Agrobacterium-Mediated Genetic Transformation of Larix kaempferi (Lamb.) Carr. Embryogenic Cell Suspension Cultures and Expression Analysis of Exogenous Genes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Culture Conditions
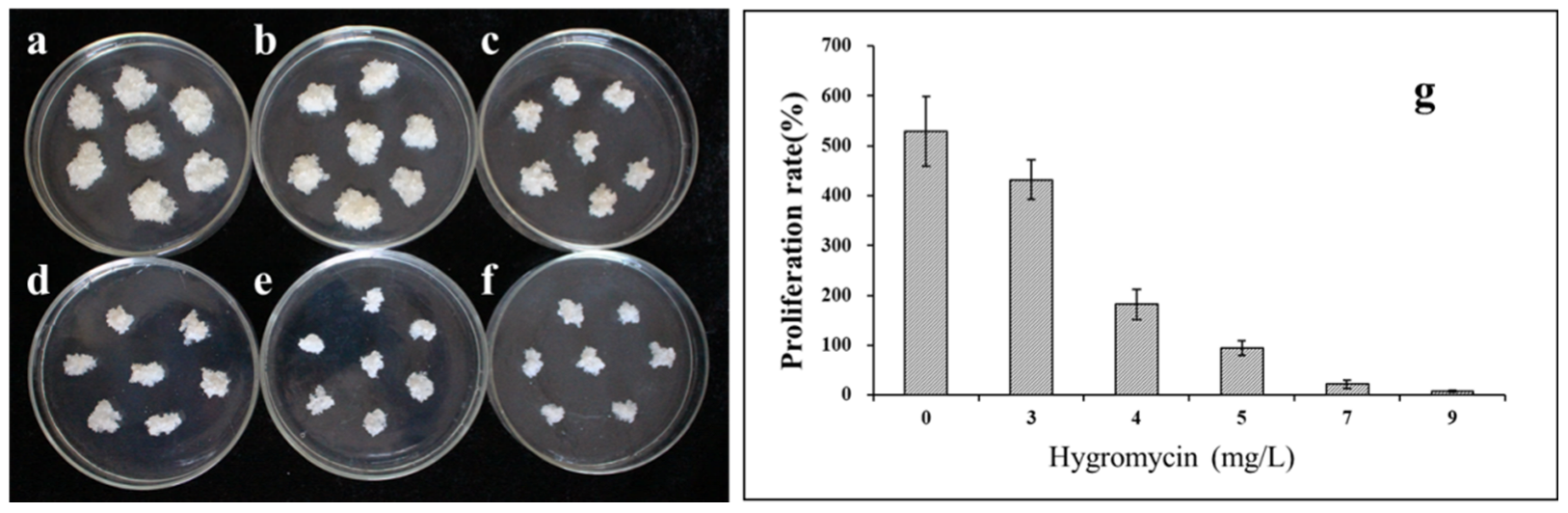
2.2. Hygromycin Sensitivity of Embryogenic Line S287
2.3. Agrobacterium Strains and Binary Vectors
2.4. Agrobacterium-Mediated Transformation
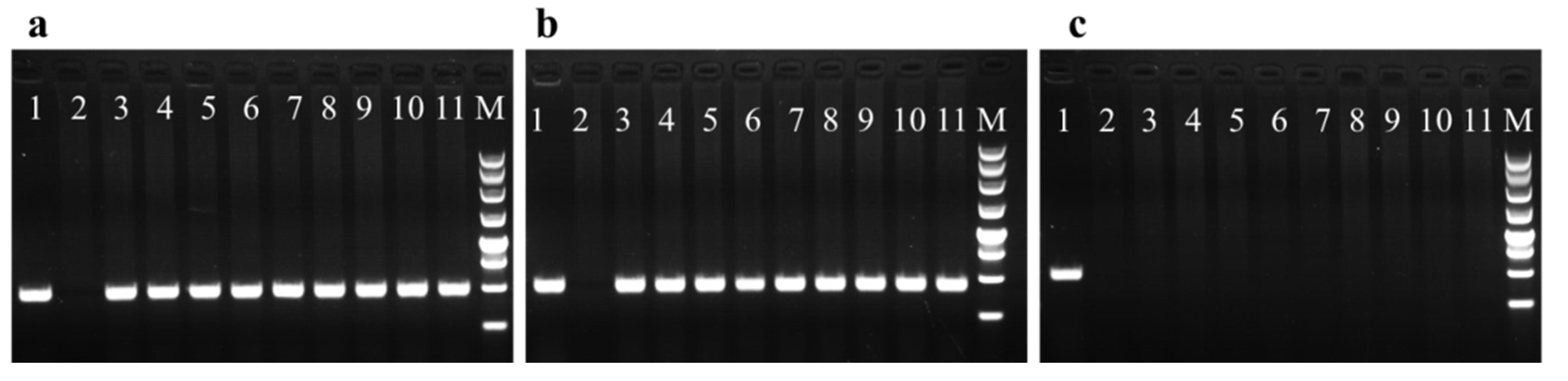
2.5. PCR Detection
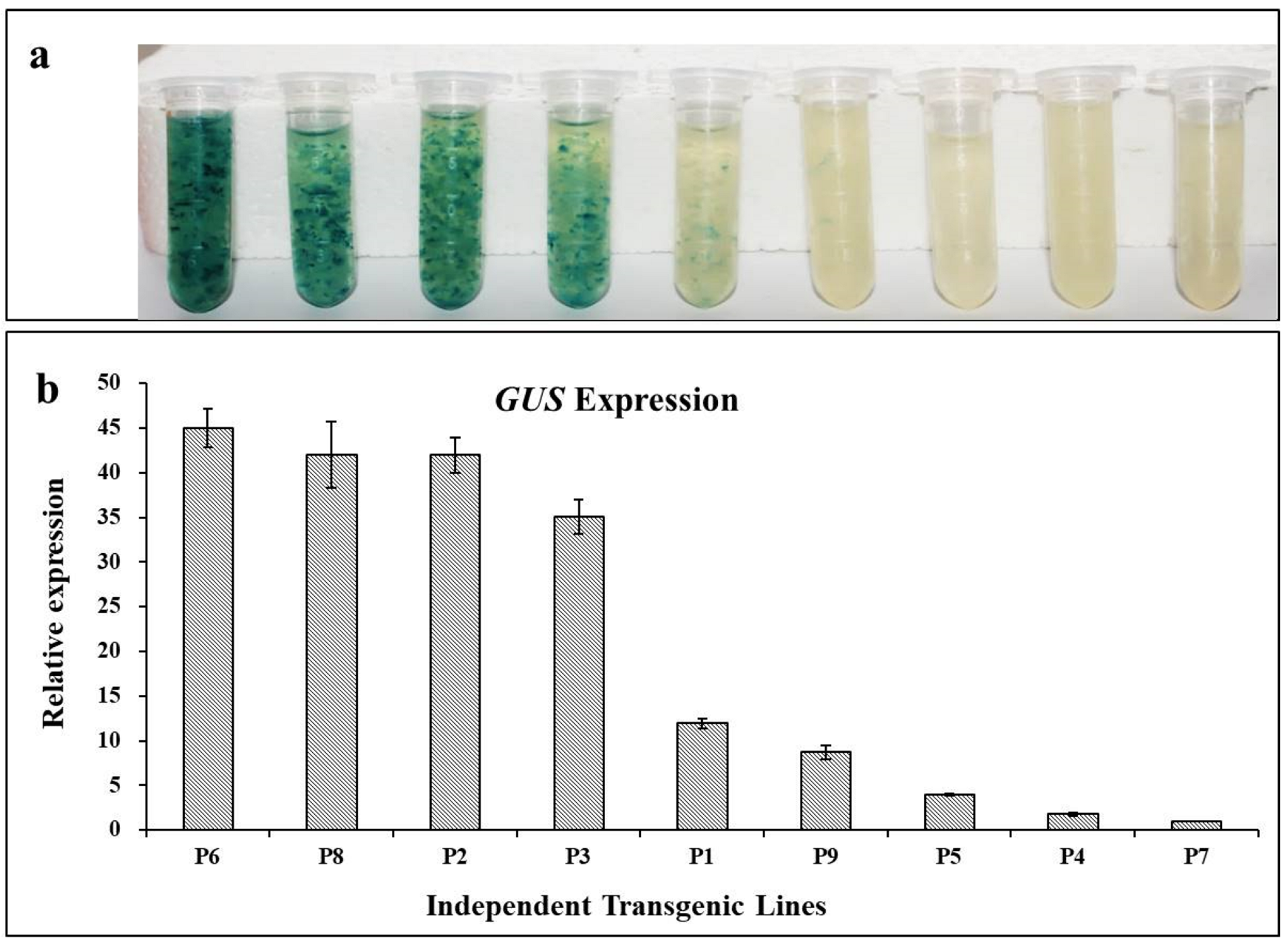
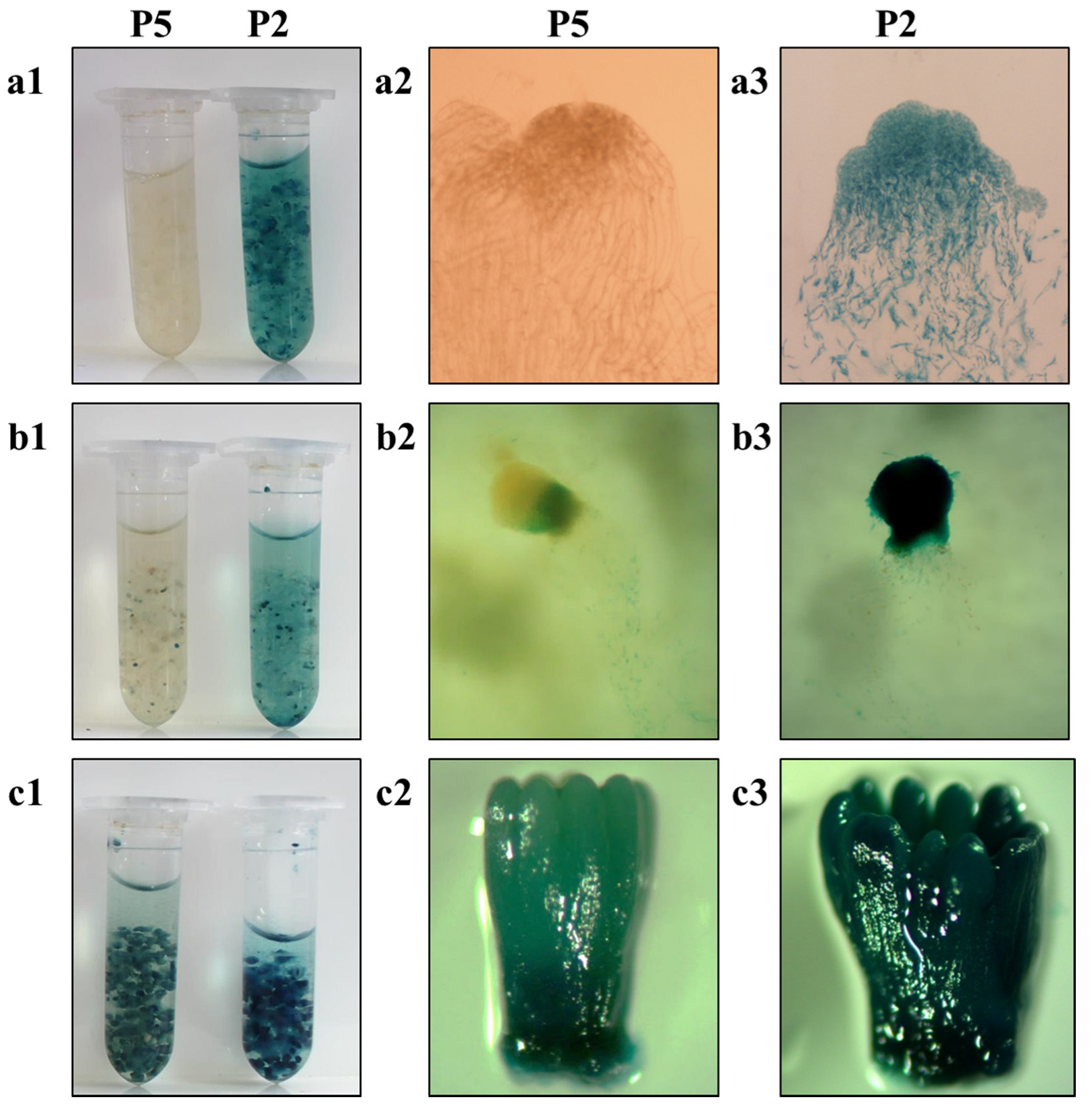
2.6. Histochemical GUS Assay
2.7. Gene Expression Analysis by Quantitative Real-Time PCR
3. Results
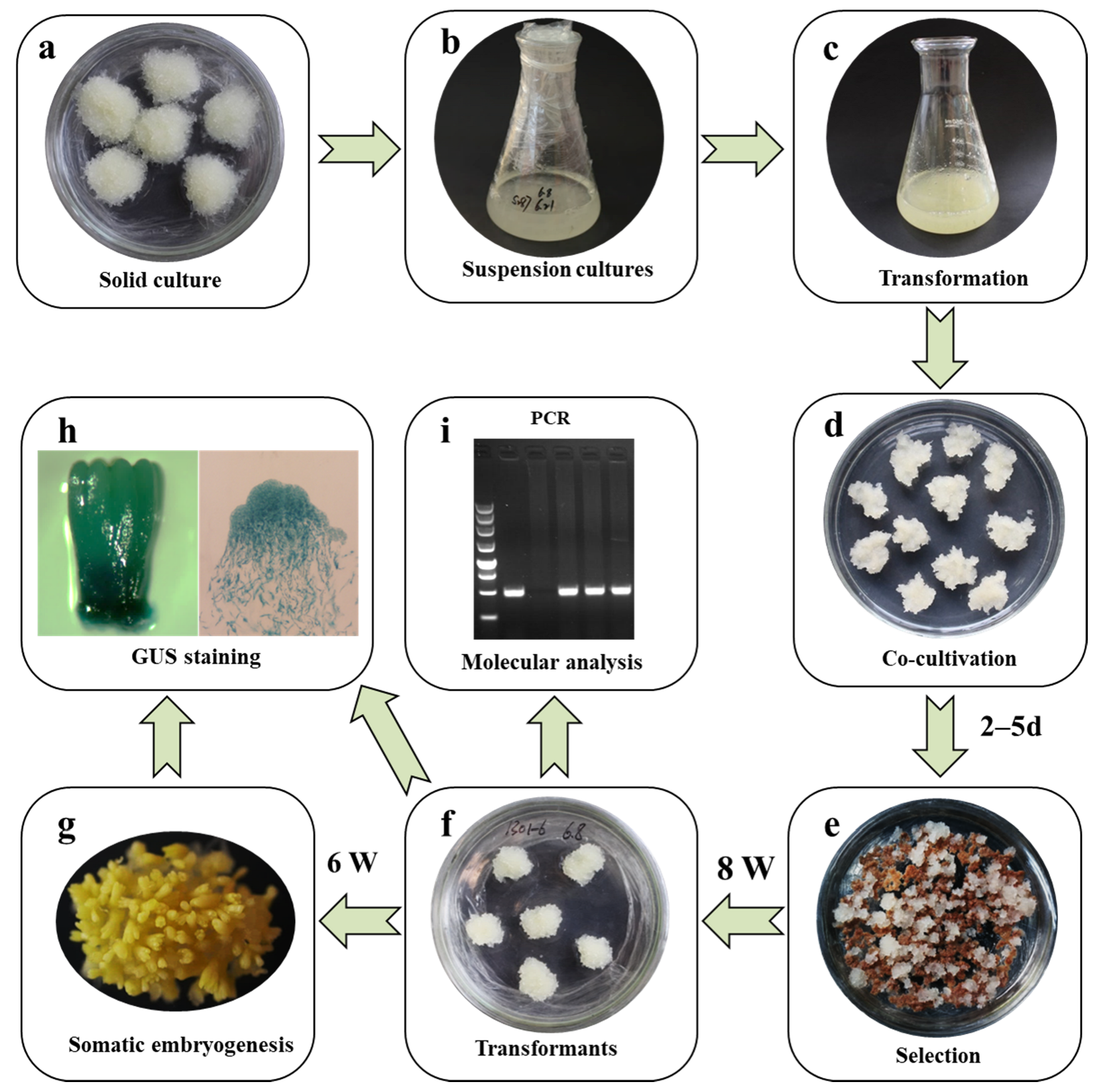
3.1. Optimization of the Transformation Procedure
3.2. Selection of Transformed Embryogenic Lines
3.3. Molecular Analysis of the Transformed Lines
3.4. GUS Assay and Gus Transcript Abundance in Stably Transformed Lines
3.5. HPT Transcript Abundance in Stable Transformation Lines
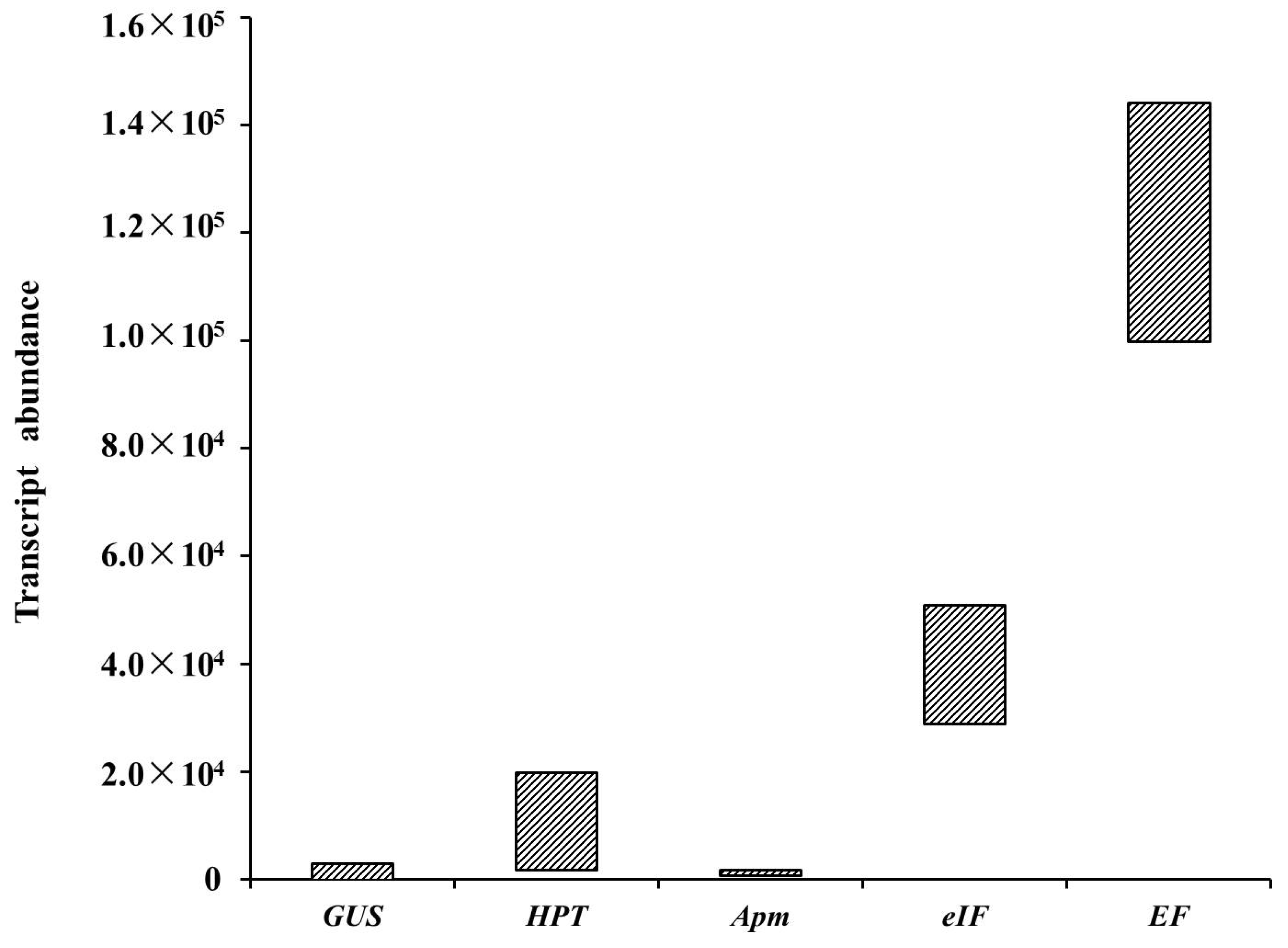
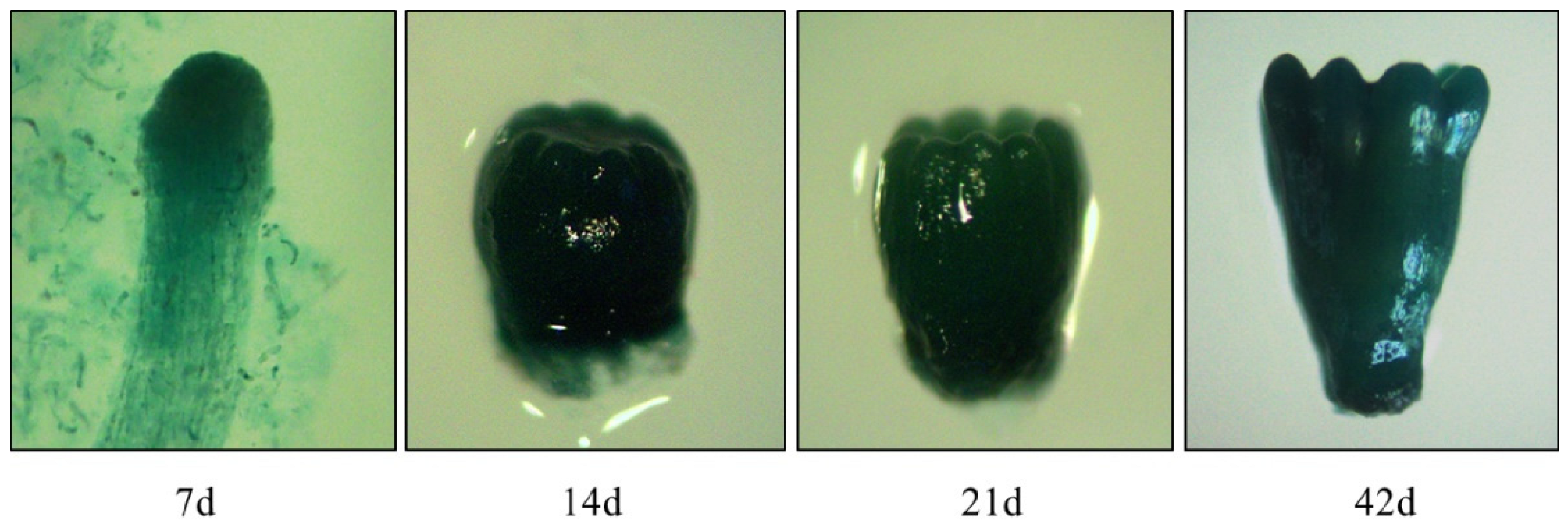
3.6. GUS Transcript Abundance under the Control of the LaTCTP Promoter during Somatic Embryogenesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pâques, L.E.; Foffová, E.; Heinze, B.; Lelu-Walter, M.A.; Liesebach, M.; Philippe, G. Larches (Larix sp.). In Forest Tree Breeding in Europe: Current State-of-the-Art and Perspectives, Managing Forest Ecosystems; Pâques, L.E., Ed.; Springer: Dordrecht, The Netherlands, 2013; Volume 25, pp. 13–122. [Google Scholar]
- Marie-Anne, L.; Pilate, G. Transgenic in Larix. In Molecular Biology of Woody Plants; Jain, S.M., Minocha, S.C., Eds.; Springer: Dordrecht, The Netherlands, 2000; Volume 2, pp. 119–134. [Google Scholar]
- Zhang, L.F.; Li, W.F.; Xu, H.Y.; Qi, L.W.; Han, S.Y. Cloning and characterization of four differentially expressed cDNAs encoding NFYA homologs involved in responses to ABA during somatic embryogenesis in Japanese larch (Larix leptolepis). Plant Cell Tissue Organ Cult. PCTOC 2014, 117, 293–304. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Han, S.; Li, X.; Qi, L. Transcriptome profiling and in silico analysis of somatic embryos in Japanese larch (Larix leptolepis). Plant Cell Rep. 2012, 31, 1637–1657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.F.; Li, W.F.; Han, S.Y.; Yang, W.H.; Qi, L.W. cDNA cloning, genomic organization and expression analysis during somatic embryogenesis of the translationally controlled tumor protein (TCTP) gene from Japanese larch (Larix leptolepis). Gene 2013, 529, 150–158. [Google Scholar] [CrossRef]
- Zhang, L.F.; Lan, Q.; Han, S.Y.; Qi, L.W. A GH3-like gene, LaGH3, isolated from hybrid larch (Larix leptolepis × Larix olgensis) is regulated by auxin and abscisic acid during somatic embryogenesis. Trees 2019, 33, 1723–1732. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Han, S.; Wu, T.; Li, X.; Li, W.; Qi, L. Genome-wide identification of microRNAs in larch and stage-specific modulation of 11 conserved microRNAs and their targets during somatic embryogenesis. Planta 2012, 236, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, S.; Han, S.; Li, X.; Tong, Z.; Qi, L. Deciphering small noncoding RNAs during the transition from dormant embryo to germinated embryo in larches (Larix leptolepis). PLoS ONE 2013, 8, e81452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Zhang, S.G.; Li, S.G.; Han, S.Y.; Li, W.F.; Li, X.M.; Qi, L.W. Regulation of synchronism by abscisic-acid-responsive small noncoding RNAs during somatic embryogenesis in larch (Larix leptolepis). Plant Cell Tissue Organ Cult. PCTOC 2014, 116, 361–370. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, J.; Han, S.; Yang, W.; Li, W.; Wei, H.; Li, X.; Qi, L. Four abiotic stress-induced miRNA families differentially regulated in the embryogenic and non-embryogenic callus tissues of Larix leptolepis. Biochem. Biophys. Res. Commun. 2010, 398, 355–360. [Google Scholar] [CrossRef]
- Sarmast, M.K. Genetic transformation and somaclonal variation in conifers. Plant Biotechnol. Rep. 2016, 10, 309–325. [Google Scholar] [CrossRef]
- Tang, W.; Newton, R. Genetic transformation of conifers and its application in forest biotechnology. Plant Cell Rep. 2003, 22, 1–15. [Google Scholar] [CrossRef]
- Klimaszewska, K.; Devantier, Y.; Lachance, D.; Lelu, M.A.; Charest, P.J. Larix laricina (tamarack): Somatic embryogenesis and genetic transformation. Can. J. For. Res. 1997, 27, 538–550. [Google Scholar] [CrossRef]
- Klimaszewska, K.; Lachance, D.; Pelletier, G.; Lelu, M.A.; Séguin, A. Regeneration of transgenic Picea glauca, P. Mariana, and P. abies after cocultivation of embryogenic tissue with Agrobacterium tumefaciens. Vitr. Cell. Dev. Biol. Plant 2001, 37, 748–755. [Google Scholar] [CrossRef]
- Newton, R.J.; Bloom, J.C.; Bivans, D.H.; Mohan Jain, S. Stable genetic transformation of conifers. Phytomorphology 2001, 51, 421–434. [Google Scholar]
- Wenck, A.R.; Quinn, M.; Whetten, R.W.; Pullman, G.; Sederoff, R. High-efficiency Agrobacterium-mediated transformation of Norway spruce (Picea abies) and loblolly pine (Pinus taeda). Plant Mol. Biol. 1999, 39, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.M.; Ordás, R.J. Stable Agrobacterium-mediated transformation of maritime pine based on kanamycin selection. Sci. World J. 2013, 2013, 681792. [Google Scholar] [CrossRef]
- Lee, H.; Moon, H.K.; Park, S.Y. Agrobacterium-mediated Transformation via Somatic Embryogenesis System in Korean fir (Abies koreana Wil.), A Korean Native Conifer. Korean J. Plant Res. 2014, 27, 242–248. [Google Scholar] [CrossRef][Green Version]
- Levée, V.; Lelu, M.A.; Jouanin, L.; Cornu, D.; Pilate, G. Agrobacterium tumefaciens-mediated transformation of hybrid larch (Larix kaempferi × L. decidua) and transgenic plant regenerationn. Plant Cell Rep. 1997, 16, 680–685. [Google Scholar] [CrossRef]
- Levee, V.; Garin, E.; Klimaszewska, K.; Seguin, A. Stable genetic transformation of white pine (Pinus strobus L.) after cocultivation of embryogenic tissues with Agrobacterium tumefaciens. Mol. Breed. 1999, 5, 429–440. [Google Scholar] [CrossRef]
- Li, Z.X.; Li, S.G.; Zhang, L.F.; Han, S.Y.; Li, W.F.; Xu, H.Y.; Yang, W.H.; Liu, Y.L.; Fan, Y.R.; Qi, L.W. Over-expression of miR166a inhibits cotyledon formation in somatic embryos and promotes lateral root development in seedlings of Larix leptolepis. Plant Cell Tissue Organ Cult. PCTOC 2016, 127, 461–473. [Google Scholar] [CrossRef]
- Ewald, D.; Weckwerth, W.; Naujoks, G.; Zocher, R. Formation of Embryo-like Structures in Tissue Cultures of Different Yew Species. J. Plant Physiol. 1995, 147, 139–143. [Google Scholar] [CrossRef]
- Li, S.G.; Li, W.f.; Han, S.Y.; Yang, W.H.; Qi, L.W. Stage-specific regulation of four HD-ZIP III transcription factors during polar pattern formation in Larix leptolepis somatic embryos. Gene 2013, 522, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Ewald, D.; Kretzschmar, U.; Chen, Y. Continuous micropropagation of juvenile larch from different species via adventitious bud formation. Biol. Plant. 1997, 39, 321–329. [Google Scholar] [CrossRef]
- Song, Y.; Bai, X.; Dong, S.; Yang, Y.; Dong, H.; Wang, N.; Zhang, H.; Li, S. Stable and Efficient Agrobacterium-Mediated Genetic Transformation of Larch Using Embryogenic Callus. Front. Plant Sci. 2020, 11, 584492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yan, S.; An, P.; Cao, Q.; Wang, C.; Wang, J.; Zhang, H.; Zheng, L. Embryogenic callus induction from immature zygotic embryos and genetic transformation of Larix kaempferi 3x Larix gmelinii 9. PLoS ONE 2021, 16, e0258654. [Google Scholar] [CrossRef]
- Bommer, U.A.; Thiele, B.J. The translationally controlled tumour protein (TCTP). Int. J. Biochem. Cell Biol. 2004, 36, 379–385. [Google Scholar] [CrossRef]
- Masura, S.S.; Parveez, G.K.; Ti, L.L. Isolation and characterization of an oil palm constitutive promoter derived from a translationally control tumor protein (TCTP) gene. Plant Physiol. Biochem. 2011, 49, 701–708. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Seong, E.S.; Lim, J.D.; Heo, K.; Kim, M.J.; Chung, I.M.; Juvik, J.A.; Yu, C.Y. Agrobacterium-mediated transformation of Codonopsis lanceolata using the γ-TMT gene. Plant Cell Tissue Organ Cult. PCTOC 2008, 95, 265–274. [Google Scholar] [CrossRef]
- Mishra, S.; Sangwan, R.S.; Bansal, S.; Sangwan, N.S. Efficient genetic transformation of Withania coagulans (Stocks) Dunal mediated by Agrobacterium tumefaciens from leaf explants of in vitro multiple shoot culture. Protoplasma 2013, 250, 451–458. [Google Scholar] [CrossRef]
- Vancanneyt, G.; Schmidt, R.; O’Connor-Sanchez, A.; Willmitzer, L.; Rocha-Sosa, M. Construction of an intron-containing marker gene: Splicing of the intron in transgenic plants and its use in monitoring early events in Agrobacterium-mediated plant transformation. Mol. Gen. Genet. MGG 1990, 220, 245–250. [Google Scholar] [CrossRef]

| Primer Name | Sequence (5′-3′) | Application |
|---|---|---|
| HPT-F | 5′-AGCGAGAGCCTGACCTATTG-3′ | PCR analysis for HPT |
| HPT-R | 5′-CTCCATACAAGCCAACCACG-3′ | PCR analysis for HPT |
| virG-F | 5′-ACGAAGTCCGATGTTCCAAT-3′ | PCR analysis for virG |
| virG-R | 5′-TCAGCGTCAAAGAAATAGCC-3′ | PCR analysis for virG |
| GUS-F | 5′-GTCAATGTAATGTTCTGCGACG-3′ | PCR analysis for GUS |
| GUS-R | 5′-GAAGTTCATGCCAGTCCAGC-3′ | PCR analysis for GUS |
| qEF-F | 5′-GACTGTACCTGTTGGTCGTG-3′ | Real-time PCR for EF |
| qEF-R | 5′-CCTCCAGCAGAGCTTCAT-3′ | Real-time PCR for EF |
| qeIF-F | 5′-CCTGCCCTTTGGGTTACT-3′ | Real-time PCR for eIF |
| qeIF-R | 5′-TTCAGTGTTACGAGGATGCTAA-3′ | Real-time PCR for eIF |
| qAPm-F | 5′-TGACCCTGGAAAGCCTATC-3′ | Real-time PCR for APm |
| qAPm-R | 5′-AGCCGAAGAGTCCCTGTTA-3′ | Real-time PCR for APm |
| qHPT-F | 5′-CTCCAACAATGTCCTGAC-3′ | Real-time PCR for HPT |
| qHPT-R | 5′-TCTGCTGCTCCATACAAGC-3′ | Real-time PCR for HPT |
| qGUS-F | 5′-AAGCGTGGTGATGTGGAGTA-3′ | Real-time PCR for GUS |
| qGUS-R | 5′-CGTAATAACGGTTCAGGCAC-3′ | Real-time PCR for GUS |
| qTCTP-F | 5′-ATTGTCAGAGGAACGCCA-3′ | Real-time PCR for TCTP |
| qTCTP-R | 5′-CATAGACCCATCATCGTGC-3′ | Real-time PCR for TCTP |
| pCAMBIA1301 | Number of Hygromycin-Resistant Cell Lines | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Total | 183 | 213 | 216 |
| per gram fresh weight | 19.9 | 20.7 | 18.5 |
| Lines | GUS | HPT | EF | eIF | APm |
|---|---|---|---|---|---|
| P1 | 4.47 × 102 | 1.11 × 104 | 9.97 × 104 | 3.35 × 104 | 7.35 × 102 |
| P2 | 2.01 × 103 | 1.35 × 104 | 1.27 × 105 | 4.08 × 104 | 9.06 × 102 |
| P3 | 1.25 × 103 | 1.27 × 104 | 9.50 × 104 | 2.88 × 104 | 6.78 × 102 |
| P4 | 7.36 × 101 | 1.78 × 104 | 1.11 × 105 | 3.62 × 104 | 8.45 × 102 |
| P5 | 1.82 × 102 | 1.80 × 103 | 1.22 × 105 | 3.60 × 104 | 1.14 × 103 |
| P6 | 2.09 × 103 | 1.45 × 104 | 1.23 × 105 | 3.64 × 104 | 1.17 × 103 |
| P7 | 3.81 × 101 | 4.27 × 103 | 1.01 × 105 | 3.09 × 104 | 8.46 × 102 |
| P8 | 2.89 × 103 | 1.98 × 104 | 1.83 × 105 | 5.08 × 104 | 1.71 × 103 |
| P9 | 4.70 × 102 | 1.59 × 104 | 1.44 × 105 | 4.06 × 104 | 1.15 × 103 |
| 35S::GUS-1 | 2.05 × 103 | 2.81 × 104 | 1.15 × 105 | 3.37 × 104 | 9.64 × 102 |
| 35S::GUS-2 | 5.94 × 102 | 2.67 × 104 | 1.02 × 105 | 2.39 × 104 | 6.81 × 102 |
| Lines | GUS | TCTP |
|---|---|---|
| 7-1 | 1.63 × 102 | 3.72 × 105 |
| 7-2 | 4.85 × 102 | 1.91 × 105 |
| 7-3 | 2.59 × 102 | 2.61 × 105 |
| 7-4 | 3.10 × 102 | 2.46 × 105 |
| 7-5 | 1.38 × 102 | 3.55 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, S.; Zhang, L.; Han, S.; Qi, L. Agrobacterium-Mediated Genetic Transformation of Larix kaempferi (Lamb.) Carr. Embryogenic Cell Suspension Cultures and Expression Analysis of Exogenous Genes. Forests 2022, 13, 1436. https://doi.org/10.3390/f13091436
Dang S, Zhang L, Han S, Qi L. Agrobacterium-Mediated Genetic Transformation of Larix kaempferi (Lamb.) Carr. Embryogenic Cell Suspension Cultures and Expression Analysis of Exogenous Genes. Forests. 2022; 13(9):1436. https://doi.org/10.3390/f13091436
Chicago/Turabian StyleDang, Shaofei, Lifeng Zhang, Suying Han, and Liwang Qi. 2022. "Agrobacterium-Mediated Genetic Transformation of Larix kaempferi (Lamb.) Carr. Embryogenic Cell Suspension Cultures and Expression Analysis of Exogenous Genes" Forests 13, no. 9: 1436. https://doi.org/10.3390/f13091436
APA StyleDang, S., Zhang, L., Han, S., & Qi, L. (2022). Agrobacterium-Mediated Genetic Transformation of Larix kaempferi (Lamb.) Carr. Embryogenic Cell Suspension Cultures and Expression Analysis of Exogenous Genes. Forests, 13(9), 1436. https://doi.org/10.3390/f13091436







