholar] [CrossRef]
- Chen, Z.; Jian, Y.; Wu, Q.; Wu, J.; Sheng, W.; Jiang, S.; Shehla, N.; Aman, S.; Wang, W. Cyclocarya paliurus (Batalin) Iljinskaja: Botany, Ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2022, 285, 114912. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fu, X.; Shang, X.; Yang, W.; Fang, S. Natural population structure and genetic differentiation for heterodicogamous plant: Cyclocarya paliurus (Batal.) Iljinskaja (Juglandaceae). Tree Genet. Genomes 2017, 13, 80. [Google Scholar] [CrossRef]
- Fang, S.; Wang, J.; Wei, Z.; Zhu, Z. Methods to break seed dormancy in Cyclocarya paliurus (Batal.) Iljinskaja. Sci. Hortic-Amst. 2006, 110, 305–309. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, M.; Qiao, Y.; Li, R.; Alkan, N.; Chen, J.; Chen, F. Cyclocarya paliurus Reprograms the Flavonoid Biosynthesis Pathway against Colletotrichum fructicola. Front. Plant Sci. 2022, 13, 933484. [Google Scholar] [CrossRef]
- Weidemüller, P.; Kholmatov, M.; Petsalaki, E.; Zaugg, J.B. Transcription factors: Bridge between cell signaling and gene regulation. Proteomics 2021, 21, e2000034. [Google Scholar] [CrossRef]
- Hobert, O. Gene regulation by transcription factors and microRNAs. Science 2008, 319, 1785–1786. [Google Scholar] [CrossRef] [PubMed]
- Renkawitz, R. Transcription Factors and Regulation of Gene Expression; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1886–1890. [Google Scholar]
- Puranik, S.; Sahu, P.P.; Srivastava, P.S.; Prasad, M. NAC proteins: Regulation and role in stress tolerance. Trends Plant Sci. 2012, 17, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; He, F.; An, Y.; Zhang, N.; Fan, S.; Tang, M.; Li, K. Genome-Wide Identification and Expression Analysis of Salt Tolerance-Associated WRKY Family Genes in Cyclocarya paliurus. Forests 2023, 14, 1771. [Google Scholar] [CrossRef]
- Lemmer, I.L.; Willemsen, N.; Hilal, N.; Bartelt, A. A guide to understanding endoplasmic reticulum stress in metabolic disorders. Mol. Metab. 2021, 47, 101169. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tang, J.; Srivastava, R.; Bassham, D.C.; Howell, S.H. The Transcription Factor bZIP60 Links the Unfolded Protein Response to the Heat Stress Response in Maize. Plant Cell 2020, 32, 3559–3575. [Google Scholar] [CrossRef]
- Hwang, J.; Qi, L. Quality Control in the Endoplasmic Reticulum: Crosstalk between ERAD and UPR pathways. Trends Biochem. Sci. 2018, 43, 593–605. [Google Scholar] [CrossRef]
- Rellmann, Y.; Eidhof, E.; Dreier, R. Review: ER stress-induced cell death in osteoarthritic cartilage. Cell Signal 2021, 78, 109880. [Google Scholar] [CrossRef]
- Read, A.; Schröder, M. The Unfolded Protein Response: An Overview. Biology 2021, 10, 384. [Google Scholar] [CrossRef]
- Wiseman, R.L.; Mesgarzadeh, J.S.; Hendershot, L.M. Reshaping endoplasmic reticulum quality control through the unfolded protein response. Mol. Cell 2022, 82, 1477–1491. [Google Scholar] [CrossRef]
- Wan, S.; Jiang, L. Endoplasmic reticulum (ER) stress and the unfolded protein response (UPR) in plants. Protoplasma 2016, 253, 753–764. [Google Scholar] [CrossRef]
- Srivastava, R.; Deng, Y.; Howell, S.H. Stress sensing in plants by an ER stress sensor/transducer, bZIP28. Front. Plant Sci. 2014, 5, 59. [Google Scholar] [CrossRef] [PubMed]
- Iwata, Y.; Ashida, M.; Hasegawa, C.; Tabara, K.; Mishiba, K.; Koizumi, N. Activation of the Arabidopsis membrane-bound transcription factor bZIP28 is mediated by site-2 protease, but not site-1 protease. Plant J. Cell Mol. Biol. 2017, 91, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.A.; Mukhtar, M.S.; Blanco, F.; Boatwright, J.L.; Moreno, I.; Jordan, M.R.; Chen, Y.; Brandizzi, F.; Dong, X.; Orellana, A.; et al. IRE1/bZIP60-mediated unfolded protein response plays distinct roles in plant immunity and abiotic stress responses. PLoS ONE 2012, 7, e31944. [Google Scholar] [CrossRef] [PubMed]
- Howell, S.H. Evolution of the unfolded protein response in plants. Plant Cell Environ. 2021, 44, 2625–2635. [Google Scholar] [CrossRef]
- Nakamura, M.; Nozaki, M.; Iwata, Y.; Koizumi, N.; Sato, Y. THESEUS1 is involved in tunicamycin-induced root growth inhibition, ectopic lignin deposition, and cell wall damage-induced unfolded protein response. Plant Biotechnol. 2022, 39, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Messias Sandes, J.; Nascimento Moura, D.M.; Divina da Silva Santiago, M.; Barbosa de Lima, G.; Cabral Filho, P.E.; da Cunha Gonçalves de Albuquerque, S.; de Paiva Cavalcanti, M.; Fontes, A.; Bressan Queiroz Figueiredo, R.C. The effects of endoplasmic reticulum stressors, tunicamycin and dithiothreitol on Trypanosoma cruzi. Exp. Cell Res. 2019, 383, 111560. [Google Scholar] [CrossRef] [PubMed]
- Back, S.H.; Schröder, M.; Lee, K.; Zhang, K.; Kaufman, R.J. ER stress signaling by regulated splicing: IRE1/HAC1/XBP1. Methods 2005, 35, 395–416. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, S.; Lu, S.; Liu, J. Site-1 protease cleavage site is important for the ER stress-induced activation of membrane-associated transcription factor bZIP28 in Arabidopsis. Sci. China Life Sci. 2015, 58, 270–275. [Google Scholar] [CrossRef]
- Paridon, A.; Fox, A.; Alvero, A.B. Detection of Unfolded Protein Response by Polymerase Chain Reaction. Methods Mol. Biol. 2021, 2255, 13–20. [Google Scholar] [CrossRef]
- Nagashima, Y.; Iwata, Y.; Ashida, M.; Mishiba, K.; Koizumi, N. Exogenous salicylic acid activates two signaling arms of the unfolded protein response in Arabidopsis. Plant Cell Physiol. 2014, 55, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kaitheri Kandoth, P. Tomato bZIP60 mRNA undergoes splicing in endoplasmic reticulum stress and in response to environmental stresses. Plant Physiol. Bioch 2021, 160, 397–403. [Google Scholar] [CrossRef]
- Lu, S.; Yang, Z.; Sun, L.; Sun, L.; Song, Z.; Liu, J. Conservation of IRE1-Regulated bZIP74 mRNA Unconventional Splicing in Rice (Oryza sativa L.) Involved in ER Stress Responses. Mol. Plant 2012, 5, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Pan, X.; Yu, Y.; Wang, W.; Zhang, F.; Ge, Y.; Shen, X.; Shen, F.; Liu, X. De novo characterization of the Anthurium transcriptome and analysis of its digital gene expression under cold stress. Bmc Genom. 2013, 14, 827. [Google Scholar] [CrossRef]
- Davidson, N.M.; Oshlack, A. Corset: Enabling differential gene expression analysis for de novo assembled transcriptomes. Genome Biol. 2014, 15, 410. [Google Scholar] [CrossRef]
- Huang, Q.; Sun, M.; Yan, P. Pathway and Network Analysis of Differentially Expressed Genes in Transcriptomes. Methods Mol. Biol. 2018, 1751, 35–55. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Yang, Z.T.; Fan, S.X.; Li, R.; Huang, T.M.; An, Y.; Guo, Z.Q.; Li, F.; Yi, Y.; Li, K. The optimal reference gene validation in Cyclocarya paliurus (Batal.) Iljinskaja under environmental stresses. Agron. J. 2022, 114, 2044–2055. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, L.; Jin, J.; Du, Z.; Li, J. Genome-wide identification and analysis of bZIP gene family reveal their roles during development and drought stress in Wheel Wingnut (Cyclocarya paliurus). BMC Genom. 2022, 23, 743. [Google Scholar] [CrossRef]
- Deng, Y.; Humbert, S.; Liu, J.; Srivastava, R.; Rothstein, S.J.; Howell, S.H. Heat induces the splicing by IRE1 of a mRNA encoding a transcription factor involved in the unfolded protein response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 7247–7252. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Fan, S.; Wang, J.; An, Y.; Guo, Z.; Li, K.; Liu, J. The plasma membrane-associated transcription factor NAC091 regulates unfolded protein response in Arabidopsis thaliana. Plant Sci. 2023, 334, 111777. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Shen, M.; Liu, S.; Yu, Q.; Chen, Y.; Xie, J. Ameliorative effect of Cyclocarya paliurus polysaccharides against carbon tetrachloride induced oxidative stress in liver and kidney of mice. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 135, 111014. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Shang, X.; Ding, H.; Cao, Y.; Fang, S. Natural variations in flavonoids and triterpenoids of Cyclocarya paliurus leaves. J. For. Res. 2021, 32, 805–814. [Google Scholar] [CrossRef]
- Genome-Wide Identification and Expression Analysis of bZIP Gene Family in Carthamus tinctorius L.—Scientific Reports. Available online: https://www.nature.com/articles/s41598-020-72390-z (accessed on 14 September 2023).
- Yang, Z.; Sun, J.; Chen, Y.; Zhu, P.; Zhang, L.; Wu, S.; Ma, D.; Cao, Q.; Li, Z.; Xu, T. Genome-wide identification, structural and gene expression analysis of the bZIP transcription factor family in sweet potato wild relative Ipomoea trifida. BMC Genet. 2019, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- LIU, J.; SRIVASTAVA, R.; HOWELL, S.H. Stress-induced expression of an activated form of AtbZIP17 provides protection from salt stress in Arabidopsis. Plant Cell Environ. 2008, 31, 1735–1743. [Google Scholar] [CrossRef]
- Yang, O.; Popova, O.V.; Süthoff, U.; Lüking, I.; Dietz, K.; Golldack, D. The Arabidopsis basic leucine zipper transcription factor AtbZIP24 regulates complex transcriptional networks involved in abiotic stress resistance. Gene 2009, 436, 45–55. [Google Scholar] [CrossRef]
- Baoxiang, W.; Yan, L.; Yifeng, W.; Jingfang, L.; Zhiguang, S.; Ming, C.; Yungao, X.; Bo, X.; Bo, Y.; Jian, L.; et al. OsbZIP72 Is Involved in Transcriptional Gene-Regulation Pathway of Abscisic Acid Signal Transduction by Activating Rice High-Affinity Potassium Transporter OsHKT1;1. Rice Sci. 2021, 28, 257–267. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Y.; Wang, X. bZIP transcription factor OsbZIP52/RISBZ5: A potential negative regulator of cold and drought stress response in rice. Planta 2012, 235, 1157–1169. [Google Scholar] [CrossRef]
- Chakraborty, R.; Baek, J.H.; Bae, E.Y.; Kim, W.; Lee, S.Y.; Kim, M.G. Comparison and contrast of plant, yeast, and mammalian ER stress and UPR. Appl. Biol. Chem. 2016, 59, 337–347. [Google Scholar] [CrossRef]
- Iwata, Y.; Koizumi, N. An Arabidopsis transcription factor, AtbZIP60, regulates the endoplasmic reticulum stress response in a manner unique to plants. Proc. Natl. Acad. Sci. USA 2005, 102, 5280–5285. [Google Scholar] [CrossRef]
- Nagashima, Y.; Mishiba, K.; Suzuki, E.; Shimada, Y.; Iwata, Y.; Koizumi, N. Arabidopsis IRE1 catalyses unconventional splicing of bZIP60 mRNA to produce the active transcription factor. Sci. Rep. 2011, 1, 29. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.; Pincus, D.; Korennykh, A.; Schuck, S.; El-Samad, H.; Walter, P. Homeostatic adaptation to endoplasmic reticulum stress depends on Ire1 kinase activity. J. Cell Biol. 2011, 193, 171–184. [Google Scholar] [CrossRef]
- Siwecka, N.; Rozpędek-Kamińska, W.; Wawrzynkiewicz, A.; Pytel, D.; Diehl, J.A.; Majsterek, I. The Structure, Activation and Signaling of IRE1 and Its Role in Determining Cell Fate. Biomedicines 2021, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Almanza, A.; Mnich, K.; Blomme, A.; Robinson, C.M.; Rodriguez-Blanco, G.; Kierszniowska, S.; McGrath, E.P.; Le Gallo, M.; Pilalis, E.; Swinnen, J.V.; et al. Regulated IRE1α-dependent decay (RIDD)-mediated reprograming of lipid metabolism in cancer. Nat. Commun. 2022, 13, 2493. [Google Scholar] [CrossRef]
- Park, S.; Kang, T.; So, J. Roles of XBP1s in Transcriptional Regulation of Target Genes. Biomedicines 2021, 9, 791. [Google Scholar] [CrossRef]
- Maurel, M.; Chevet, E.; Tavernier, J.; Gerlo, S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem. Sci. 2014, 39, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Bukau, B.; Weissman, J.; Horwich, A. Molecular Chaperones and Protein Quality Control. Cell 2006, 125, 443–451. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).