Abstract
Self-incompatibility (SI) is a common strategy to avoid inbreeding and, consequently, keep genetic diversity within a species. In its mechanism, pollen rejection happens in the style when the single multiallelic locus (SFB in prunus species) of the haploid pollen matches one of the S-alleles existing in the diploid pistil. The SFB gene for the pollen S gene has been identified in many Prunus species. However, Japanese apricot is a species with a typical gametophytic self-incompatibility (GSI), and its SFB alleles available are limited, although they are required for studying GSI. Therefore, we used an AS-PCR amplification method, sequencing, and the pair primers SFB-C1F and Pm-Vb designed based on the conserved region of the Prunus SFB gene to identify SFB genotypes of 48 Japanese apricot (P. mume) accessions. Eleven novel SFB alleles were isolated from these accessions and shared typical structural features with SFB alleles from other Prunus species. These novel SFB alleles were uniquely expressed in pollen. Hence, we concluded that these 11 PmSFB were pollen S determinants of P. mume. This current study offers the novel SFB genes of the P. mume S locus, which could be a useful potential resource for studies on pollen SI mechanisms.
1. Introduction
Self-incompatibility (SI) is the most widespread strategy that flowering plants use to avoid self-fertilization and promote outcrossing [1,2], which has been identified in almost half of blooming plants [3]. However, this system has only been studied in a limited number of families in which the fundamental molecular and genetic aspects are involved, and they have only been slightly characterized in detail in the gametophytic self-incompatibility (GSI) system, and in the sporophytic self-incompatibility (SSI) system [4]. In SSI, the genotype of the diploid parental plant (sporophyte), which acts as pollen donor, regulates the incompatibility type, while in GSI, the genotype of the haploid pollen itself (gametophyte) controls its incompatibility system [5,6].
However, GSI is the most prevalent in Plantaginaceae, Solanaceae, and Rosaceae, in which it is controlled via a single multiallelic locus (S locus) containing two related genes: the female part (pistil) pistil determinant S-ribonuclease gene (S-RNase gene) and the male part (pollen) pollen S determinant (s) encoding an F-box protein [7,8,9,10]. The male part determinant is termed as SLF (S-locus F-box) in Plantaginaceae and in Solanaceae [11,12]; SFBB (S-locus F-box brothers) in the subtribe Malinae (Pyrus in Rosaceae) [13]; and SFB (S haplotype-specific F-box protein) in Prunus (Rosaceae) [14,15,16].
Subsequent to the first identification of S-RNase in the Solanaceae family [17], a key candidate gene for the male part was discovered in several Prunus species named SFB, including almond [18,19], sweet cherry [20,21,22,23,24], P. armeniaca [16,25,26,27], and P. mume [20,28,29]. However, SFB features including pollen-specific expression, a high level of allelic polymorphism, and a close physical proximity to S-RNase, are suitable for the pollen S gene [18,28,30]. The physical relationship of SFB gene with S-RNase has been established in other Prunus species including P. mume [28,31] and sweet and sour cherries [20,32]. Subsequently, the physical distance between SFB and S-RNase alleles in sweet cherry [24,33,34], Japanese plum [35,36], apricot [16,25,27], and Chinese cherry [37] has been established. One of the largest genus in the Rosaceae family is Prunus L., with over 200 species of evergreen and deciduous trees and shrubs that produce valuable fruits and nuts, [38], and most of them exhibit Gametophytic System (GSI).
Japanese apricot is an important stone fruit and an ornamental tree of this genus, that originates from China [39], which is sympathetic to typical GSI species [29]. P. mume blooms very early in the spring when pollination is severely restricted by several constraints (such as available insects, weather, and pollinizers). Thus, it is very important to determine the correct S haplotypes of cultivars/accessions. In P. mume, numerous S-RNase alleles have been reported [29,40,41,42] and Entani et al. [28] first discovered its pollen gene (S haplotype-specific F-box protein); then, other researchers reported the SFB alleles in a few cultivars [43]. To explore the GSI system in P. mume, the availability of SFB genes is sufficient, and its genotyping in several accessions are required. In this study, we identified the SFB genotype and novel SFB alleles of forty-eight P. mume accessions. These might contribute to the improvement of SFB alleles’ basic data, which would be useful for the in-depth research aimed at understanding the self-(in)compatibility mechanism.
2. Materials and Methods
2.1. Materials
Forty-eight P. mume accessions from the National Field Gene-Bank for Japanese apricot in Nanjing, China were used in this research (Table 1). However, young fresh leaves of each accession were collected in spring, while styles, and pollen were collected from ‘Sichuanbaimei’ in winter. All of these plant materials were stored at −80 degrees Celsius until use.

Table 1.
SFB genotypes of 48 P. mume accessions.
2.2. Methods
2.2.1. DNA and RNA Extraction
The total genomic DNA from each of the 48 P. mume accessions was extracted from young fresh leaves using a modified CTAB method [44], treated with RNase (TaKaRa, Kyoto, Japan), and incubated at 37 °C for an hour. Subsequently, the extracted DNA concentration was determined with a BioPhotometer (Eppendorf, Hamburg, Germany), and its integrity was verified via electrophoresis.
Total RNA was extracted from young fresh leaves, styles, and pollen of ‘Sichuanbaimei’ according to Tao R. et al. [45], and then treated with gDNA Eraser (TaKaRa). The extracted RNA concentration was determined with a BioPhotometer, and its integrity was checked via electrophoresis.
2.2.2. PCR Amplification of SFB Alleles
An AS-PCR amplification method was used according to the protocols outlined in Junxia. X. et al. [46], to amplify SFB alleles using the primer pair SFB-C1F[RTTCGRTTTCTDTTTACRTG] [31] and Pm-Vb[ATCCAAGCAAGTTCTTGAAACA] [43]. However, PCR was carried out in a 25 µL reaction volume having 70 ng of genomic DNA, 2.0 μL of 10× PCR buffer (TaKaRa), 1.5 mM MgCl2, 0.15 mM dNTPs, 0.1 μM of each primer, and 1 U of Taq DNA polymerase (TaKaRa) in a PTC-100 thermal cycler (MJ Research, Cambridge, MA, USA). Then, a program of 35 cycles at 94 °C for 1 min, 54 °C for 1 min, and 72 °C for 1 min 30 s, with an initial denaturation of 94 °C for 3 min, and a final extension of 72 °C for 10 min, were used to run PCRs. PCR products were separated by 1.5% agarose gel electrophoresis in a 1× TAE buffer, and we observed the bands under ultraviolet light.
2.2.3. RT-PCR Amplification
The extracted RNA from ‘Sichuanbaimei’ tissues including leaves, pollen, and styles were reverse-transcribed into cDNAs according to the method of the reverse transcription kit (TaKaRa, Japan). However, RT-PCR was carried out using primers Pru-C2 and PCE-R, and cDNAs as templates following the manufacturer’s protocol (TaKaRa, Japan). Specific reactions and procedures were carried out according to the provided descriptions in Junxia X et al. [47]. Primers for the PCR amplification were SFB-C1F and Pm-Vb as above. As in references, RT-PCR was also performed with an actin gene-specific primer pair, ActF1[ATGGTGAGGATATTCAACCC] [18], and ActRI[CTTCCTGTGGACAATGGATGG] [18]. RT-PCR products were separated by 1.5% agarose gel electrophoresis in a 1× TAE buffer, and we observed the bands under ultraviolet light.
2.2.4. PCR Products Sequencing
A DNA purification kit (TaKaRa, Japan) was used to extract/isolate all PCR products and cDNA fragments from 1.2% agarose gels. Refined target products were cloned into the 007 Simple Vector Kit according to the manufacturer’s recommendations and transformed into an Escherichia coli DH5α cell. At least three positive samples of each target clones were sequenced by Kinco Biotechnology Co., Ltd. Company (Nanjing, China) to acquire accurate and correct sequences and avoid PCR amplification errors.
2.2.5. Sequence and Phylogenetic Analysis
Homology searches were performed using BLAST version +2.6.0 (Altschul, S.F., New York, NY, USA, http://www.ncbi.nlm.nih.gov/BLAST/, accessed on 27 August 2021) [48] program from the National Center for Biotechnology Information (NCBI), and we also determined whether a sequenced gene was a new SFB gene. SFB gene nucleotide sequence and amino acid sequence alignments were performed using DNAMAN Version V8 software (Lynnon Biosoft, Foster City, CA, USA, https://www.bioz.com/result/doap2%20proteins%20dnaman%20version%208%200%20software/product/Lynnon%20corporation, accessed on 27 August 2021) [49], MEGA X (Kumar, S., Philadelphia, PA, USA, www.megasoftware.net (accessed on 27 August 2021), and Jalview version 2.11.2.0 (Waterhouse, A, Dundee, UK, https://www.jalview.org/download, accessed on 27 August 2021). The online MEME tool: https://meme-suite.org/meme (accessed on 27 August 2021) [50], was used to analyze the proteins’ conserved motifs distribution. Using MEGA X [51] software by the neighbor-joining approach [52], and Figtree (version V1.4.4) software (Rambaut, A, Edinburgh, UK, http://tree.bio.ed.ac.uk/, accessed on 27 August 2021), a phylogenetic tree was generated based on the putative amino acid sequences of the S-locus F-box genes in Prunus.
3. Results
3.1. SFB Alleles Identification from P. mume Accessions
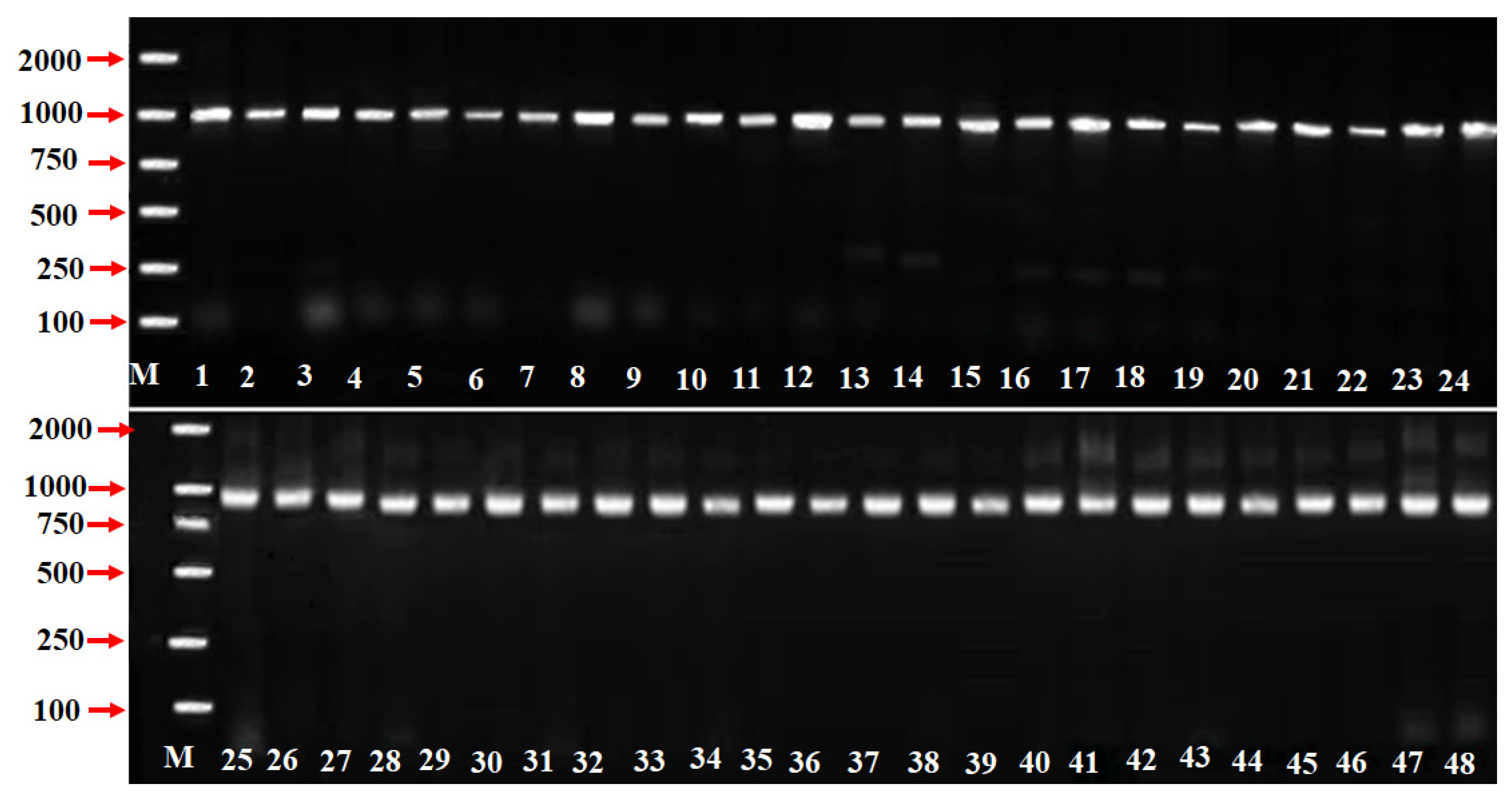
The Prunus SFB primer pairs SFB-C1F and Pm-Vb were designed from conserved regions of Prunus SFB including the F-box motif and a downstream region of variable HVb, respectively. However, only one PCR amplification fragment of approximately 1000 bp was obtained from each P. mume accession (Figure 1). To identify, the SFB alleles from the forty-eight P. mume germplasm resources (accessions), a further analysis of the sequences was performed. According to the DNA homologous sequence analysis using DNAMAN (Version V6; Lynnon Biosoft) software [49], these sequences were categorized/classified into 25 types. Moreover, there were 11 novel SFB alleles sharing a high homology with the SFB alleles identified in other Prunus species, which were named pollen-specific SFB (PmSFB). The sequences of these 11 new SFB alleles were logged to the NCBI database by our research group, and their accession numbers were as follows: PmSFB44 (MW186460), PmSFB45 (MW186461), PmSFB46 (MW186462), PmSFB48 (MW186464), PmSFB50 (MW186466), PmSFB52 (MW186468), PmSFB54 (MW186470), PmSFB56 (MW186472), PmSFB57 (MW786959), PmSFB58 (MW786960), and PmSFB59 (MW786961). The other PmSFB alleles had previously been reported. The detailed information is accessible in Table 1.
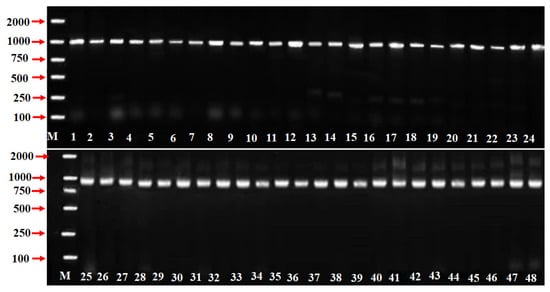
Figure 1.
SFB alleles AS-PCR amplification in 48 P. mume accessions. Lane M = 2000 bp ladder marker, lane 1 to lane 48 represent the accessions in Table 1. Schemes follow the same formatting.
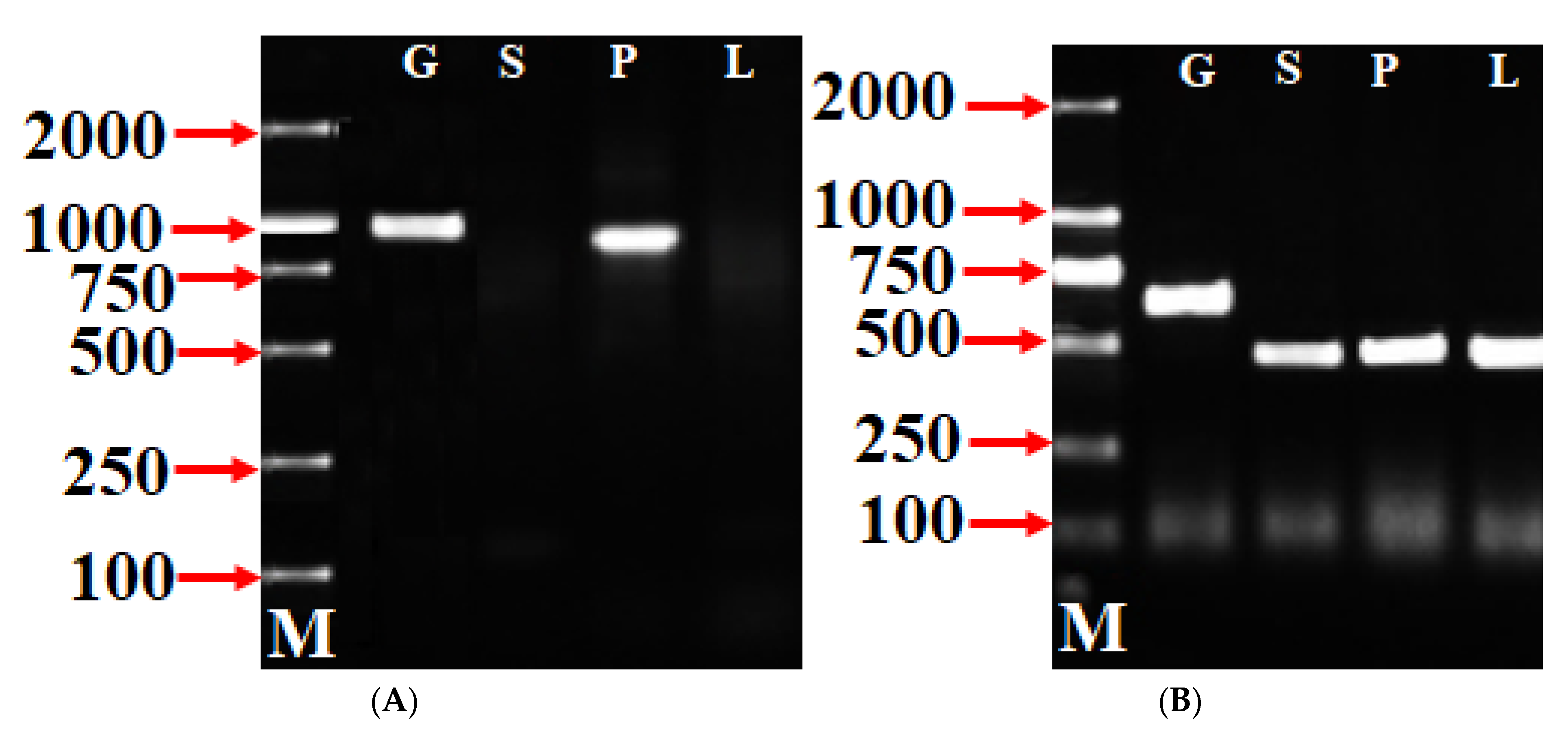
3.2. Specific Expression Analysis of SFB Alleles
To authenticate the Pm SFB isolated/identified in this study, an RT-PCR was conducted with the cDNAs of pollen, leaves, and styles of ‘Sichuanbaimei’ using SFB-C1F and Pm-Vb to explore the expression patterns of the PmSFB genes. The RT-PCR analysis of the actin gene was carried out using ActF1 and ActRI as positive controls (Figure 2B). Figure 2A (PmSFB) shows that the RT-PCR of pollen cDNA produced a DNA fragment of the same size as that of genomic DNA, but no fragment was amplified from leaf cDNA and style cDNA. The actin-gene-amplified fragments were obtained from genomic DNA, leaf cDNA, style cDNA, and pollen cDNA (Figure 2B). The fragments from leaf cDNA, style cDNA, and pollen cDNA had the same size, and they were shorter compared to the fragment from genomic DNA (the size of the fragment from genomic DNA was different compared to that of the fragments from pollen, style, and leaf) (Figure 2B), implying that the RNA preparations were free from genomic DNA. Consequently, these findings prove that the extracted RNA used in this study was exempted from genomic DNA contamination. As in the instance of other Prunus SFB alleles [53], the PmSFB alleles were exclusively expressed in pollen. The sequencing findings and a comparative analysis of the sequences showed that the amplification fragment of the SFB gene from pollen was identified as two SFB genes.
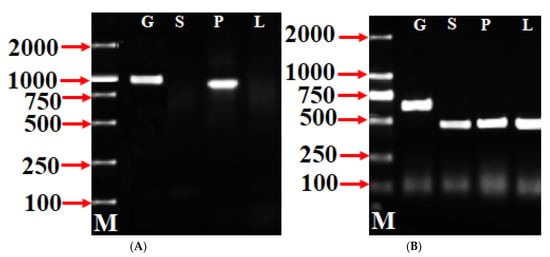
Figure 2.
PmSFB (A) and actin gene (B) expression analysis in pollen (P), styles (S), and leaves (L), respectively, of ‘Sichuanbaimei’. Lane G = genomic DNA; lane M = 2 kb DNA marker. The actin gene was used as positive control.
3.3. Comparative Analysis of SFB Alleles Identified in P. mume and Other Prunus Species
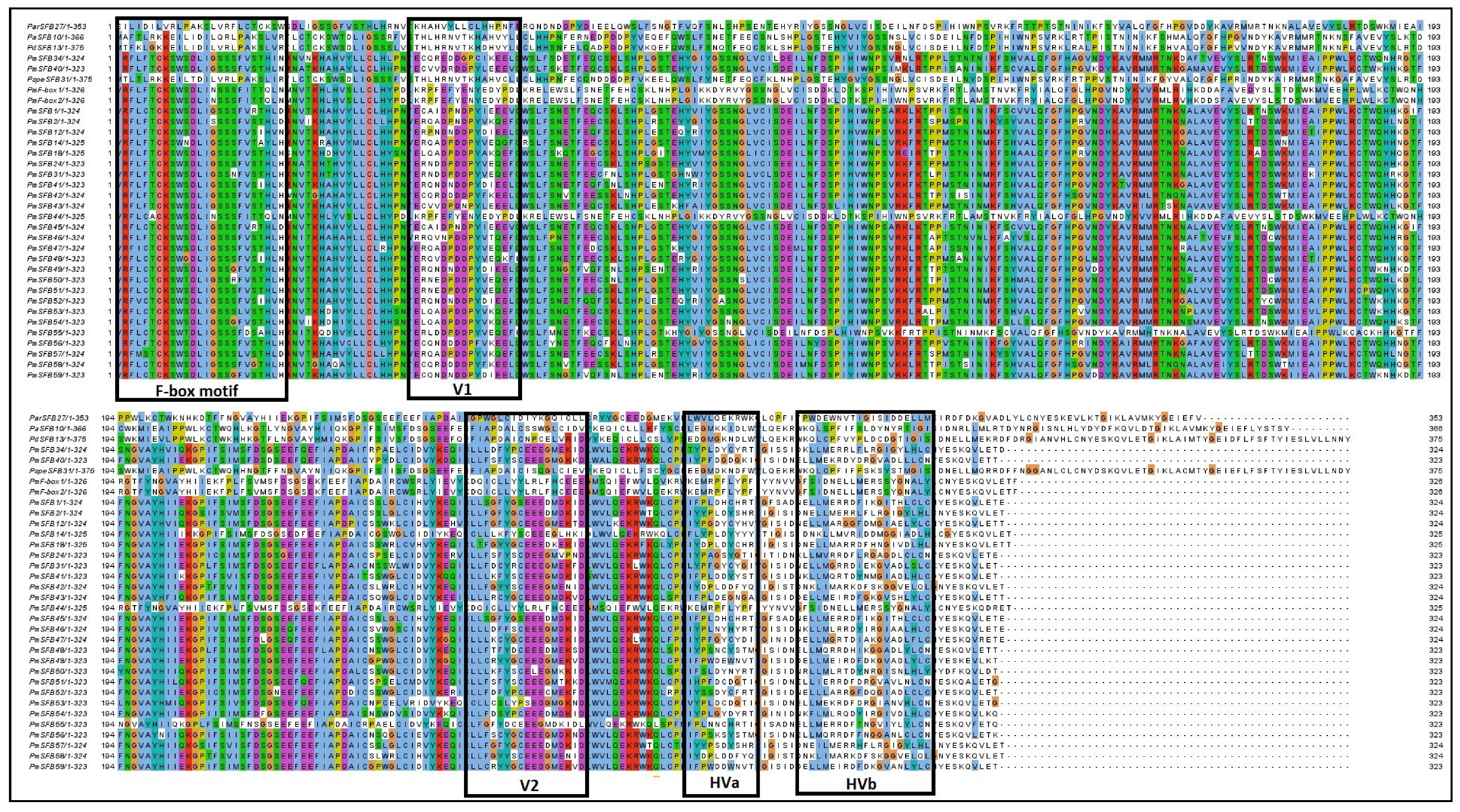
Ikeda et al. [21] were the first to characterize the structural features of the PmSFB gene based on a comparative analysis of the amino acid sequences. In the current study, the new 11 SFB alleles were similar to those of others Prunus species. However, the structure/feature is described as follows: two hypervariable areas (HVa and HVb) situated in the C-terminal zone and variable domains V2 and V1 situated upstream of the nonconserved HVa and downstream of the F-box motif, respectively (Figure 3). The putative amino acid identities of Pm SFB sequences ranged from 60.06 to 96.93% (Table 2), while their identities with other Prunus species ranged from 61.03 to 98.14% (the similarity between the PmSFB alleles and other Prunus species was greater than the identities of the PmSFB alleles with each other for the Japanese apricot accessions) (Table 3).

Figure 3.
The amino acid sequences of Prunus armeniaca (Par), Prunus avium (Pa), Prunus dulcis (Pd), Prunus speciosa (Pspe), and Prunus mume (Pm) SFB genes source comparison. The dark areas show conserved residues and dots indicate gaps. The F-box domain, variable regions (V1 regions, V2 regions), and hypervariable regions (HVa regions and HVb regions) are, respectively, circled with boxes and marked.

Table 2.
Homologies (%) of deduced amino acid sequences of PmSFB alleles.

Table 3.
Homologies (%) of deduced amino acid sequences of PmSFB alleles with other Prunus species.
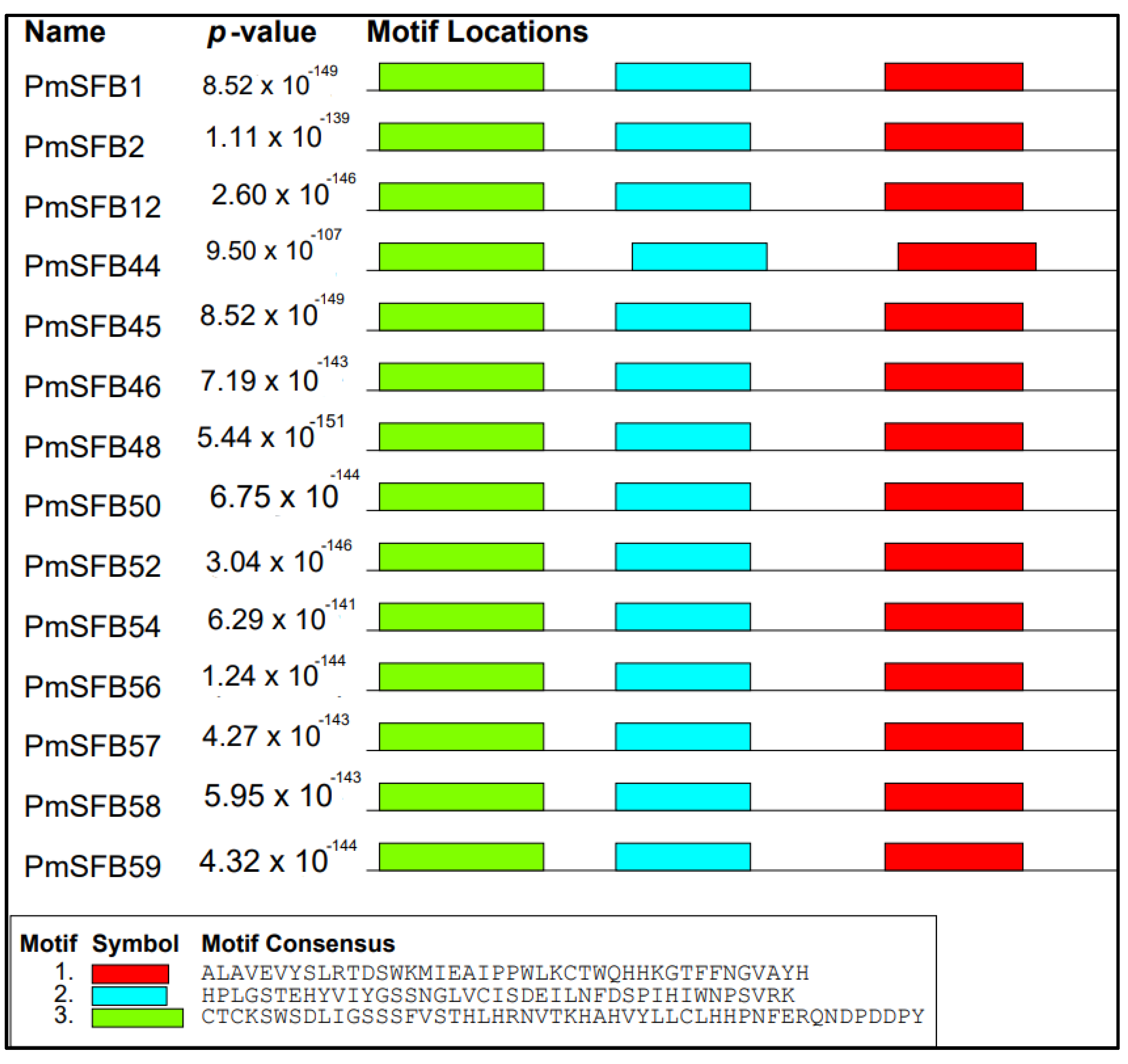
3.4. Conserved Motifs Analysis
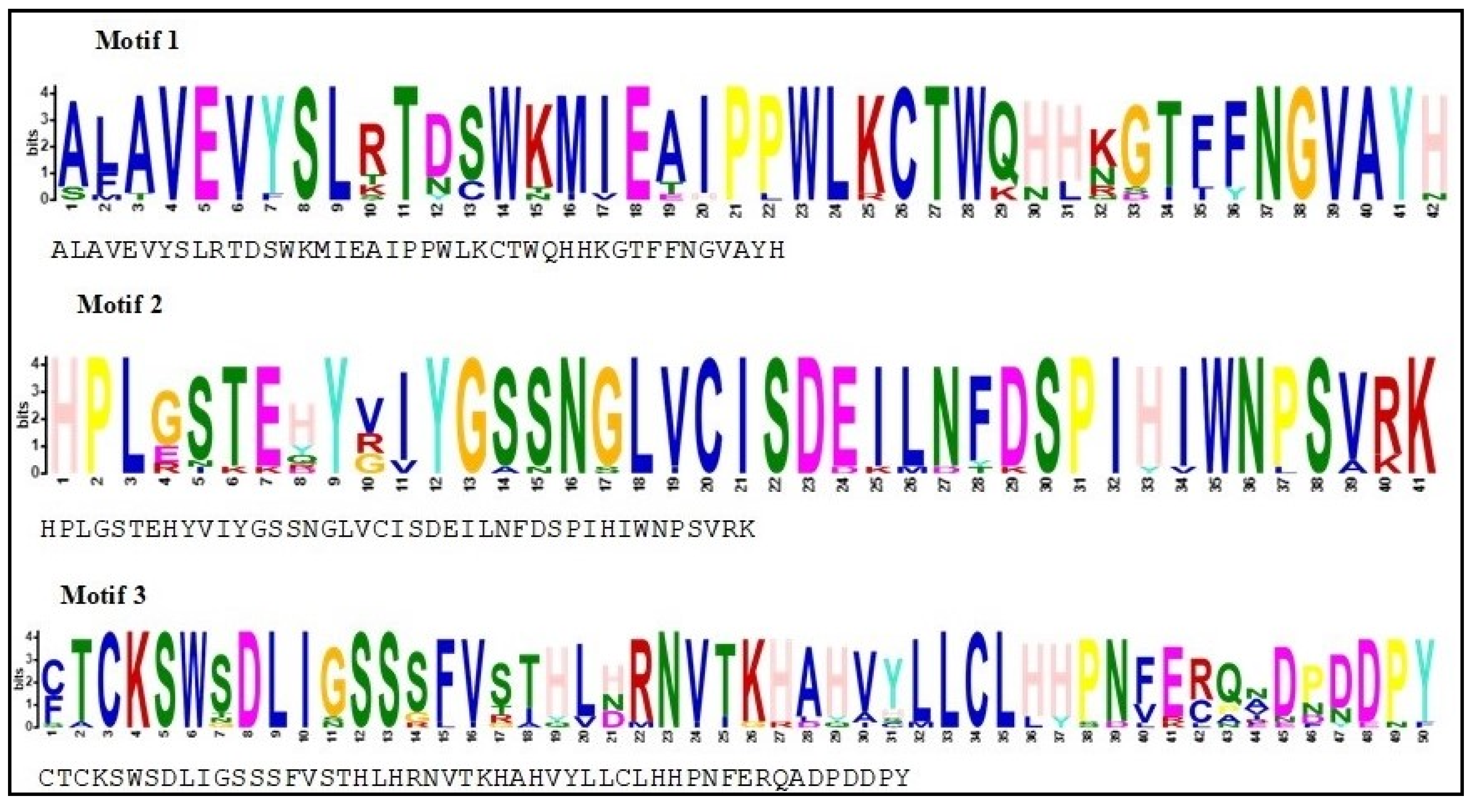
To acquire the motif structural composition, arrangement/order of the conserved motifs of PmSFB alleles, fourteen protein sequences of the SFB genes in Japanese apricot, including the eleven newly identified in this work, were examined for a conserved motif distribution analysis using MEME. The results revealed that the studied gene sequences shared exactly the same direction/order/organization for at least three most common motif structures (3, 2, 1) (Figure 4). Moreover, summary sequence LOGO and regular expression of each motif shared by SFB proteins displayed in Figure 5. The distance from motifs 3 and 2 in each amino acid sequence was approximately the same, as between motifs 2 and 1. The above results suggested that these proteins in P. mume shared a similar structure and function.
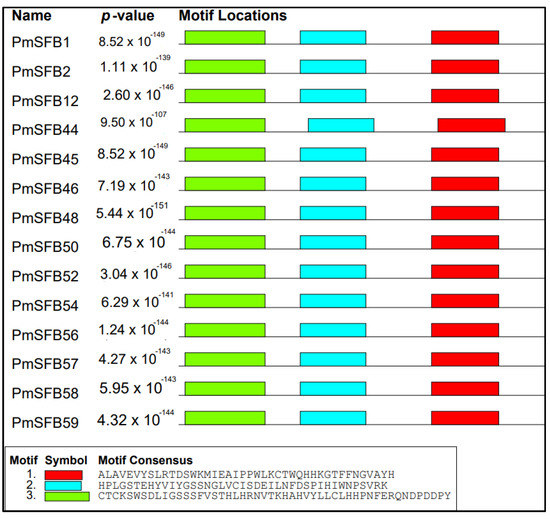
Figure 4.
Motif distribution of SFB proteins in Japanese apricot. Each motif is denoted by a number in the colored box.
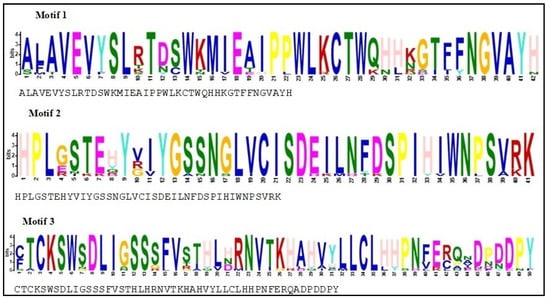
Figure 5.
Summary sequence LOGO and regular expression of each motif shared by SFB proteins in Japanese apricot. At each location in the motif, the sequence logo features stacks of letters. The ‘information content’ of that place in the motif in bits is the whole height of the stack. The likelihood of the letter at that location multiplied by the overall information content of the stack determines the height of individual letters in a stack. The motif is described by the black letter below the sequence logo, which is a regular expression (RE). The RE includes all letters with observed frequencies greater than 0.2; less-frequent letters are not included.
3.5. Phylogenetic Analysis of Pollen S genes (SLFL, SFB) in Prunus Species
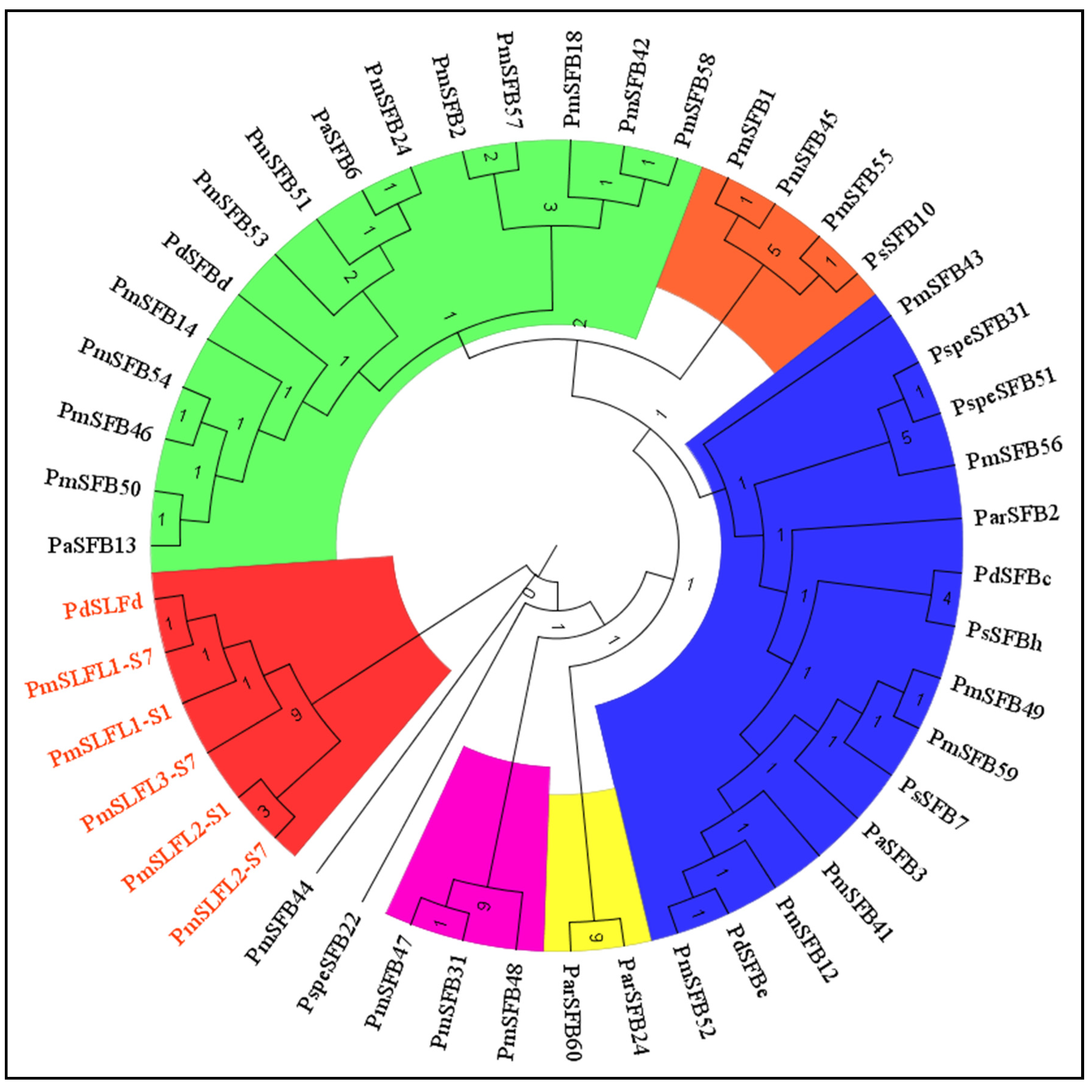
To further explore the relationship between S-locus F-box genes in Prunus species, we constructed a phylogenetic tree by the neighbor-joining method using the amino acid sequences of 47 S pollen genes, including the SLFL and SFB genes, from different Prunus species (Figure 6). The results indicated that the SFB alleles clustered together, whereas all SLFL alleles clustered together (SFB alleles from different Prunus species grouped together, and the same for the SLFL genes). Hence, the S-locus F-box genes in Prunus species shared a high similarity. In addition, the newly identified 11 PmSFB alleles were placed within the SFB group and displayed a significant similarity with other Prunus SFB alleles. These findings indicated that the novel PmSFB alleles were orthologs of the SFB alleles in diverse Prunus species.

Figure 6.
The phylogenetic tree was constructed based on the amino acid sequence using the neighbor-joining method of 47 S-locus F-box genes (SFB and SLFL) from, P. armeniaca (Par), P. avium (Pa), P. dulcis (Pd), P. salicina (Ps), P. speciosa (Pspe), and P. mume (Pm). GenBank accession numbers: ParSFB1 (AY587563), ParSFB2 (AY587562), PaSFB1 (AY805048), PdSFBa (AB092966), PdSFBd (AB081648), PdSFBc (AB079776), PmSLFL1-S1 (AB092623), PmSLFL1-S7 (AB092624), PmSLFL2-S1 (AB092625), PmSLFL2-S7 (AB092626), PmSLFL3-S7 (AB092627), PdSLFd (AB101660), PaSLF1-S1 (AB360339), PaSLF1-S2 (AB360340), PsSFBb (AB252412), PsSFBc (AB280792), PspeSFB1 (HM347508), and PspeSFB22 (HM347509); PmSFB1 (AB101440), PmSFB12 (JQ356586), PmSFB42 (JQ356581), PmSFB43 (JQ356578), PmSFB41 (JQ356593), PmSFB40 (JQ356585), and PmSFB accession numbers are detailed in the text.
4. Discussion
In previous studies, it has been reported that P. salicina [54], P. armeniaca, P. mume, and their cultivars/varieties are diploid [55]. Theoretically, these P. mume accessions should result in two amplified bands of SFB alleles. However, all the 48 accessions possessed two distinct sequences of SFB alleles (Table 2) using the Prunus SFB primer pair (SFB-C1F and Pm-Vb), which confirmed the exactness/correctness or accuracy of this pair of primers.
In Antirrhinum, it was first discovered that F-box genes (AhSLF) were physically connected to S-RNase and expressed only in pollen [30]. Yamane et al. [31] established a method for the molecular typing of P. mume SFB genes to determine the S haplotype utilizing a PmSFB probe and genomic DNA blots, while Zhang et al. [35] determined the SFB alleles of P. salicina (Japanese plum) cultivars using specific primers designed based on the hypervariable regions of PsSFB. Based on the PCR-amplified region, Vaughan et al. [23] established a rapid method to determine the S genotypes of sweet cherry cultivars. However, we successfully determined/identified the SFB alleles of 48 Japanese apricot accessions via the method based on AS-PCR amplification, which was less demanding in terms of instrumentation, more advantageous, and convenient.
The putative amino acid sequences of the eleven novel PmSFB alleles contained two hypervariable regions (HVa and HVb), two variable regions (V1 and V2), the F-box motif, and the same size as other Prunus SFB [43,56,57,58]. Furthermore, the motifs’ structure was conserved among SFB alleles proteins, which could be important for the function and structure of SFB genes. In previous studies, SFB was reported to be a gene that recognized and protected the self S-RNase through some types of modification in peach (SC) [53]; others proposed that in S-RNase-based GSI, the ubiquitin/26S proteasome proteolytic pathway played a major role in nonself/self-pollen discrimination [18,28,30]. However, the four variable regions including HVa, HVb, V1, and V2 of SFB may play a crucial role in the haplotype-specific interaction mechanism with S-RNase for the discrimination of nonself/self-S-RNase.
Because the F-box motif is required for the formation of a complex termed SCF for the degradation of proteins, it is essential for the discrimination of self/nonself-pollen [56]. In P. mume, and P. avium, the self-compatible phenotypes have been reported to be associated to the insertion/deletions (indels) in SFB variable regions, which produce a frameshift in translation, resulting in a nonfunctional truncated amino acid/protein [56,59]. Likewise, in some cultivars of apricot (P. armeniaca), a 358 bp insertion was found in the SFBc gene located upstream from the HVa hypervariable region, resulting in the expression of a truncated protein; this gene alteration is associated with self-incompatibility breakdown [26]. On the other hand, Orlando Marchesano B.M. et al. (2022) [16] reported that in apricots, self-compatibility was related to a transposable element insertion within the coding sequence of SFB [16]. Furthermore, peach (P. persica) is a common self-compatible species [57], and its SFB mutation version was found in self-incompatible Prunus species, conferring SC to some of their cultivars [60,61]. However, these suggest that SFB alleles are essential in GSI study in general, specially its four variable regions.
For the Pm SFB alleles, the intraspecific amino acid identities were lower than interspecific identities when comparing with other Prunus SFBs. However, this could indicate the evolution of intraspecific identities in Prunus. The phylogenetic tree suggested that the newly identified SFB alleles in P. mume were orthologs rather than paralogs of SFB alleles from other Prunus species.
5. Conclusions
In this study, we identified the SFB genotypes of 48 Japanese apricot accessions, including 11 novel SFB alleles. Each accession possessed two distinct SFB alleles, the same as most of Prunus. The eleven new SFB alleles had the same typical features as SFB alleles from other Prunus species. The findings of this current study will enhance the accessible data/information on SFB alleles of Prunus and P. mume, which are required for the study of the self-(in)compatibility mechanism.
Author Contributions
Z.G. conceived and designed the study. G.H., D.C., C.M. and X.H. performed the experiment. G.H. and D.C. analyzed the data. D.C. and G.H. wrote the whole manuscript. C.M., X.H., Z.N. and K.O.O. assisted with the sample collection. K.O.O., S.I. and F.H. corrected the English language. S.I., F.H., T.S., B.K. and K.O.O. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Key Research and Development Program of China (2018YFD1000107), the project of Jiangsu Key Research on Seed Industry (JBGS (2021)019)) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), for the materials collection, data analysis, and experiment.
Data Availability Statement
All data analyzed or generated during this study were comprised in this manuscript. Eleven new Pm SFB from this study were logged/submitted to the NCBI database (GenBank) under accession numbers: MW186460(PmSFB44), MW186461(PmSFB45), MW186462(PmSFB46), MW186464(PmSFB48), MW186466(PmSFB50), MW186468(PmSFB52), MW186470(PmSFB54), MW186472(PmSFB56), MW786959(PmSFB57), MW786960(PmSFB58), MW786961(PmSFB59).
Acknowledgments
We thank all researchers for their contribution in this study.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PmSFB | SFB allele of Prunus mume |
| bp | Base pairs |
| SC | Self-compatibility |
| SI | Self-incompatibility |
| PCR | Polymerase chain reaction |
| AS-PCR | Allele-specific polymerase chain reaction |
| RT-PCR | Reverse-transcription polymerase chain reaction |
| CTAB | Cetyltrimethylammonium bromide |
References
- Muñoz-Sanz, J.V.; Zuriaga, E.; Cruz-García, F.; McClure, B.; Romero, C. Self-(in) compatibility systems: Target traits for crop-production, plant breeding, and biotechnology. Front. Plant Sci. 2020, 11, 195. [Google Scholar] [CrossRef]
- Nasrallah, J.B. Self-incompatibility in the Brassicaceae: Regulation and mechanism of self-recognition. Curr. Top. Dev. Biol. 2019, 131, 435–452. [Google Scholar] [PubMed]
- Raduski, A.R.; Haney, E.B.; Igić, B. The expression of self-incompatibility in angiosperms is bimodal. Evol. Int. J. Org. Evol. 2012, 66, 1275–1283. [Google Scholar] [CrossRef]
- Bedinger, P.A.; Broz, A.K.; Tovar-Mendez, A.; McClure, B. Pollen-pistil interactions and their role in mate selection. Plant Physiol. 2017, 173, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Hiscock, S.J.; Tabah, D.A. The different mechanisms of sporophytic self–incompatibility. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2003, 358, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.B.; Meagher, T.R.; Gibbs, P.E. Do s genes or deleterious recessives control late-acting self-incompatibility in Handroanthus heptaphyllus (Bignoniaceae)? A diallel study with four full-sib progeny arrays. Ann. Bot. 2021, 127, 723–736. [Google Scholar] [CrossRef]
- Aguiar, B.; Vieira, J.; Cunha, A.E.; Fonseca, N.A.; Iezzoni, A.; van Nocker, S.; Vieira, C.P. Convergent evolution at the gametophytic self-incompatibility system in Malus and Prunus. PLoS ONE 2015, 10, e0126138. [Google Scholar] [CrossRef]
- Sheick, R.; Serra, S.; Tillman, J.; Luby, J.; Evans, K.; Musacchi, S. Characterization of a novel S-RNase allele and genotyping of new apple cultivars. Sci. Hortic. 2020, 273, 109630. [Google Scholar] [CrossRef]
- Morimoto, T. Insights into the Evolution and Establishment of the Prunus-Specific Self-Incompatibility Recognition Mechanism. Ph.D. Thesis, Kyoto University, Kyoto, Japan, 2017. [Google Scholar]
- Yamane, H.; Tao, R. Molecular and Developmental Biology: Self-incompatibility. In The Prunus Mume Genome; Springer: Cham, Switzerland, 2019; pp. 119–135. [Google Scholar]
- Newbigin, E.D.; Joshua, T.P.; Kohn, R. RNase-Based Self-Incompatibility: Puzzled by Pollen S. Plant Cell 2008, 20, 2286–2292. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, C.; Zhang, B.; Tang, F.; Li, F.; Liao, Q.; Tang, D.; Peng, Z.; Jia, Y.; Gao, M. A nonS-locus F-box gene breaks self-incompatibility in diploid potatoes. Nat. Commun. 2021, 12, 4142. [Google Scholar] [CrossRef]
- Claessen, H.; Keulemans, W.; Van de Poel, B.; De Storme, N. Finding a compatible partner: Self-incompatibility in European pear (Pyrus communis); molecular control, genetic determination, and impact on fertilization and fruit set. Front. Plant Sci. 2019, 10, 407. [Google Scholar] [CrossRef]
- Morimoto, T.; Akagi, T.; Tao, R. Evolutionary analysis of genes for S-RNase-based self-incompatibility reveals S locus duplications in the ancestral Rosaceae. Hortic. J. 2015, 84, 233–242. [Google Scholar] [CrossRef]
- Meng, X.; Sun, P.; Kao, T. S-RNase-based self-incompatibility in Petunia inflata. Ann. Bot. 2011, 108, 637–646. [Google Scholar] [CrossRef]
- Orlando Marchesano, B.M.; Chiozzotto, R.; Baccichet, I.; Bassi, D.; Cirilli, M. Development of an HRMA-Based Marker Assisted Selection (MAS) Approach for Cost-Effective Genotyping of S and M Loci Controlling Self-Compatibility in Apricot (Prunus armeniaca L.). Genes 2022, 13, 548. [Google Scholar] [CrossRef]
- McClure, B.A.; Haring, V.; Ebert, P.R.; Anderson, M.A.; Simpson, R.J.; Sakiyama, F.; Clarke, A.E. Style self-incompatibility gene products of Nicotlana alata are ribonucleases. Nature 1989, 342, 955–957. [Google Scholar] [CrossRef]
- Ushijima, K.; Sassa, H.; Dandekar, A.M.; Gradziel, T.M.; Tao, R.; Hirano, H. Structural and transcriptional analysis of the self-incompatibility locus of almond: Identification of a pollen-expressed F-box gene with haplotype-specific polymorphism. Plant Cell 2003, 15, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Goonetilleke, S.N.; Croxford, A.E.; March, T.J.; Wirthensohn, M.G.; Hrmova, M.; Mather, D.E. Variation among S-locus haplotypes and among stylar RNases in almond. Sci. Rep. 2020, 10, 583. [Google Scholar] [CrossRef] [PubMed]
- Yamane, H.; Ikeda, K.; Ushijima, K.; Sassa, H.; Tao, R. A pollen-expressed gene for a novel protein with an F-box motif that is very tightly linked to a gene for S-RNase in two species of cherry, Prunus cerasus and P. avium. Plant Cell Physiol. 2003, 44, 764–769. [Google Scholar] [CrossRef]
- Ikeda, K.; Igic, B.; Ushijima, K.; Yamane, H.; Hauck, N.R.; Nakano, R.; Sassa, H.; Iezzoni, A.F.; Kohn, J.R.; Tao, R. Primary structural features of the S haplotype-specific F-box protein, SFB, in Prunus. Sex. Plant Reprod. 2004, 16, 235–243. [Google Scholar] [CrossRef]
- Sonneveld, T.; Tobutt, K.R.; Vaughan, S.P.; Robbins, T.P. Loss of pollen-S function in two self-compatible selections of Prunus avium is associated with deletion/mutation of an S haplotype–specific F-Box gene. Plant Cell 2005, 17, 37–51. [Google Scholar] [CrossRef]
- Vaughan, S.; Russell, K.; Sargent, D.; Tobutt, K. Isolation of S-locus F-box alleles in Prunus avium and their application in a novel method to determine self-incompatibility genotype. Theor. Appl. Genet. 2006, 112, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Duan, X.; Wu, C.; Yu, J.; Liu, C.; Wang, J.; Zhang, X.; Yan, G.; Jiang, F.; Li, T. Ubiquitination of S4-RNase by S-LOCUS F-BOX LIKE2 contributes to self-compatibility of Sweet Cherry ‘Lapins’. Plant Physiol. 2020, 184, 1702–1716. [Google Scholar] [CrossRef]
- Romero, C.; Vilanova, S.; Burgos, L.; Martinez-Calvo, J.; Vicente, M.; Llácer, G.; Badenes, M.L. Analysis of the S-locus structure in Prunus armeniaca L. Identification of S-haplotype specific S-RNase and F-box genes. Plant Mol. Biol. 2004, 56, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, S.; Badenes, M.L.; Burgos, L.; Martínez-Calvo, J.; Llácer, G.; Romero, C. Self-compatibility of two apricot selections is associated with two pollen-part mutations of different nature. Plant Physiol. 2006, 142, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gu, C.; Zhang, S.; Zhang, S.; Wu, H.; Heng, W. Identification of S-haplotype-specific S-RNase and SFB alleles in native Chinese apricot (Prunus armeniaca L.). J. Hortic. Sci. Biotechnol. 2009, 84, 645–652. [Google Scholar] [CrossRef]
- Entani, T.; Iwano, M.; Shiba, H.; Che, F.S.; Isogai, A.; Takayama, S. Comparative analysis of the self-incompatibility (S-) locus region of Prunus mume: Identification of a pollen-expressed F-box gene with allelic diversity. Genes Cells 2003, 8, 203–213. [Google Scholar] [CrossRef]
- Hu, G.; Ni, Z.; Couliblay, D.; Gao, Z. Analysis of S Genotypes of 11 Plum Cultivars and Identification of New S Genes. J. Plant Genet. Resour. 2021, 22, 860–872. [Google Scholar]
- Lai, Z.; Ma, W.; Han, B.; Liang, L.; Zhang, Y.; Hong, G.; Xue, Y. An F-box gene linked to the self-incompatibility (S) locus of Antirrhinum is expressed specifically in pollen and tapetum. Plant Mol. Biol. 2002, 50, 29–41. [Google Scholar] [CrossRef]
- Yamane, H.; Ushijima, K.; Sassa, H.; Tao, R. The use of the S haplotype-specific F-box protein gene, SFB, as a molecular marker for S-haplotypes and self-compatibility in Japanese apricot (Prunus mume). Theor. Appl. Genet. 2003, 107, 1357–1361. [Google Scholar] [CrossRef]
- Makovics-Zsohár, N.; Halász, J. Self-incompatibility system in polyploid fruit tree species—A review. Int. J. Plant Reprod. Biol. 2016, 8, 1–10. [Google Scholar]
- Ikeda, K.; Ushijima, K.; Yamane, H.; Tao, R.; Hauck, N.R.; Sebolt, A.M.; Iezzoni, A.F. Linkage and physical distances between the S-haplotype S-RNase and SFB genes in sweet cherry. Sex. Plant Reprod. 2005, 17, 289–296. [Google Scholar] [CrossRef]
- Lomax, J. Investigating Pollen Compatibility of Commercial Sweet Cherry Cultivars by DNA Analysis. Bachelor’s Thesis, University of Tasmania, Tasmania, Australia, 2021. [Google Scholar]
- Zhang, S.-L.; Huang, S.-X.; Kitashiba, H.; Nishio, T. Identification of S-haplotype-specific F-box gene in Japanese plum (Prunus salicina Lindl.). Sex. Plant Reprod. 2007, 20, 1–8. [Google Scholar] [CrossRef]
- Abdallah, D.; Baraket, G.; Ben Mustapha, S.; Angeles Moreno, M.A.; Salhi Hannachi, A. Molecular and Evolutionary Characterization of Pollen S Determinant (SFB Alleles) in Four Diploid and Hexaploid Plum Species (Prunus spp.). Biochem. Genet. 2021, 59, 42–61. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Wu, J.; Zhang, S.-J.; Yang, Y.-N.; Wu, H.-Q.; Khan, M.A.; Zhang, S.-L.; Liu, Q.-Z. Molecular analysis of eight SFB alleles and a new SFB-like gene in Prunus pseudocerasus and Prunus speciosa. Tree Genet. Genomes 2011, 7, 891–902. [Google Scholar] [CrossRef]
- Chin, S.-W.; Shaw, J.; Haberle, R.; Wen, J.; Potter, D. Diversification of almonds, peaches, plums and cherries—Molecular systematics and biogeographic history of Prunus (Rosaceae). Mol. Phylogenet. Evol. 2014, 76, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Pan, Z.; Hayat, F.; Bai, Y.; Coulibaly, D.; Ali, S.; Ni, X.; Shi, T.; Gao, Z. Comprehensive transcriptome profiling to identify genes involved in pistil abortion of Japanese apricot. Physiol. Mol. Biol. Plants 2021, 27, 1191–1204. [Google Scholar] [CrossRef]
- Yaegaki, H.; Shimada, T.; Moriguchi, T.; Hayama, H.; Haji, T.; Yamaguchi, M. Molecular characterization of S-RNase genes and S-genotypes in the Japanese apricot (Prunus mume Sieb. et Zucc.). Sex. Plant Reprod. 2001, 13, 251–257. [Google Scholar] [CrossRef]
- Habu, T.; Matsumoto, D.; Fukuta, K.; Esumi, T.; Tao, R.; Yaegaki, H.; Yamaguchi, M.; Matsuda, M.; Konishi, T.; Kitajima, A. Cloning and characterization of twelve S-RNase alleles in Japanese apricot (Prunus mume Sieb. et Zucc.). J. Jpn. Soc. Hortic. Sci. 2008, 77, 374–381. [Google Scholar] [CrossRef][Green Version]
- Xu, J.; Gao, Z.; Zhang, Z. Identification of S-genotypes and novel S-RNase alleles in Japanese apricot cultivars native to China. Sci. Hortic. 2010, 123, 459–463. [Google Scholar] [CrossRef]
- Wang, P.; Gao, Z.; Ni, Z.; Zhuang, W.; Zhang, Z. Isolation and identification of new pollen-specific SFB genes in Japanese apricot (Prunus mume). Genet. Mol. Res. 2013, 12, 3286–3295. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tong, Z.; Zhang, Z.; Chen, Q.; Jiang, L.; Zhao, J. Improved method of DNA extraction from young leaves of wild peach. Jiangsu Agric. Sci. 2006, 5, 66–69. [Google Scholar]
- Tao, R.; Yamane, H.; Sugiura, A.; Murayama, H.; Sassa, H.; Mori, H. Molecular typing of S-alleles through identification, characterization and cDNA cloning for S-RNases in sweet cherry. J. Am. Soc. Hortic. Sci. 1999, 124, 224–233. [Google Scholar] [CrossRef]
- Xu, J.; Gao, Z.; Hou, J.; Wang, S.; Zhang, Z. Optimization of Guomei AS-PCR reaction system. Jiangsu Agric. Sci. 2008, 2, 69–71. [Google Scholar]
- Junxia, X. S Genotype Identification and New S Gene Cloning of Plum Varieties Native to China. Thesis of Mater, Nanjing Agricultural University, Nanjing, China, 2011. [Google Scholar]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in bipolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1994, 2, 28–36. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Chen, Q.; Meng, D.; Gu, Z.; Li, W.; Yuan, H.; Duan, X.; Yang, Q.; Li, Y.; Li, T. SLFL genes participate in the ubiquitination and degradation reaction of S-RNase in self-compatible peach. Front. Plant Sci. 2018, 9, 227. [Google Scholar] [CrossRef]
- Abdallah, D.; Baraket, G.; Perez, V.; Ben Mustapha, S.; Salhi-Hannachi, A.; Hormaza, J.I. Analysis of self-incompatibility and genetic diversity in diploid and hexaploid plum genotypes. Front. Plant Sci. 2019, 10, 896. [Google Scholar] [CrossRef]
- Zhang, J. Study and Use Advance about Plum and Apricot Resource, 5th ed.; China Forestry Publishing House: Beijing, China, 2008. [Google Scholar]
- Ushijima, K.; Yamane, H.; Watari, A.; Kakehi, E.; Ikeda, K.; Hauck, N.R.; Iezzoni, A.F.; Tao, R. The S haplotype-specific F-box protein gene, SFB, is defective in self-compatible haplotypes of Prunus avium and P. mume. Plant J. 2004, 39, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, D.; Baraket, G.; Perez, V.; Hannachi, A.S.; Hormaza, J.I. Self-compatibility in peach [Prunus persica (L.) Batsch]: Patterns of diversity surrounding the S-locus and analysis of SFB alleles. Hortic. Res. 2020, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.H.R. Transcriptome Analysis of Self- and Cross-pollinated Pistils of Japanese Apricot (Prunus mume Sieb. et Zucc.). J. Jpn. Soc. Hort. Sci. 2014, 82, 95–107. [Google Scholar]
- Yamane, H.; Tao, R. Molecular basis of self-(in) compatibility and current status of S-genotyping in Rosaceous fruit trees. J. Jpn. Soc. Hortic. Sci. 2009, 78, 137–157. [Google Scholar] [CrossRef]
- Tao, R.; Watari, A.; Hanada, T.; Habu, T.; Yaegaki, H.; Yamaguchi, M.; Yamane, H. Self-compatible peach (Prunus persica) has mutant versions of the S haplotypes found in self-incompatible Prunus species. Plant Mol. Biol. 2007, 63, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Sanz, J.V.; Zuriaga, E.; López, I.; Badenes, M.L.; Romero, C. Self-(in) compatibility in apricot germplasm is controlled by two major loci, S and M. BMC Plant Biol. 2017, 17, 82. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).