Exogenous Spermidine Alleviated Waterlogging Damages in Two Varieties of Camellia oleifera
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Experimental Setup
2.3. Growth Analysis
2.4. Photosynthesis Analysis
2.5. Water Status
2.6. Root Activity Measurement
2.7. Proteins, Carbohydrates, and Proline Determination
2.8. Hydrogen Peroxide (H2O2), Hydroxyl Radical (•OH), and Malondialdehyde (MDA) Measurements
2.9. Antioxidant Activities Analysis
2.10. Principal Component Analysis
2.11. Data Analysis
3. Results
3.1. Phenotypic Traits
3.2. Photosynthesis
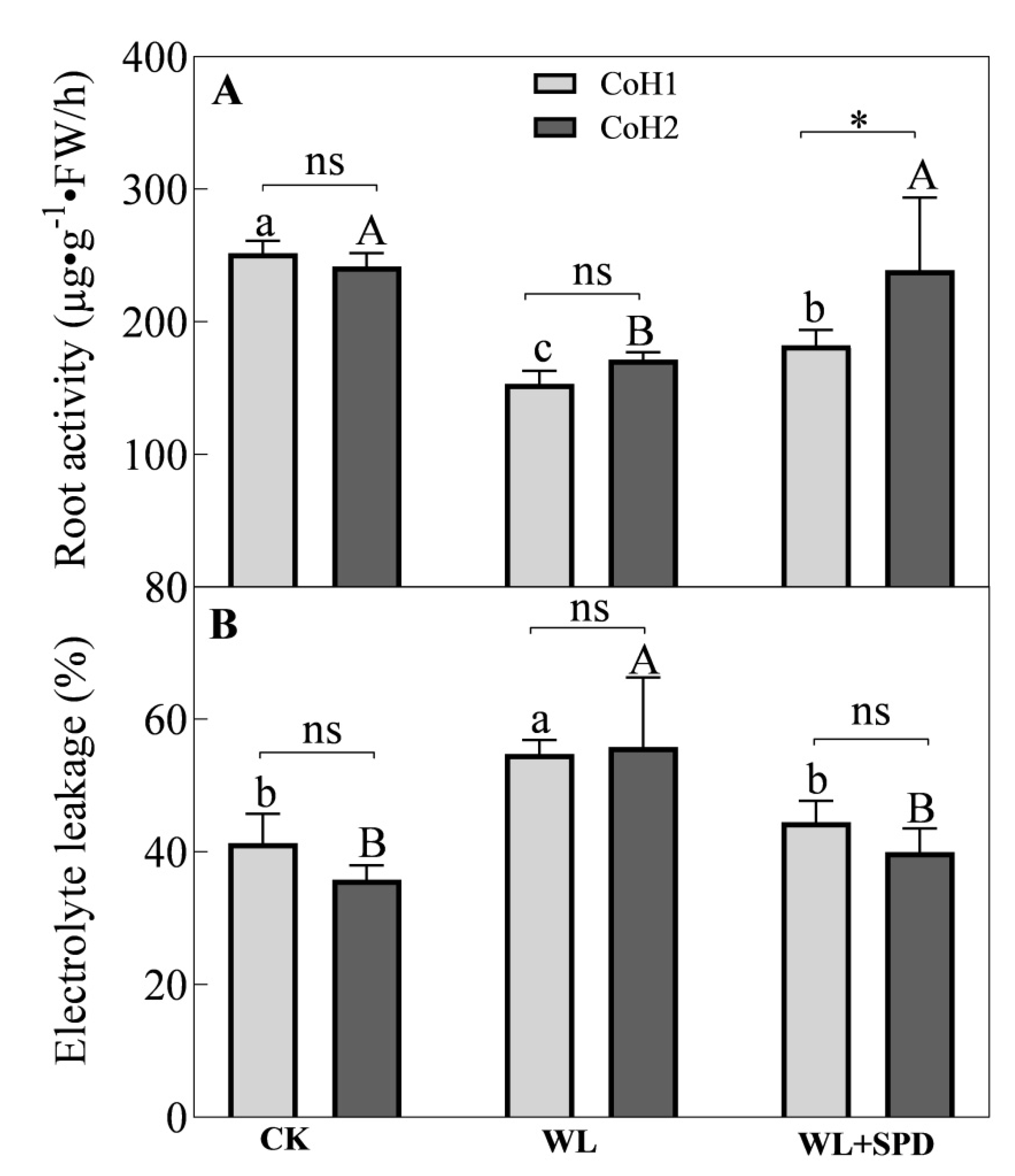
3.3. Water Status and Root Activity
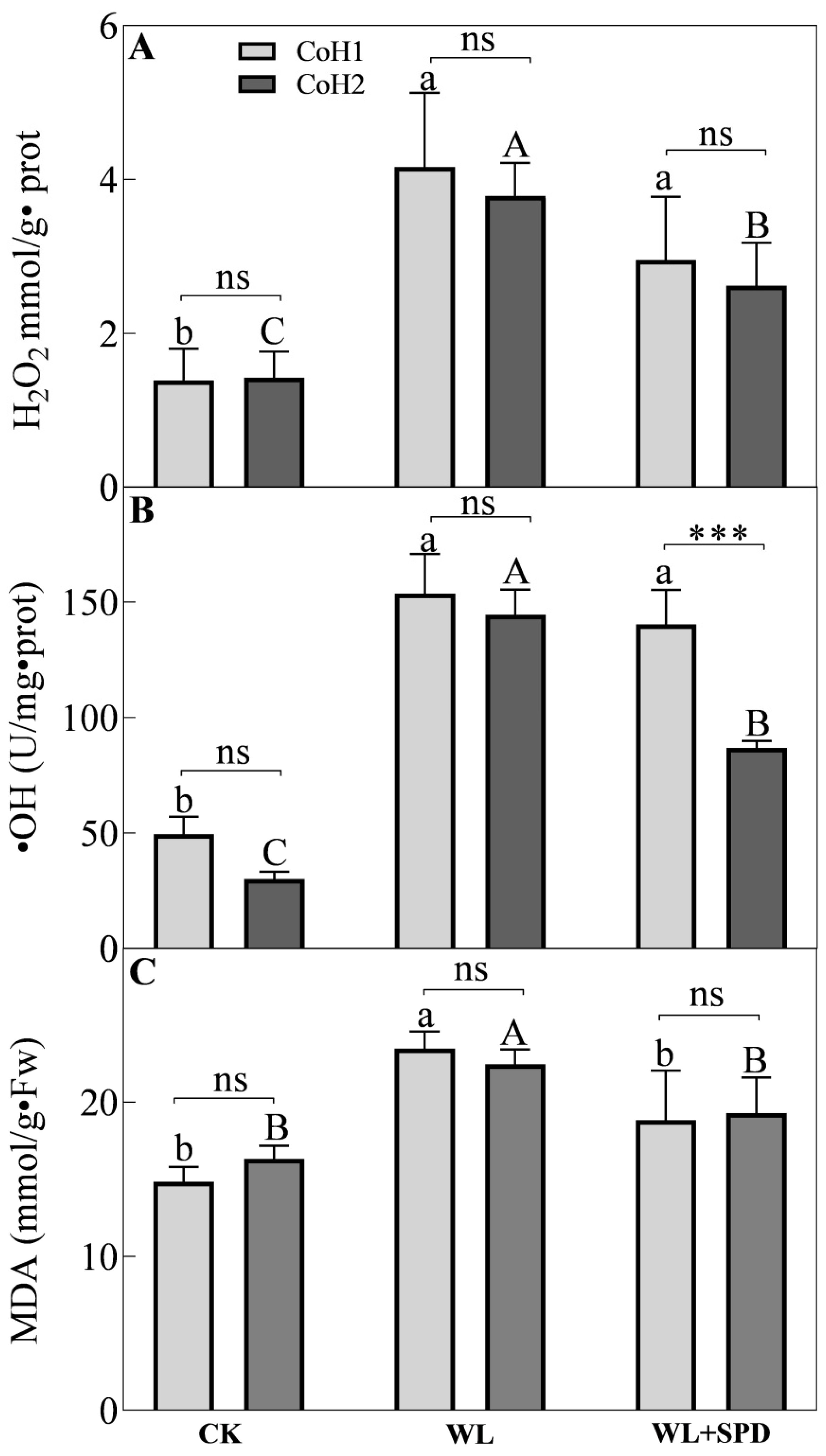
3.4. ROS Accumulation and Lipid Peroxidation
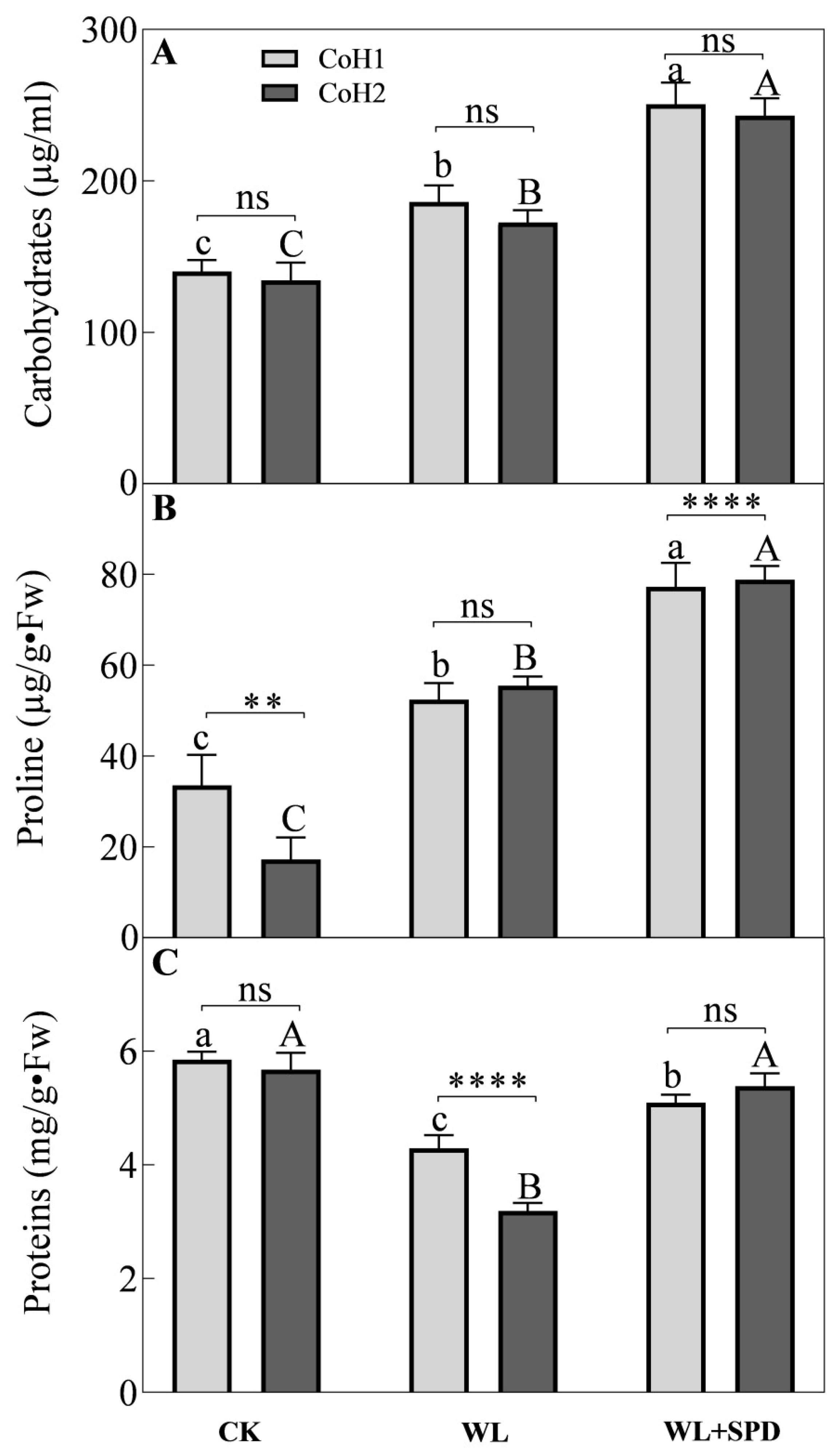
3.5. Osmoprotectants Accumulation and Soluble Proteins
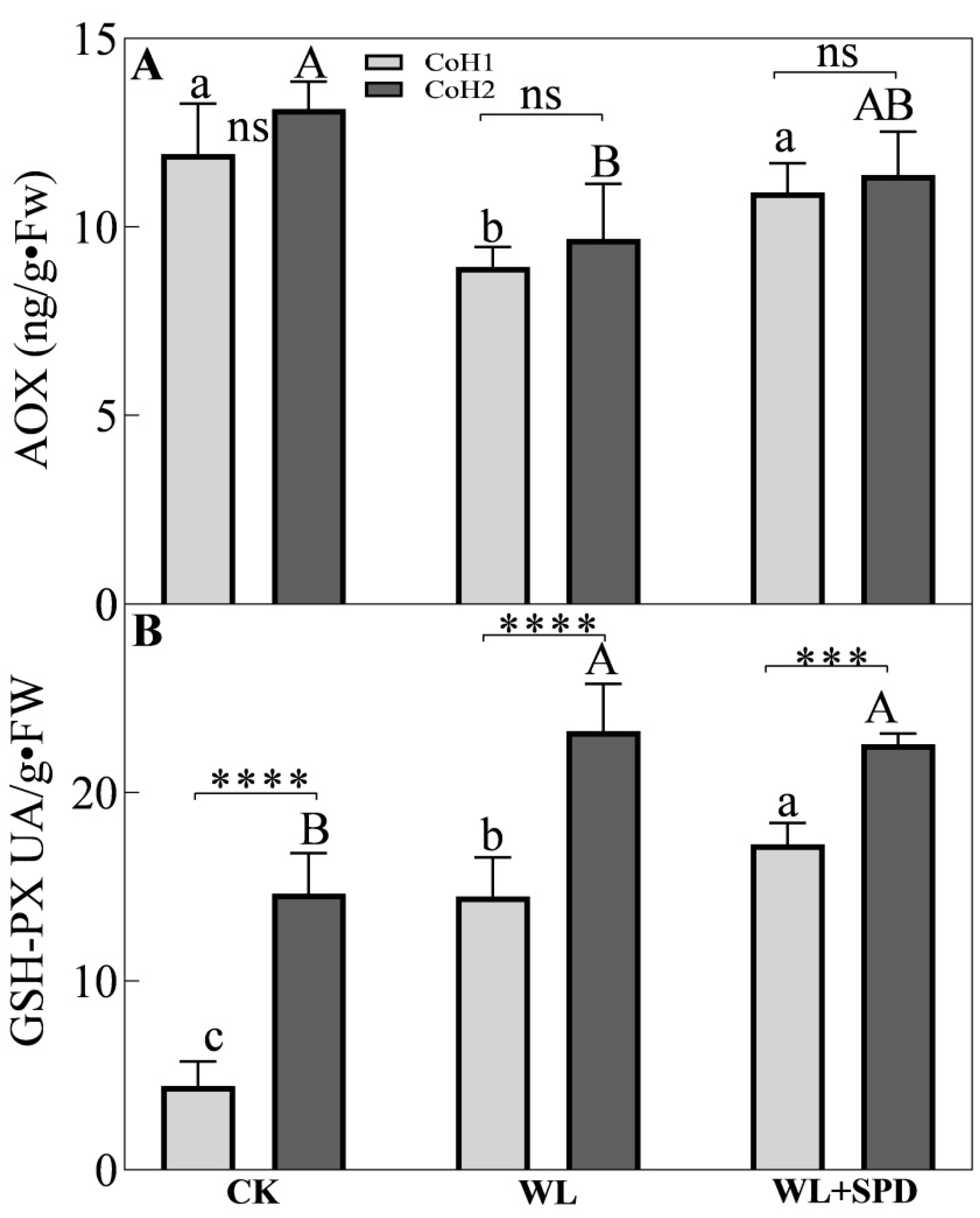
3.6. Antioxidant Enzymatic Activities
3.7. PCA Results
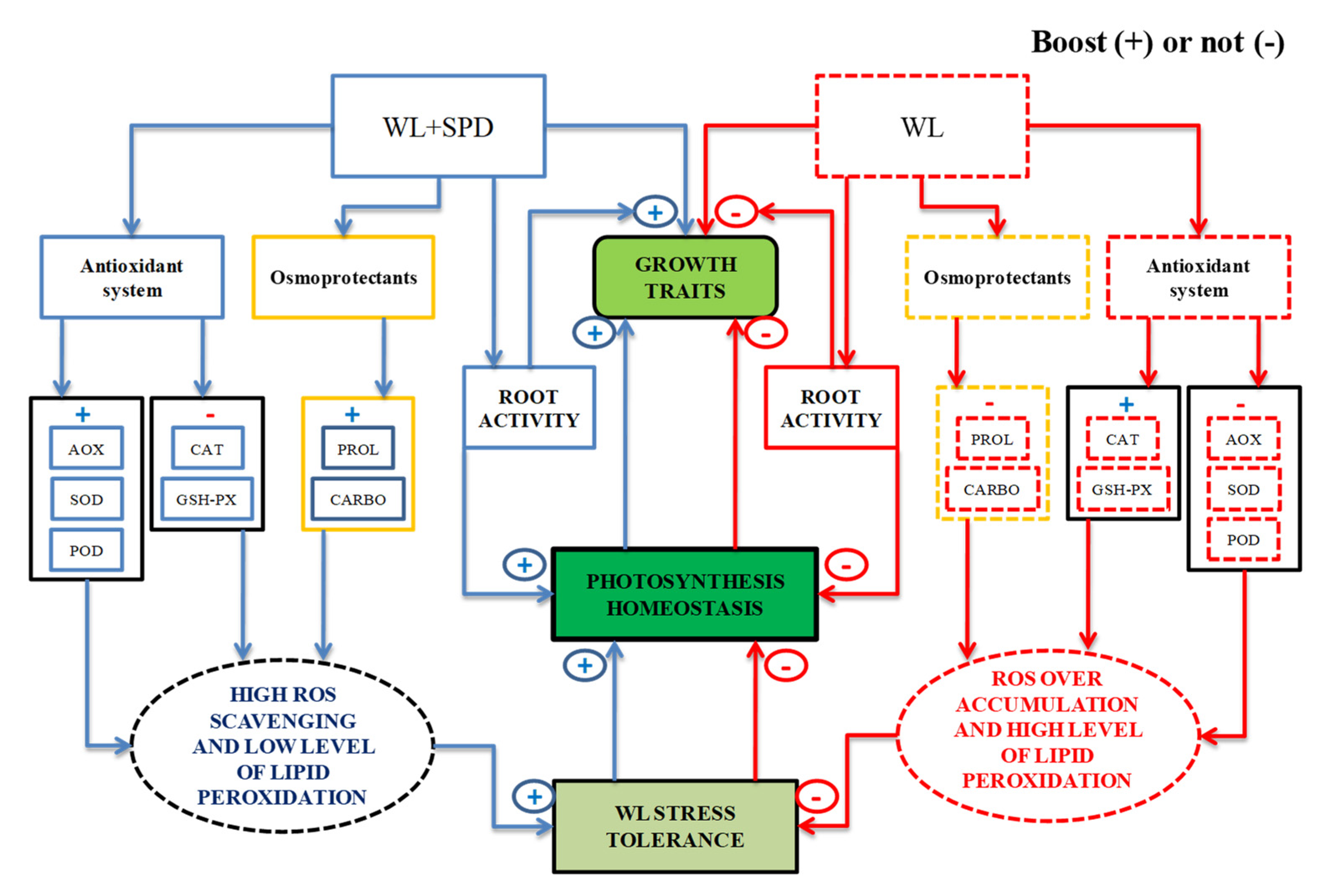
4. Discussion
4.1. Spermidine Affected Root Activity and Morphological Traits of C. oleifera
4.2. Photosynthesis in Both Varieties of C. oleifera Was Significantly Increased by SPD under WL
4.3. SPD Application Alleviated Oxidative Stress in C. oleifera, Particularly in CoH2
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaur, G.; Singh, G.; Motavalli, P.P.; Nelson, K.A.; Orlowski, J.M.; Golden, B.R. Impacts and management strategies for crop production in waterlogged or flooded soils: A review. Agron. J. 2020, 112, 1475–1501. [Google Scholar] [CrossRef]
- Zheng, J.; Ying, Q.; Fang, C.; Sun, N.; Si, M.; Yang, J.; Zhu, B.; Ruan, Y.L.; Zhu, Z.; He, Y. Alternative oxidase pathway is likely involved in waterlogging tolerance of watermelon. Sci. Hortic. 2021, 278, 109831. [Google Scholar] [CrossRef]
- Dagar, J.C.; Minhas, P.S. Introduction. In Agroforestry for the Management of Waterlogged Saline Soils and Poor-Quality Waters; Advances in Agroforestry; Dagar, J., Minhas, P., Eds.; Springer: New Delhi, India, 2016; Volume 13. [Google Scholar]
- Wollmer, A.C.; Pitann, B.; Mühling, K.H. Timing of waterlogging is crucial for the development of micronutrient deficiencies or toxicities in winter wheat and rapeseed. J. Plant Growth Regul. 2019, 38, 824–830. [Google Scholar] [CrossRef]
- Irfan, M.; Hayat, S.; Hayat, Q.; Afroz, S.; Ahmad, A. Physiological and biochemical changes in plants under waterlogging. Protoplasma 2010, 241, 3–17. [Google Scholar] [CrossRef]
- Zaidi, P.H.; Rafique, S.; Rai, P.K.; Singh, N.N.; Srinivasan, G. Tolerance to excess moisture in maize (Zea mays L.): Susceptible crop stages and identification of tolerant genotypes. Field Crops Res. 2004, 90, 189–202. [Google Scholar] [CrossRef]
- Oosterhuis, D.M.; Scott, H.D.; Hampton, R.E.; Wullschleger, S.D. Physiological responses of two soybeans [Glycine max (L.) Merr] cultivars to short-term flooding. Environ. Exp. Bot. 1990, 30, 85–92. [Google Scholar] [CrossRef]
- Griffin, J.L.; Saxton, A.M. Response of solid-seeded soybean to flood irrigation. ii. Flood duration. Agron. J. 1988, 80, 885–888. [Google Scholar] [CrossRef]
- Gu, X.; Xue, L.; Lu, L.; Xiao, J.; Song, G.; Xie, M.; Zhang, H. Melatonin enhances the waterlogging tolerance of Prunus persica by modulating antioxidant metabolism and anaerobic respiration. J. Plant Growth Regul. 2020, 40, 2178–2190. [Google Scholar] [CrossRef]
- Liu, M.; Chu, M.; Ding, Y.; Wang, S.; Liu, Z.; Tang, S.; Ding, C.; Li, G. Exogenous spermidine alleviates oxidative damage and reduce yield loss in rice submerged at tillering stage. Front. Plant Sci. 2015, 6, 919. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Polyamines and abiotic stress tolerance in plants. Plant Signal. Behav. 2010, 5, 26–33. [Google Scholar] [CrossRef]
- Zhou, L.; Cheng, W.; Hou, H.; Peng, R.; Hai, N.; Bian, Z.; Jiao, C.; Wang, C. Antioxidative responses and morpho-anatomical alterations for coping with flood-induced hypoxic stress in grass Pea (Lathyrus sativus L.) in comparison with Pea (Pisum sativum). J. Plant Growth Regul. 2016, 35, 690–700. [Google Scholar] [CrossRef]
- Arbona, V.; Gómez-Cadenas, A. Hormonal modulation of citrus responses to flooding. J. Plant Growth Regul. 2008, 27, 241–250. [Google Scholar] [CrossRef]
- Li, S.; Jin, H.; Zhang, Q. The effect of exogenous spermidine concentration on polyamine metabolism and salt tolerance in zoysia grass (Zoysia japonica Steud) subjected to short-term salinity stress. Front. Plant Sci. 2016, 7, 1221. [Google Scholar]
- Yiu, J.C.; Liu, C.W.; Fang, D.Y.T.; Lai, Y.S. Waterlogging tolerance of Welsh onion (Allium fistulosum L.) enhanced by exogenous spermidine and spermine. Plant Physiol. Biochem. 2009, 47, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, S.; Guo, S.; Sun, Y. Effects of exogenous spermidine on the photosynthesis of Cucumis sativus L. seedlings under rhizosphere hypoxia stress. Front. Agric. China 2008, 2, 55. [Google Scholar] [CrossRef]
- Puyang, X.; An, M.; Xu, L.; Han, L.; Zhang, X. Protective effect of exogenous spermidine on ion and polyamine metabolism in Kentucky bluegrass under salinity stress. Hortic. Environ. Biotechnol. 2016, 57, 11–19. [Google Scholar] [CrossRef]
- Chaydarreh, K.C.; Lin, X.; Guan, L.; Yun, H.; Gu, J.; Hu, C. Utilization of tea oil camellia (Camellia oleifera Abel.) shells as alternative raw materials for manufacturing particleboard. Ind. Crops Prod. 2021, 161, 113221. [Google Scholar] [CrossRef]
- Ma, L.; Rao, X.; Chen, X. Waterlogging tolerance of 57 plant species grown hydroponically. HortScience 2019, 54, 749–753. [Google Scholar] [CrossRef]
- Cao, X.; Jiang, F.; Wang, X.; Zang, Y.; Wu, Z. Comprehensive evaluation and screening for chilling-tolerance in tomato lines at the seedling stage. Euphytica 2015, 205, 569–584. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Y.; Miao, L.F. Comparative physiological and proteomic responses to drought stress in two poplar species originating from different altitudes. Physiol. Plant. 2010, 139, 388–400. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Fan, J.; Hu, Z.; Xie, Y.; Chan, Z.; Chen, K.; Amombo, E. Alleviation of cold damage to photosystem II and metabolisms by melatonin in Bermudagrass. Front. Plant Sci. 2015, 6, 925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Huang, G.Q.; Bian, X.M.; Zhao, Q.G. Effects of nitrogen fertilization and root separation on the plant growth and grain yield of maize and its rhizosphere microorganisms. Ying Yong Sheng Tai Xue Bao 2012, 23, 3369–3376. [Google Scholar] [PubMed]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Cisse, E.H.M.; Miao, L.F.; Yang, F.; Huang, J.F.; Li, D.D.; Zhang, J. Gly betaine surpasses melatonin to improve salt tolerance in Dalbergia odorifera. Front. Plant Sci. 2021, 12, 588847. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Song, R.; Ritonga, F.N.; Yu, H.; Ding, C.; Zhao, X. Effects of exogenous antioxidant melatonin on physiological and biochemical characteristics of Populus cathayana × canadansis ‘Xin Lin 1′ under salt and alkaline stress. Forests 2022, 13, 1283. [Google Scholar] [CrossRef]
- Salah, A.; Zhan, M.; Cao, C.; Han, Y.; Ling, L.; Liu, Z.; Li, P.; Ye, M.; Jiang, Y. γ-Aminobutyric acid promotes chloroplast ultra structure, antioxidant capacity, and growth of waterlogged maize seedlings. Sci. Rep. 2019, 9, 484. [Google Scholar] [CrossRef]
- Leng, X.; Xue, L.; Wang, J.; Li, S.; Yang, Z.; Ren, H.; Yao, X.; Wu, Z.; Li, J. Physiological responses of Handeliodendron bodinieri (Levl.) Rehd. to exogenous calcium supply under drought Stress. Forests 2020, 11, 69. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Yoshida, N.; Fujita, M. Spermidine pretreatment enhances heat tolerance in rice seedlings through modulating antioxidative and glyoxalase systems. Plant Growth Regul. 2014, 73, 31–44. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 01945. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Sharif, R.; Xu, X.; Chen, X. Mechanisms of waterlogging tolerance in plants: Research progress and prospects. Front. Plant Sci. 2021, 11, 627331. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Responses of woody plants to flooding and salinity. Tree Physiol. 1997, 17, 490. [Google Scholar] [CrossRef]
- Ashraf, M. Relationships between growth and gas exchange characteristics in some salt-tolerant amphidiploid Brassica species in relation to their diploid parents. Environ. Exp. Bot. 2001, 45, 155–163. [Google Scholar] [CrossRef]
- Borrell, A.; Carbonell, L.; Farras, R.; Puig-Parellada, P.; Tiburcio, A.F. Polyamines inhibit lipid peroxidation in senescing oat leaves. Physiol. Plant. 1997, 99, 385–390. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, H.; Li, L.; Liu, X.; Chen, L.; Chen, W.; Ding, Y. Exogenous spermidine enhances the photosynthetic and anti-oxidant capacity of rice under heat stress during early grain-filling period. Funct. Plant Biol. 2018, 45, 911–921. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef]
- Duan, J.; Li, J.; Guo, S.; Kang, Y. Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J. Plant Physiol. 2008, 165, 1620–1635. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Shen, H.; Pan, Y.; Guo, B.; Lv, C.; Xu, R. Elucidating the hypoxic stress response in barley (Hordeum vulgare L.) during waterlogging: A proteomics approach. Sci. Rep. 2018, 8, 9655. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Xie, C.; Zhang, H.; Arnao, M.; Ali, M.; Ali, Q.; Muhammad, I.; Shalmani, A.; Nawaz, M.; Chen, P.; et al. Melatonin and its effects on plant systems. Molecules 2018, 23, 2352. [Google Scholar] [CrossRef] [PubMed]
- Cvetkovska, M.; Vanlerberghe, G.C. Alternative oxidase modulates leaf mitochondrial concentrations of superoxide and nitric oxide. New Phytol. 2012, 195, 32–39. [Google Scholar] [CrossRef]
- Vanlerberghe, G.C.; McIntosh, L. Alternative oxidase: From gene to function. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 703–734. [Google Scholar] [CrossRef]


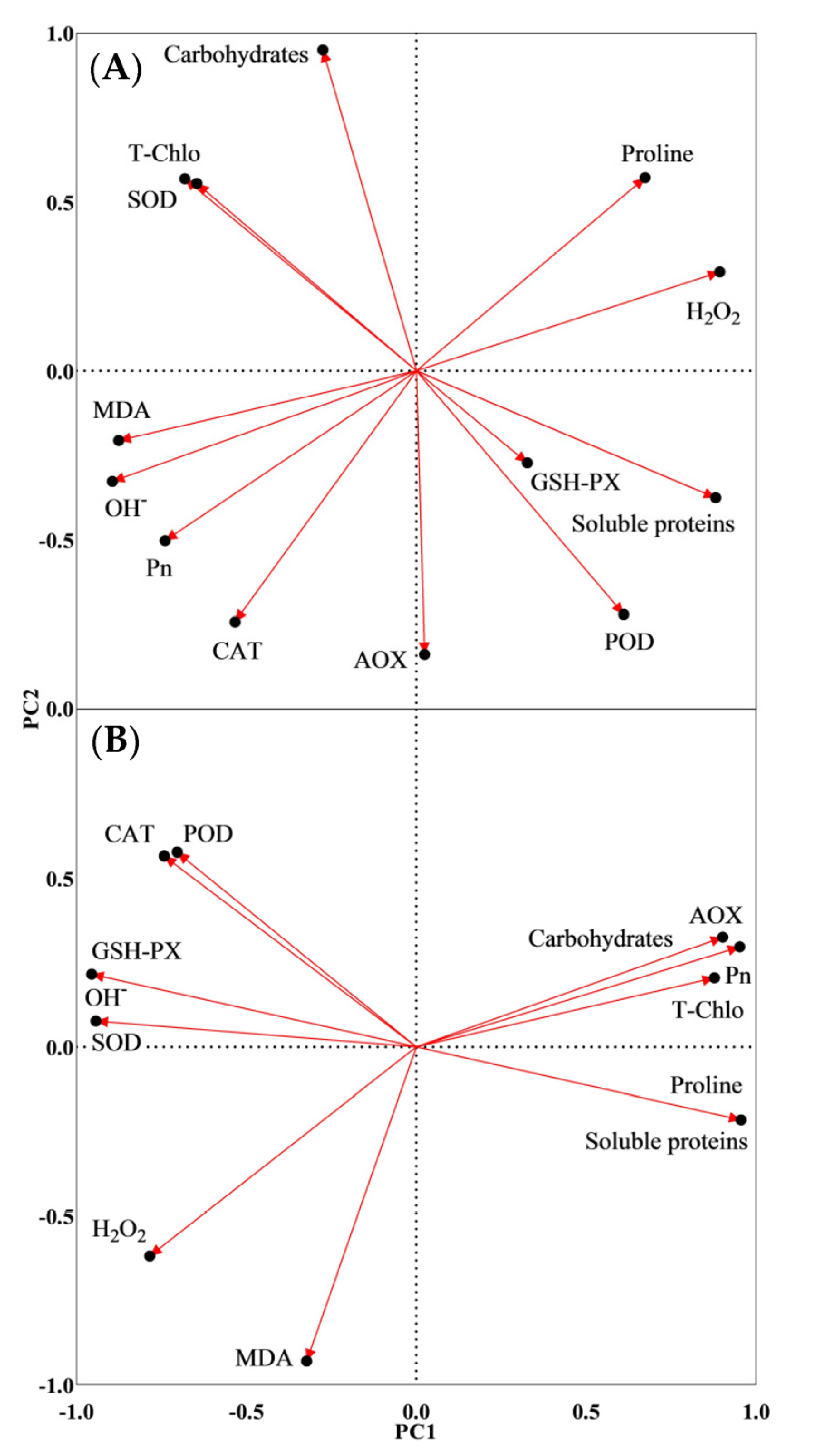

| Control | WL + 0.1 mM SPD | WL + 0.5 mM SPD | WL + 1 mM SPD | |
|---|---|---|---|---|
| Basal diameter of stem | 1 | 0 | 0.403 | 0.06 |
| Plant height | 0 | 0.71 | 1 | 0.79 |
| Number of leaves | 1 | 0.55 | 0.82 | 0 |
| Mean | 0.67 | 0.42 | 0.74 | 0.28 |
| Variety | Treatments | Number of Leaves Increment | Plant Height Increment (cm) | Basal Diameter of Stem Increment (mm) | Below-Ground Fresh Weight (g) | Above-Ground Fresh Weight (g) |
|---|---|---|---|---|---|---|
| C. oleifera (CoH1) | CK | 11.50 ± 1.29 a | 11.00 ± 1.77 a | 3.07 ± 0.55 ab | 13.48 ± 3.26 ab | 5.60 ± 1.02 ab |
| WL | 6.25 ± 1.25 c | 4.82 ± 0.85 c | 2.63 ± 0.36 ab | 9.48 ± 2.17 b | 4.60 ± 1.50 b | |
| WL + SPD | 7.75 ± 1.05 bc | 5.42 ± 1.97 c | 2.84 ± 0.84 ab | 9.65 ± 1.44 b | 4.41 ± 1.32 b | |
| C. oleifera (CoH2) | CK | 12.00 ± 1.41 a | 7.87 ± 1.65 b | 3.48 ± 0.76 a | 17.01 ± 2.77 a | 7.05 ± 1.75 a |
| WL | 8.25 ± 1.70 bc | 7.05 ± 1.05 b | 2.37 ± 0.44 b | 8.85 ± 1.06 b | 4.81 ± 0.75 ab | |
| WL + SPD | 9.75 ± 1.70 ab | 9.00 ± 1.47 ab | 2.84 ± 0.33 ab | 10.65 ± 1.76 ab | 5.00 ± 0.70 ab |
| Variety | Treatments | T-Chl (µg/g.Fw) | Chl-a (µg/g.Fw) | Chl-b (µg/g.Fw) | Car (µg/g.Fw) | Pn (μmol·m−2·s−1) | Gs (mol·m−2·s−1) | Ci (μmol·mol−1) | Trr (mmol·m−2·s−1) | Wue (µmol·mmol−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| C. oleifera (CoH1) | CK | 813.56 ± 13.1 b | 606.7 ± 8.23 b | 206.8 ± 16.30 c | 202.50 ± 11.40 b | 5.77 ± 0.99 b | 0.10 ± 0.020 b | 396.00 ± 31.17 d | 3.64 ± 0.84 b | 1.58 ± 0.43 c |
| WL | 432.42 ± 17.6 e | 317.1 ± 5.03 f | 115.3 ± 4.06 f | 132.90 ± 8.50 e | 2.76 ± 0.90 e | 0.04 ± 0.009 d | 403.50 ± 40.21 c | 1.74 ± 0.42 f | 1.75 ± 0.95 a | |
| WL + SPD | 739.06 ± 22.3 c | 546.7 ± 12.60 d | 192.2 ± 7.31 d | 187.03 ± 13.01 c | 3.76 ± 0.61 d | 0.05 ± 0.010 cd | 389.00 ± 16.98 e | 2.24 ± 0.54 e | 1.67 ± 0.28 b | |
| C. oleifera (CoH2) | CK | 900.83 ± 26.4 a | 654.2 ± 20.01 a | 246.6 ± 9.13 a | 231.70 ± 8.80 a | 7.35 ± 1.64 a | 0.13 ± 0.014 a | 354.66 ± 41.13 f | 4.1 ± 1.09 a | 1.78 ± 0.98 a |
| WL | 552.45 ± 17.4 d | 393.3 ± 7.36 e | 159.1 ± 14.11 e | 132.15 ± 4.21 e | 3.53 ± 0.58 d | 0.06 ± 0.010 c | 436.57 ± 23.56 b | 2.37 ± 0.76 d | 1.64 ± 0.65 bc | |
| WL + SPD | 795.37 ± 10.3 c | 559.6 ± 5.12 c | 235.6 ± 8.03 b | 162.82 ± 3.83 d | 4.06 ± 0.40 c | 0.09 ± 0.012 b | 445.80 ± 38.92 a | 3.41 ± 1.29 c | 1.34 ± 0.59 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cisse, E.-H.M.; Huang, J.-F.; Li, D.-D.; Miao, L.-F.; Xiang, L.-S.; Yang, F. Exogenous Spermidine Alleviated Waterlogging Damages in Two Varieties of Camellia oleifera. Forests 2023, 14, 91. https://doi.org/10.3390/f14010091
Cisse E-HM, Huang J-F, Li D-D, Miao L-F, Xiang L-S, Yang F. Exogenous Spermidine Alleviated Waterlogging Damages in Two Varieties of Camellia oleifera. Forests. 2023; 14(1):91. https://doi.org/10.3390/f14010091
Chicago/Turabian StyleCisse, El-Hadji Malick, Jin-Fu Huang, Da-Dong Li, Ling-Feng Miao, Li-Shan Xiang, and Fan Yang. 2023. "Exogenous Spermidine Alleviated Waterlogging Damages in Two Varieties of Camellia oleifera" Forests 14, no. 1: 91. https://doi.org/10.3390/f14010091
APA StyleCisse, E.-H. M., Huang, J.-F., Li, D.-D., Miao, L.-F., Xiang, L.-S., & Yang, F. (2023). Exogenous Spermidine Alleviated Waterlogging Damages in Two Varieties of Camellia oleifera. Forests, 14(1), 91. https://doi.org/10.3390/f14010091







