Variation of Fertility and Phenological Synchronization in Cunninghamia lanceolata Seed Orchard: Implications for Seed Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Investigated Seed Orchard
2.2. Flowering Survey
2.3. Phenological Data Collection
2.4. Evaluation of Seed Production
2.5. Data Analysis
3. Results
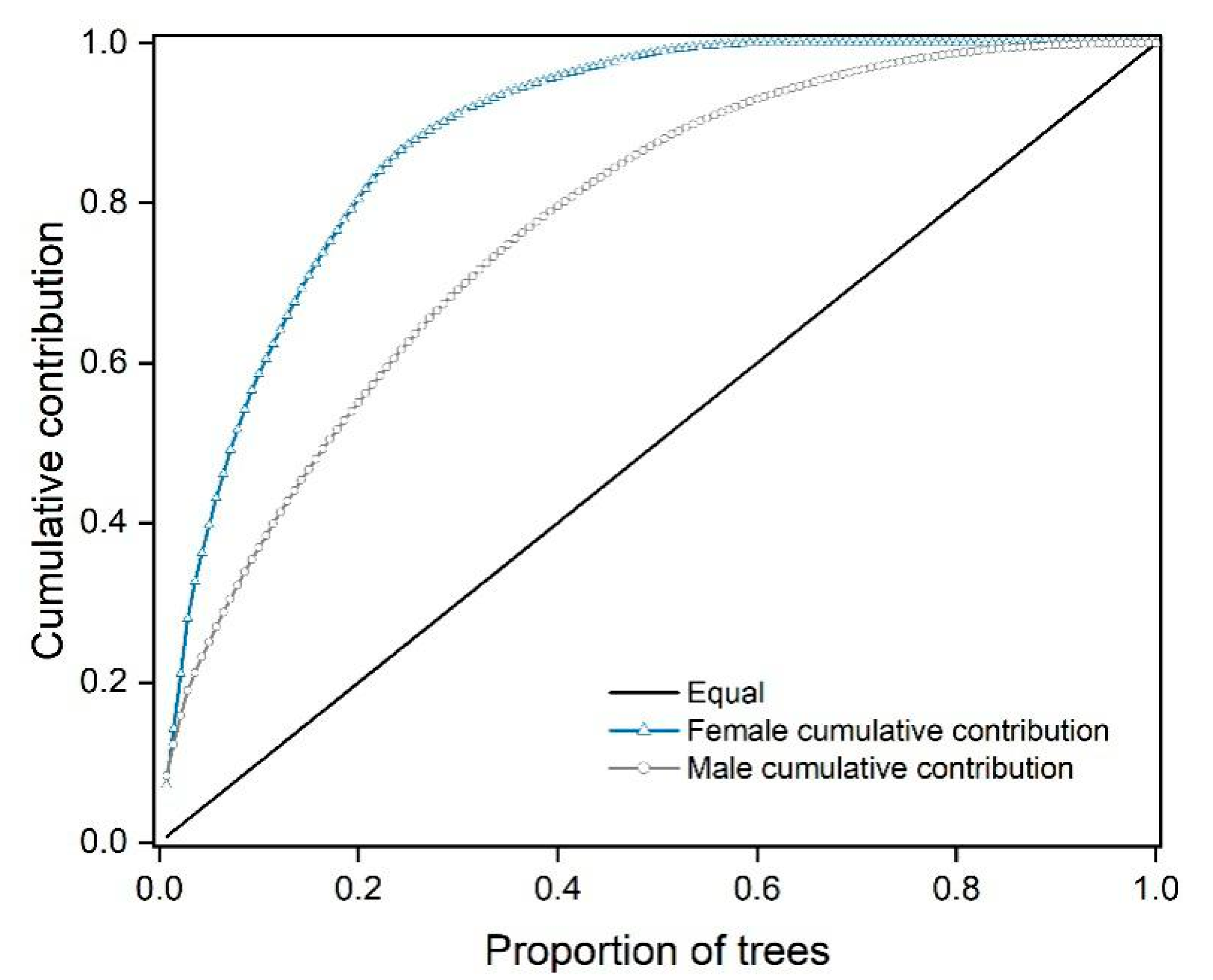
3.1. Fertility Variation in the CSO
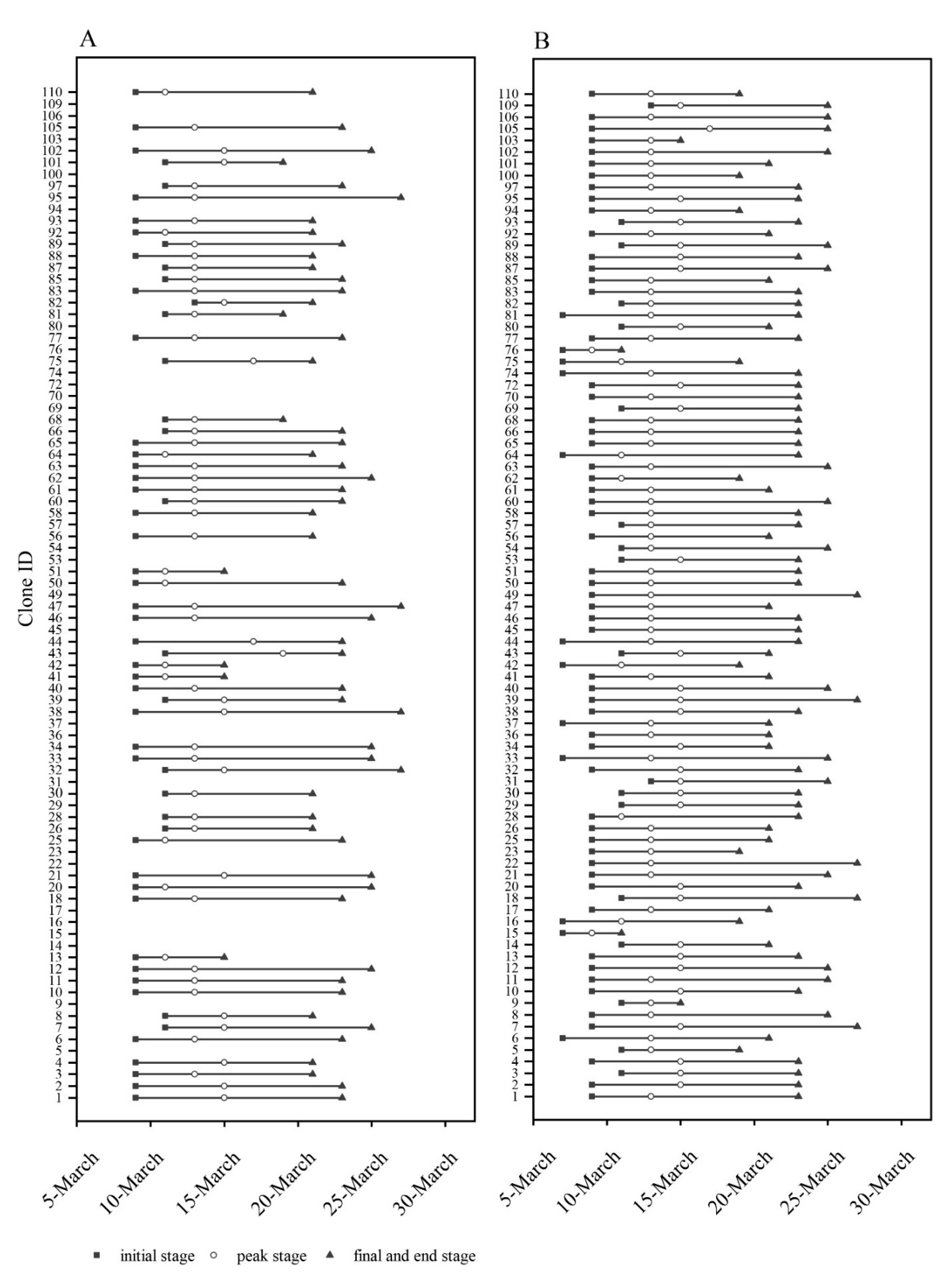
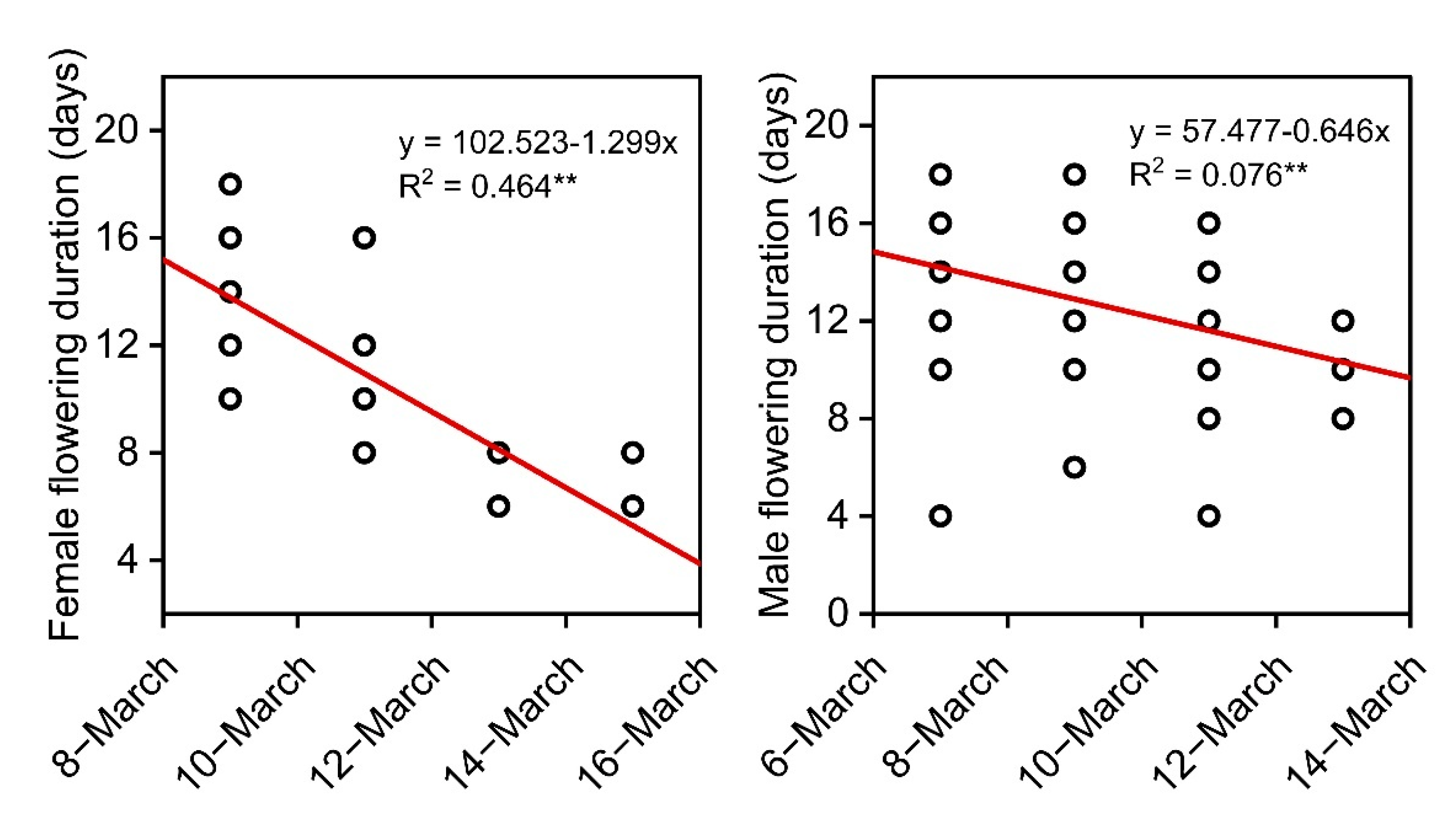
3.2. Genetic Variation in Reproductive Phenology
3.3. Phenological Synchronization
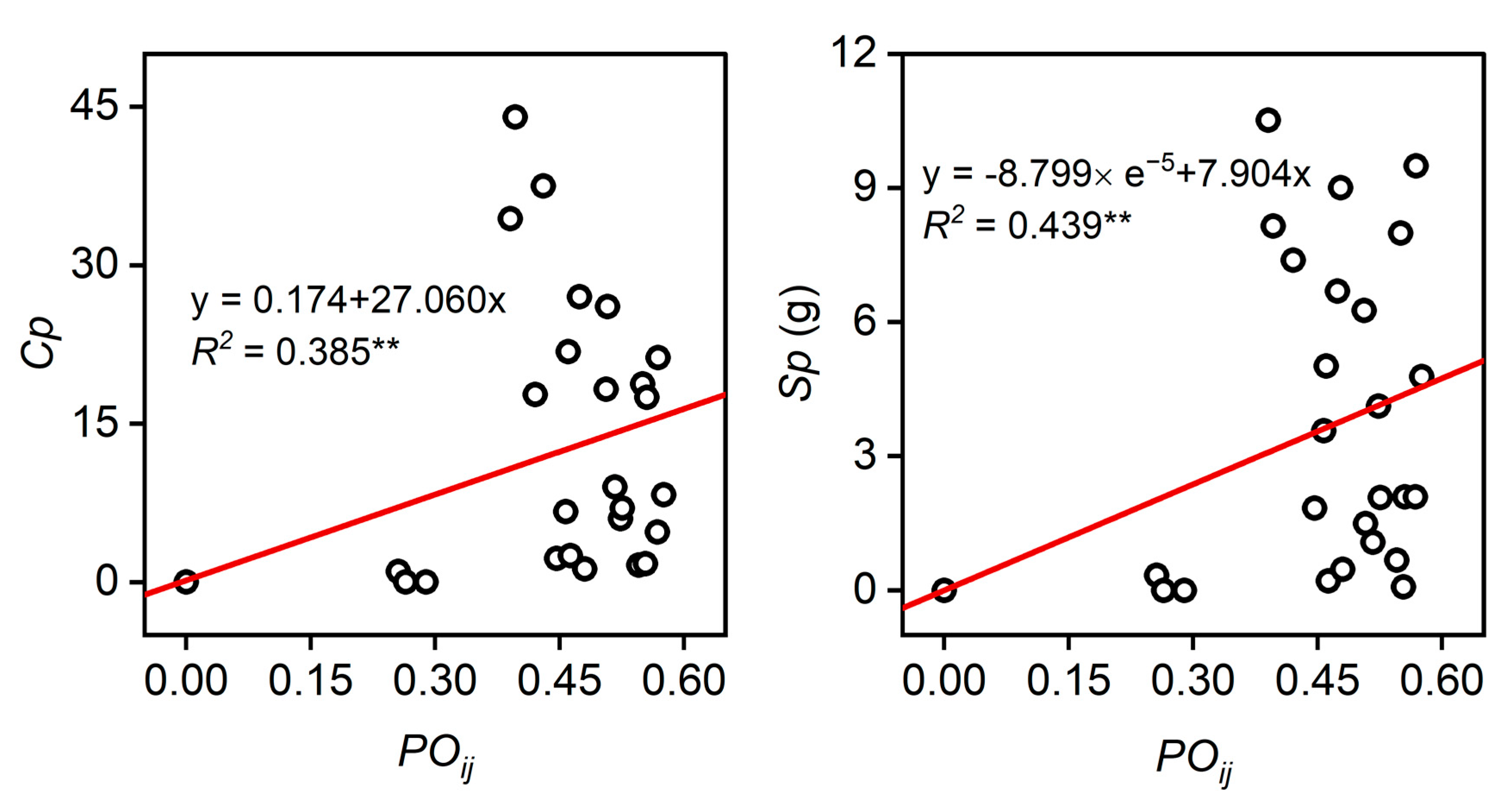
3.4. Relationships between Seed Production, Fertility, and Synchronization
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CSO | Clonal seed orchard | FDf | Female flower duration |
| Nm | Number of male flowers | FDm | Male flower duration |
| Nf | Number of female flowers | FDm | Flowering duration of males |
| ISf | Initial stage of female flower | FDf | Flowering duration of females |
| PSf | Peak stage of female flower | F_E | Early individuals began receptive |
| FSf | Final stage of female flower | F_L | Late individuals began receptive |
| ESf | End stage of female flower | M_L | Late individuals began shedding pollen |
| ISm | Initial stage of male flower | The clonal broad-sense heritability | |
| PSm | Peak stage of male flower | Nr | Relative effective number of parents |
| FSm | Final stage of male flower | Nrf | Relative effective numbers of female |
| ESm | End stage of male flower | Nrm | Relative effective numbers of male |
| Cp | Cone production | POij | The phenological synchronization index |
| Sp | Seed production | M_E | Early individuals began shedding pollen |
| SOsc | Seed output of a single cone | CVm | The coefficients of variation for the male strobilus |
| TSW | Thousand-seed weight | CVf | The coefficients of variation for the female strobilus |
| ψf | Variation in female fertility | Npf | The effective number of female gametic parents |
| ψm | Variation in male fertility | Npm | The effective number of male gametic parents |
| ψ | The sibling coefficient | F_M | “Middle” in terms of the timing of their receptivity |
| Np | Effective number of parents | M_M | “Middle” in terms of the timing of their pollen shedding |
| DBH | Diameter at breast height | r | The correlation between female and male strobilus production |
References
- Askew, G.R. Estimation of gamete pool compositions in clonal seed orchards. Silvae Genet. 1988, 37, 227–232. [Google Scholar]
- Muñoz-Gutiérrez, L.; Vargas-Hernández, J.J.; López-Upton, J.; Ramírez-Herrera, C.; Jiménez-Casas, M.; Aldrete, A. Variation in reproductive phenology in a Pinus patula seed orchard and risk of genetic contamination from nearby natural stands. New For. 2019, 50, 1027–1041. [Google Scholar] [CrossRef]
- Kang, K.; Bilir, N. Seed Orchards (Establishment, Management and Genetics); CRN Promotion and Press: Ankara, Turkey, 2021. [Google Scholar]
- Korecký, J.; El-Kassaby, Y. Pollination dynamics variation in a Douglas-fir seed orchard as revealed by microsatellite analysis. Silva Fenn. 2016, 50, 1682. [Google Scholar] [CrossRef]
- Pupin, S.; Sebbenn, A.M.; Cambuim, J.; da Silva, A.M.; Zaruma, D.U.; Silva, P.H.; Rosse, L.N.; Souza, I.C.; Marino, C.L.; Moraes, M.L. Effects of pollen contamination and non-random mating on inbreeding and outbreeding depression in a seedling seed orchard of Eucalyptus urophylla. For. Ecol. Manag. 2019, 437, 272–281. [Google Scholar] [CrossRef]
- Kartikawati, N.K. Fertility variation of Melaleuca cajuput subsp cajuputi and its implication seed orchard management. Indones. J. For. Res. 2016, 3, 83–94. [Google Scholar] [CrossRef]
- Yuan, H.; Li, Z.; Fang, P.; Li, W.; Li, Y. Variation and Stability in Female Strobili Production of a First-Generation Clonal Seed Orchard of Chinese Pine (Pinus tabuliformis). Silvae Genet. 2014, 63, 41–47. [Google Scholar] [CrossRef][Green Version]
- Bila, A.D.; Lindgren, D. Fertility variation in Milletia sthuhlmannii, Brachystegia spiciformis, Brachystegia bohemii and Leucaena leucocephala and its effects on relatedness in seeds. For. Genet. 1998, 5, 119–129. [Google Scholar]
- Chen, X.; Shen, X.; Yang, P.; Pan, Q.; Li, W. A study on characteristics of reproductive phenology of Chinese fir seed orchard. J. Beijing For. Univ. 1995, 17, 10–18. [Google Scholar]
- Kjær, E.D. Estimation of effective population number in aPicea abies(Karst.) seed orchard based on flower assessment. Scand. J. For. Res. 1996, 11, 111–121. [Google Scholar] [CrossRef]
- Lindgren, D.; Gea, L.; Jefferson, P. Loss of genetic diversity monitored by status number. Silvae Genet. 1996, 45, 52–58. [Google Scholar]
- Kang, K.S.; Mullin, T.J. Variation in Clone Fertility and its Effect on the Gene Diversity of Seeds From a Seed Orchard of Chamaecyparis obtusa in Korea. Silvae Genet. 2007, 56, 134–137. [Google Scholar] [CrossRef][Green Version]
- Kang, K.; Bila, A.; Harju, A.; Lindgren, D. Estimation of fertility variation in forest tree populations. For. Int. J. For. Res. 2003, 76, 329–344. [Google Scholar] [CrossRef]
- Bilir, N.; Kang, K.-S.; Lindgren, D. Fertility variation in six populations of Brutian pine (Pinus brutia Ten.) over altitudinal ranges. Euphytica 2005, 141, 163–168. [Google Scholar] [CrossRef]
- Kang, K.; Lindgren, D. Fertility variation and its effect on the relatedness of seeds in Pinus densiflora, Pinus thunbergii and Pinus koraiensis clonal seed orchards. Silvae Genet. 1998, 47, 196–201. [Google Scholar]
- Bilir, N.; Kang, K.-S. Fertility variation, seed collection and gene diversity in natural stands of Taurus cedar (Cedrus libani). Eur. J. For. Res. 2020, 140, 199–208. [Google Scholar] [CrossRef]
- Alexander, L.W.; Woeste, K.E. Phenology, dichogamy, and floral synchronization in a northern red oak (Quercus rubra) seed orchard. Can. J. For. Res. 2016, 46, 629–636. [Google Scholar] [CrossRef]
- Muñoz-Gutiérrez, L.; Vargas-Hernández, J.J.; López-Upton, J.; Ramírez-Herrera, C.; Jiménez-Casas, M. Clonal variation in phenological synchronization and cone production in a Pinus patula seed orchard. Silvae Genet. 2020, 69, 130–138. [Google Scholar] [CrossRef]
- Burczyk, J.; Chalupka, W. Flowering and cone production variability and its effect on parental balance in a Scots pine clonal seed orchard. Ann. For. Sci. 1997, 54, 129–144. [Google Scholar] [CrossRef]
- Bian, L.; Huang, D.; Zhang, X.; Tong, X.; Ye, D.; Jisen, S. Analysis on flowering phenology and synchronization indexed of Chinese fir clonal archive. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2020, 44, 207–212. [Google Scholar]
- Gömöry, D.; Bruchánik, R.; Longauer, R. Fertility variation and flowering asynchrony in Pinus sylvestris: Consequences for the genetic structure of progeny in seed orchards. For. Ecol. Manag. 2003, 174, 117–126. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, R.; Song, P.; Zhou, Z. Flowering phenology and synchronization of clones among plant ages in a seed orchard of Schima superba. For. Res. 2017, 30, 551–558. [Google Scholar]
- Yang, H.; Zhang, R.; Zhou, Z. Pollen dispersal, mating patterns and pollen contamination in an insect-pollinated seed orchard of Schima superba Gardn. et Champ. New For. 2017, 48, 431–444. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Li, Y. Variation in Floral Phenological Synchronization in a Clonal Seed Orchard of Pinus tabuliformis in Northeast of China. Silvae Genet. 2012, 61, 133–142. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.-Q.; Kirschbaum, M.U.; Hou, Z.; Guo, Z. Carbon stock changes in successive rotations of Chinese fir (Cunninghamia lanceolata (lamb) hook) plantations. For. Ecol. Manag. 2004, 202, 131–147. [Google Scholar] [CrossRef]
- Wang, R.; Hu, D.; Zheng, H.; Liu, W.; Liang, R.; Yang, B. Genetic variation analysis of flowering phenology in the 2.5 generation seed orchard of Cunninghamia laceolata. J. Southwest For. Univ. 2013, 33, 25–29, 43. [Google Scholar]
- Chen, X.; Li, W.; Pan, Q.; Yang, M. Studies on spacial distribution and the spread distance of pollen in Chinese fir seed orchards. J. Beijing For. Univ. 1996, 18, 24–30. [Google Scholar]
- Corp, I. IBM SPSS Statistics for Windows, Version 27.0; IBM Corporation: Armonk, NY, USA, 2020. [Google Scholar]
- Wright, J.W. Introduction to Forest Genetics; Academic Press, Inc.: New York, NY, USA, 1976. [Google Scholar] [CrossRef]
- Kang, K.-S.; El-Kassaby, Y. Considerations of correlated fertility between genders on genetic diversity: The Pinus densiflora seed orchard as a model. Theor. Appl. Genet. 2002, 105, 1183–1189. [Google Scholar] [CrossRef]
- Kang, K.S.; Lindgren, D.; Mullin, T.J. Prediction of genetic gain and gene diversity in seed orchard crops under alternative management strategies. Theor. Appl. Genet. 2001, 103, 1099–1107. [Google Scholar] [CrossRef]
- Lindgren, D.; Mullin, T.J. Relatedness and status number in seed orchard crops. Can. J. For. Res. 1998, 28, 276–283. [Google Scholar] [CrossRef]
- Suraj, P.; Nagabhushana, K.; Kamalakannan, R.; Varghese, M. Impact of fertility variation on genetic diversity and phenotypic traits in second generation seed production areas and clonal seed orchards of Eucalyptus camaldulensis. Silvae Genet. 2019, 68, 29–40. [Google Scholar] [CrossRef]
- Chaisurisri, K.; El-Kassaby, Y. Estimation of clonal contribution to cone and seed crops in a Sitka spruce seed orchard. Ann. Des Sci. For. 1993, 50, 461–467. [Google Scholar] [CrossRef][Green Version]
- Askew, G.R.; Blush, D. Short note: An index of phenological overlap in flowering for clonal conifer seed orchards. Silvae Genet. 1990, 39, 168–171. [Google Scholar]
- Kamalakannan, R.; Varghese, M.; Suraj, P.G.; Arutselvan, T. Options for converting a clone trial of Eucalyptus camaldulensis into a clonal seed orchard considering gain, fertility and effective clone number. J. For. Res. 2015, 27, 51–57. [Google Scholar] [CrossRef]
- El-Kassaby, Y.; Ritland, K. The relationship of outcrossing rate to reproductive phenology and supplemental mass pollination in a douglas-fir seed orchard. Silvae Genet. 1986, 35, 240–244. [Google Scholar]
- Cheng, L.; Chen, Q.; Chen, S.; Dai, J.; Dong, L.; Chen, X.; Tan, W.; Chen, D.; Huang, K.; Li, K.; et al. Study on cone and seed qualities among different Cunninghamia lanceolata clones. Guangxi For. Sci. 2021, 50, 1–7. [Google Scholar]

| Origin | Longitude (°) | Latitude (°) | Collection No. |
|---|---|---|---|
| Fushun forest farm, Sichuan Province, China | 104.85 | 29.01 | EQ48, EQ60, EQ59, EQ61, EQ58, EQ46, EQ41, EQ42, EQ43 |
| Gongxian seed orchard, Sichuan Province, China | 104.71 | 28.45 | T91, T72, T73 |
| Hongya state-own forest farm, Sichuan Province, China | 103.26 | 29.70 | T209, T204, T205, T206, T217, T218, T164 |
| Nanchuan forest farm, Chongqing Province, China | 107.14 | 29.14 | T313, T301, T312, T314, T311, T315, T316, T317, T309, T308, T307, T319, T320, T321, T305, T334, T336, T335, T328 |
| Xuyong forest farm, Sichuan Province, China | 105.44 | 28.16 | T358, T359, T367, T361, T360, T372, T363, T378, EQ22, EQ23, T382, T379, EQ24, EQ25, T380, EQ27, T312R, EQ28, EQ21, EQ29, T376 |
| Yuejiang forest farm, Sichuan Province, China | 104.52 | 28.44 | T2, T3, T6, T7, T5, T8, T13, T12, T10, T9, T14, T15, T16, T17 |
| Junlian forest farm, Sichuan Province, China | 104.50 | 28.16 | T101, T113, T114, T115, T119, T120, T105, T103, T109, T121, T122, T107, T106, T112 |
| Source | Traits | df | Type III SS | Mean Square | F | Hc2 |
|---|---|---|---|---|---|---|
| Provenance | Nm | 6 | 37,855.434 | 6309.239 | 0.230 | |
| Nf | 6 | 3618.721 | 603.120 | 1.750 | ||
| Clone (provenance) | Nm | 79 | 1,770,227.261 | 22,407.940 | 0.808 | 0.396 |
| Nf | 79 | 35,415.797 | 448.301 | 1.285 | 0.318 | |
| Error | Nm | 55 | 1,507,138.583 | 27,402.520 | ||
| Nf | 55 | 18,952.333 | 344.588 |
| Source | Traits | df | Type III SS | Mean Square | F | Hc2 |
|---|---|---|---|---|---|---|
| Provenance | ISm | 6 | 10.056 | 1.676 | 1.398 | |
| FDm | 6 | 57.599 | 9.600 | 2.040 | ||
| ISf | 6 | 2.739 | 0.457 | 0.132 | ||
| FDf | 6 | 25.332 | 4.222 | 0.300 | ||
| Clone (provenance) | ISm | 79 | 173.713 | 2.199 | 1.834 * | 0.433 |
| FDm | 79 | 1025.777 | 12.985 | 2.760 ** | 0.476 | |
| ISf | 55 | 80.768 | 1.469 | 0.424 | 0.272 | |
| FDf | 55 | 556.817 | 10.124 | 0.720 | 0.356 | |
| Error | ISm | 52 | 62.333 | 1.199 | ||
| FDm | 52 | 244.667 | 4.705 | |||
| ISf | 20 | 69.333 | 3.467 | |||
| FDf | 20 | 281.333 | 14.067 |
| Male | Female | ||
|---|---|---|---|
| Early Phenological Type | Middle Phenological Type | Late Phenological Type | |
| Early phenological type | 0.197 a | 0.354 c | 0.266 b |
| Middle phenological type | 0.489 e | 0.461 e | 0.333 c |
| Late phenological type | 0.164 a | 0.381 d | 0.288 b |
| Source | Trait | df | Type III SS | Mean Square | F | Hc2 |
|---|---|---|---|---|---|---|
| Provenance | Cp | 5 | 1539.855 | 307.971 | 0.708 | |
| Sp | 5 | 365.211 | 73.042 | 2.383 | ||
| TSW | 5 | 13.747 | 2.749 | 3.740 * | ||
| SOsc | 5 | 0.437 | 0.087 | 2.548 | ||
| Clone (provenance) | Cp | 40 | 18,319.518 | 457.988 | 1.053 | 0.381 |
| Sp | 40 | 2410.360 | 60.259 | 1.966 | 0.368 | |
| TSW | 40 | 127.04 | 3.176 | 4.320 ** | 0.477 | |
| SOsc | 40 | 1.891 | 0.047 | 1.377 | 0.280 | |
| Error | Cp | 18 | 7825.833 | 434.769 | ||
| Sp | 14 | 429.149 | 30.653 | |||
| TSW | 13 | 9.557 | 0.735 | |||
| SOsc | 14 | 0.481 | 0.034 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Huang, X.; Liu, Y.; Zhu, P.; Zhu, Y.; Li, F.; Yao, J.; Chen, L.; Yang, H. Variation of Fertility and Phenological Synchronization in Cunninghamia lanceolata Seed Orchard: Implications for Seed Production. Forests 2022, 13, 1571. https://doi.org/10.3390/f13101571
Xie J, Huang X, Liu Y, Zhu P, Zhu Y, Li F, Yao J, Chen L, Yang H. Variation of Fertility and Phenological Synchronization in Cunninghamia lanceolata Seed Orchard: Implications for Seed Production. Forests. 2022; 13(10):1571. https://doi.org/10.3390/f13101571
Chicago/Turabian StyleXie, Jiaxin, Xin Huang, Yingquan Liu, Peng Zhu, Yuanwei Zhu, Fengqing Li, Jiabao Yao, Lianghua Chen, and Hanbo Yang. 2022. "Variation of Fertility and Phenological Synchronization in Cunninghamia lanceolata Seed Orchard: Implications for Seed Production" Forests 13, no. 10: 1571. https://doi.org/10.3390/f13101571
APA StyleXie, J., Huang, X., Liu, Y., Zhu, P., Zhu, Y., Li, F., Yao, J., Chen, L., & Yang, H. (2022). Variation of Fertility and Phenological Synchronization in Cunninghamia lanceolata Seed Orchard: Implications for Seed Production. Forests, 13(10), 1571. https://doi.org/10.3390/f13101571





