Abstract
A new subgenus of the genus Chrysolina Motschulsky, 1860, endemic to Yunnan Province of China, Ch. (Volosatik subgen. nov.) is described. All species inhabit highland forests. Seven species, including four species new to science, are recognized: Chrysolina fascinatrix Lopatin, 1998, Ch. liqingzhaoae Daccordi et Ge, 2011, Ch. wangi Lopatin, 2005, Ch. igori sp. nov., Ch. marinae sp. nov., Ch. genriki sp. nov., and Ch. ilyakabaki sp. nov. The representatives of this new subgenus are characterised by a small, broadly oval, convex, shining body with metallic dorsum, with maxillary palpus narrow, similar in both sexes, with pronotal lateral impressions wide and shallow or obsolete, without numerous large punctures, with elytral puncture rows paired, mostly regular, intervals flat, with tarsomeres 1–3 with entire sole, rather narrow in both sexes, with aedeagus bearing sensilla of different types ventro-laterally near apex, flagellum narrow. Photographs of habitus and male aedeagus are presented.
Keywords:
Coleoptera; Chrysomelidae; Chrysomelinae; Chrysolina; new subgenus; new species; taxonomy; China; Yunnan 1. Introduction
urn:lsid:zoobank.org:pub:F1D5994D-3850-42E9-80A3-D4B0BBE4FDD9 (accessed on 29 December 2022)
Yunnan Province is the most southwestern province of China, bordering Laos and Vietnam to the south, Myanmar to the west, Tibet Autonomous Region and Sichuan Province to the north, Guizhou Province and Guangxi Zhuang Autonomous Region to the east.
The fauna of insects and other invertebrates of Yunnan province has great diversity and originality [1,2,3,4,5]. As for the genus Chrysolina Motschulsky, 1860, the subgenus Ch. (Timarchomela Achard, 1922) was described from this province, seven species of which are endemic to this province, as well as a number of species from other groups [6]. A total of 23 species of Chrysolina are known in Yunnan Province [6]. This is very small for such a large mountainous region with a favorable climate and a variety of landscapes. This indicates that the species of the genus Chrysolina are not yet sufficiently studied in Yunnan. This article aims to partially fill this gap in knowledge.
In 1998, Lopatin [7] described from Yunnan a very peculiar, unlike anything Ch. fascinatrix Lopatin, 1998. Later, Ch wangi Lopatin, 2005 and Ch. liqingzhaoae Daccordi, Ge, 2011 were described from this Province [8,9]. However, the authors did not see any similarities between them. The present study of comparative morphology has shown the close relationship between these species. As a result of studying additional materials, four more new species from this group were found. The species of this group are very close to each other in external morphology. Only the study of male genitalia often makes it possible to accurately identify species. A taxonomic review of this group of species is the subject of this article. In view of the originality of this group and its good separation from other groups within the genus Chrysolina, I propose to consider it as a new subgenus. Since the original descriptions of Ch fascinatrix, Ch. liqingzhaoae, and Ch. wangi did not include many characters important for subgenus and species identification [6], they are redescribed below, based on the type and additional specimens.
2. Materials and Methods
Exact label data are cited for all specimens. The examined materials are deposited in the following collections:
ZIN: Zoological Institute of the Russian Academy of Sciences, St.-Petersburg, Russia (B. A. Korotyaev, A. G. Moseyko and S.V. Andreeva);
BC: the author’s collection, Zelenograd, Russia;
DC: Mauro Daccordi collection, Verona, Italy;
RC: Pavel V. Romantsov collection, St.-Petersburg, Russia.
First of all, sensilla on the male aedeagus of the most numerous and widespread species, Ch. fascinatrix, was studied. Scanning electron microscope study was carried out using Leica EM CPD300 (Leica Microsystems, Wetzlar, Germany), S150A Sputter Coater (Edwards, West Sussex, UK), and TESCAN MIRA 3 LMH (TESCAN, Brno, Czech Republic), preparation of samples for microscopic analysis—with stereomicroscope Leica S9D (Leica Microsystems, Wetzlar, Germany).
Then, the morphological variability of Ch. fascinatrix, as the most variable species, was studied. 41 specimens (27 males and 14 females) were examined. On this basis, the limits of intraspecific variability were found for further taxonomic revision. The following characters were studied: (1) number of punctures in the 5th elytral row, (2) diameter of puncture in 5th elytral row near mid-length, (3) length of aedeagus in lateral view, absolute and relative to body length, (4) extension of the aedeagus in the direction from the base to the apex (the ratio of the greatest width before apex to the smallest width at base).
Finally, a taxonomic revision was carried out.
Holotypes of all new species are deposited in ZIN.
The holotypes of the new species were soaked, the aedeagus was dissected, and the characters of the underside of the thorax and abdomen, the soles of the tarsi, and the pygidium were studied. Then these specimens were placed (glued) on cardboard plates for photography and better preservation. The morphology description plan and terminology of structural details follow Bieńkowski [6]. Photographs were made by a Nikon D 90 digital camera, combined with Tamron SP 70–300 mm F/4–5.6 and inverted Vega-12B 2.8–90 lenses, using Helicon Remote software. Images of the same objects at different focal planes were combined using Helicon Focus software.
3. Results
3.1. Electron Microscopic Study of the Sensilla on the Aedeagus
An electron microscopic study of the aedeagus was made on non-type specimens of Ch. fascinatrix (Figure 1) Aedeagus on the ventral side at the very apex, more apically than apical denticles, bears sensilla campaniformia (diameter 2.0 μM); behind the apex on latero-ventral side bears a group of trichoid sensilla (about 85–135 μM long, maximum diameter 6 μM) (Figure 1a). From this group of sensilla towards the base of the aedeagus there is an area occupied by basiconic sensilla (1.7 μM long, maximum diameter 2.8 μM) (Figure 1b). There are also microscopic pores (diameter 0.6 μM) in this area.
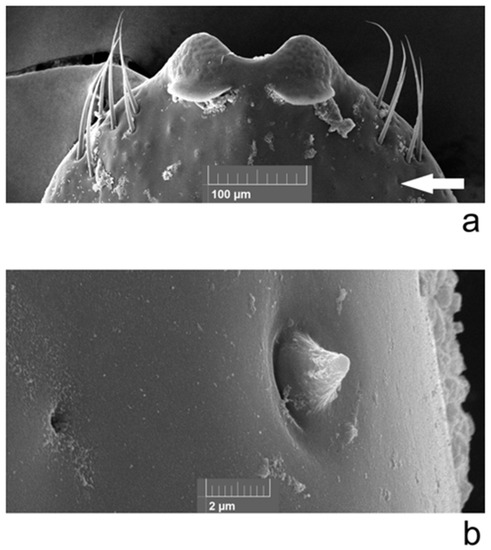
Figure 1.
Chrysolina fascinatrix, male aedeagus, ventral view: (a) trichoid sensilla (arrow shows location of basiconic sensilla and pores); (b) pore (left) and basiconic sensilla (right).
3.2. Study of the Morphological Variability of Chrysolina Fascinatrix
3.2.1. Number of Punctures in the 5th Elytral Row
According to the number of punctures, two groups are distinguished: from 9 to 15 and from 20 to 30 punctures in a row (Figure 2). A difference between females and males is not found.
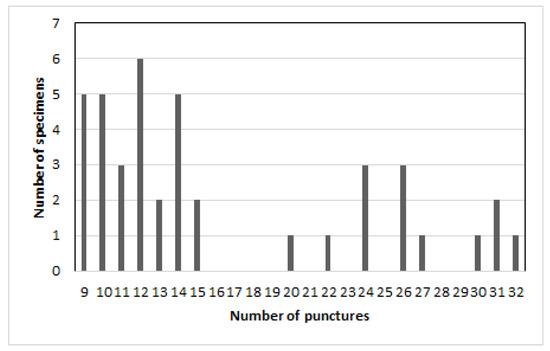
Figure 2.
Chrysolina fascinatrix, number of punctures in 5th elytral row.
3.2.2. Diameter of Puncture in 5th Elytral Row near Mid-Length
The diameter of punctures varies by almost five times from about 0.02 to 0.1 mm with a predominance of average values (Figure 3). Difference between females and males is not found.
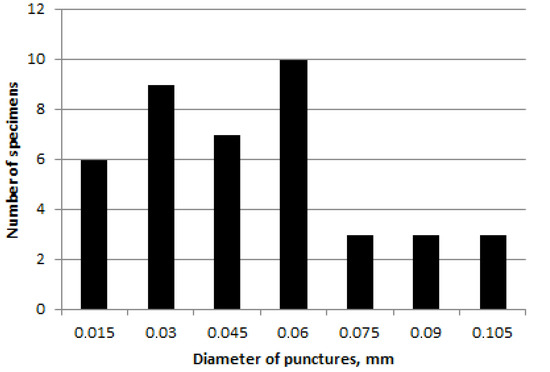
Figure 3.
Chrysolina fascinatrix, diameter of punctures in 5th elytral row.
3.2.3. Length of Aedeagus in Lateral View
The length of the aedeagus varies considerably from 1.5 to 2.1 mm with a predominance of average values (Figure 4 and Figure 5). However, the relative length remains approximately the same, about 0.3 X of the body length.
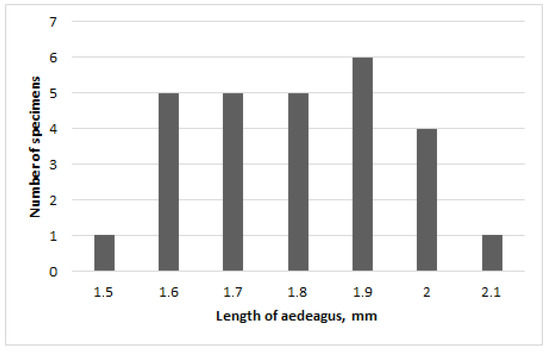
Figure 4.
Chrysolina fascinatrix, length of male aedeagus.
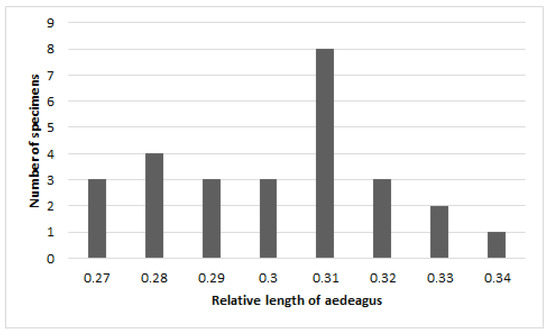
Figure 5.
Chrysolina fascinatrix, relative length of male aedeagus.
3.2.4. Extension of the Aedeagus in the Direction from the Base to the Apex (the Ratio of the Greatest Width before the Apex to the Smallest Width at the Base)
The shape of the aedeagus, namely the degree of its expansion towards the apex, varies considerably: from almost parallel-sided to one and a half times widened at the apex, with a predominance of average values (Figure 6).
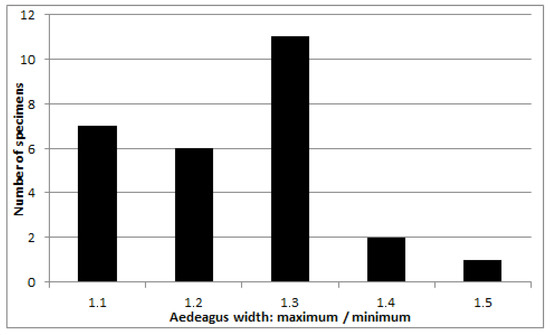
Figure 6.
Chrysolina fascinatrix, male aedeagus, the ratio of the greatest width before apex to the smallest width at base.
3.3. Chrysolina (Volosatik Bieńkowski Subgen. nov.)
urn:lsid:zoobank.org:act:3923965F-3B7F-46DB-895D-8AA90074892F (accessed on 29 December 2022).
Description. Body oval, convex, 5.1–7.4 mm long.
Body more or less shining, entirely metallic, differently colored but never with combination of metallic green and purple spots and stripes; with antennae, maxillary palpi and legs dark, wholly rufous, or partly rufous.
Last maxillary palpomere barrel-shaped, 1.2–1.5 X as long as wide, 1–1.3 X wider than penultimate one, almost similar in both sexes.
Antennal insertion 2–3 X closer to clypeus than to eye.
Pronotum without anterior setiferous pores; with anterior side marginated and ciliate. Pronotum with shallow to obsolete, broad impression; impression smooth or with some moderately large punctures. Lateral callus, convex.
Prothoracic hypomeron, weakly convex, with obsolete lateral impression, smooth or filled with weak wrinkles, without basal fold.
Metasternum, entirely marginated anteriorly.
Elytron with obsolete humeral callus or without callus. Elytral puncture rows paired, regular in most species except Ch. wangi. Abbreviated scutellar row present. All intervals, flat.
Elytral epipleuron inclined outside, visible along entire length in lateral view, apically with rather sparse to very few setae.
Hind wings, absent.
Pygidium with broad impression in basal ½, and convex, without impression in apical ½.
Last abdominal sternite, simple, evenly convex in both sexes.
Tarsomeres 1–3 with entire sole in both sexes, narrow in female, slightly broadened in male. Claw tarsomere without denticles.
Aedeagus with apex trapezoidal or roundly trapezoidal, or rounded, bearing sensilla of different types ventro–laterally near apex. Flagellum narrow, exposed.
Distribution: China (NW Yunnan).
Type species: Chrysolina fascinatrix Lopatin, 1998
Etymology. The subgenus is named after the Russian word meaning “bearing hairs”—according to the main distinguishing feature of the subgenus, rather rare in the genus Chrysolina. Grammatical gender: masculine.
Differential diagnosis.
Chrysolina jiangi Lopatin, 2006 and Ch. geae Lopatin, 2006 (both from Sichuan, not assigned to any subgenus) resembles the members of the present subgenus (especially Ch. fascinatrix—by the aedeagus structure) but differ in elytral punctures mostly irregular, and pronotal lateral impression narrow and deep [6].
Chrysolina (Pezocrosita) roborowskii (Jacobson, 1895) from Tibet is also similar to the present subgenus due to small size (4.9–5.4 mm long), bronze dorsal color, narrow last maxillary palpomere, absence of anterior setiferous pores of pronotum, slight lateral impression of pronotum, absence of prothoracic basal fold, presence of regular rows of elytral punctures, absence of hind wings, absence of depression in apical half of pygidium, simple and convex last abdominal sternite, complete sole of tarsomeres 1–3 in both sexes, weakly widened tarsi of male, aedeagus with apical denticles ventrally, and narrow flagellum. Chrysolina roborowskii represents a separate species group formally assigned to the subgenus Ch. (Pezocrosita Jacobson, 1901). The subgenus Pezocrosita is very heterogeneous, includes many clearly unrelated groups of species, and Ch. roborowskii is morphologically far from the type species of the subgenus [6]. I do not include Ch. roborowskii in the subgenus Ch. (Volosatik nov.) because this species is characterized by the following significant differences: elytral puncture rows are equidistant, scutellar puncture row absents, apical sensilla of aedeagus absent.
3.3.1. Chrysolina (Volosatik) fascinatrix Lopatin, 1998
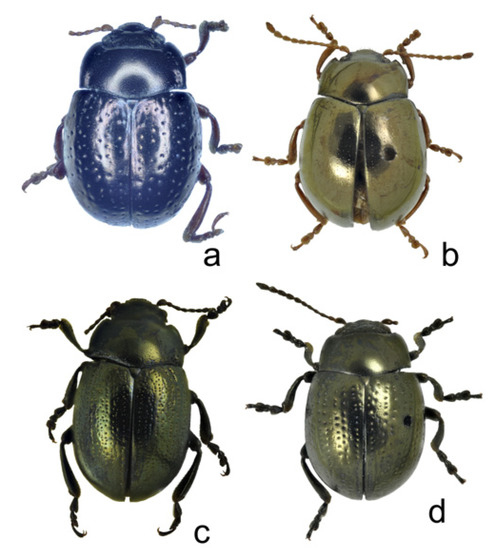
Figure 7.
Chrysolina, total dorsal view: (a) Ch. fascinatrix, holotype, male; (b) Ch. liqingzhaoae, paratype, male; (c) Ch. wangi, holotype, male; (d) Ch. marinae sp. nov., holotype, male.
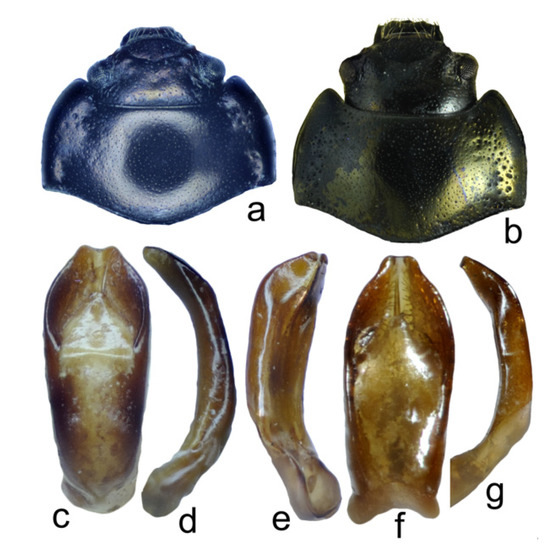
Figure 8.
Chrysolina, structural details: (a) Ch. fascinatrix, holotype, male, head and pronotum; (b) Ch. wangi, holotype, male, head and pronotum; (c) Ch. fascinatrix, holotype, male, aedeagus, dorsal view; (d) the same, lateral view; (e) Ch. wangi, holotype, male, aedeagus, latero-ventral view; (f) the same, dorsal view; (g) the same, lateral view.
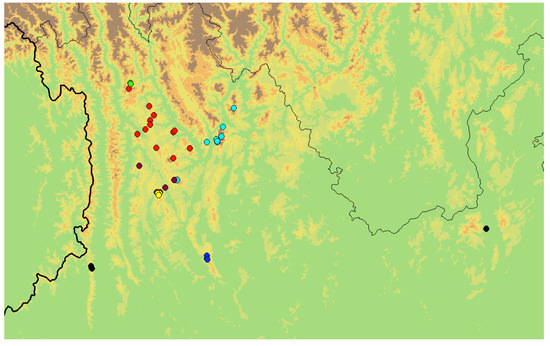
Figure 9.
Distribution of the species of the subgenus Chrysolina (Volosatik): (green) Ch. igori sp. nov.; (red) Ch. fascinatrix; (light blue) Ch. liqingzhaoae; (purple) Ch. marinae sp. nov.; (yellow) Ch. genriki sp. nov.; (black) Ch. wangi; (dark blue) Ch. ilyakabaki sp. nov.
Chrysolina fascinatrix Lopatin, 1998: 831.
Type locality. China, NW Yunnan, 20 km E Weixi.
Redescription. Body broadly oval, convex. Dorsal side, dark metallic, differently colored: violet, golden, blue, brassy, coppery, bronze, green, or black with weak bronze tint, or head and pronotum coppery (or brassy), elytra green. Antennae, entirely rufous, or black with antennomeres 1 and 2 rufous below, or entirely black. Legs, entirely rufous, or with femora mostly dark metallic, and rest parts rufous, or dark metallic with tarsi black, or entirely black. Head and pronotum sericeous, shining, shagreen and micropunctulate, elytra very shining, smooth, micropunctulate, or dorsum entirely very shining, almost smooth, micropunctulate and obsoletely reticulate.
Last maxillary palpomere, barrel-shaped, tapering equally towards base and apex, or with narrow base and broad apex, 1.3–1.5 X as long as wide, 1.3–1.4 X longer and 1.2–1.3 X wider than penultimate palpomere, similar in both sexes.
Antenna inserted 3 X closer to clypeus than to eye.
Pronotum 1.8–1.9 X as wide as long, with rounded lateral sides, broadest at mid-length, narrower at the apex than at the base, or broadest basally. Lateral callus convex along entire length, separated from disc by broad shallow impression filled with sparse, not numerous (about 12–20) moderately large punctures. Disc evenly covered by fine (0.02 mm) numerous punctures. Pronotum, anteriorly marginated and ciliate. Anterior setiferous pore of pronotum, absent.
Prothoracic hypomeron almost flat (slightly convex), with smooth obsolete lateral impression, without wrinkles. Basal fold of prothoracic hypomeron, absent.
Metasternum marginated anteriorly.
Elytron with obsolete humeral callus. Elytral punctures arranged in abbreviated scutellar row and 10 regular entire rows; rows 2–3, 4–5, 6–7, and 8–9 paired. Punctures from small (about 0.02 mm) to large (0.10 mm), foveiform. The 5th row consists of 9–32 punctures. Intervals flat. Broad intervals between paired rows covered by irregular punctures (0.03–0.04 mm) in some specimens.
Elytral epipleuron inclined outside, visible along entire length in lateral view; with few setae apically.
Hind wings, absent.
Tarsomeres 1–3 narrow, with entire sole in both sexes, with fore- and mid-tarsomeres 1 and 3 slightly broadened in males.
Claw tarsomere without denticles beneath.
Pygidium convex, without impression in apical 1/2, and with shallow impression in basal 1/2.
Last abdominal sternite, simple, convex in both sexes, with apical margin straight in males, arc-shaped in females.
Aedeagus arc-shaped, depressed dorso-ventrally, slightly or distinctly broadened laterally towards apical orifice, with apical margin trapezoidal, shortly drawn at the top and provided with shallow emargination, with cover plate triangular, developed in basal 1/3–2/3 of apical orifice, with two obtuse or acute apical denticles on ventral side, with numerous latero-ventral sensilla of different types at apex. Flagellum narrow, exposed.
Body length 5.4–6.8 mm (males), 7.1–7.6 mm (females).
The total number of specimens examined: 45.
Type material examined: holotype: China, NW Yunnan, 20 km O Weixi, 3300 m, 13.VIII.1996, A. Zamotailov, A. Miroschnikov leg.: male (ZIN).
Additional specimens examined: China, Yunnan, SW Guoditang, 6.4 km ENE of Weixi City, 27°12′38″ N/99°21′22″ E, H = 3235 m, 29.V.2016, I. Kabak, G. Davidian leg.: 2 males, 2 females (BC); China, Yunnan, NW Lijiang, W Chang J., NW Jinzhuang, 4 km N Ana Vill., 27°10′45″ N/99°39′47″ E, H = 3490 m, 20.V.2017, I. Belousov, G. Davidian, I. Kabak leg.: 1 male, 2 females (BC); China, Yunnan, W Lijiang, W Yangtze, near plateau, NW of Dashitou Vill., 27°00′11″ N/99°50′24″ E, H = 3610 m, 3.VI.2018, I. Belousov, I. Kabak leg.: 4 males, 1 female (BC), 1 male, 1 female (RC); China, Yunnan, NNE Weixi City, 2.75 km SW Gejupo Vill., 27°21′43″ N/99°26′57″ E, H = 3150 m, 7.VI.2015, I. Belousov, I. Kabak, G. Davidian leg.: 1 female (BC); China, Yunnan, NNE Weixi City, river tributary of Lapugou River, 5.2 km ENE Jizong, 27°27′36″ N/99°23′53″ E, H = 3480 m, 5.VI.2015, I. Belousov, I. Kabak, G. Davidian leg.: 1 male, 1 female (BC); China, Yunnan, NE Weixi City, 4.1 km WNW Yanguangean, 27°15′32″ N/99°24′28″ E, H = 3595 m, 10.VI.2015, I. Belousov, I. Kabak, G. Davidian leg.: 4 males, 3 females (BC), 2 males (RC); China, Yunnan, NW Lijiang, W Chang J., NW Jinzhuang, 5 km SW Dalu Vill., 27°11′31″ N/99°40′39″ E, H = 3630 m, 19.V.2017, I. Belousov, G. Davidian, I. Kabak leg.: 1 male, 2 females (BC); China, Yunnan, Laojunshan Mts., 6.26 km SSW Segengsheng, 27°0′20″ N/99°28′33″ E, H = 3575 m, 6.VI.2016, I. Kabak, G. Davidian leg.: 1 female (BC); China, Yunnan, Laojunshan, watersh. riv. to Shigu and Liming, 26°53′32″ N/99°39′43″ E, H = 3795 m, 3.VI.2014, I. Belousov, I. Kabak leg.: 1 male (BC), 1 male (RC); China, Yunnan, Deqen, Weixi, Dewei Line, Mountain Range between Xiaruolisuzuxiang and Yezhizhen, 27°39′5″ N/99°10′26″ E, H = 3785 m, 13.VI.2013, I. Belousov, I. Kabak, G. Davidian leg.: 2 males (BC); China, Yunnan, Diancan Shan, 0.9 km NW Mt. Lianhuafe, 25°47′39″ N/100°01′40″ E, H = 3675 m, 28.V.2019, I. Belousov, G. Davidian, I. Kabak leg.: 3 males (RC); China, Yunnan, Diancan Shan, 0.8 km ENE Mt. Wutaifeng, 25°49′27″ N/100°01′40″ E, H = 3665 m, 27.V.2019, I. Belousov, G. Davidian, I. Kabak leg.: 1 male, 2 females (RC); China, Yunnan, NE Weixi City, 5 km ESE Rilidi, 27°18′14″ N/99°24′24″ E, H = 3605 m, 9.VI.2015, I. Belousov, I. Kabak, G. Davidian leg.: 3 males, 1 female (BC).
Comments. It was originally described from a pair (male and female) from one locality, so the variability has hardly been studied. The examination of specimens from different localities showed that the species is variable in coloration of dorsal side, legs, antennae, shape of the last maxillary palpomere, number and size of elytral punctures, shape of the aedeagus.
In the original diagnosis, it was compared only with Ch. (Allohypericia) perforata pallidipes L.Medvedev, 1980 (synonym of Ch. perforata simillima Mohr, 1966), with which it has some superficial resemblance, but is not taxonomically related at all [6].
3.3.2. Chrysolina (Volosatik) liqingzhaoae Daccordi et Ge, 2011
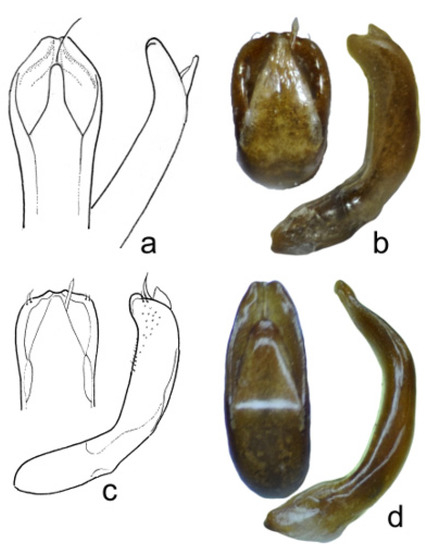
Figure 10.
Chrysolina, male aedeagus, dorsal and lateral view: (a) Ch. liqingzhaoae, paratype; (b,c) Ch. marinae sp. nov., holotype; (d) Ch. genriki sp. nov., holotype.
Chrysolina liqingzhaoae Daccordi et Ge in: Daccordi, Ge, Cui, Yang, 2011: 300.
Type locality. China, NW Yunnan, 27.07° N, 100.03° E, Yulongshan Mts.
Redescription. Body broadly oval, convex. Dorsal side, dark metallic, differently colored: bronze, brassy, golden-green, or head and pronotum bronze, elytra blue. Antennae, entirely rufous, or black with antennomeres 1 and 2 rufous below. Legs, entirely rufous or dark metallic, or femora and tibiae dark metallic with tarsi piceous or rufous. Microrelief varies: (1) dorsal side entirely very shining, smooth, micropunctulate, (2) head and pronotum sericeous shining, shagreen and micropunctulate, elytra very shining, smooth, micropunctulate, (3) dorsal sericeous shining, shagreen, and micropunctulate.
Last maxillary palpomere, barrel-shaped, tapering equally towards base and apex, or with narrow base and broad apex, 1.4–1.5 X as long as wide, 1.5–1.6 X longer and 1.2 X wider than penultimate palpomere, similar in both sexes.
Antenna inserted 3 X closer to clypeus than to eye.
Pronotum 1.8–1.9 X as wide as long, with rounded lateral sides, broadest at mid-length, narrower at the apex than at the base. Lateral callus convex along entire length, separated from disc by broad shallow impression filled with sparse, not numerous (about 5–15) moderately large punctures or without them. Disc evenly covered by fine (0.02 mm) numerous punctures. Pronotum anteriorly marginated and ciliate. Anterior setiferous pore of pronotum absent.
Prothoracic hypomeron almost flat (slightly convex), with smooth obsolete lateral impression, without wrinkles or with obsolete irregular wrinkles. Basal fold of prothoracic hypomeron, absent.
Metasternum marginated anteriorly.
Elytron with obsolete humeral callus. Elytral punctures are arranged in abbreviated scutellar row and 10 regular entire rows; rows 2–3, 4–5, 6–7, and 8–9 paired. In males, punctures moderately large (0.06 mm) or fine (0.03 mm), in females, punctures fine (0.02–0.03 mm), 5th row consists of 21–30 punctures. In the specimens with fine punctures, elytral rows hardly traced in posterior 1/2 of elytron. Intervals flat.
Elytral epipleuron inclined outside, visible along entire length in lateral view; with few setae apically.
Hind wings, absent.
Tarsomeres 1–3 narrow, with entire sole in both sexes, with fore- and mid-tarsomeres 1 and 3 slightly broadened in males.
Claw tarsomere without denticles beneath.
Pygidium convex, without impression in apical 1/2, and with shallow impression in basal 1/2.
Last abdominal sternite, simple, convex in both sexes, with apical margin straight in males, arc-shaped in females.
Aedeagus arc-shaped, depressed dorso-ventrally, distinctly broadened laterally towards apical orifice, with apical margin trapezoidal, not drawn at the top, but provided with shallow emargination, with cover plate elongated triangularly, developed almost up to apex, with two acute apical denticles on ventral side, with numerous latero-ventral sensilla at apex. Flagellum narrow, exposed.
Body length 5.1–7.3 mm.
The total number of specimens examined: 17.
Type material examined: paratypes: China, Yunnan, Baju (50 km N of Lijiang), Jendek leg.: 1 male (DC); China, Yunnan, Yulong Mts., 27.10 N/100.13 E, H = 3900 m, 16–19.VI.1993, Bolm leg.: 1 female (BC).
Additional specimens examined: China, Yunnan, Laojunshan, watershed Yushi and Chongjiang r., 26°39′22″ N/99°41′3″ E, H = 4020 m, 22.VI.2014, I. Belousov, I. Kabak leg.: 3 males, 2 females (BC), 5 males (RC); China, Yunnan, Laojunshan, lake, sources river near Xishiyan, 26°39′15″ N/99°41′60″ E, H = 3935 m, 23.VI.2014, I. Belousov, I. Kabak leg.: 1 male, 1 female (BC); China, Yunnan, N Lijiang, W Maguwa, 4.2 km SE Shanggaohan Vill., 27°26′33″ N/100°19′27″ E, H = 4055 m, 24.V.2017, I. Belousov, G. Davidian, I. Kabak leg.: 1 male, 2 females (BC).
Comments. The examination of specimens from different localities showed that the species is variable in coloration of dorsal side, legs, antennae, shape of the last maxillary palpomere, number and size of elytral punctures.
In the original diagnosis [9], it was compared only with Ch. claripes Lopatin, 2002, with which it has some superficial resemblance, but is not taxonomically related because Ch. claripes has male fore- and mid-tarsomeres 1–3 very broad, elytral puncture rows hardly visible, elytral surface slightly wrinkled, prothoracic hypomeron distinctly wrinkled laterally [6].
3.3.3. Chrysolina (Volosatik) wangi Lopatin, 2005
Chrysolina wangi Lopatin, 2005: 570.
Type locality. China, Yunnan, NNE of Kunming, 15 km NE of Dongchuan, N of Daohe Shan.
Redescription. Body broadly oval, convex. Dorsal side, black with bronze reflection. Antennae, black with antennomeres 1 and 2 rufous below. Legs, black with bronze reflection. Dorsum, shagreen and micropunctulate, moderately shining.
Last maxillary palpomere, barrel-shaped, with narrow base and broad apex, 1.2 X as long as wide, 1.2 X longer than penultimate palpomere, as wide as latter, similar in both sexes.
Antenna inserted 2 X closer to clypeus than to eye.
Pronotum 1.9 X as wide as long, with rounded lateral sides, broadest at mid-length, narrower at the apex than at the base or broadest basally. Lateral callus convex along entire length, separated from disc by broad shallow impression filled with sparse, not numerous (about 8–10) moderately large punctures or without them. Disc evenly covered by fine (0.02 mm) numerous punctures. Pronotum anteriorly marginated and ciliate. Anterior setiferous pore of pronotum, absent.
Prothoracic hypomeron almost flat (slightly convex), with smooth obsolete lateral impression, without wrinkles or with obsolete irregular wrinkles. Basal fold of prothoracic hypomeron, absent.
Metasternum marginated anteriorly.
Elytron with obsolete humeral callus or without callus at all. Elytral punctures arranged in abbreviated scutellar row and 10 partly irregular rows; rows 2–3, 4–5, 6–7, and 8–9 paired. Punctures fine (0.03 mm). In males, 5th row consists of 25 punctures, rows hardly visible, especially posteriorly. In females, elytral punctures mostly irregular, rows hardly traced here and there, mostly at base. Intervals flat.
Elytral epipleuron inclined outside, visible along entire length in lateral view; with few setae apically.
Hind wings, absent.
Tarsomeres 1–3 narrow, with entire sole in both sexes, with fore- and mid-tarsomeres 1 and 3 slightly broadened in males. Claw tarsomere without denticles beneath.
Pygidium convex, without impression in apical 1/2, and with shallow impression in basal 1/2.
Last abdominal sternite, simple, convex in both sexes, with apical margin straight in males, arc-shaped in females.
Aedeagus arc-shaped, depressed dorso-ventrally, distinctly broadened laterally towards apical orifice, with apical margin trapezoidal, drawn at the top, and provided with shallow emargination, with cover plate triangular, developed in basal 1/2 of apical orifice, with two obtuse apical denticles on ventral side, with numerous latero-ventral sensilla of different types at apex. Flagellum, narrow, exposed.
Body length 5.9–7.3 mm.
The total number of specimens examined: 6.
Type material examined: holotype: China, Yunnan, NNE of Kunming, 15 km NE of Dongchuan, N of Daohe Shan, 3200–3600 m, 23.IV.2001, I. Belousov, Korolev. leg.: male (ZIN).
Additional specimens examined: China, W Yunnan, SSW Liuku, 25°41′31″ N/98°45′47″ E, H = 3545 m, 19.V.2006, I. Belousov, I. Kabak leg.: 1 male, 2 females (BC); China, W Yunnan, SSW Liuku, 25°41′13″ N/98°46′03″ E, H = 3815 m, 20.V.2006, I. Belousov, I. Kabak leg.: 1 male (BC); China, W Yunnan, SW Liuku, 25°42′48″ N/98°45′33″ E, H = 3575 m, 24.V.2006, I. Belousov, I. Kabak leg.: 1 male (BC).
Comments. According to the original diagnosis [8], it belongs to the group of wingless metallic species of the fauna of China, from which it differs in the shape of the aedeagus. Such a diagnosis says nothing: there are a lot of wingless species in China, and all of them differ in the shape of the aedeagus.
Due to the complex features, the species occupies a fairly isolated position among the species in China [6]. Nevertheless, I attribute it to the present subgenus due to: a narrow barrel-shaped last maxillary palpomere, shallow and wide lateral impression of the pronotum, narrow tarsi with an entire sole, pygidium without impression apically, simple last abdominal sternite, the shape of the aedeagus (similar to that in Ch. fascinatrix and Ch. liqingzhaoae), and especially the sensilla at its apex latero-ventrally.
The type locality of Ch. wangi is located far to the east of the range of other species of the present subgenus and even from the localities of all known non-type specimens of Ch. wangi. This may indicate an erroneous type label. However, new collections are necessary to confirm this assumption.
3.3.4. Chrysolina (Volosatik) igori Bieńkowski, sp. nov.
urn:lsid:zoobank.org:act:F1F8F384-AD0D-48D3-B8E1-1BC89A7105C8 (accessed on 29 December 2022) Figure 9, Figure 11b,c, Figure 12 and Figure 13a.
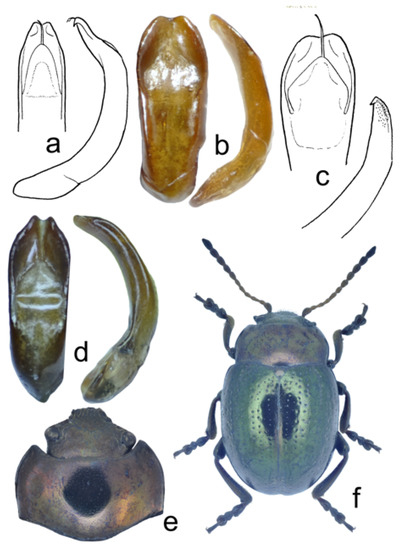
Figure 11.
Chrysolina, structural details: (a) Ch. genriki sp. nov., holotype, aedeagus dorsal and lateral view; (b,c) Ch. igori sp. nov., holotype, aedeagus, dorsal and lateral view; (d–f) Ch. ilyakabaki sp. nov., holotype: (d) aedeagus, dorsal and lateral view; (e) head and pronotum, dorsal view; (f) total dorsal view.

Figure 12.
Chrysolina igori sp. nov., type locality. Photo by I.A. Belousov.
Type locality. China, NW Yunnan, Deqen, Tuoxia Highway, Mt. Range between Xiaruolisuzuxiang and Yezhizhen 27°42′50″ N/99°11′27″ E
Description. Holotype (male). Body, broadly oval, convex. Dorsal side, dark bronze. Antennae, black with antennomeres 1 and 2 rufous below apically. Legs, entirely black. Head and pronotum sericeous, shining, shagreen and micropunctulate, elytra very shining, smooth, micropunctulate.
Last maxillary palpomere barrel-shaped, tapering equally towards base and apex, 1.4 X as long as wide, 1.3 X longer and 1.2 X wider than penultimate palpomere.
Antennae are inserted 3 X closer to clypeus than to eye.
Pronotum 1.9 X as wide as long, with rounded lateral sides, broadest basally. Lateral callus convex along entire length, separated from disc by broad shallow impression without large punctures. Disc, lateral impression, and callus evenly covered by fine (0.02 mm) numerous punctures. Pronotum anteriorly marginated and ciliate. Anterior setiferous pore of pronotum absent.
Prothoracic hypomeron almost flat (slightly convex), with smooth obsolete lateral impression, without wrinkles. Basal fold of prothoracic hypomeron, absent.
Metasternum marginated anteriorly.
Elytron with obsolete humeral callus. Elytral punctures arranged in abbreviated scutellar row and 10 regular entire rows; rows 2–3, 4–5, 6–7, and 8–9 paired. Rows 6 and 7 abbreviated anteriorly and posteriorly. Punctures large (0.08 mm), foveiform. The 5th row consists of 13–14 punctures on right and left elytron, respectively. Intervals flat.
Elytral epipleuron inclined outside, visible along entire length in lateral view; with single setae apically.
Hind wings, absent.
Tarsomeres 1–3 narrow, with entire sole, with fore- and mid-tarsomeres 1 and 3 slightly broadened. Claw tarsomere without denticles beneath.
Pygidium convex, without impression in apical 1/2, and with shallow impression in basal 1/2.
Last abdominal sternite, simple, convex, with apical margin straight.
Aedeagus arc-shaped, depressed dorso-ventrally, slightly broadened laterally towards apical orifice, with apical margin roundly elongated, not drawn at the top, but provided with shallow apical emargination, with cover plate triangular, developed in basal 2/3 of apical orifice, with two acute apical denticles on ventral side, with numerous latero-ventral pores at apex, but without setae. Flagellum, narrow, exposed.
Body length 6.5 mm.
Variability. Dorsum, dark metallic: green, violet, or head and pronotum brassy, elytra green. Pronotal lateral impression with few moderately large punctures. Fifth elytral row consists of 10–17 punctures, rows 6 and 7 entire. In some males, aedeagus distinctly broadened laterally towards apical orifice. Female tarsomeres 1–3 narrow, with entire sole. Body length, 5.9–6.4 mm (males), 6.3–7.4 mm (females).
Differential diagnosis. Among the species of the present subgenus, Ch. igori is morphologically close to Ch. fascinatrix, but differs in the shape of the apex of the aedeagus: apical margin roundly elongated but never drawn at the top, and pronotum broadest basally.
The total number of specimens examined: 16.
Type material examined: holotype (male): China, Yunnan, Deqen, Tuoxia Highway, Mt. Range between Xiaruolisuzuxiang and Yezhizhen 27°42′50″ N/99°11′27″ E, H = 4025 m, 11.VI.2013, I. Belousov, I. Kabak, G. Davidian leg.: male (ZIN); paratypes: the same label as holotype: 5 males, 6 females (ZIN, BC); China, Yunnan, Deqen, Tuoxia Highway, Mt. Range between Xiaruolisuzuxiang and Yezhizhen 27°42′11″ N/99°11′31″ E, H = 4250 m, 12.VI.2013, I. Belousov, I. Kabak, G. Davidian leg.: 1 male, 3 females (BC).
Etymology. The new species is named after Igor A. Belousov (All-Russian Research Institute of Plant Protection, St. Petersburg), a well-known Russian entomologist, expert in Carabidae, who collected many new species of leaf beetles during his expeditions to China.
Biological notes. One female was found with embryos in the abdominal cavity. The embryos had a developed head with ocelli, legs, thoracic and abdominal segments of the body with chaetotaxy. This indicates ovoviviparity in this species.
3.3.5. Chrysolina (Volosatik) marinae Bieńkowski, sp. nov.
urn:lsid:zoobank.org:act:624D6A81-47F0-4778-874B-1B8D5F6CFB0E (accessed on 29 December 2022)
Type locality. China, NW Yunnan, NE Lanping City, 6.4 km W Guanping Village, 26°31′ N/99°31′0″ E.
Description. Holotype (male). Body, broadly oval, convex. Dorsal side, dark brassy. Antennae, piceous with antennomeres 1 and 2 rufous below. Legs, entirely black. Head sericeous, shining, shagreen and micropunctulate, pronotum and elytra very shining, smooth, micropunctulate.
Last maxillary palpomere barrel-shaped, tapering equally towards base and apex, 1.3 X as long as wide, 1.2 X longer and 1.3 X wider than penultimate palpomere.
Antennae inserted 2.5 X closer to clypeus than to eye.
Pronotum 2.0 X as wide as long, with rounded lateral sides, broadest basally. Lateral callus convex along entire length, separated from disc by broad shallow impression with 1–2 moderately large punctures. Disc, impression and callus evenly covered by fine (0.02 mm) numerous punctures. Pronotum anteriorly marginated and ciliate. Anterior setiferous pore of pronotum absent.
Prothoracic hypomeron almost flat (slightly convex), with weak lateral impression covered by obsolete wrinkles. Basal fold of prothoracic hypomeron, absent.
Metasternum marginated anteriorly.
Elytron without humeral callus. Elytral punctures arranged in abbreviated scutellar row and 10 regular entire rows; rows 2–3, 4–5, 6–7, and 8–9 paired. Punctures moderately large (0.05 mm), foveiform. The 5th row consists of 19 punctures. Intervals, flat.
Elytral epipleuron inclined outside, visible along entire length in lateral view; with few setae apically.
Hind wings, absent.
Tarsomeres 1–3 narrow, with entire sole. Claw tarsomere without denticles beneath.
Pygidium convex, without impression in apical 1/2, and with shallow impression in basal 1/2.
Last abdominal sternite, simple, convex, with apical margin straight.
Aedeagus arc-shaped, depressed dorso-ventrally, slightly broadened laterally towards apical orifice, with apical margin broadly truncate, very shortly drawn at the top and provided with emargination, with cover plate elongated triangularly, reaching apex of apical orifice, without apical denticles on ventral side, with numerous latero-ventral sensilla at apex. Flagellum, narrow, exposed.
Body length, 6.1 mm.
Variability. Body length, 5.6–6.4 mm (males), 6.3–7.2 mm (females). Body dorsally dark metallic: bronze, or head brassy, pronotum and elytra dark blue. Femora and tibiae, bronze, tarsi rufous or piceous. Antennae, wholly dark, or with rufous base, or wholly rufous. Pronotum, shining or sericeous. Elytral fifth puncture row consists of 11–30 punctures, which are 0.03–0.09 mm wide. Female tarsomeres, 1–3 narrow, with entire sole.
Differential diagnosis. Chrysolina marinae differs from other members of the present subgenus in the shape of the apex of the aedeagus, which is broadly truncate, but not narrowed at sides of apical orifice and devoid of apical denticles.
The total number of specimens examined: 21.
Type material examined: holotype: China, Yunnan, NE Lanping City, 6.4 km W Guanping Village, 26°31′ N/99°31′0″ E, H = 3455 m, 28.V.2015, I. Belousov, I. Kabak, G. Davidian leg.: male (ZIN); paratypes: China, Yunnan, N Lanping, 11.3 km SW of Hexi, 26°48′34″ N/99°17′16″ E, H = 3600 m, 10.VI.2016, I. Kabak, G. Davidian leg.: 1 male (ZIN); China, Yunnan, Laojunshan, s-ces of Yushi r., Shangzhuping, 26°39′5″ N/99°40′6″ E, H = 3465 m, 21.VI.2014, I. Belousov, I. Kabak leg.: 3 males, 1 female (BC); China, Yunnan, Laojunshan, Dajdugeng Vill., Yushi riv., 26°34′8″ N/99°34′21″ E, H = 2425 m, 21.VI.2014, I. Belousov, I. Kabak leg.: 1 male (RC); China, Yunnan, NE Lanping, SE Xinshanchang, 26°31′13″ N/99°28′47″ E, H = 3325 m, 18.VI.2014, I. Belousov, I. Kabak leg.: 6 males, 4 females (ZIN, BC); China, Yunnan, NE Lanping, Xinshanchang vic., 26°31′17″ N/99°28′57″ E, H = 3240 m, 18.VI.2014, I. Belousov, I. Kabak leg.: 4 males (RC).
Etymology. The new species is named after my wife, Marina J. Orlova-Bienkowskaja (A.N. Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences, Moscow), who has been helping me with my work for over 30 years.
3.3.6. Chrysolina (Volosatik) genriki Bieńkowski, sp. nov.
rn:lsid:zoobank.org:act:A8F6D8B1-43EC-4A6C-9EA3-5B9F8F52C20E (accessed on 29 December 2022) Figure 9, Figure 10d, Figure 11a and Figure 13b.
Type locality. China, NW Yunnan, NE Lanping, SE Xinshanchang, 26°30′37″ N/99°29′26″ E.
Description. Holotype (male). Body, broadly oval, convex. Dorsal side, dark bronze. Antennae, piceous with antennomeres 1 and 2 rufous below apically. Legs, dark bronze with tarsi piceous. Head sericeous, shining, shagreen and micropunctulate, pronotum and elytra very shining, smooth, micropunctulate.
Last maxillary palpomere barrel-shaped, tapering equally towards base and apex, 1.5 X as long as wide, 1.2 X longer and 1.3 X wider than penultimate palpomere.
Antennae inserted 2.3 X closer to clypeus than to eyes.
Pronotum 1.9 X as wide as long, with rounded lateral sides, broadest basally. Lateral callus convex along entire length, separated from disc by broad shallow impression with few large punctures. Disc, lateral impression, and callus evenly covered by fine (0.02 mm) numerous punctures. Pronotum anteriorly marginated and ciliate. Anterior setiferous pore of pronotum, absent.
Prothoracic hypomeron almost flat (slightly convex), with smooth obsolete lateral impression, without wrinkles. Basal fold of prothoracic hypomeron, absent.
Metasternum marginated anteriorly.
Elytron with obsolete humeral callus. Elytral punctures arranged in abbreviated scutellar row and 10 regular entire rows; rows 2–3, 4–5, 6–7, and 8–9 paired. Punctures moderately large (0.06 mm). The 5th row consists of 16 punctures. Intervals, flat.
Elytral epipleuron inclined outside, visible along entire length in lateral view; with few setae apically.
Hind wings, absent.
Tarsomeres 1–3 narrow, with entire sole, with fore- and mid-tarsomeres 1 and 3 slightly broadened. Claw tarsomere without denticles beneath.
Pygidium convex, without impression in apical 1/2, and with shallow impression in basal 1/2.
Last abdominal sternite, simple, convex, with apical margin straight.
Aedeagus arc-shaped, depressed dorso-ventrally, with apical part bent up, parallel-sided up to apical orifice, and then slightly narrowed, not drawn at the top, but provided with shallow apical emargination, with cover plate elongated triangularly, developed in basal 2/3 of apical orifice, with two small apical denticles on ventral side, with latero-ventral pores at apex, but without setae. Flagellum, narrow, exposed. Body length, 5.5 mm.
Variability. Fifth elytral row consists of 16–29 punctures. Female tarsomeres 1–3 narrow, with entire sole. Body length, 6.2 mm (male), 6.4–7.3 mm (females).
Differential diagnosis. Among the species of the present subgenus, Ch. genriki is morphologically close to Ch. igori, but differs in the shape of the aedeagus: it is parallel-sided up to apical orifice, but not broadened.
The total number of specimens examined: 7.
Type material examined: holotype: China, Yunnan, NE Lanping, SE Xinshanchang, 26°30′37″ N/99°29′26″ E, H = 3780 m, 19.VI.2014, I. Belousov, I. Kabak leg.: male (ZIN); paratypes: the same label as holotype: 1 female (ZIN), 1 female (RC), 1 female (BC); China, Yunnan, NE Lanping City, 0.95 km NNE Xuebangshan Mt., 26°29′14″ N/99°30′8″ E, H = 4035 m, 29.V.2015, I. Belousov, I. Kabak leg., G. Davidian: 1 male, 1 female (BC); China, Yunnan, NE Lanping City, 7 km W Guanping Vill., 26°30′39″ N/99°30′28″ E, H = 3650 m, 29.V.2015, I. Belousov, I. Kabak leg., G. Davidian: 1 male (RC).
Etymology. The new species is named after Genrik E. Davidian (All-Russian Research Institute of Plant Protection, St. Petersburg), a well-known Russian entomologist, expert in Curculionidae, who collected many new species of leaf beetles during his expeditions to China.
3.3.7. Chrysolina (Volosatik) ilyakabaki Bieńkowski, sp. nov.
urn:lsid:zoobank.org:act:40472358-A198-41C9-BD11-C19692546DBF (accessed on 29 December 2022)

Figure 14.
Chrysolina ilyakabaki sp. nov., type locality. Photo by I.I. Kabak.
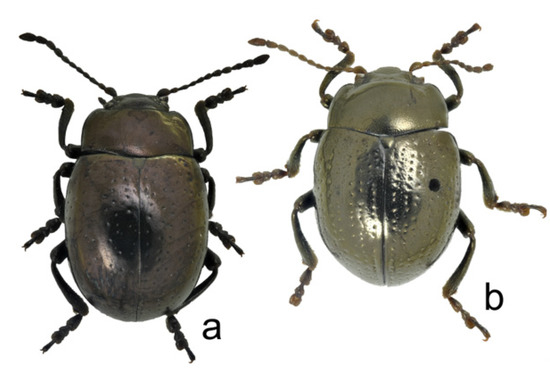
Figure 13.
Chrysolina, total dorsal view: (a) Ch. igori sp. nov., holotype, male; (b) Ch. genriki sp. nov., holotype, male.
Type locality. China, Yunnan, Diancan Shan, 0.9 km NW Mt. Lianhuafe, 25°47′39″ N/100°01′40″ E.
Description. Holotype (male). Body, broadly oval, convex. Dorsal side, metallic, two-colored: head, pronotum, and scutellum coppery, elytra green. Antennae, rufous with antennomeres 7–11th slightly darkened. Femora bronze, tibiae and tarsi rufous with bronze reflection. Dorsum, shagreen and micropunctulate, moderately shining.
Last maxillary palpomere barrel-shaped, with narrow base and broad apex, 1.4 X as long as wide, 1.3 X longer than penultimate palpomere, 1.2 X broader than latter.
Antennae inserted 3 X closer to clypeus than to eyes.
Pronotum 1.8 X as wide as long, with rounded lateral sides, broadest at mid-length, narrower at the apex than at the base or broadest basally. Lateral callus convex along entire length, separated from disc by broad shallow impression without moderately large punctures. Disc evenly covered by fine (0.02 mm) numerous punctures. Pronotum anteriorly marginated and ciliate. Anterior setiferous pore of pronotum, absent.
Prothoracic hypomeron almost flat (slightly convex), with smooth obsolete lateral impression, without wrinkles. Basal fold of prothoracic hypomeron, absent.
Metasternum marginated anteriorly.
Elytron without humeral callus. Elytral punctures arranged in abbreviated scutellar row and 10 regular rows; rows 2–3, 4–5, 6–7, and 8–9 paired. Punctures large (0.08 mm). 5th row consists of 16 punctures. Intervals flat, covered by sparse, fine (0.02 mm) setae.
Elytral epipleuron inclined outside, visible along entire length in lateral view; with few setae apically.
Hind wings, absent.
Tarsomeres 1–3 narrow, with entire sole, with fore- and mid-tarsomeres 1 and 3 slightly broadened. Claw tarsomere without denticles beneath.
Pygidium convex, without impression in apical 1/2, and with shallow impression in basal 1/2.
Last abdominal sternite, simple, convex, with apical margin straight.
Aedeagus arc-shaped, depressed dorso-ventrally, parallel-sided, not broadened laterally towards apical orifice, with apical margin trapezoidal, drawn at the top, and provided with deep apical emargination, with cover plate triangular, developed in basal 1/2 of apical orifice, without apical denticles on ventral side (they replaced by simple swellings), without median longitudinal keel at apex on ventral side (keel replaced by paired black stripes), with numerous latero-ventral sensilla at apex. Flagellum narrow, exposed.
Body length, 6.2 mm.
Variability. Males 6.2–6.4 mm long, females 6.6–7.7 mm long. In females, tarsomeres 1–3 narrow, with entire sole. One male with head and pronotum golden green, elytra coppery, and one male entirely golden green from above. One male with tibiae black, and one male with legs entirely black. Fifth elytral row consists of 12–25 punctures.
Differential diagnosis. The new species is morphologically closest to Ch. fascinatrix, Ch. liqingzhaoae, and Ch. wangi. It differs from all of them in the two-color coloration of the dorsal side (copper head and pronotum, green elytra) in most specimens, and in deep emargination at the apex of aedeagus. It differs from Ch. fascinatrix and Ch. liqingzhaoae in the absence of denticles at the apex of the aedeagus (instead of them there are simple small swellings), from Ch. wangi in entirely regular and distinct puncture rows of elytra, in the absence of large punctures in the intervals of elytra and in the pronotal lateral impressions, and the absence of a longitudinal carina at the ventral side of aedeagus near apex, from Ch. liqingzhaoae and Ch. wangi in the aedeagus not broadened towards the apex, from Ch. liqingzhaoae in a short cover plate of the apical orifice of the aedeagus.
The total number of specimens examined: 8.
Type material examined: holotype: China, Yunnan, Diancan Shan, 0.9 km NW Mt. Lianhuafe, 25°47′39″ N/100°01′40″ E, H = 3675 m, 28.V.2019, I. Belousov, G. Davidian, I. Kabak leg.: male (ZIN); paratypes: with the same label as holotype: 2 males (RC); China, Yunnan, Diancan Shan, 0.8 km ENE Mt. Wutaifeng, 25°49′27″ N/100°01′40″ E, H = 3665 m, 27.V.2019, I. Belousov, G. Davidian, I. Kabak leg.: 1 male (BC), 1 female (RC); China, Yunnan, Diancan Shan, 0.4 km S Mt. Lianhuafeng, 25°47′03″ N/100°02′00″ E, H = 3775 m, 29.V.2019, I. Belousov, G. Davidian, I. Kabak leg.: 2 males, 1 female (RC).
Etymology. The new species is named after Ilya I. Kabak (All-Russian Research Institute of Plant Protection, St. Petersburg), a well-known Russian entomologist, expert in Carabidae, who collected many new species of leaf beetles during his expeditions to China.
3.4. Diagnostic Features of the Species
The species of the subgenus are externally variable and rather close to each other. All species differ in the details of the structure of the male aedeagus. Identification of females not associated with males is impossible in some cases. Differences in species have the character of combinatorics. Thus, the table with diagnostic features is more convenient for species identification than the dichotomous key (Table 1).

Table 1.
Diagnostic characters of the species.
3.5. Biological Notes
Species of the subgenus Ch. (Volosatik) inhabit the belt of alpine coniferous forests, occurring under the forest canopy and in open meadow areas there. These species live in the herbaceous layer. Data on the host plants are not yet available, as is the case for most Chinese highland Chrysolina species. Most likely, beetles and larvae feed on the leaves of herbaceous plants, like all species of this genus in which host plants are known. For at least one of the studied species, namely Ch. igori, ovoviviparity was established. This is characteristic of the species of the genus Chrysolina living in high-mountain and high-latitude conditions with a lack of heat [10].
4. Discussion
The species of this subgenus have a distinctive feature: the presence of sensilla of different types on the latero-ventral side at the apex of the male aedeagus. This character has not been described by previous authors [7,8,9]. I am practically unaware of other species of the large genus Chrysolina in which the aedeagus is provided with sensilla, with the exception of a single species (Ch. sichuanica Lopatin, 2002), also from China [6,11]. In general, setae near the apex of aedeagus are found in different groups of Clytrinae and Cryptocephalinae, but not in Chrysomelinae [12]. Among the world diversity of the subfamily Chrysomelinae, sensilla on the aedeagus are found only in representatives of two subgenera of the genus Gonioctena Chervolat, 1843, namely G. (Asiphytodecta Chen, 1935) and G. (Brachyphytodecta Bechyně, 1948) [13,14,15,16]. It is interesting that these species of the genus Gonioctena inhabit only southern China and Indochina, that is, close to the range of the subgenus Ch. (Volosatik).
Among the species of the subgenus Ch. (Volosatik), the most numerous and widespread species, Ch. fascinatrix, also has the greatest variability in morphological characters. In this species, the details of the external morphology and the structure of the aedeagus of the male are subject to great variability. Most of the features have a variability close to the statistical normal distribution. A gap is present only by the number of punctures in the 5th row of elytra. In the absence of a hiatus for all other features, including the structure of the aedeagus, I do not attach any importance to this difference as a specific one.
Contrary to Lopatin’s [17] statement, the male aedeagus, like other characters, is subject to variability. Aside from Ch. fascinatrix, significant intraspecific variability in the shape of the aedeagus was shown before for different species of the genus Chrysolina [6,18,19].
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
I am grateful to the curators: B. A. Korotyaev, A. G. Moseyko and S.V. Andreeva (ZIN) for generously allowing me to borrow material from this Museum, to I.A. Belousov, G.E. Davidian, I.I. Kabak, M. Daccordi and P.V. Romantsov, who presented material at my disposal, to I.A. Belousov and I.I. Kabak for providing color photographs of type localities, to A.N. Neretina for help with work on the electron microscope, to M. Daccordi for the valuable comments, and to my youngest son S.A. Bienkowski for computer processing of the photographs. Electron microscope study was conducted using Joint Usage Center “Instrumental methods in ecology” at the IEE RAS.
Conflicts of Interest
The author declares no conflict of interest.
References
- Belousov, I.A.; Kabak, I.I. Revision of the genus Kozlovites Jeannel, 1935 with description of a new genus of the tribe Trechini (Coleoptera: Carabidae). Far East. Entomol. 2016, 308, 1–32. [Google Scholar]
- Beron, P. High altitude Isopoda‚ Arachnida and Myriapoda in the Old World (supplementa and corrigenda 2008–2016). Hist. Nat. Bulg. 2016, 23, 141–155. [Google Scholar] [CrossRef]
- Davidian, G.E. A new genus and new species of the weevil Tribe Blosyrini Lacordaire, 1863 (Coleoptera, Curculionidae: Entiminae) from the Sino-Tibetan Mountains. Entomol. Rev. 2020, 100, 1157–1189. [Google Scholar] [CrossRef]
- Deuve, T. Carabus of the World. Collect. Systématiques 2021, 30, 1–652. [Google Scholar]
- Medvedev, G.S. A contribution to the taxonomy and morphology of the tribe Blaptini (Coleoptera, Tenebrionidae). Entomol. Rev. 2007, 87, 181–214. [Google Scholar] [CrossRef]
- Bieńkowski, A.O. Chrysolina of the World—2019 (Coleoptera: Chrysomelidae). Taxonomic Review; Mukhametov G.V. Publishing House: Livny, Russia, 2019; 920p. [Google Scholar]
- Lopatin, I.K. Contribution to the systematics of leaf beetles of the subfamily Chrysomelinae (Coleoptera, Chrysomelidae). Entomol. Rev. 1998, 77, 826–835. (In Russian) [Google Scholar]
- Lopatin, I.K. New species of leaf beetles (Coleoptera, Chrysomelidae) from China. IV. Entomol. Rev. 2005, 84, 569–575. (In Russian) [Google Scholar]
- Daccordi, M.; Ge, S.-Q.; Cui, J.-Z.; Yang, X.-K. New species of Chrysolina Motschulsky, 1860 from Southwest China (Coleoptera, Chrysomelidae, Chrysomelinae). Nouv. Rev. Entomol. (NS) 2011, 26, 291–316. [Google Scholar]
- Dubeshko, L.N.; Medvedev, L.N. Ecology of Leaf-Beetles of Siberia and the Far East; Irkutsk University Publishing House: Irkutsk, Russia, 1989; 224p. (In Russian) [Google Scholar]
- Lopatin, I.K. New species of the leaf-beetle subfamily Chrysomelinae (Coleoptera, Chrysomelidae) from China. I. Entomol. Rev. 2002, 81, 111–120. (In Russian) [Google Scholar]
- Warchałowski, A. Chrysomelidae. The Leaf-Beetles of Europe and the Mediterranean Area; Natura optima dux Foundation: Warsaw, Poland, 2003; 600p. [Google Scholar]
- Cho, H.-W. Two new species of the Gonioctena mauroi species-group from China (Coleoptera: Chrysomelidae: Chrysomelinae). Acta Entomol. Mus. Nat. Pragae 2017, 57, 173–181. [Google Scholar] [CrossRef][Green Version]
- Cho, H.-W. Definition of the Gonioctena subgeminata species group (Coleoptera, Chrysomelidae, Chrysomelinae), with descriptions of two new species from China and Vietnam. ZooKeys 2021, 1032, 79–90. [Google Scholar] [CrossRef]
- Cho, H.-W.; Borowiec, L. On the genus Gonioctena Chevrolat (Coleoptera: Chrysomelidae: Chrysomelinae), with descriptions of seven new species from the Oriental region and Palaearctic China. Zootaxa 2016, 4067, 168–184. [Google Scholar] [CrossRef]
- Cho, H.-W.; Borowiec, L. Revision of the Gonioctena fl avoplagiata species-group (Coleoptera: Chrysomelidae: Chrysomelinae), with descriptions of two new species from China and Laos. Acta Entomol. Musei Natl. Pragae 2016, 56, 755–768. [Google Scholar]
- Lopatin, I.K. Book review: V.F. Palij, G.A. Avanesova “Flea-beetles—Coleoptera, Chrysomelidae, Halticinae (Guide to genera and harmful species)”. Zool. J. 1976, 55, 1915–1917. (In Russian) [Google Scholar]
- Ge, S.-Q.; Daccordi, M.; Yang, X.-K. Two new species of Chrysolina Motschulsky from China, with redescription of C. kozlovi Lopatin and notes on the genus Doeberlia Warchałowski (Coleoptera: Chrysomelidae: Chrysomelinae). Acta Entomol. Musei Natl. Pragae 2009, 49, 805–818. [Google Scholar]
- Kippenberg, H. Diversity of aedeagus shape in Slovenian populations of Chrysolina purpurascens (Germar) (Chrysomelinae). In New Development in the Biology of Chrysomelidae; Jolivet, P., Santiago-Blay, J., Schmitt, M., Eds.; SPB Academic Publishing: The Hague, The Netherlands, 2004; pp. 659–665. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).