Dendroremediation Potential of Six Quercus Species to Polluted Soil in Historic Copper Mining Sites
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Preparation
2.2. Plant Cultivation
2.3. Photosynthetic Parameters
2.4. Element Determination
2.5. Analytic Hierarchy Process and Entropy Weighted Method
2.6. Calculation and Statistical Analysis
3. Results
3.1. Plant Growth and Biomass Production
3.2. Photosynthesis and Foliar Pigments
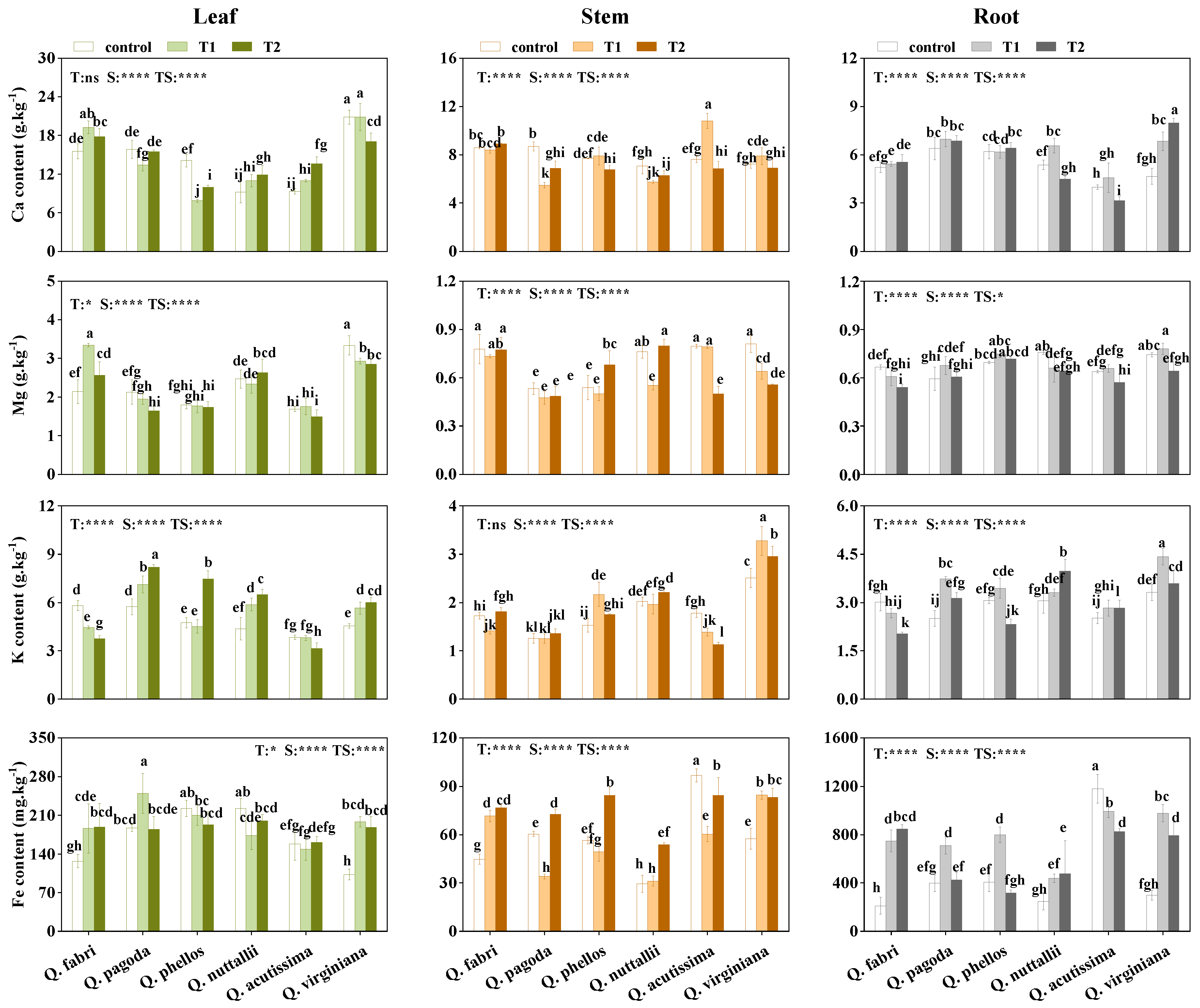
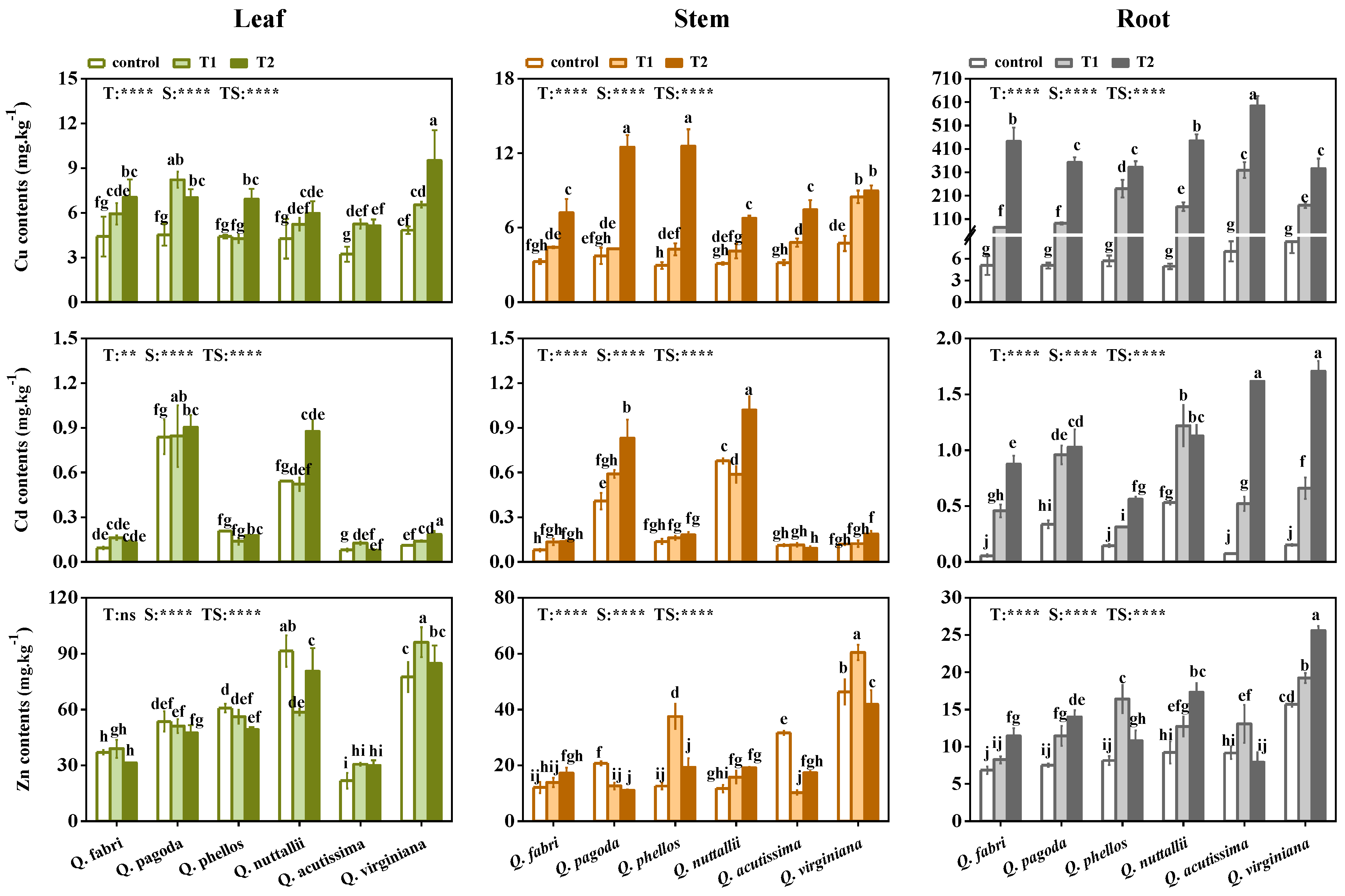
3.3. Accumulation of Nutrient Elements in Plants
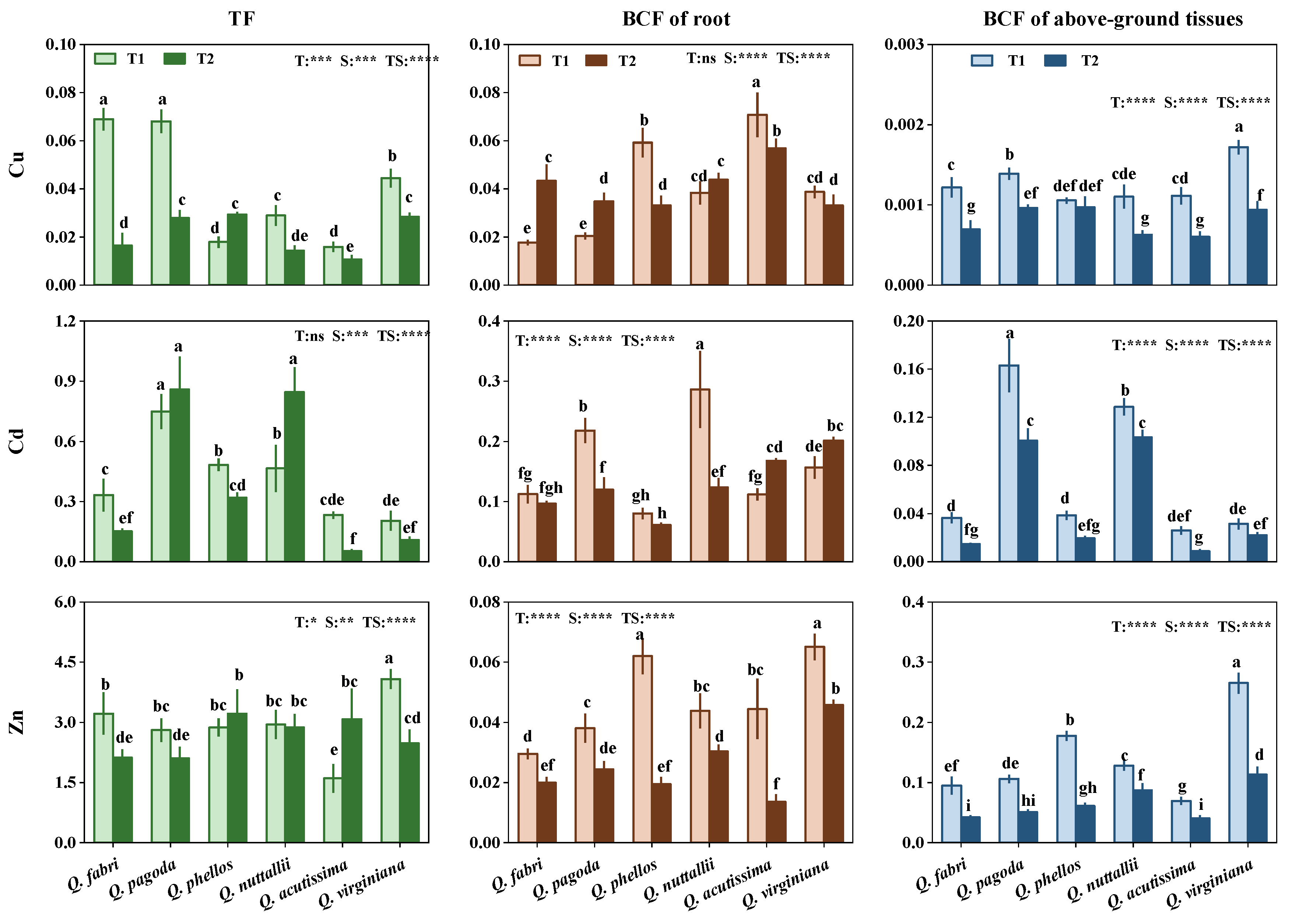
3.4. Bioconcentration and Translocation of HMs in Plants
4. Discussion
4.1. Quercus spp. Tolerance to HMs
4.2. Elements Accumulation and Distribution in Plants
4.3. Phytoremediation Potential of Quercus Species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, L.; Ji, B.; Hu, Y.H.; Liu, R.Q.; Sun, W. A review on in situ phytoremediation of mine. Chemosphere 2017, 184, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.M.; Li, Y.; Li, F.R.; Li, C.M.; Liu, K.H. The effects of EDTA on plant growth and manganese (Mn) accumulation in Polygonum pubescens Blume cultured in unexplored soil, mining soil and tailing soil from the Pingle Mn mine, China. Ecotoxicol. Environ. Saf. 2019, 173, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ordiales, E.; Cienfuegos, P.; Roqueni, N.; Covelli, S.; Flor-Blanco, G.; Fontolan, G.; Loredo, J. Historical accumulation of potentially toxic trace elements resulting from mining activities in estuarine salt marshes sediments of the Asturias coastline (northern Spain). Environ. Sci. Pollut. Res. 2019, 26, 3115–3128. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shaheen, S.M.; Jiang, Y.; Li, R.; Slaný, M.; Kwon, B.N.; Rinklebe, J.; Zhang, Z. Fe/Mn- and P-modified drinking water treatment residuals decreased Cu and Pb phytoavailability and uptake in a mining soil. J. Hazard. Mater. 2021, 403, 123628. [Google Scholar] [CrossRef]
- Adiansyah, J.S.; Rosano, M.; Vink, S.; Keir, G. A framework for a sustainable approach to mine tailings management: Disposal strategies. J. Clean. Prod. 2015, 108, 1050–1062. [Google Scholar] [CrossRef]
- Yin, G.; Li, G.; Wei, Z.; Wan, L.; Shui, G.; Jing, X. Stability analysis of a copper tailings dam via laboratory model tests: A Chinese case study. Miner. Eng. 2011, 24, 122–130. [Google Scholar] [CrossRef]
- Milla-Moreno, E.; Guy, R.D. Growth response, uptake and mobilization of metals in native plant species on tailings at a Chilean copper mine. Int. J. Phytoremediat. 2021, 23, 539–547. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, W.H.; Tian, J.; Liu, X.M.; Liu, F.W.; Wang, G.L.; Di, X.Y. Effects of different amendments on water-stable aggregates and organic carbon components in a reclaimed soil. Chin. J. Soil Sci. 2022, 53, 392–402. [Google Scholar]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.Y.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Tang, C.F.; Chen, Y.H.; Zhang, Q.N.; Li, J.B.; Zhang, F.Y.; Liu, Z.M. Effects of peat on plant growth and lead and zinc phytostabilization from lead-zinc mine tailing in southern China: Screening plant species resisting and accumulating metals. Ecotoxicol. Environ. Saf. 2019, 176, 42–49. [Google Scholar] [CrossRef]
- Zou, T.; Li, T.; Zhang, X.; Yu, H.; Huang, H. Lead accumulation and phytostabilization potential of dominant plant species growing in a lead–zinc mine tailing. Environ. Earth. Sci. 2012, 65, 621–630. [Google Scholar] [CrossRef]
- Heckenroth, A.; Rabier, J.; Dutoit, T.; Torre, F.; Prudent, P.; Laffont-Schwob, I. Selection of native plants with phytoremediation potential for highly contaminated Mediterranean soil restoration: Tools for a non-destructive and integrative approach. J. Environ. Manag. 2016, 183, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Khan, Q.M.; Sessitsch, A. Endophytic bacteria: Prospects and applications for the phytoremediation of organic pollutants. Chemosphere 2014, 117, 232–242. [Google Scholar] [CrossRef]
- Wu, T.; Wang, G.; Wu, Q.; Cheng, X.; Yu, M.; Wang, W.; Yu, X. Patterns of leaf nitrogen and phosphorus stoichiometry among Quercus acutissima provenances across China. Ecol. Complex. 2014, 17, 32–39. [Google Scholar] [CrossRef]
- Yang, B.S.; He, F.; Zhao, X.X.; Wang, H.; Xu, X.H.; He, X.H.; Zhu, Y.D. Composition and function of soil fungal community during the establishment of Quercus acutissima (Carruth.) seedlings in a Cd contaminated soil. J. Environ. Manag. 2019, 246, 150–156. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Robinson, B.H.; Gunthardt-Goerg, M.S.; Schulin, R. Metal uptake and allocation in trees grown on contaminated land: Implications for biomass production. Int. J. Phytorem. 2013, 15, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Gogorcena, Y.; Larbi, A.; Andaluz, S.; Carpena, R.O.; Abadia, A.; Abadia, J. Effects of cadmium on cork oak (Quercus suber L.) plants grown in hydroponics. Tree Physiol. 2011, 31, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.J.; Ma, C.X.; Xiao, J.; Li, X.G.; Wang, S.F.; Chen, G.C. Co-planting of Quercus nuttallii, Quercus pagoda with Solanum nigrum enhanced their phytoremediation potential to multi-metal contaminated soil. Int. J. Phytoremediat. 2021, 2, 1104–1112. [Google Scholar] [CrossRef]
- Xiao, J.; Salam, M.M.A.; Chen, G.C. Evaluation of dendroremediation potential of ten Quercus spp. for heavy metals contaminated soil-a three-year field trial. Sci. Total Environ. 2022, 851, 158232. [Google Scholar]
- Zhao, X.L.; Zheng, L.Y.; Xia, X.L.; Yin, W.L.; Lei, J.P.; Shi, S.Q.; Shi, X.; Li, H.Q.; Li, Q.H.; Wei, Y.; et al. Responses and acclimation of Chinese cork oak (Quercus variabilis Bl.) to metal stress: The inducible antimony tolerance in oak trees. Environ. Sci. Pollut. Res. 2015, 22, 11456–11466. [Google Scholar] [CrossRef]
- Shi, X.; Wang, S.F.; Sun, H.J.; Chen, Y.T.; Wang, D.X.; Pan, H.W.; Zou, Y.Z.; Liu, J.F.; Zheng, L.Y.; Zhao, X.L.; et al. Comparative of Quercus spp. and Salix spp. for phytoremediation of Pb/Zn mine tailings. Environ. Sci. Pollut. Res. 2017, 24, 3400–3411. [Google Scholar] [CrossRef] [PubMed]
- Rinklebe, J.; Antoniadis, V.; Shaheen, S.M.; Rosche, O.; Altermann, M. Health risk assessment of potentially toxic elements in soils along the Central Elbe River, Germany. Environ. Int. 2019, 126, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Sozoniuk, M.; Nowak, M.; Dudziak, K.; Bulak, P.; Leśniowska-Nowak, J.; Kowalczyk, K. Antioxidative system response of pedunculate oak (Quercus robur L.) seedlings to Cd exposure. Physiol. Mol. Biol. Plants 2019, 25, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.N.; Tan, Q.; Zhang, F.; Ma, C.X.; Xiao, J.; Chen, G.C. Phytoremediation potential evaluation of multiple Salix clones for heavy metals (Cd, Zn and Pb) in flooded soils. Sci. Total Environ. 2022, 813, 152482. [Google Scholar] [CrossRef]
- Cao, Y.N.; Ma, C.X.; Chen, H.J.; Zhang, J.F.; White, J.C.; Chen, G.C.; Xing, B.S. Xylem-based long-distance transport and phloem remobilization of copper in Salix integra Thunb. J. Hazard. Mater. 2020, 392, 122428. [Google Scholar] [CrossRef]
- Metwally, A.; Safronova, V.I.; Belimov, A.A.; Dietz, K.J. Genotypic variation of the response to cadmium toxicity in Pisum sativum L. J. Exp. Bot. 2005, 56, 167–178. [Google Scholar] [CrossRef]
- Kim, I.S.; Kang, K.H.; Johnson-Green, P.; Lee, E.J. Investigation of heavy metal accumulation in Polygonum thunbergii for phytoextraction. Environ. Pollut. 2003, 126, 235–243. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.D.; Zhou, Q.X.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- de Souza, S.C.R.; de Andrade, S.A.L.; de Souza, L.A.; Schiavinato, M.A. Lead tolerance and phytoremediation potential of Brazilian leguminous tree species at the seedling stage. J. Environ. Manag. 2012, 110, 299–307. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Yang, H.H.; Li, X.X.; Cui, Z.J. Physiological responses of Suaeda glauca and Arabidopsis thaliana in phytoremediation of heavy metals. J. Environ. Manag. 2018, 223, 132–139. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef] [PubMed]
- Shanying, H.; Xiaoe, Y.; Zhenli, H.; Baligar, V.C. Morphological and physiological responses of plants to cadmium toxicity: A review. Pedosphere 2017, 27, 421–438. [Google Scholar]
- Shabir, R.; Abbas, G.; Saqib, M.; Shahid, M.; Shah, G.M.; Akram, M.; Niazi, N.K.; Naeem, M.A.; Hussain, M.; Ashraf, F. Cadmium tolerance and phytoremediation potential of acacia (Acacia nilotica L.) under salinity stress. Int. J. Phytoremediat. 2018, 20, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, S.; Chen, Y.; Xu, Q.; Sun, H.; An, R.; Lu, X.; Lu, Y.; Fan, S. Tolerance and vegetation restoration prospect of seedlings of five oak species for Pb/Zn mine tailing. Chin. J. Appl. Ecol. 2019, 30, 4091–4098. [Google Scholar]
- Meneguelli-Souza, A.C.; Vitória, A.P.; Vieira, T.O.; Degli-Esposti, M.S.O.; Souza, C.M.M. Ecophysiological responses of Eichhornia crassipes (mart.) Solms to As5+ under different stress conditions. Photosynthetica 2016, 54, 243–250. [Google Scholar] [CrossRef]
- Zeng, J.; Li, X.Y.; Wang, X.X.; Zhang, K.H.; Wang, Y.; Kang, H.Y.; Chen, G.D.; Lan, T.; Zhang, Z.W.; Yuan, S.; et al. Cadmium and lead mixtures are less toxic to the Chinese medicinal plant Ligusticum chuanxiong Hort. Than either metal alone. Ecotoxicol. Environ. Saf. 2020, 193, 110342. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 1–908. [Google Scholar] [CrossRef]
- Jin, M.F.; You, M.X.; Lan, Q.Q.; Cai, L.Y.; Lin, M.Z. Effect of copper on the photosynthesis and growth of Eichhornia crassipes. Plant Biol. 2021, 23, 777–784. [Google Scholar] [CrossRef]
- Leal-Alvarado, D.A.; Espadas-Gil, F.; Sáenz-Carbonell, L.; Talavera-May, C.; Santamerfa, J.M. Lead accumulation reduces photosynthesis in the lead hyper-accumulator Salvinia minima Baker by affecting the cell membrane and inducing stomatal closure. Aquat. Toxicol. 2016, 171, 37–47. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annual Review of Plant. Physiology 1982, 33, 317–345. [Google Scholar]
- Raven, J.A. Interactions between above and below ground plant structures: Mechanisms and ecosystem services. Front. Agric. Sci. Eng. 2022, 9, 197–213. [Google Scholar]
- Burges, A.; Alkorta, I.; Epelde, L.; Garbisu, C. From phytoremediation of soil contaminants to phytomanagement of ecosystem services in metal contaminated sites. Int. J. Phytoremediat. 2018, 20, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Zhivotovsky, O.P.; Kuzovkina, J.A.; Schulthess, C.P.; Morris, T.; Pettinelli, D.; Ge, M. Hydroponic screening of willows (Salix L.) for lead tolerance and accumulation. Int. J. Phytoremediat. 2010, 13, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Lux, A.; Šottníková, A.; Opatrná, J.; Greger, M. Differences in structure of adventitious roots in Salix clones with contrasting characteristics of cadmium accumulation and sensitivity. Physiol. Plant 2004, 120, 537–545. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Song, M.Y.; Zhang, S.; Cai, Z.Q.; Lei, Y.B. Unravelling community assemblages through multi-element stoichiometry in plant leaves and roots across primary successional stages in a glacier retreat area. Plant Soil 2018, 428, 291–305. [Google Scholar] [CrossRef]
- Huang, D.; Wang, D.M.; Ren, Y. Using leaf nutrient stoichiometry as an indicator of flood tolerance and eutrophication in the riparian zone of the Lijang River. Ecol. Indic. 2019, 98, 821–829. [Google Scholar] [CrossRef]
- Han, W.X.; Fang, J.Y.; Reich, P.B.; Ian Woodward, F.; Wang, Z.H. Biogeography and variability of eleven mineral elements in plant leaves across gradients of climate, soil and plant functional type in China. Ecol. Lett. 2011, 14, 788–796. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kudla, J. Calcium decoding mechanisms in plants. Biochimie 2011, 93, 2054–2059. [Google Scholar] [CrossRef]
- Antoniadis, V.; Shaheen, S.M.; Stärk, H.J.; Wennrich, R.; Levizou, E.; Merbach, I.; Rinklebe, J. Phytoremediation potential of twelve wild plant species for toxic elements in a contaminated soil. Environ. Int. 2021, 146, 106233. [Google Scholar] [CrossRef]
- Krämer, U. Metal hyperaccumulation in plants. Annu. Rev. Plant Biol. 2010, 61, 517–534. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Element in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Jeyakumar, P.; Loganathan, P.; Sivakumaran, S.; Anderson, C.W.N.; McLaren, R.G. Bioavailability of copper and zinc to poplar and microorganisms in a biosolids-amended soil. Soil Res. 2010, 48, 459–469. [Google Scholar] [CrossRef]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation—A review. Earth Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Saaty, T.L. Fundamentals of decision making and priority theory with the analytic hierarchy process. Anal. Hierarchy Process 2000, 6. [Google Scholar]

| Treatments | pH | OM (g·kg−1) | TN (g·kg−1) | TP (g·kg−1) | Heavy Metal Content (mg·kg−1) | Risk Screening Value (mg·kg−1) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Cd | Zn | Cu | Cd | Zn | |||||
| control | 6.9 ± 0.1 | 43.1 ± 2.5 | 1.7 ± 0.4 | 0.5 ± 0.05 | 71 ± 12 | 0.4 ± 0.0 | 99 ± 10 | 100 | 0.3 | 250 |
| T1 | 7.2 ± 0.1 | 32.0 ± 1.3 | 1.2 ± 0.2 | 1.4 ± 0.05 | 4366 ± 201 | 3.8 ± 0.3 | 270 ± 10 | |||
| T2 | 7.4 ± 0.2 | 23.4 ± 3.9 | 0.9 ± 0.1 | 3.0 ± 0.15 | 9839 ± 212 | 8.5 ± 0.3 | 562 ± 14 | |||
| Species | Treatments | Above-Ground Biomass | Root Biomass | Total Biomass | TI |
|---|---|---|---|---|---|
| Q. fabri | Control | 28.3 1.7 | 28.3 1.6 | 56.6 0.2 | -- |
| T1 | 14.2 2.7 | 17.4 1.8 | 31.7 1.2 | 0.56 0.02 | |
| T2 | 14.4 1.0 | 14.9 1.4 | 29.3 0.6 | 0.52 0.01 | |
| Q. pagoda | Control | 18.9 1.8 | 14.8 2.6 | 33.7 3.0 | -- |
| T1 | 12.6 1.7 | 8.7 2.9 | 21.3 4.7 | 0.64 0.16 | |
| T2 | 18.0 1.7 | 18.2 4.1 | 36.2 5.8 | 1.09 0.27 | |
| Q. phellos | Control | 52.3 2.7 | 41.8 1.2 | 95.6 1.7 | -- |
| T1 | 38.3 7.3 | 20.4 2.1 | 58.7 5.3 | 0.61 0.07 | |
| T2 | 37.4 5.6 | 31.3 0.9 | 68.7 6.3 | 0.72 0.05 | |
| Q. nuttallii | Control | 23.5 4.2 | 12.1 3.5 | 35.6 6.9 | -- |
| T1 | 30.4 7.0 | 13.9 1.7 | 44.3 8.4 | 1.29 0.42 | |
| T2 | 26.6 3.3 | 15.6 1.7 | 42.2 5.0 | 1.21 0.23 | |
| Q. acutissima | Control | 26.1 5.3 | 28.5 6.2 | 54.5 0.9 | -- |
| T1 | 23.4 5.2 | 28.4 1.1 | 51.8 4.2 | 0.95 0.09 | |
| T2 | 28.5 7.6 | 30.2 5.1 | 58.8 2.7 | 1.08 0.04 | |
| Q. virginiana | Control | 27.3 1.8 | 19.9 1.8 | 47.4 3.1 | -- |
| T1 | 25.7 4.0 | 16.0 2.8 | 41.7 4.5 | 0.88 0.04 | |
| T2 | 24.5 1.0 | 21.7 4.9 | 46.2 4.8 | 0.98 0.11 | |
| Significances | T | ** | **** | **** | ns |
| S | **** | **** | **** | * | |
| T×S | ** | **** | **** | ns |
| Quercus Species | Treatments | Chl a | Chl b | Chl (a + b) | Car |
|---|---|---|---|---|---|
| Q. fabri | Control | 2.68 0.15 | 0.87 0.05 | 3.55 0.20 | 0.49 0.03 |
| T1 | 3.41 0.15 | 1.05 0.04 | 4.46 0.19 | 0.57 0.02 | |
| T2 | 3.14 0.27 | 0.99 0.09 | 4.13 0.36 | 0.53 0.04 | |
| Q. pagoda | Control | 2.24 0.11 | 0.74 0.05 | 2.98 0.16 | 0.40 0.01 |
| T1 | 2.26 0.03 | 0.72 0.01 | 2.98 0.04 | 0.42 0.00 | |
| T2 | 1.74 0.09 | 0.57 0.01 | 2.30 0.08 | 0.32 0.03 | |
| Q. phellos | Control | 2.83 0.22 | 0.87 0.09 | 3.69 0.30 | 0.52 0.03 |
| T1 | 2.54 0.17 | 0.80 0.06 | 3.34 0.22 | 0.49 0.03 | |
| T2 | 1.95 0.05 | 0.58 0.04 | 2.54 0.09 | 0.37 0.01 | |
| Q. nuttallii | Control | 1.34 0.10 | 0.42 0.04 | 1.76 0.13 | 0.26 0.02 |
| T1 | 1.79 0.17 | 0.61 0.06 | 2.40 0.22 | 0.35 0.03 | |
| T2 | 1.61 0.07 | 0.52 0.03 | 2.12 0.10 | 0.31 0.02 | |
| Q. acutissima | Control | 1.32 0.03 | 0.41 0.03 | 1.73 0.05 | 0.27 0.01 |
| T1 | 1.97 0.09 | 0.61 0.03 | 2.58 0.12 | 0.37 0.02 | |
| T2 | 1.64 0.13 | 0.53 0.01 | 2.17 0.13 | 0.33 0.03 | |
| Q. virginiana | Control | 2.38 0.14 | 0.81 0.05 | 3.19 0.19 | 0.47 0.03 |
| T1 | 2.74 0.12 | 0.87 0.04 | 3.61 0.16 | 0.50 0.02 | |
| T2 | 2.59 0.13 | 0.84 0.07 | 3.43 0.19 | 0.49 0.01 | |
| Significances | T | **** | **** | **** | **** |
| S | **** | **** | **** | **** | |
| T×S | **** | **** | **** | **** |
| Quercus Species | Plant Growth | Photosynthesis | Metal Accumulation | Importance Weight | ||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 | |
| Q. fabri | 0.268 | 0.037 | 1.00 | 1.00 | 0.049 | 0.138 | 0.383 | 0.355 |
| Q. pagoda | 0.000 | 0.144 | 0.27 | 0.40 | 0.293 | 0.233 | 0.204 | 0.256 |
| Q. phellos | 0.870 | 0.913 | 0.16 | 0.14 | 0.342 | 0.228 | 0.441 | 0.398 |
| Q. nuttallii | 0.715 | 0.430 | 0.18 | 0.30 | 0.447 | 0.390 | 0.449 | 0.375 |
| Q. acutissima | 0.629 | 0.705 | 0.08 | 0.03 | 0.305 | 0.482 | 0.333 | 0.416 |
| Q. virginiana | 0.611 | 0.507 | 0.77 | 0.68 | 0.582 | 0.555 | 0.645 | 0.576 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Yu, L.; Dang, N.; Sun, L.; Zhang, P.; Cao, J.; Chen, G. Dendroremediation Potential of Six Quercus Species to Polluted Soil in Historic Copper Mining Sites. Forests 2023, 14, 62. https://doi.org/10.3390/f14010062
Cao Y, Yu L, Dang N, Sun L, Zhang P, Cao J, Chen G. Dendroremediation Potential of Six Quercus Species to Polluted Soil in Historic Copper Mining Sites. Forests. 2023; 14(1):62. https://doi.org/10.3390/f14010062
Chicago/Turabian StyleCao, Yini, Liangqian Yu, Ning Dang, Lixiang Sun, Pingxuan Zhang, Jiwu Cao, and Guangcai Chen. 2023. "Dendroremediation Potential of Six Quercus Species to Polluted Soil in Historic Copper Mining Sites" Forests 14, no. 1: 62. https://doi.org/10.3390/f14010062
APA StyleCao, Y., Yu, L., Dang, N., Sun, L., Zhang, P., Cao, J., & Chen, G. (2023). Dendroremediation Potential of Six Quercus Species to Polluted Soil in Historic Copper Mining Sites. Forests, 14(1), 62. https://doi.org/10.3390/f14010062







