Spatial Distribution of Lepidoptera in Forest Ecosystems of Central European Russia: Studies Using Beer Traps
Abstract
1. Introduction
2. Materials and Methods
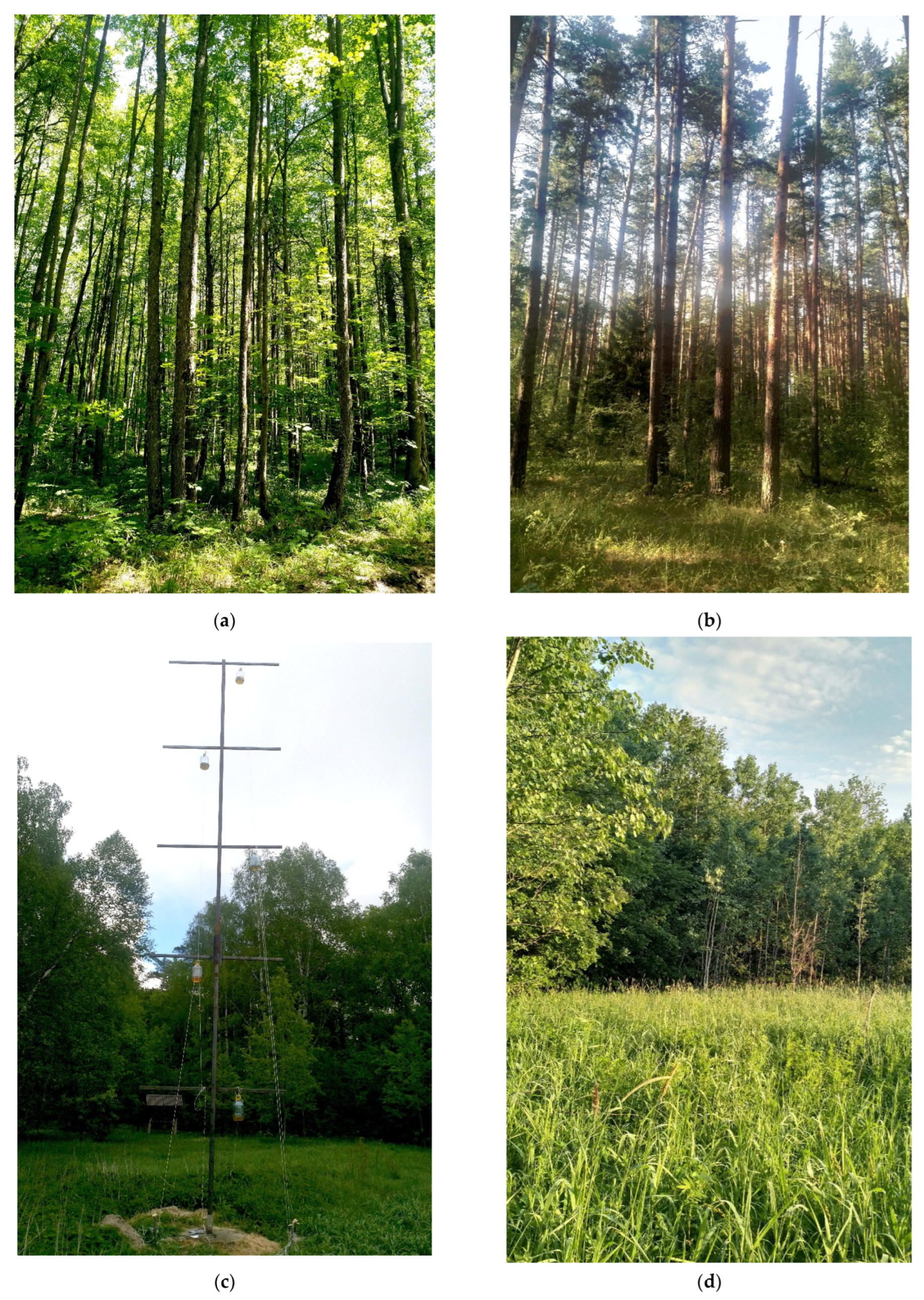
2.1. Study Area and Design of Studies
- (1)
- Vertical distribution in deciduous forests
- (2)
- Vertical distribution in pine forests
- (3)
- Vertical distribution in clearings in forests
- (4)
- The impact of the glades on the distribution
2.2. Data Analyses
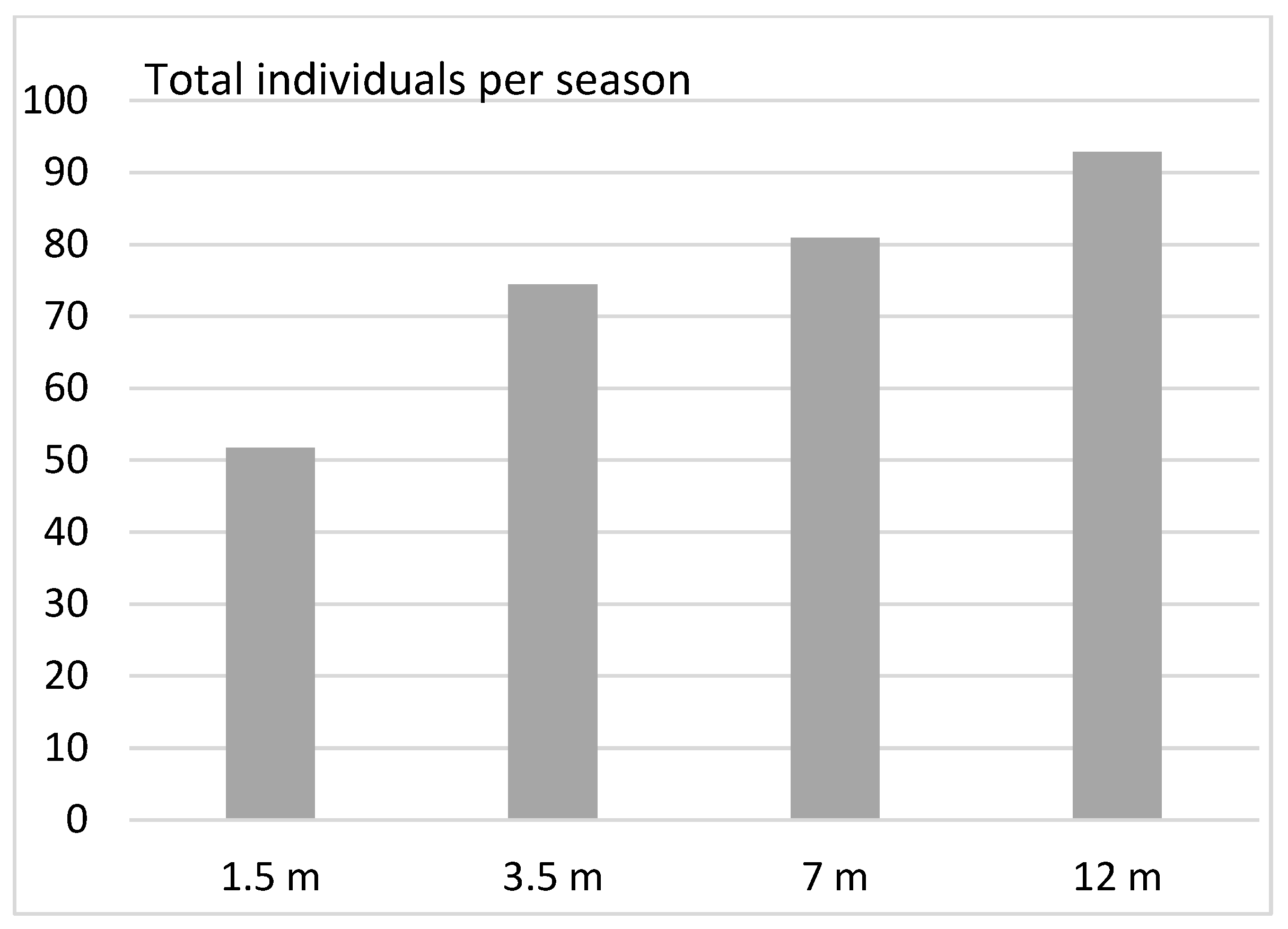
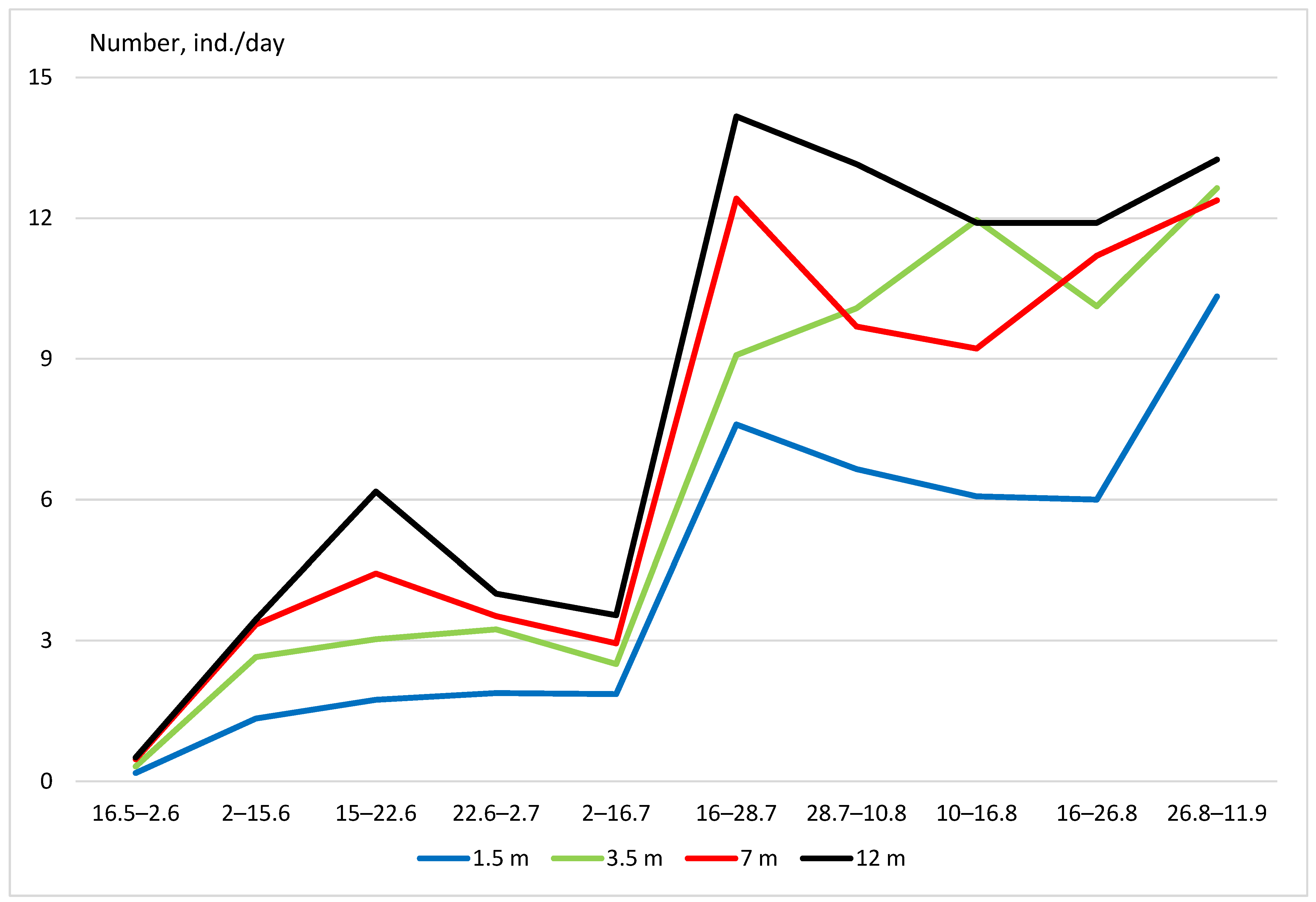
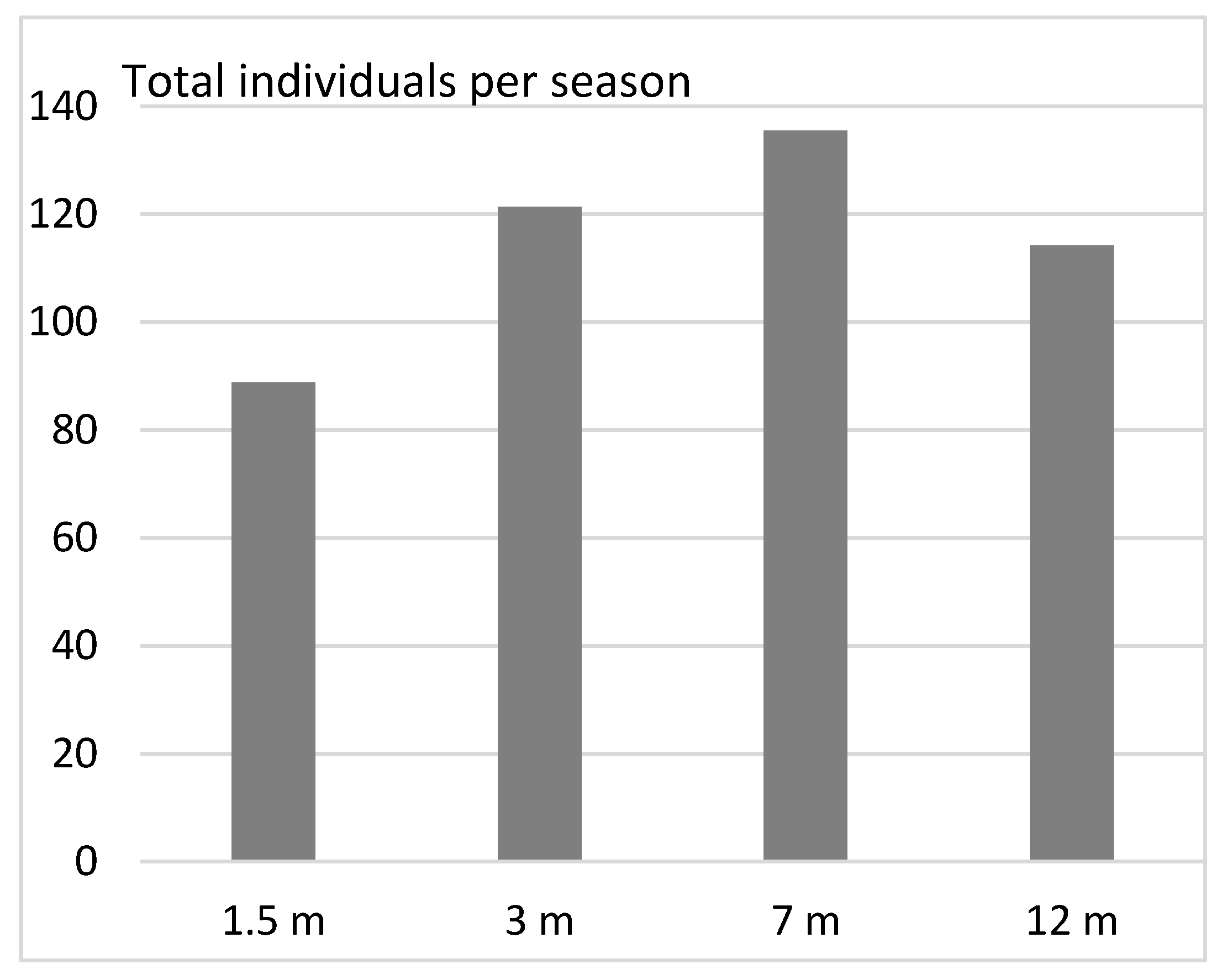
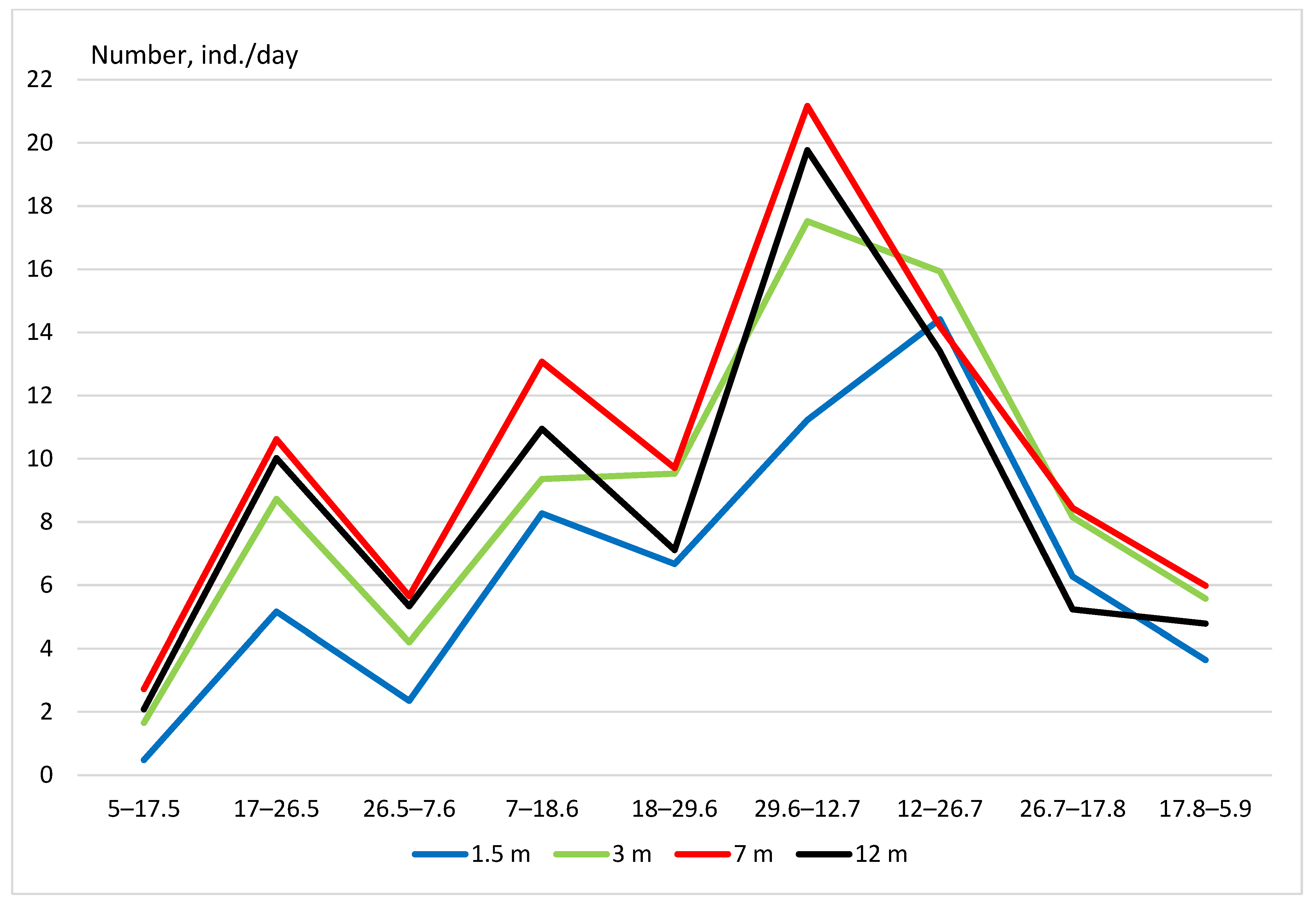
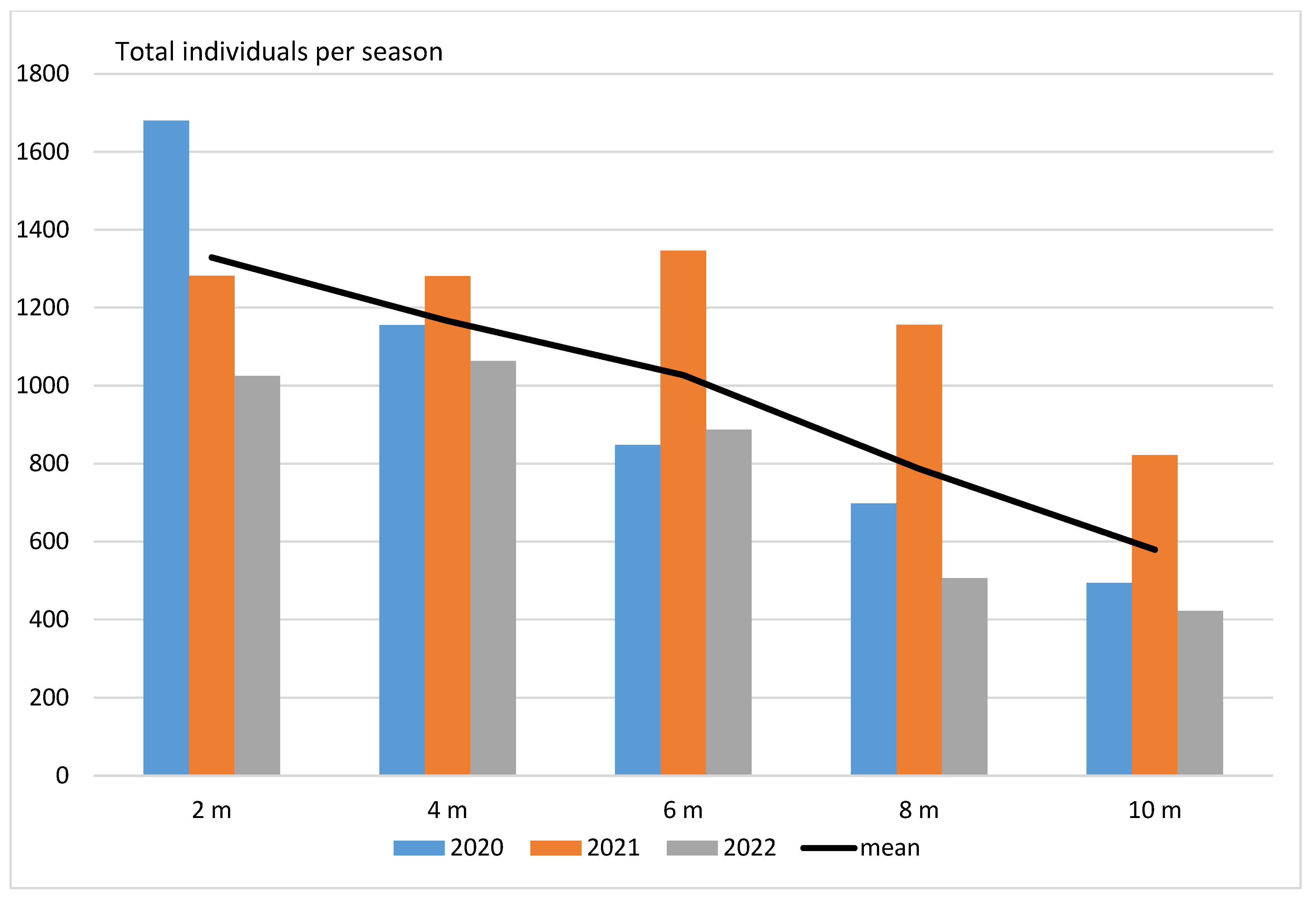
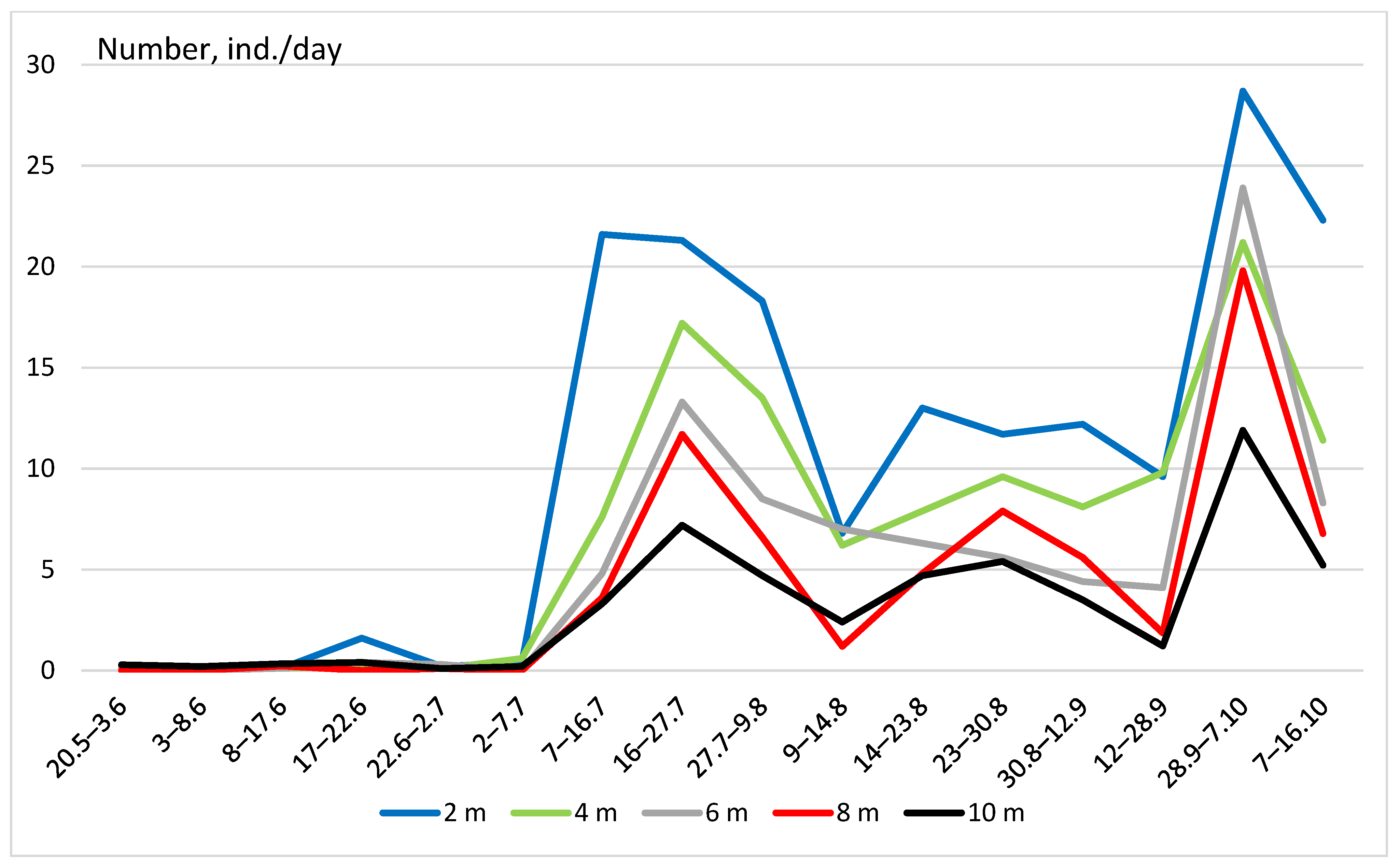
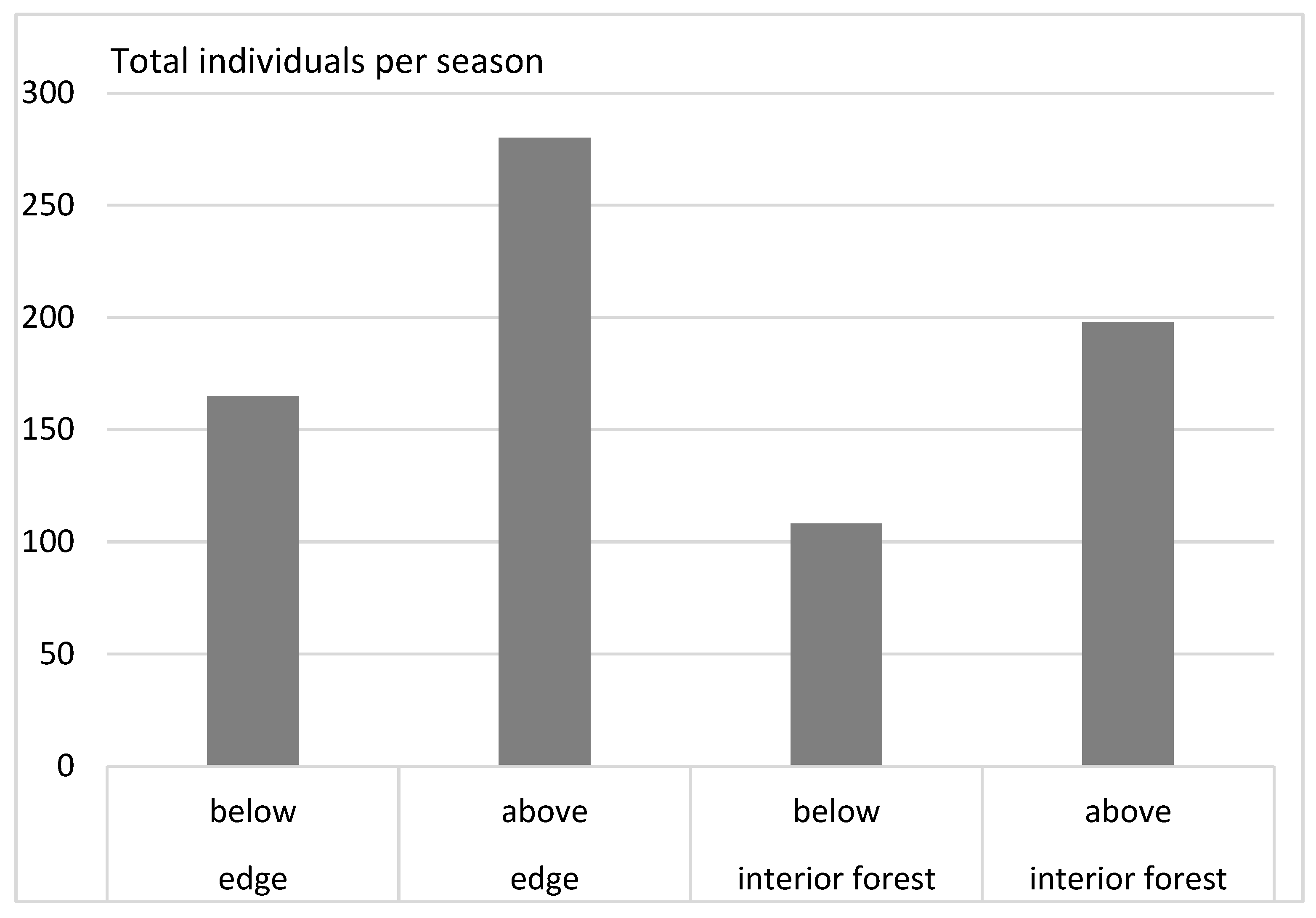
3. Results
- (1)
- Vertical distribution in deciduous forests
- (2)
- Vertical distribution in pine forests
- (3)
- Vertical distribution in glades in forests
- (4)
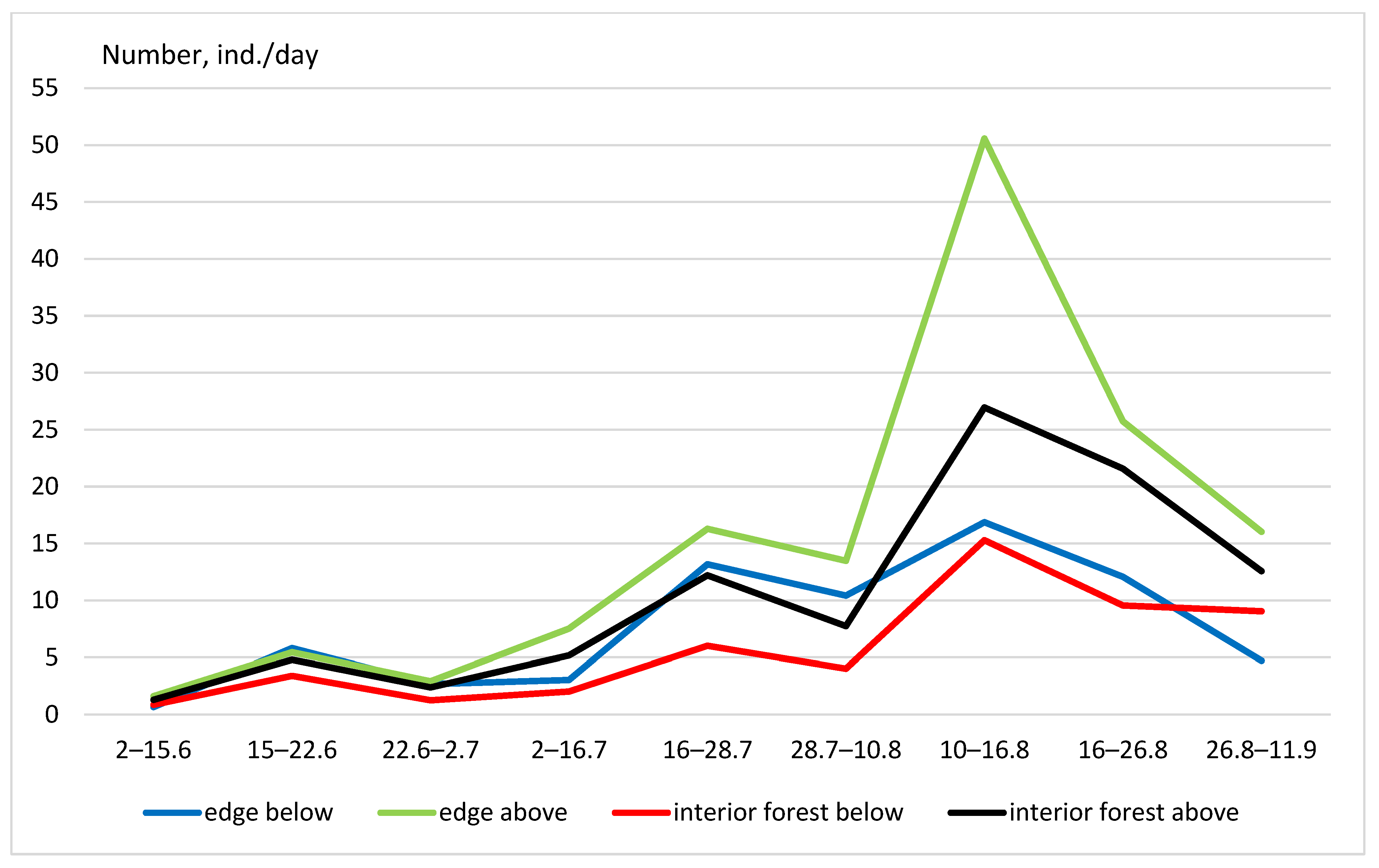
- The impact of glades on the distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perry, K.I.; Wallin, K.F.; Wenzel, J.W.; Herms, D.A. Forest disturbance and arthropods: Small-scale canopy gaps drive invertebrate community structure and composition. Ecosphere 2018, 9, e02463. [Google Scholar] [CrossRef]
- Ahissa, L.; Akpatou, B.K.; Bohoussou, H.K.; Kadjo, B.; Koné, I. Species composition and community structure of terrestrial small mammals in Tanoé-Ehy Swamp Forest (South-East Ivory Coast): Implication for conservation. Nat. Conserv. Res. 2020, 5, 53–63. [Google Scholar] [CrossRef]
- Caminha-Paiva, D.; Gomes, V.M.; Alves, M.J.P.; Rosa, D.C.P.; Santiago, J.C.; Negreiros, D.; Fernandes, G.W. Floristic mosaics of the threatened Brazilian campo rupestre. Nat. Conserv. Res. 2022, 7, 10–18. [Google Scholar] [CrossRef]
- Zhukova, Y.O.; Yorkina, N.V.; Budakova, V.S.; Kunakh, O.M. The small-scale variation of herb-layer community structure in a riparian mixed forest. Biosyst. Divers. 2020, 28, 390–398. [Google Scholar] [CrossRef]
- Pesotskaya, V.V.; Chaplygina, A.B.; Shupova, T.V.; Kratenko, R.I. Fruit and berry plants of forest belts as a factor of species diversity of ornithofauna during the breeding season and autumn migration period. Biosyst. Divers. 2020, 28, 290–297. [Google Scholar] [CrossRef]
- Nekrich, A. Key factors determining scales of burned areas in state Victoria (Australia) and province Alberta (Canada) during 1980–2019. J. Wildl. Biodivers. 2022, 6, 87–99. [Google Scholar] [CrossRef]
- Anselmo, L.; Rizzioli, B. Side threats: Further possible effects of warming on the high alpine narrow endemic Carabus cychroides (Coleoptera: Carabidae). Nat. Conserv. Res. 2022, 7, 88–94. [Google Scholar] [CrossRef]
- Ruczynski, I.; Barton, K.A. Seasonal changes and the influence of tree species and ambient temperature on the fission-fusion dynamics of tree-roosting bats. Behav. Ecol. Sociobiol. 2020, 74, 63. [Google Scholar] [CrossRef]
- Popkova, T.V.; Zryanin, V.A.; Ruchin, A.B. The ant fauna (Hymenoptera: Formicidae) of the Mordovia State Nature Reserve, Russia. Nat. Conserv. Res. 2021, 6, 45–57. [Google Scholar] [CrossRef]
- Dedyukhin, S.V. Fauna and biotopic distribution of weevils (Coleoptera: Curculionoidea) of the Zhiguli State Nature Reserve, Russia. Nat. Conserv. Res. 2022, 7, 55–69. [Google Scholar] [CrossRef]
- Teshome, M.; Asfaw, Z.; Mohammed, M. Pattern of functional diversity along the elevation gradient in the dry evergreen Afromontane forest of Hararghe Highland, Southeast Ethiopia. Biosyst. Divers. 2020, 28, 257–264. [Google Scholar] [CrossRef]
- Polevoi, A.V. Fungus gnats (Diptera: Bolitophilidae, Diadocidiidae, Keroplatidae, Mycetophilidae) in the Kostomuksha State Nature Reserve, Russia. Nat. Conserv. Res. 2021, 6 (Suppl. 1), 5–16. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V. Vertical stratification of beetles in deciduous forest communities in the Centre of European Russia. Diversity 2021, 13, 508. [Google Scholar] [CrossRef]
- Ruchin, A.; Egorov, L. On the distribution of Coleoptera in forests and open areas (center of the European part of Russia): A study using beer traps. J. Wildl. Biodivers. 2023, 7. [Google Scholar] [CrossRef]
- Basset, Y.; Hammond, P.M.; Barrios, H.; Holloway, J.D.; Miller, S.E. Vertical stratification of arthropod assemblages. In Arthropods of Tropical Forests: Spatio-Temporal Dynamics and Resource Use in the Canopy; Basset, Y., Ed.; Cambridge University Press: Cambridge, UK, 2003; pp. 17–27. [Google Scholar]
- Lowman, M.D.; Wittman, P.K. Forest canopies: Methods, hypotheses, and future directions. Annu. Rev. Ecol. Syst. 1996, 27, 55–81. [Google Scholar] [CrossRef]
- Gossner, M.M.; Struwe, J.-F.; Sturm, S.; Max, S.; McCutcheon, M.; Weisser, W.W.; Zytynska, S.E. Searching for the Optimal Sampling Solution: Variation in Invertebrate Communities, Sample Condition and DNA Quality. PLoS ONE 2016, 11, e0148247. [Google Scholar] [CrossRef]
- Puker, A.; Correa, C.M.A.; Silva, A.S.; Silva, J.V.O.; Korasaki, V.; Grossi, P.C. Effects of fruit-baited trap height on flower and leaf chafer scarab beetles sampling in Amazon rainforest. Entomol. Sci. 2020, 23, 245–255. [Google Scholar] [CrossRef]
- Gruppe, A.; Schubert, H. The spatial distribution and plant specificity of Neuropterida in different forest sites in Southern Germany (Raphidioptera and Neuroptera). Beiträge Entomol. 2001, 51, 517–527. [Google Scholar] [CrossRef]
- Preisser, E.; Smith, D.C.; Lowman, M.D. Canopy and ground level insect distribution in a temperate forest. Selbyana 1998, 19, 141–146. [Google Scholar]
- Ulyshen, M.D. Arthropod vertical stratification in temperate deciduous forests: Implications for conservation-oriented management. For. Ecol. Manag. 2011, 261, 1479–1489. [Google Scholar] [CrossRef]
- Dvořák, L.; Dvořáková, K.; Oboňa, J.; Ruchin, A.B. Selected Diptera families caught with beer traps in the Republic of Mordovia (Russia). Nat. Conserv. Res. 2020, 5, 65–77. [Google Scholar] [CrossRef]
- Giovanni, F.; Mei, M.; Cerretti, P. Vertical stratification of selected Hymenoptera in a remnant forest of the Po Plain (Italy, Lombardy) (Hymenoptera: Ampulicidae, Crabronidae, Sphecidae). Fragm. Entomol. 2017, 49, 71–77. [Google Scholar] [CrossRef]
- Ruchin, A.B. Seasonal dynamics and spatial distribution of lepidopterans in selected locations in Mordovia, Russia. Biodiversitas 2021, 22, 2569–2575. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Esin, M.N. Seasonal dynamics of Diptera in individual biotopes in the center of the European part of Russia. Biosyst. Divers. 2021, 29, 374–379. [Google Scholar] [CrossRef]
- Birtele, D.; Hardersen, S. Analysis of vertical stratification of Syrphidae (Diptera) in an oak-hornbeam forest in northern Italy. Ecol. Res. 2012, 27, 755–763. [Google Scholar] [CrossRef]
- Duelli, P.; Obrist, M.K.; Flückiger, P.F. Forest edges are biodiversity. Acta Zool. Acad. Sci. Hung. 2002, 48 (Suppl. 2), 75–87. [Google Scholar]
- Procházka, J.; Cizek, L.; Schlaghamerský, J. Vertical stratification of scolytine beetles in temperate forests. Insect Conserv. Divers 2018, 11, 534–544. [Google Scholar] [CrossRef]
- Charles, E.; Basset, Y. Vertical stratification of leaf-beetle assemblages (Coleoptera: Chrysomelidae) in two forest types in Panama. J. Trop. Ecol. 2005, 21, 329–336. [Google Scholar] [CrossRef]
- Kato, M.; Inoue, T.; Hamid, A.A.; Nagamitsu, T.; Merdek, M.B.; Nona, A.R.; Itino, T.; Yamane, S.; Yumoto, T. Seasonality and vertical structure of light-attracted insect communities in a dipterocarp forest in Sarawak. Popul. Ecol. 1995, 37, 59–79. [Google Scholar] [CrossRef]
- de Souza Amorim, D.; Brown, B.V.; Boscolo, D.; Ale-Rocha, R.; Alvarez-Garcia, D.M.; Balbi, M.I.P.A.; Barbosa, A.d.M.; Capellari, R.S.; de Carvalho, C.J.B.; Couri, M.S.; et al. Vertical stratification of insect abundance and species richness in an Amazonian tropical forest. Sci. Rep. 2022, 12, 1734. [Google Scholar] [CrossRef]
- Freitas, A.V.L.; Iserhard, C.A.; Santos, J.P.; Carreira, J.Y.O.; Ribeiro, D.B.; Melo, D.H.A.; Rosa, A.H.B.; Marini-Filho, O.J.; Accacio, G.M.; Uehara-Prado, M. Studies with butterfly bait traps: An overview. Rev. Colomb. Entomol. 2014, 40, 203–212. [Google Scholar]
- Checa, M.F.; Rodríguez, J.; Willmott, K.R.; Liger, B. Microclimate variability significantly affects the composition, abundance and phenology of butterfly communities in a highly threatened Neotropical dry forest. Fla. Entomol. 2014, 97, 1–13. [Google Scholar] [CrossRef]
- Luk, C.L.; Hadi, U.K.; Ziegler, T.; Waltert, M. Vertical and horizontal habitats of fruit-feeding butterflies (Lepidoptera) on Siberut, Mentawai Islands, Indonesia. Ecotropica 2011, 17, 79–90. [Google Scholar]
- Santos, J.; Iserhard, C.; Carreira, J.; Freitas, A. Monitoring fruit-feeding butterfly assemblages in two vertical strata in seasonal Atlantic forest: Temporal species turnover is lower in the canopy. J. Trop. Ecol. 2017, 33, 345–355. [Google Scholar] [CrossRef]
- Araujo, P.F.; Freitas, A.V.L.; Gonçalves, G.A.S.; Ribeiro, D.B. Vertical stratification on a small scale: The distribution of fruit-feeding butterflies in a semi-deciduous Atlantic forest in Brazil. Stud. Neotrop. Fauna Environ. 2021, 56, 10–39. [Google Scholar] [CrossRef]
- Mohamed, R.; Rosmidi, F.H.; Adanan, N.A.; Ahmad, A.; Abdullah, M.T. Vertical Stratification of Fruit-Feeding Butterflies in Tasik Kenyir. In Greater Kenyir Landscapes; Abdullah, M., Mohammad, A., Nor Zalipah, M., Safiih Lola, M., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Schulze, C.H.; Linsenmair, K.E.; Fiedler, K. Understorey versus canopy: Patterns of vertical stratification and diversity among Lepidoptera in a Bornean rain forest. In Tropical Forest Canopies: Ecology and Management. Forestry Sciences; Linsenmair, K.E., Davis, A.J., Fiala, B., Speight, M.R., Eds.; Springer: Dordrecht, The Netherlands, 2001; Volume 69. [Google Scholar] [CrossRef]
- DeVries, P.J.; Walla, T.R.; Greeney, H.F. Species diversity in spatial and temporal dimensions of fruit-feeding butterflies from two Ecuadorian rainforests. Biol. J. Linn. Soc. 1999, 68, 333–353. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A. Seasonal activity of Coleoptera attracted by fermental crown traps in forest ecosystems of Central Russia. Ecol. Quest. 2021, 32, 37–53. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; MacGowan, I.; Makarkin, V.N.; Antropov, A.V.; Gornostaev, N.G.; Khapugin, A.A.; Dvořák, L.; Esin, M.N. Post-fire insect fauna explored by crown fermental traps in forests of the European Russia. Sci. Rep. 2021, 11, 21334. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; Semishin, G.B. Fauna of click beetles (Coleoptera: Elateridae) in the interfluve of Rivers Moksha and Sura, Republic of Mordovia, Russia. Biodiversitas 2018, 19, 1352–1365. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A.; Vikhrev, N.E.; Esin, M.N. The use of simple crown traps for the insects collection. Nat. Conserv. Res. 2020, 5, 87–108. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A. Usage of fermental traps for studying the species diversity of Coleoptera. Insects 2021, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Landolt, P.J.; Alfaro, J.F. Trapping Lacanobia subjuncta, Xestia nigrum, and Mamestra configurata (Lepidoptera: Noctuidae) with acetic acid and 3-methyl-1-butanol in controlled release dispensers. Environ. Entomol. 2001, 30, 656–662. [Google Scholar] [CrossRef]
- Nishida, R. Sequestration of defensive substances from plants by Lepidoptera. Annu. Rev. Entomol. 2002, 47, 57–92. [Google Scholar] [CrossRef] [PubMed]
- Utrio, P.; Eriksson, K. Volatile fermentation products as attractants for Macrolepidoptera. Ann. Zool. Fenn. 1977, 14, 98–104. [Google Scholar]
- Daily, G.C.; Ehrlich, P.R. Preservation of biodiversity in small rainforest patches: Rapid evaluations using butterfly trapping. Biodivers. Conserv. 1995, 4, 35–55. [Google Scholar] [CrossRef]
- Landolt, P.J.; Higbee, B.S. Both sexes of the true armyworm (Lepidoptera: Noctuidae) trapped with the feeding attractant composed of acetic acid and 3-methyl-1-butanol. Florida Entomol. 2002, 85, 182–186. [Google Scholar] [CrossRef]
- Süssenbach, D.; Fiedler, K. Noctuid moths attracted to fruit baits: Testing models and methods of estimating species diversity. Nota Lepidopterol. 1999, 22, 115–154. [Google Scholar]
- Walla, T.R.; Engen, S.; DeVries, P.J.; Lande, R. Modeling vertical beta-diversity in tropical butterfly communities. Oikos 2004, 107, 610–618. [Google Scholar] [CrossRef]
- Dell’Aglio, D.D.; Mena, S.; Mauxion, R.; McMillan, W.O.; Montgomery, S.H. Divergence in Heliconius flight behaviour is associated with local adaptation to different forest structures. J. Anim. Ecol. 2022, 91, 727–737. [Google Scholar] [CrossRef]
- Sutton, S.L.; Ash, C.P.J.; Grundy, A. The vertical stratification of flying insects in lowland rain forests of Panama, Papua New-Guinea and Brunei. Zool. J. Linn. Soc. 1983, 78, 287–297. [Google Scholar] [CrossRef]
- Brehm, G.; Axmacher, J.C. A comparison of manual and automatic moth sampling methods (Lepidoptera: Arctiidae, Geometridae) in a rain forest in Costa Rica. Environ. Entomol. 2006, 35, 757–764. [Google Scholar] [CrossRef]
- Baldocchi, D.; Collineau, S. The physical nature of solar radiation in heterogeneous canopies: Spatial and temporal attributes. In Exploitation of Environmental Heterogeneity by Plants; Caldwell, M.M., Pearcy, R.W., Eds.; Academic Press: New York, NY, USA, 1994; pp. 21–71. [Google Scholar]
- Grimmond, C.S.B.; Robeson, S.M.; Schoof, J.T. Spatial variability of micro-climatic conditions within a mid-latitude deciduous forest. Clim. Res. 2000, 15, 137–149. [Google Scholar] [CrossRef]
- Ste-Marie, C.; Paré, D.; Gagnon, D. The Contrasting Effects of Aspen and Jack Pine on Soil Nutritional Properties Depend on Parent Material. Ecosystems 2007, 10, 1299–1310. [Google Scholar] [CrossRef]
- Vesterdal, L.; Raulund-Rasmussen, K. Forest floor chemistry under seven tree species along a soil fertility gradient. Can. J. For. Res. 1998, 28, 1636–1647. [Google Scholar] [CrossRef]
- Darsono Riwidiharso, E.; Santoso, S.; Sudiana, E.; Yani, E.; Nasution, E.K.; Aprilliana, H.; Chasanah, T. Insect diversity in various distances to forest edge in small nature reserve: A case study of Bantarbolang Nature Reserve, Central Java, Indonesia. Biodiversitas 2020, 21, 4821–4828. [Google Scholar]
- Fortin, M.; Mauffette, Y. Forest edge effects on the biological performance of the forest tent caterpillar (Lepidoptera: Lasiocampidae) in sugar maple stands. Écoscience 2001, 8, 164–172. [Google Scholar] [CrossRef]
- Dodonov, P.; Harper, K.A.; Silva-Matos, D.M. The role of edge contrast and forest structure in edge influence: Vegetation and microclimate at edges in the Brazilian cerrado. Plant Ecol. 2013, 214, 1345–1359. [Google Scholar] [CrossRef]
- Mathew, G. Insect biodiversity in tropical forests: A study with reference to butterflies and moths (Insecta: Lepidoptera) in the Silent Valley National Park (Kerala). Adv. For. Res. India 1994, 11, 134–171. [Google Scholar]
- Kuussaari, M.; Heliölä, J.; Luoto, M.; Pöyry, J. Determinants of local species richness of diurnal Lepidoptera in boreal agricultural landscapes. Agric. Ecosyst. Environ. 2007, 122, 366–376. [Google Scholar] [CrossRef]
- Melo, D.H.A.; Duarte, M.; Mielke, O.H.H.; Robbins, R.K.; Freitas, A.V.L. Butterflies (Lepidoptera: Papilionoidea) of an urban park in northeastern Brazil. Biota Neotrop. 2019, 19, e20180614. [Google Scholar] [CrossRef]
- Gornostaev, N.G.; Ruchin, A.B.; Esin, M.N.; Kulikov, A.M. Seasonal Dynamics of Fruit Flies (Diptera: Drosophilidae) in Forests of the European Russia. Insects 2022, 13, 751. [Google Scholar] [CrossRef]
- Chick, L.D.; Strickler, S.; Perez, A.; Martin, R.A.; Diamond, S.E. Urban heat islands advance the timing of reproduction in a social insect. J. Therm. Biol. 2019, 80, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Minin, A.A.; Ananin, A.A.; Buyvolov, Y.A.; Larin, E.G.; Lebedev, P.A.; Polikarpova, N.V.; Prokosheva, I.V.; Rudenko, M.I.; Sapelnikova, I.I.; Fedotova, V.G.; et al. Recommendations to unify phenological observations in Russia. Nat. Conserv. Res. 2020, 5, 89–110. [Google Scholar] [CrossRef]
- Ribeiro, D.B.; Prado, P.I.; Brown, K.S.; Freitas, A.V.L. Temporal diversity patterns and phenology in fruit-feeding butterflies in the Atlantic forest. Biotropica 2010, 42, 710–716. [Google Scholar] [CrossRef]
- Grøtan, V.; Lande, R.; Engen, S.; Sæther, B.E.; DeVries, P.J. Seasonal cycles of species diversity and similarity in a tropical butterfly community. J. Anim. Ecol. 2012, 81, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Con, V.Q.; Lien, V.V. Seasonal dynamics of butterfly (Lepidoptera, Rhopalocera) abundance in the Tropical Rain Forest of Vietnam. Entmol. Rev. 2015, 95, 578–582. [Google Scholar] [CrossRef]
- Scalco, V.W.; de Morais, A.B.B.; Romanowski, H.P.; Mega, N.O. Population dynamics of the swallowtail butterfly Battus polystictus polystictus (Butler) (Lepidoptera: Papilionidae) with Notes on Its Natural History. Neotrop. Entomol. 2016, 45, 33–43. [Google Scholar] [CrossRef]
- Altermatt, F. Temperature-related shifts in butterfly phenology depend on the habitat. Glob. Chang. Biol. 2012, 18, 2429–2438. [Google Scholar] [CrossRef]
- Zografou, K.; Kati, V.; Grill, A.; Wilson, R.J.; Tzirkalli, E.; Pamperis, L.N.; Halley, J.M. Signals of climate change in butterfly communities in a mediterranean protected area. PLoS ONE 2014, 9, e87245. [Google Scholar] [CrossRef] [PubMed]
- Colom, P.; Traveset, A.; Carreras, D.; Stefanescu, C. Spatio-temporal responses of butterflies to global warming on a Mediterranean island over two decades. Ecol. Entomol. 2021, 46, 262–272. [Google Scholar] [CrossRef]
- Laaksonen, J.; Laaksonen, T.; Itamies, J.; Rytkonen, S.; Valimaki, P. A new efficient bait-trap model for Lepidoptera surveys-the “Oulu” model. Entomol. Fenn. 2006, 17, 153–160. [Google Scholar] [CrossRef]
- Pettersson, L.B.; Franzén, M. Comparing wine-based and beer-based baits for moth trapping: A field experiment. Entomol. Tidskr. 2008, 129, 129–134. [Google Scholar]
- Kirby, K.J.; Buckley, G.P.; Mills, J. Biodiversity implications of coppice decline, transformations to high forest and coppice restoration in British woodland. Folia Geobot 2017, 52, 5–13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruchin, A.B. Spatial Distribution of Lepidoptera in Forest Ecosystems of Central European Russia: Studies Using Beer Traps. Forests 2023, 14, 680. https://doi.org/10.3390/f14040680
Ruchin AB. Spatial Distribution of Lepidoptera in Forest Ecosystems of Central European Russia: Studies Using Beer Traps. Forests. 2023; 14(4):680. https://doi.org/10.3390/f14040680
Chicago/Turabian StyleRuchin, Alexander B. 2023. "Spatial Distribution of Lepidoptera in Forest Ecosystems of Central European Russia: Studies Using Beer Traps" Forests 14, no. 4: 680. https://doi.org/10.3390/f14040680
APA StyleRuchin, A. B. (2023). Spatial Distribution of Lepidoptera in Forest Ecosystems of Central European Russia: Studies Using Beer Traps. Forests, 14(4), 680. https://doi.org/10.3390/f14040680





