Abstract
Studying the effect of surface ozone (O3) and water stress on tree growth is important for planning sustainable forest management and forest ecology. In the present study, a 22-year long time series (1998–2019) on basal area increment (BAI) and fructification severity of European beech (Fagus sylvatica L.) and Norway spruce (Picea abies (L.) H.Karst.) at five forest sites in Western Germany (Rhineland Palatinate) was investigated to evaluate how it correlates with drought and stomatal O3 fluxes (PODY) with an hourly threshold of uptake (Y) to represent the detoxification capacity of trees (POD1, with Y = 1 nmol O3 m−2 s−1). Between 1998 and 2019, POD1 declined over time by on average 0.31 mmol m−2 year−1. The BAI showed no significant trend at all sites, except in Leisel where a slight decline was observed over time (−0.37 cm2 per year, p < 0.05). A random forest analysis showed that the soil water content and daytime O3 mean concentration were the best predictors of BAI at all sites. The highest mean score of fructification was observed during the dry years, while low level or no fructification was observed in most humid years. Combined effects of drought and O3 pollution mostly influence tree growth decline for European beech and Norway spruce.
1. Introduction
Effects of ozone pollution are prevalent in forests throughout the growing season around the world [1,2,3,4,5,6]. Tropospheric ozone (O3) is leading serious environmental problems in Europe [7], as well as in other parts of the Northern Hemisphere e.g., in North America and South Asia [8]. The adverse effects of O3 on forest can be visible foliar O3 injury [9,10,11,12], a reduction of growth [13,14], or the sluggishness or impairment of the regulation ability of leaf stomata [11,12]. Some investigations on O3 effects on tree biomass were carried out worldwide [15,16,17,18] including sites in Germany [19]. However, tree responses to O3 under real-world conditions remain under-investigated [18,20], due to the difficulties to disentangle O3 by other meteorological effects [21]. The total biomass of trees is estimated to be reduced by 7% under the current O3 mean concentrations (40 ppb on average) and by 17% at mean O3 concentrations expected by 2100 (97 ppb based on a meta-analysis) compared to preindustrial O3 levels in North Hemisphere (about 10 ppb) [18].
In Europe, the total stem volume increment for the whole European area declined by 13 million m3 over 178 million hectares between 2005 and 2010 [22], and models predict severe beech growth declines by 2090, ranging from 20% to more than 50%, depending on the climate change scenario and the geographical region [23]. A decline in the growth of basal area was also observed in Switzerland where O3 induced a 19.5% growth reduction at national level [21,24], in France [25], Austria [26], Italy and Spain [2,27,28], and in Germany [29] mainly due to rising air temperature (on average, + 1.5 °C per decade). Drought, increasing age, rising background O3 levels, and reduced nitrogen deposition are some of the environmental factors limiting growth, however the contribution of each factor is rarely quantified [21]. According to [27,30,31], at the lower and mid-range elevation of tree species, there is often a negative relationship between growth and air temperature, while a positive relationship between precipitation and growth is often reported. Drought, due to rising air temperature and reduced total precipitation, affected different ecosystems during last decades [32,33,34]. The increase of fructification during the last decades has been also suggested by [35] as factors explaining the growth decline, e.g., for the decline observed in beech forests in Switzerland [21].
To derive critical levels, the dose-response relationships between tree biomass and O3 concentrations were established under fumigation experiments and/or in open top chambers with young trees [36,37,38]. To date, the biomass reduction is evaluated from fumigation experiments with O3-free air as reference [36,37,38]. The biomass losses are thus purely theoretical, i.e., not representative of real world conditions, as such conditions did not exist in pre-industrial times. The dose–response relationships and associated critical levels were calculated for European beech and for Norway spruce [38,39,40]: 5.2 mmol O3 m−2 per leaf area for European beech (4% biomass reduction) and 9.2 mmol O3 m−2 per leaf area for Norway spruce (2% biomass reduction). Looking at the forest responses to O3, the Phytotoxic Ozone Dose, defined as the amount of O3 absorbed into the leaves or needles through stomata over the growing season, and above an hourly threshold Y (PODY), integrates the effects of climatic factors and vegetation characteristics on O3 uptake [41], such as air temperature, soil moisture, solar radiation, phenology, and wind speed. To investigate real-world forest-response indicators, such as tree growth, PODY is more realistic compared to the exposure-based approach i.e., the accumulated ozone over threshold of 40 ppb; AOT40 [42].
In the present paper, we investigated the influence of tropospheric O3 (by using the PODY metric) and fructification on the radial growth of Rhineland-Palatinate forests over the time period 1998–2019. In this study, we hypothesized that: surface O3, drought, and fructification have negative impacts on radial tree growth over the 22-year time period under field conditions. Furthermore, the question is investigated whether a quantitative estimation or differentiation of the effects of O3 flux or water stress is possible.
2. Materials and Methods
2.1. Site Description
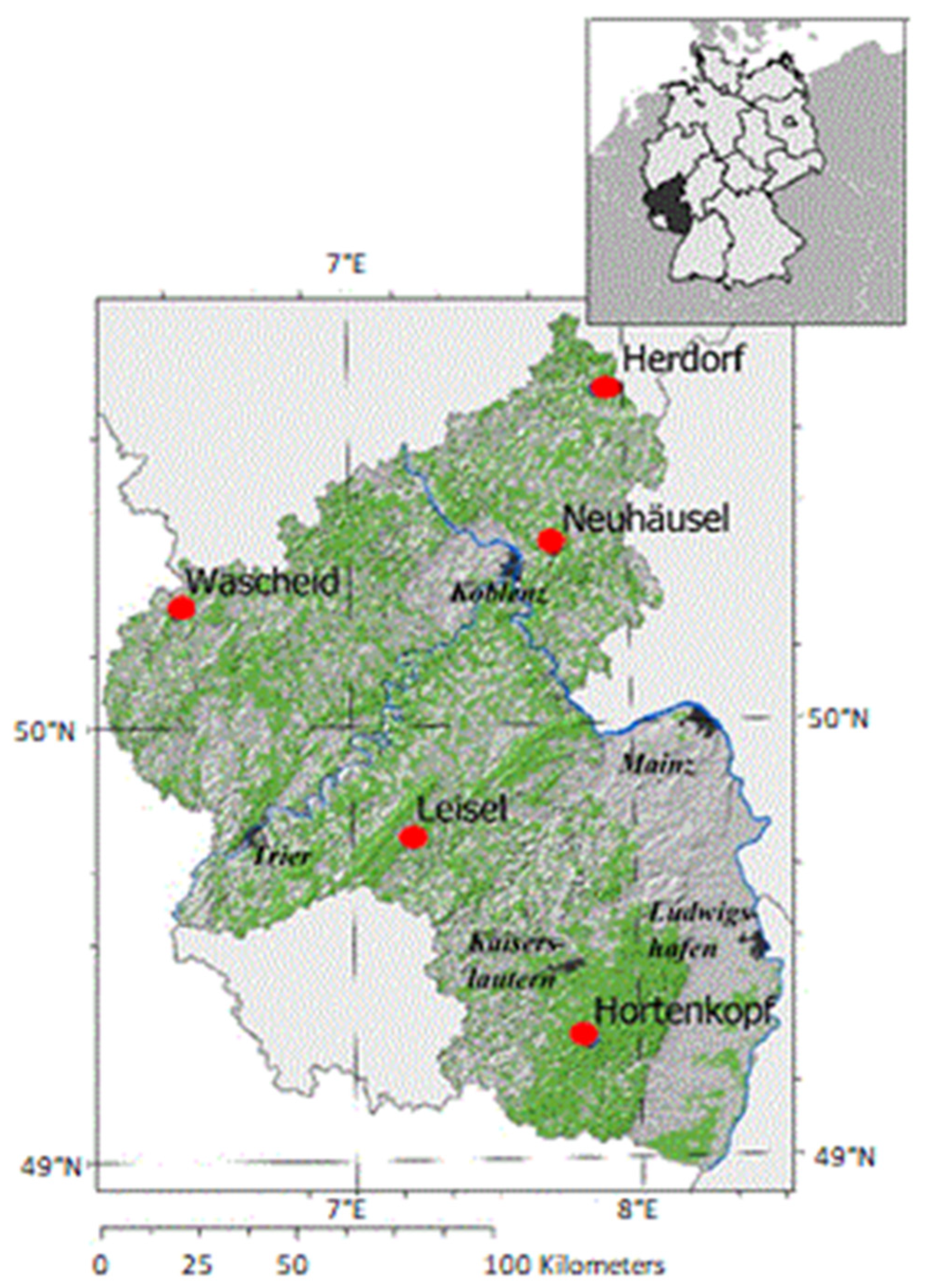
The study was performed in a network of five permanent low-altitude mountain range forest sites (Hortenkopf, Neuhäusel, Herdorf, Leisel, and Wascheid) in Western Germany (Figure 1, Table 1). The investigations focused on the most frequent tree species found in Western Germany, i.e., Norway spruce (Picea abies) and European beech (Fagus sylvatica) showing growth reduction and extensive mortality since few years [43]. The long-term measurements (1998–2019) of air pollutants concentrations and meteorological parameters were performed in open field monitoring stations surrounded by forests in Rhineland-Palatinate nearby ICP (International Co-operative program) Level II Plots. The forest plots are managed as ICP Level II plots according to the ICP Forest criteria [44]. The Environmental Agency of Germany’s federal state Rhineland-Palatinate operates the ZIMEN (Zentrales Immissionmessnetz Rheinland-Pfalz) network gathering meteorological and air quality monitoring stations [45] since 1978. In total, there are 26 monitoring stations in urban, rural and forest areas of the federal state Rhineland-Palatinate to monitor meteorological parameters such as wind speed and wind direction at a height of 10 m, air temperature (T; °C), relative humidity (RH; %), precipitation (p; mm), vapor pressure deficit (VPD; kPa) and global radiation (GR; W m−2). These parameters were recorded at standard height above ground level (i.e., 1 m for p, and 3 m above ground level for GR, T and RH needed for VPD calculation). The monitoring stations continuously record the concentrations of O3, nitrogen dioxide (NO2), nitrogen monoxide (NO), sulfur dioxide (SO2) and methane (CH4) in ambient air at a height of 3 m above the ground level [46,47]. Meteorological data for the five forest sites were gathered from all stations within 1 km2 around each site (so-called raster data) and processed by the climatic competency center Rhineland Palatinate (Klimakompetenzzentrum Rheinland-Pfalz) to generate InterMet data [48]. The atmospheric water balance (AWB; mm) was calculated from the difference between the daily sum of precipitation (p) and daily potential evapotranspiration (PET) during the time series. Moreover, drought (mm) was defined by the sum of daily negative AWB [46] for which the soil water supply decreased below 50% of the field capacity [46].
Figure 1.
Geographical location (red circles) of the twinned forest sites (Level II) and nearby meteorological and air quality monitoring ZIMEN stations in Rhineland-Palatinate (Germany) [46,47].

Table 1.
The main characteristics of ZIMEN stations and their nearby ICP Forest Level II forest plots [47].
2.2. Methods
2.2.1. Radial Growth and Basal Area Increment
The radial growth of trees was measured by using permanent girth band at 1.30 m height, according to the ICP Forest Manual Citation methodology [49], representing the Diameter at Breast Height (DBH). Radial growth data from 70 trees of European beech (Fagus sylvatica) and 250 trees of Norway spruce (Picea abies) were analyzed (Table 2).

Table 2.
Extent of the dataset from 1998–2019.
Annual forest growth, linked to the Basal Area Increment (BAI) of each tree, is the change in cross-sectional area at breast height associated with each annual ring [50]. When BAI is estimated from ring-width series, a value for the tree diameter is required. This diameter is ideally measured in the field but can also be estimated as the sum of the annual ring widths. Basal area was calculated with the help of Equation (1).
where DBH is the diameter at breast height before start of the growing season (in cm). For a discrete year Y, BAI = Basal area (Y)–Basal area (Y − 1). The BAI was taken as a proxy for radial growth of the sampled trees [21,51,52,53] which represent the whole forest stand.
2.2.2. Fructification
Fructification has an influence on biomass growth [54]. In beech, annual fructification was determined by visual assessment of fruit scars backwards on the branches collected for nutrient analysis. In Norway spruce cones were estimated during the annual visual assessment [49]. Finally, the annual fructification severity was recorded as four scores (0 = absent, 1 = scarce, 2 = common and 3 = abundant fructification) for selected tree sample (Table 2). If the data could not be recorded, because not easily recognisable and assessable due to obstacles, they are recorded as missing values (Table S1). In the case of the Hortenkopf site, fructification data are only available since 2008. In contrast to the metric increment data, the fructification observations are on an ordinal scale level. For correlation analysis, we calculated for each plot and each year an averaged fructification severity (mean fructification scoring) by joining all trees of the plot.
2.2.3. Ozone Flux
The Phytotoxic Ozone Dose (PODY, in mmol m−2 per leaf area) with an hourly uptake threshold Y = 1 nmol O3 m−2 per leaf area s−1, as recommended by CLRTAP (2017) [36], was calculated from O3 concentrations and meteorological measurements for the five forest sites in western Germany by using the DO3SE model [55] and applying the Continental Central European parameterization for European beech and Norway Spruce [36]. The methodology is described in detail in [47]. This DO3SE model has been already validated against in-situ observations for different forest species at several locations across Europe, with correlation coefficients ranging from 0.65 to 0.80 (e.g., Nunn et al., 2005; Fares et al., 2014) [56,57]. The yearly POD1 values, calculated at the 5 sites between 1998 and 2019, are presented in Table S2.
2.2.4. Comparison to Actual Yield Tables
Bender et al. (2015) [58] suggested the year 1980 as reference for O3 levels to estimate more realistic yield losses (Table S3). To assess the trees growth between 1998 and 2019 within our plots, the growth data were compared with the yield tables provided by the Northwest German Forest Research Institute [59]. As growth performance, we considered the annual BAI calculated from permanent girth band measurements (Table S3). To assess the forest growth, a comparison of the BAI and DBH with yield tables was carried out during the 22-year time series. As large area yield table values do not correspond exactly with measured growth parameters, due to different competition conditions, site-specific water and nutrient balance and genotypes, the growth trends for the mean DBH and the basal area per tree were compared (Figures S3 and S4). Differing regression coefficients between the growth trend of the yield tables and the DBH and basal area values measured in the field are used as an indicator for poorer or better growth.
2.2.5. Statistical Analyses
For all data, i.e., the fructification, POD1, yield table values, the mean basal area (BA) and the mean DBH, we applied non-parametric statistical tests that are more suitable for non-normally distributed data, missing data, and extreme values, which are frequently encountered in environmental time series [60]. These tests can also be applied to small datasets whose distribution shape cannot be specified with certainty. A 10-year time series of environmental data is long enough to assess short-term trends [60,61]. To detect changes in POD1, BAI and fructification severity, the non-parametric Mann-Kendall test, coupled to the Sen’s slope estimator, was used [60] as well as the Spearman’s rho test. Statistical significance was set at p < 0.05. Statistical analyses were performed with Excel, SPSS, and R software. A principal component analysis (PCA) was used to analyze the dependence among variables, and applied to yearly data [62,62]: BAI, fructification severity, POD1, daytime mean O3 concentration, global radiation (GR), air temperature (T), soil water content (SWC), drought duration, atmospheric water balance (AWB) and the elevation of forest sites. The random forest analysis (RFA) is a method for classification [10] and was applied to rank the importance of variables in a regression or classification [47,61]. The RFA was carried out to understand and determine the importance of each variable, averaged over the growing season, in determining growth and fructification.
3. Results
3.1. POD1 and Potential Biomass Loss
The highest POD1 (29.7 mmol O3 m−2 per leaf area) was registered at Leisel in 2007, and the lowest POD1 (12.6 mmol O3 m−2 per leaf area) was measured at Hortenkopf in 2019 (Table S2). Over the period 1998–2019, POD1 significantly decreased over time: −0.56, −0.26, and −0.24 mmol m−2 year−1 in Hortenkopf, Herdorf, and Wascheid, respectively (Table 3). A not significant (p > 0.1) downward trend in POD1 was observed in Leisel (−0.11 mmol m−2 year−1).

Table 3.
Mean values, standard deviations (± SD), and annual trends (Mann-Kendall test) of stomatal ozone flux (POD1), maximum potential biomass loss (a) and potential biomass loss in beech and Norway spruce for all sites with the 1980 ozone reference (b) over the time period 1998–2019 (significance level: *** p< 0.001; * 0.01 < p < 0.05).
The potential biomass loss in beech and Norway spruce for all sites during the time period 1998–2019 are presented in Table S3, and there is no significant difference between O3 flux and biomass loss. The potential maximum reduction rate of biomass (Table 3) on beech stands (on average 15.2% ± 3.2%) is higher than on Norway spruce stands (on average 3.0% ± 0.8%). Based on POD1 values, the highest reduction rate was observed in 2000 at Hortenkopf and the lowest reduction occurred in 2018 at Herdorf (Table S3). Higher reductions were observed in wet years (i.e., 2000, 2007, 2008) and lower reductions occured in drier years, e.g., 2018 and 2019 (Table S3). Based on baseline O3 levels (year 1980), the maximum reduction rates were observed in the same years, while the averaged reduction rate are lower, i.e., 7.9% ± 3.2% for beech and 1.7% ± 0.8% for Norway spruce (Table 3).
3.2. Basal Area Increment
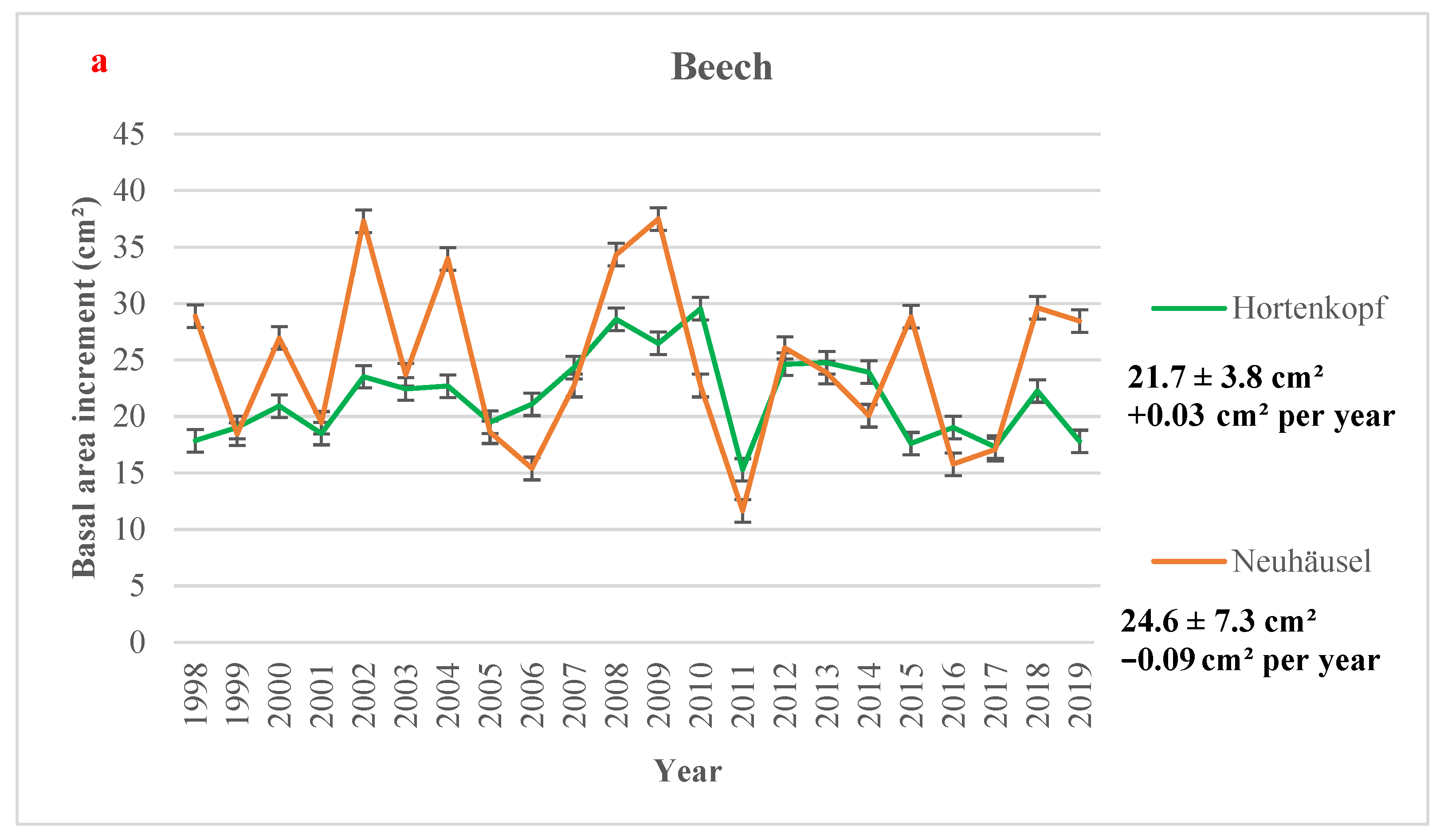
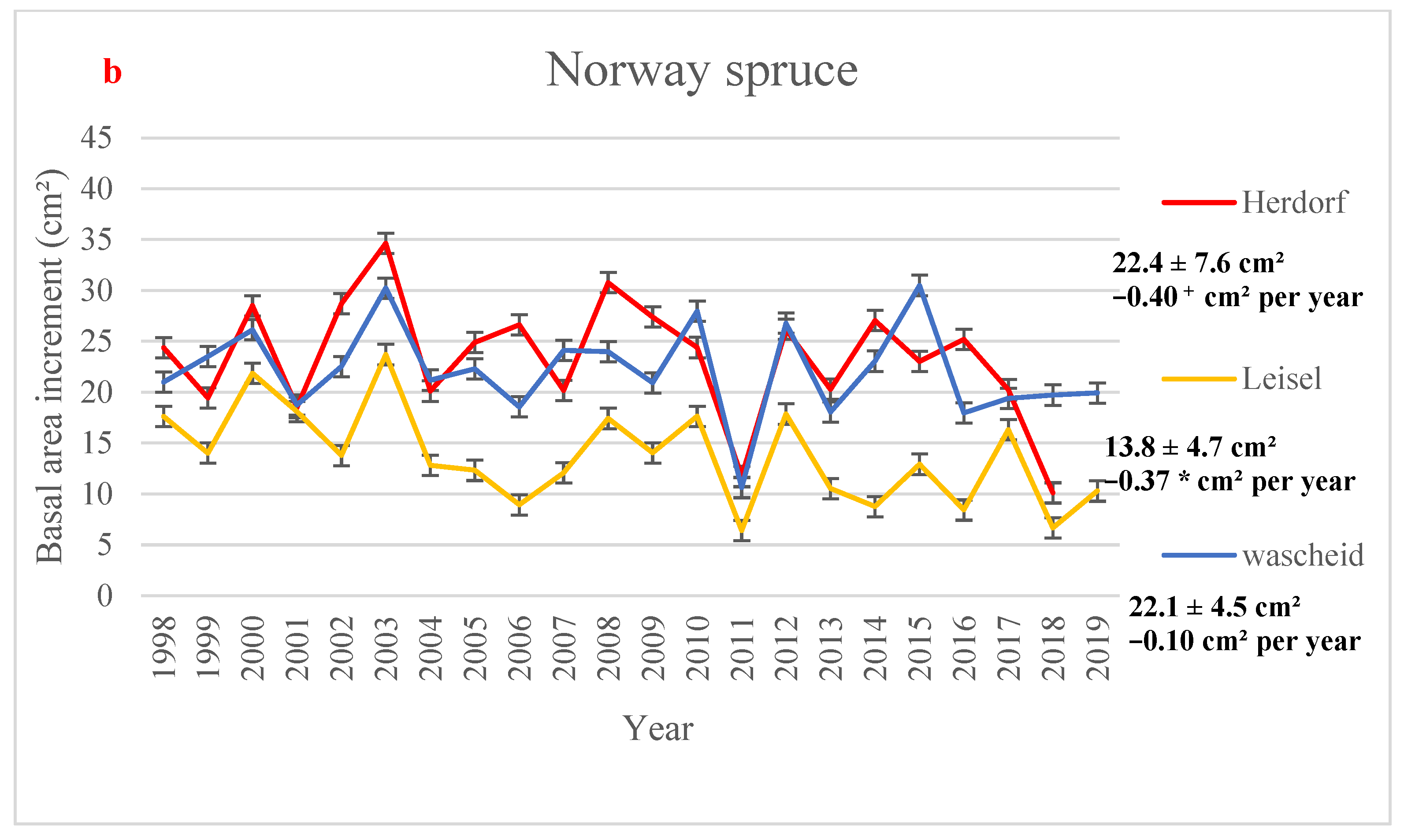
The highest yearly mean BAI value for beech sites (37.49 cm2) was observed at Neuhäusel in 2009, and the lowest value (11.66 cm2) was reported at Neuhäusel in 2011 (Figure 2a). In Norway spruce sites, the lowest value was found at the oldest site, i.e., Leisel (6.41 cm2) in 2011, and the highest growth value (34.64 cm2) was measured at Herdorf in 2003 (Figure 2b). In all sites, the lowest BAI increment is observed in 2011, one of the driest years at all forest sites (Table S7), and no significant temporal trend in BAI was observed, except in Leisel (Figure 2). Figure 2 shows higher BAI values in some wet years (e.g., 2002, 2007, 2008). Over the time period 1998–2019, BAI significantly decreased over time in Leisel (−0.37 cm2 per year) while not significant downward trends were observed in Neuhäusel, Wascheid, and Leisel (Figure 2). A slight BAI increase (p > 0.1) was found in Hortenkopf (+0.03 cm2 year−1).
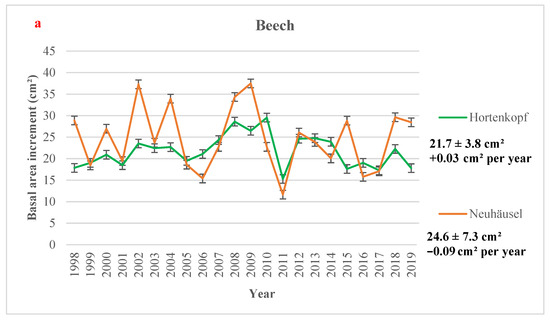
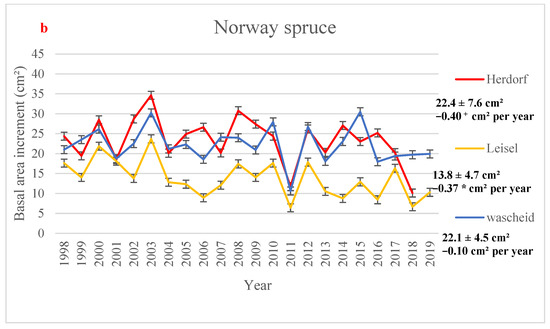
Figure 2.
Yearly mean values of basal area increment (in cm2) with 95% confidence intervals (a) European beech and (b) Norway spruce monitoring sites, averaged over the time period 1998–2019. Mean values, standard deviations (±SD), and the annual trends (Mann-Kendall test) in Basal Area Increment (in cm2 per year) is showed for all sites over the time period 1998–2019. Significance level: * 0.01 < p < 0.05; + 0.05 < p < 0.1; or p > 0.1.
3.3. Fructification
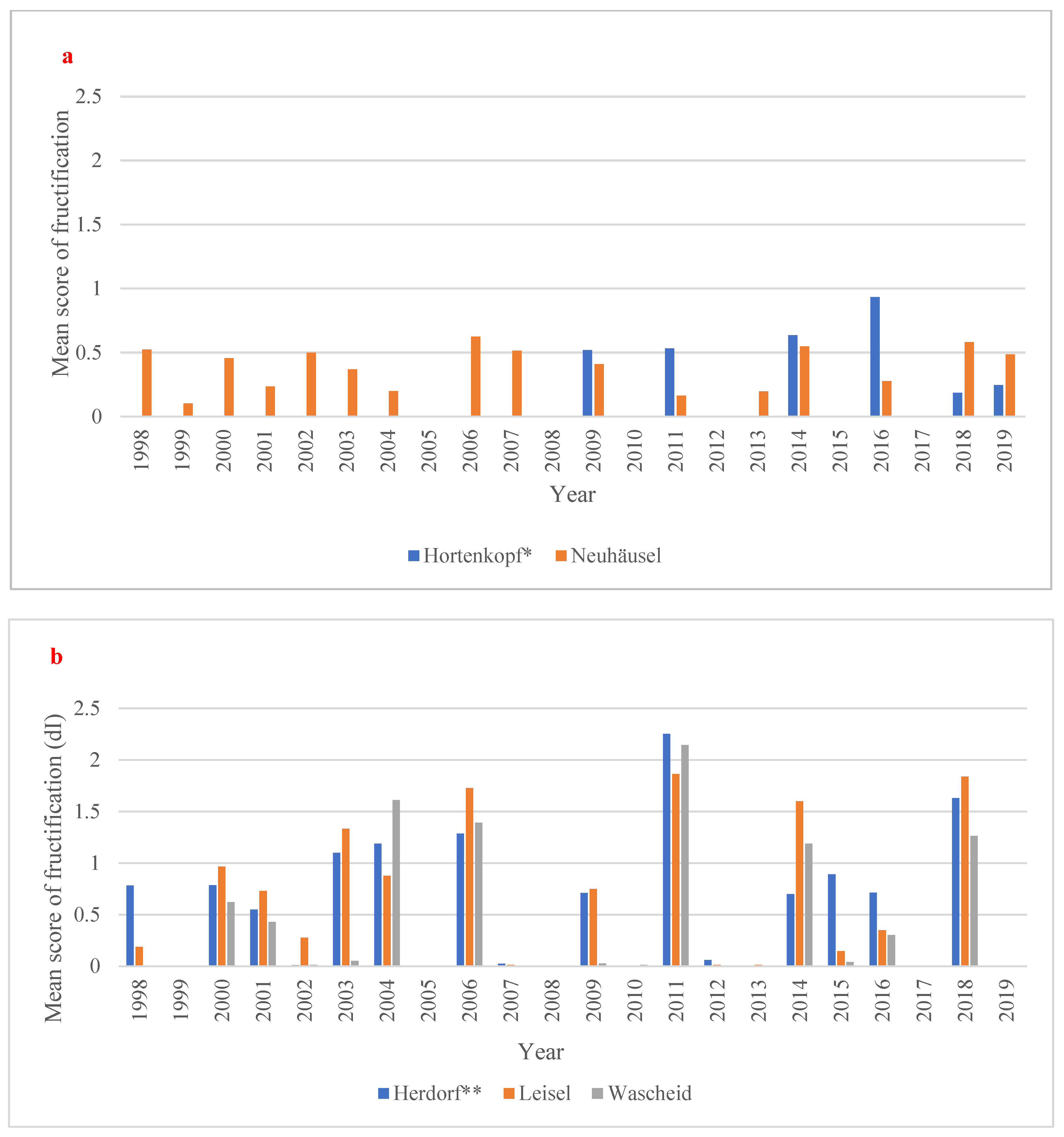
Among the European beech sites, the years 2014 and 2016 have displayed so-called “mast years” (i.e., when trees produce a bumper crop of their fruits) with the highest fructification for Hortenkopf as young beech population (Figure 3a, Table S6). Moreover, the year 2011 is a particular “mast year” with the highest number of dry days (125 days in total; Table S7a during the available data for fructification. In Neuhäusel, the year 2006 is a “mast year” (mean fructification scoring: 0.62), associated to a high level of maximum cumulative water deficit (−74.90 mm; Table S7a). Furthermore, the years 2014, 2018 and 2019 showed particular “mast years”, especially in 2018 (mean fructification scoring: 0.58) with the longest number of dry days (93 days in total; Table S6a) and the highest max cumulative water deficit (−119.80 mm; Table S7a). For Norway Spruce, the years 2003, 2004, 2006, and 2014 showed a higher fructification severity (mast years). At Leisel, the fructification in 2006 and 2014 is high (Figure 3b; Table S6). The highest mean score of fructification (=2.25) was observed in Herdorf for the year 2011 associated to a max cumulative water deficit (−67.70 mm; Table S7b). A similar observation was made by Leisel in 2011 with a high fructification scoring (=1.86) and a max cumulative water deficit (−74.70 mm; Table S7b). During the drier years (e.g., 2003, 2006, 2011, 2016, 2018 and 2019), we observed heavy fructification of spruce (on average, score of 0.60), while in most humid years (e.g., 1999, 2007, 2008 and 2010), low level or no fructification was observed (Figure 3b). Over the time period 1998–2019, a null trend in fructification severity was observed in all forest sites.
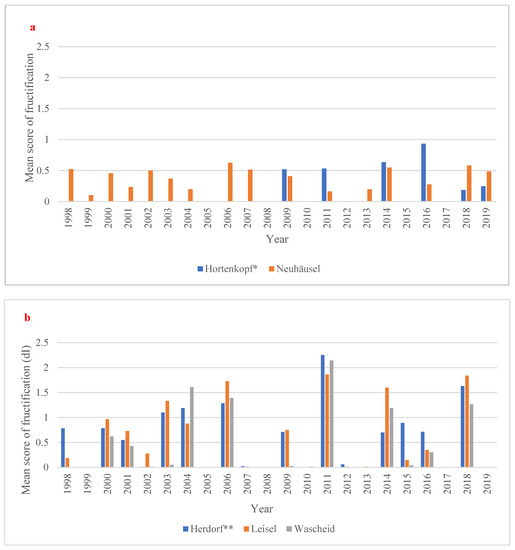
Figure 3.
Mean score of fructification (0 = absent, 1 = scarce, 2 = common, and 3 = abundant fructification, dimensionless) for (a) European beech and (b) Norway spruce sites over the time period 1998-2019. * no data fructification data from 1998 to 2007 and 2012 and 2013 available. ** no fructification data for 2019 available.
3.4. PCA Analysis
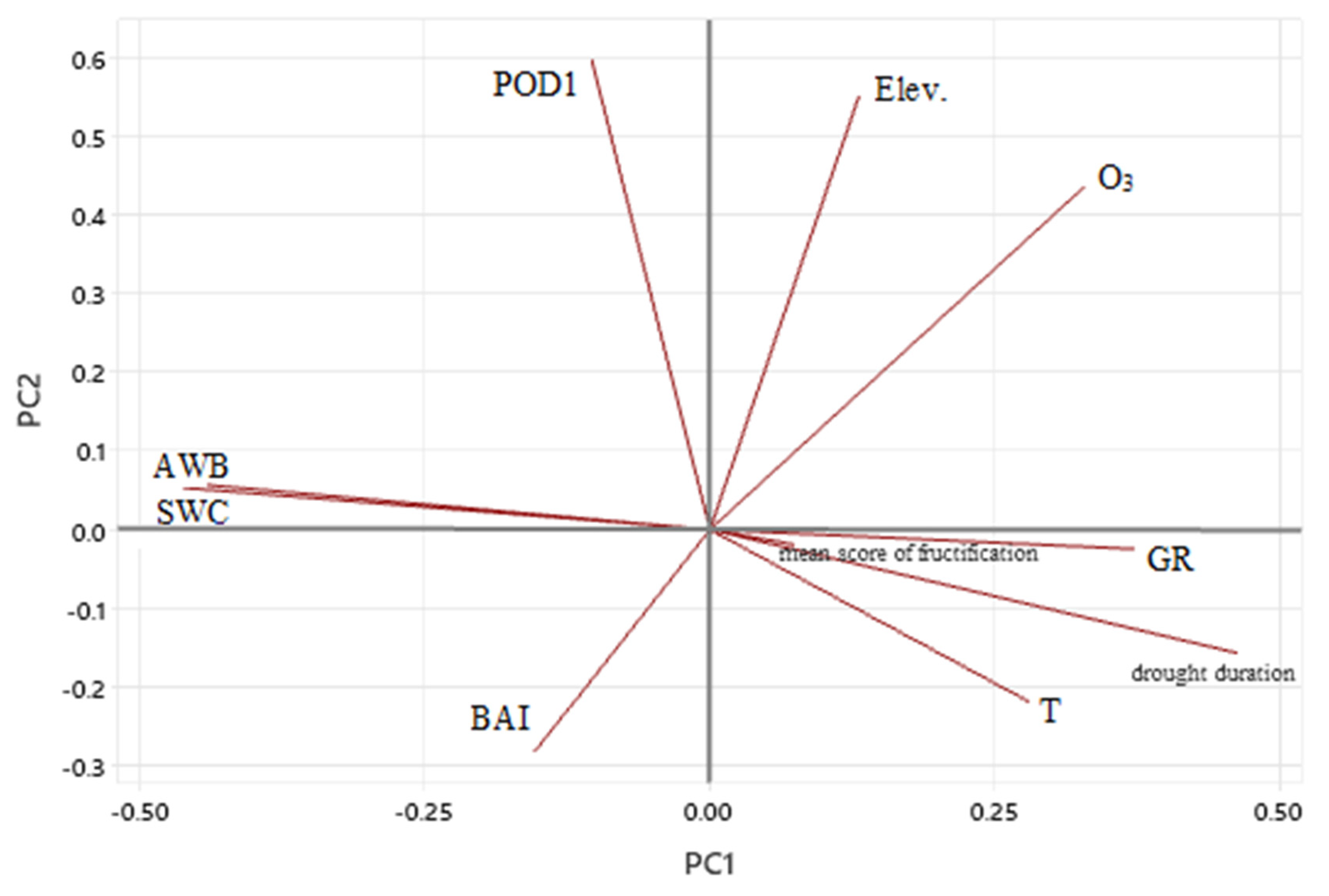
The first and second axes justified the most significant changes in eigenvalues, i.e., 34.9% and 56.4% of the accumulated variance, respectively (Tables S4 and S5). Fructification is more linked to GR, T, and drought duration than other meteorological parameters, and point in the same direction. The low angle is a sign of the high correlation between these parameters and the first axis (Figure 4). Moreover, there were very long vectors, such as drought duration and SWC, that highlight the importance and high contribution of the first axis as compared to vectors with shorter length like mean score of fructification. POD1 and O3 concentration are closer to orthogonality, which is associated with independency (Figure 4) and site elevation, and BAI is more linked to lower elevation, POD1, and a little bit more to water availability. BAI and T as well as drought duration are correlated neither to axis 1 nor to axis 2 (Figure 4).
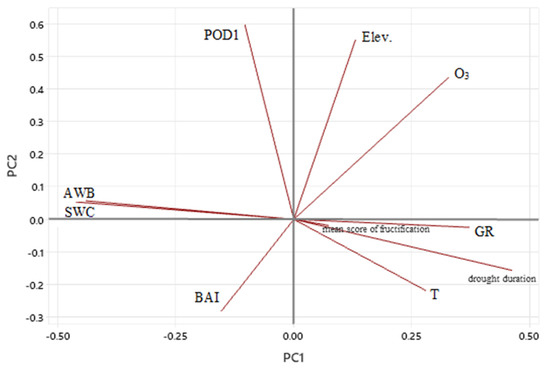
Figure 4.
Principal Component Analysis at five forest sites in Rhineland-Palatinate (Germany) over the time period 1998–2019 between following annual data: daytime ozone mean concentration (O3; ppb), Phytotoxic Ozone Dose over a threshold 1 nmol m−2 per leaf area s−1 (POD1; mmol O3 m−2), basal area increment (BAI, cm2), mean score of fructification, atmospheric water balance (AWB; mm), soil water content (SWC; %), air temperature (T; °C), global radiation (GR; W m−2), drought duration (number of days), and elevation (Elev., m above sea level).
3.5. RFA Analysis
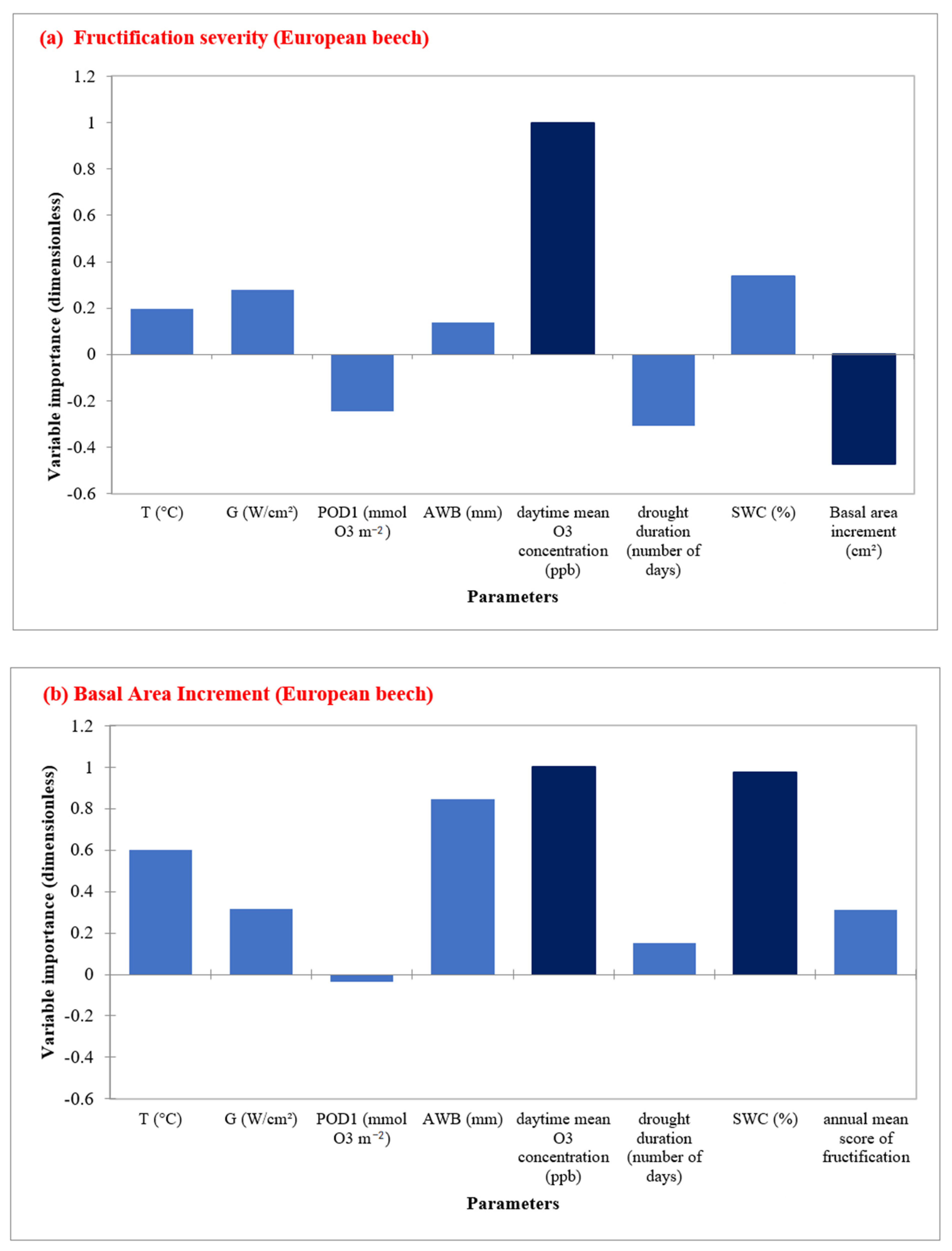
By performing a RFA for the European beech sites, the most important factors affecting fructification severity were the BAI and annual daytime O3 mean concentration (Figure 5a, Table S8), while SWC and daytime O3 mean concentrations were the most important predictors for radial growth (Figure 5b). Regarding feature importance, BAI, drought duration, and POD1 showed a negative impact on the fructification prediction. For radial growth prediction, AWB was the third important factor (Figure 5b, Table S9). For European beech, POD1 and drought duration were not important factors for radial growth.
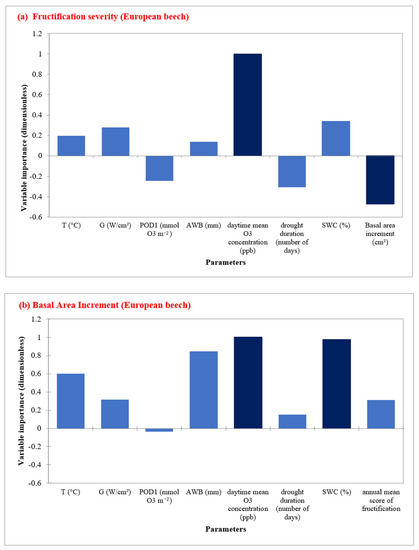
Figure 5.
Random Forest Analysis for European beech showing the most important predictors in determining (a) fructification severity, and (b) Basal area increment in five sites during the time period 1998–2019.
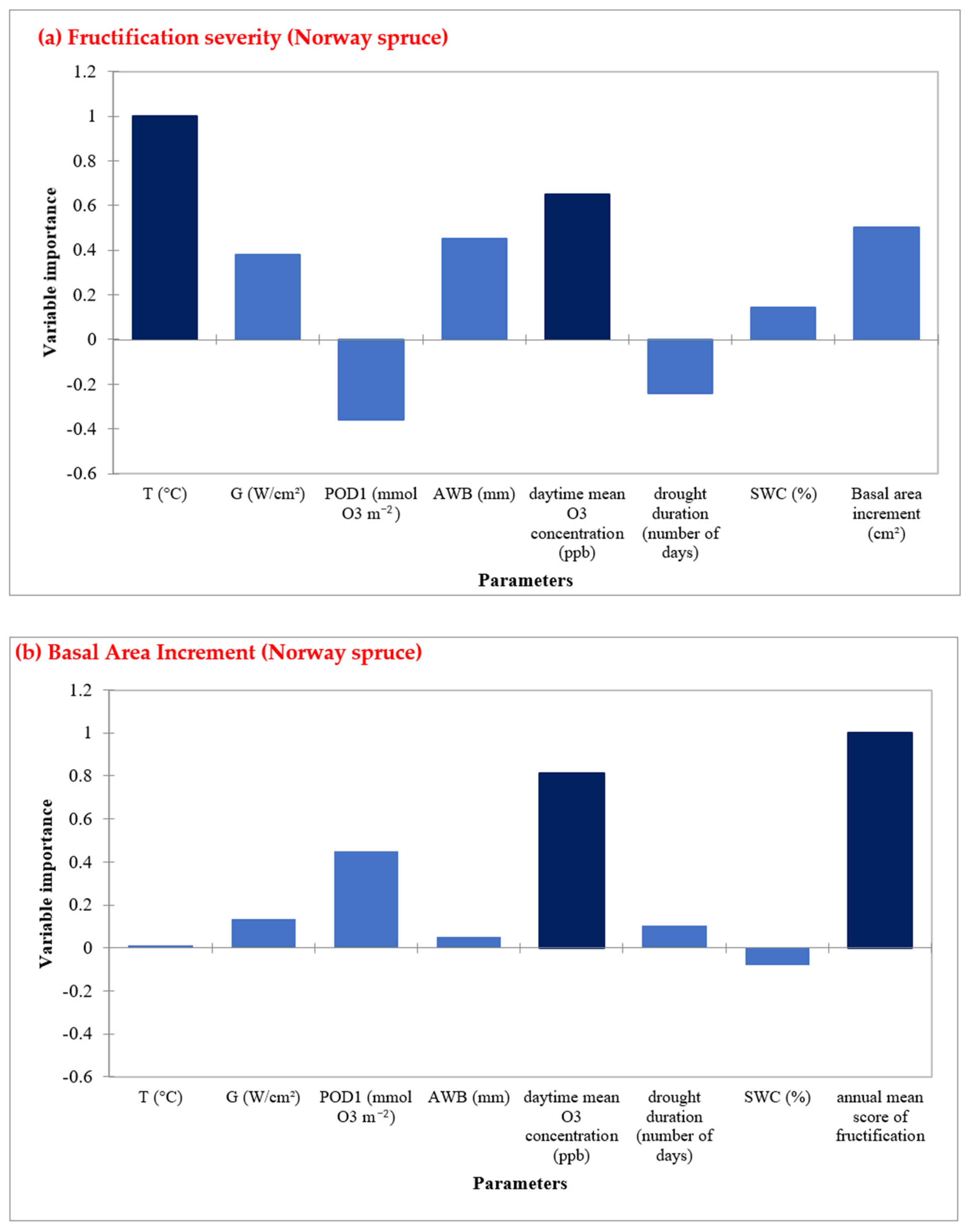
In Norway spruce sites, annual daytime O3 mean concentrations and air temperature are the most important factors in determining fructification (Figure 6a, Table S8). Moreover, fructification severity and O3 mean concentrations are the most important factors for radial growth (Figure 6b, Table S9). Similarly to European beech, drought duration and POD1 showed a negative impact on the fructification prediction. For radial growth prediction, POD1 was the third important factor (Figure 5b).
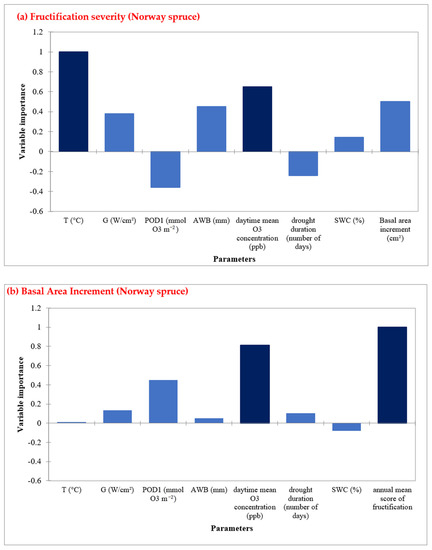
Figure 6.
Random Forest Analysis for Norway spruce, showing the most important predictors in determining (a) fructification, and (b) Basal area increment in five sites during the time period 1998–2019.
3.6. Comparison with Yield Tables
Figures S3 and S4 show the linear regression between yield table values (in 5-year steps) and measured values every year for mean DBH and basal area per tree. The slope of the trend line for measured DBH is smaller than the slope of DBH, except for spruce in Herdorf. The trend in BA is less significant compared to DBH. The slopes of BA growth are higher for the sites Herdorf and Hortenkopf, i.e., these stands grow faster than expected by yield table values (Table 4). For other sites, the slopes for measured values are smaller, i.e., they showed a slighter growth over time than expected. For both growth parameters, a slump in growth can be seen on all areas in 2011. The year 2011 was a meteorologically deviating year with severe drought in spring. The lowest growth trend can be found in Neuhäusel. At this site, the slopes of the measured and expected growth trends from the yield tables differ the most. The stands in Herdorf and Neuhäusel as well as Leisel were so severely damaged by drought stress and/or bark beetle infestation (Herdorf and Leisel) in the dry years 2019, 2020, and 2021 that the increment measurements were abandoned due to the lack of healthy dominant trees in 2020 (Herdorf) and 2021 in Leisel and Neuhäusel. It will take several decades until representative increment measurements for forest monitoring of adult stands are possible again.

Table 4.
Regression analysis of Brest Height Diameter (cm). The regression equations are the result of a trend analysis between BHD or Basal Area Increment (BAI) of the specific age and yield power (site quality class defined by height of dominant trees) which are compared with the regression equation during the same time span of measured DBH or BAI. Afterwards the significance (p ≤ 0.05) of the slopes was determined using a t-test between the Expected Growth (Yield Table) and the Measured Growth values.
4. Discussion
Between 1998 and 2019, the study revealed significant decrease in POD1 (except in Leisel) over time by on average 0.31 mmol m−2 year−1. A significant decline in BAI, i.e., a decrease in radial growth, was observed in the oldest site (Leisel, 137-year-old). A decline of annual growth in mature Fagus sylvatica trees over the last half-century was previously reported across Europe [24,27,33].
The effects of various parameters of water supply (e.g., drought, SWC, and VPD) were investigated by applying a RFA, and SWC was one of the most important predictors of BAI for all sites. These findings are in agreement with previous studies (e.g., Dohrenbusch et al., 2002 and Schäfer et al., 2019 [63,64] in Germany, Sánchez-Salguero et al., 2012 [65] in Spain and Adams and Kolb, 2005 [66] in USA). These results observed a significant reduction in growth rate under drought stress conditions. Granier et al., 2007 [67] have showed in Germany and Castagneri et al., 2014 [68] in Italy that drought and heat waves have a negative impact on trees growth. Agyei et al. (2021) [69] found with eddy covariance techniques that severe drought led to stomatal closure and less stomatal O3 flux, while less harmful non-stomatal O3 deposition on cuticula, bark, soil, and/or water as well as chemical reactions of O3 with NO and volatile organic compounds substantially increase. Particularly, European beech, predominant in lowland forests in Central Europe, showed a greater sensitivity to drought than coniferous sites (Norway spruce) [2]. Another important factor for BAI was the surface O3 mean concentration. Braun et al., 2014 [24] confirmed the growth reduction by O3 in Switzerland. Paoletti et al., 2018 [2] found that by averaging SWC and O3 concentration over more than five years, the effects on growth of European beech disappeared, and thus recommended to use fine time-resolution and to include co-factors, e.g., thinning, tree age, and size, to accurately investigate the drought and O3 effects on trees growth.
In Austria, Vospernik (2021) found that soil type and soil moisture were significant factors for tree growth decline [53]. Growth decreased at dry sites, but very moist and wet sites could result in either a decrease or an increase in growth, depending on the tree species. Picea abies and Fagus sylvatica were two tree species, which were mainly favored by mixture effects. The relationship between BAI and stem-volume increment or biomass annual production was investigated in 30 dominant European beech in northeastern France [51]. The annual growth is influenced by meteorology during the growing season, particularly drought events. To quantify decline, Livingston et al. (2017) [52] calculated changes in hemlock yearly radial growth using BAI measurements to identify periods of growth decline in eastern North American forests. The onset of growth decline periods was predominantly associated with either pest infestation or drought.
Usually the maximum potential biomass loss, due to O3 pollution, is calculated from dose-response relationships for beech and spruce published in Mapping Manual [36]. However, these losses are often high because they are related to O3 free air (charcoal filtered air) experiments, not realistic and not representative of real field conditions [6]. Therefore, a more realistic O3 baseline, established for the year 1980, was suggested to better represent the background conditions with anthropogenic sources, i.e., pollution from industry and traffic [58]. The calculated biomass losses using the dose-response function [36] is unreal high. Even the biomass losses calculated from reference data [58] led to lower but still not convincing and understandable results. This is due to two different reasons. On the one hand, the dose-response curve is related to an (unrealistic) unnatural zero point, namely ozone-free air [38], which never existed during the evolution of tree species, and on the other hand, the fumigation experiments were conducted with trees in their early juvenile phase [38], which show a completely different growth and allocation behaviour of assimilates to adult trees. Juran et al. (2018) [70] found that net ecosystem production (NEP) simulated at low, pre-industrial O3 flux rates (0.5 nmol m−2 s−1) was 24.8% higher as compared to NEP assessed at current (8.3 nmol m−2 s−1) O3 flux rates. Juran et al. (2018) [70] also found that high site-specific variability in O3 concentration affects photosynthetic carbon uptake, as a species-specific sensitivity of stomata to environmental factors is evident. The simultaneous occurrence of other stress factors, especially extreme temperatures and drought, which lead to changes in stomatal conductance and stomatal ozone flux, may also influence the correct response of trees to O3 concentration. The few epidemiological findings, especially, from Switzerland [21,71] (Braun et al., 2017, Braun et al., 2007) support these objections. The application of the dose-response relationship from such fumigation experiments leads to an overestimation of biomass losses due to O3 [14] (Cailleret et al., 2018). The potential maximum reduction rate of biomass on beech stands (on average 15.2%) is higher than on Norway spruce stands (on average 3.0%), corresponding to the differential sensitivity to O3 evidenced by different slopes of the dose-response relationships [36,38].
Comparisons of BAI and DBH with current increment tables [56] showed that increment loss has not occurred or is negligible. It should be noted, however, that the increments included in growth tables come from observations on sage plots that were observed under ambient O3 influence, i.e., already include a normal O3 exposure. Therefore, both methods are not useful for determining growth losses. Only O3 gradients, free air (face) fumigations, or growth models parameterised without or only with low O3 influence provide reasonably plausible results for the O3 influence. However, many such experimental setups hardly take into account the interactions between temperature, O3 concentration, N deposition, and drought. This complex interaction of ecological factors can only be achieved by field observations. The younger stands in Herdorf (Norway spruce) and Hortenkopf (European beech) showed a somewhat higher or similar growth trend as in the Yield tables. Cailleret et al., 2018 [14] considered O3 effects on the growth of European forests to be controversial and negligible. According to Cailleret et al., 2018 [14], physiological aspects will explain impacts caused by O3, while at the higher ecological scale of stand growth other factors, such as drought and nitrogen supply, effects of past land use and forest management, as well as acclimation of trees to site conditions and the resulting competitive conditions (population adaptations and species composition), may interfere and cause a differential response in growth behaviour.
Fructification increases with increasing air temperature and SWC. This means that, during years with high seed production (especially 2011), European beech and Norway spruce show reduced growth, which is in agreement with findings of Braun et al., 2017 and Piovesan et al., 2008 [21,28]. Moreover, other studies have reported reduced diameter and/or height growth of Norway spruce due to fructification [62,72]. However, most studies on the fructification of forest trees are based solely on estimates of the number of fruits or cones [73].
Far-reaching and threatening events, such as bark beetle infestations, or the sequence of extreme drought events in recent years (e.g., 2019 to 2021) have to a collapse of individual stands (Herdorf, Leisel and Neuhäusel). The onset of senescence was predominantly associated with either pests’ infestation or drought. Trees can be debilitated by stress and thus become more susceptible to predator attacks or environmental stress, until they have not enough energy and organic substances to defend against such attacks or environmental conditions.
5. Conclusions
Forest trees exhibit a large variation in BAI, which depends on the individual tree and its interaction with abiotic and biotic factors. Investigations of O3, drought, and fructification on tree growth are essential for forest ecology and forest management, because they determine a big part of tree growth [53]. Under stress like severe drought, trees will grow slower or not at all. Diameter at breast height measured by growth bands on Beech or Spruce is a valuable tool for dendrochronological investigations. To detect and accurately estimate trends in DBH over time, such measurements should be performed annually at monitoring ICP-forest plots on long-term. By interpreting calculated growth loss, effects of drought, fructification and O3-Flux should be considered. Depending on the investment of assimilates in growth, reproduction, defense against parasites or toxic substances (e.g., ozone), growth or storage, in a broader sense of [74], the growth parameter expressed as BAI varies and is not directly causally related to an influencing variable such as O3 flux or drought. A growth decline could be aggravated by projected climate change. To date, some tools or models exist (e.g., dose–response relationship and yield tables) to estimate the tree growth losses. Theoretically, the dose–response function [36] is not a reliable tool for mature trees, because they are established in open-top chamber experiments with young trees showing different growth performance than adult ones [14,38] and the O3 levels are not representative of field conditions. The yield tables represent real world conditions and use the BAI and age of trees to calculate growth loss. However, an assessment by inter-comparison of different approaches and tools is needed at regional scale. By including some additional co-factors (e.g., nutrient supply, biotic interactions) in form of growth models, it would be possible to have more accurate growth results. The present study showed that growth reduction at stand level can hardly be detected, although the modelled O3 fluxes allowed for site-specific differentiation (according to species, age, water, and nutrient supply). Drought led to lower O3 flux. Indeed, even if high O3 concentrations are formed by high temperature, O3 cannot penetrate the plant due to lower leaf conductivity. This means that the chemical part in the atmosphere and the physiological part at the plant level are well understood, but the ecological effects and interactions at higher levels such as at the stand level are not well understood yet [14]. A further step would involve improved, calibrated, and monitored growth models, as well as insight (partitioning) into the energy expenditure of detoxification, defense, and storage. Site-calibrated models, possibly also using stable isotope fractionation (e.g., 13C, 18O, and 2H) for partitioning carbohydrate allocation into defense, growth/accumulation, and reproduction components, may be successful in detecting the influence of drought and pollutants. Future research challenges include model development to expand the sets of site-specific biological, climatic, soil, and O3 data to refine site and species-specific growth model, as well as partitioning carbohydrate allocation. There is also an urgent need for the further development of field-based validation on mature forest stands of the O3 flux-based method to establish robust flux–effect relationships in order to provide valuable critical levels for forest protection against O3 pollution. Therefore, we recommend a large-scale epidemiological investigation applied to biomass losses as measured in real-world forests.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f13081215/s1, Table S1: Missing values for fructification at all sites during the long time series; Table S2: Accumulated O3 flux based on PAW approach with DO3SE model for each year during the long time series in all sites.; Table S3: Annual average, and standard deviation (± SD), of the potential biomass loss in beech and Norway spruce for all sites during the time period 1998-2019, (a) Potential maximum rate of reduction (%) and (b) Potential maximum rate of reduction (%) critical level compared to before 1980 (the highest values are in red and the lowest values in blue); Table S4: Eigenvectors of the PCA ordination in Figure 3; Table S5: Eigenvalue and proportions of Eigenvalues from PCA in Figure 3; Table S6: Annual fructification severity was recorded as four scores (0 = absent, 1 = scarce, 2 = common and 3 = abundant fructification) for each individual tree. For each year and site, we have calculated an averaged fructification severity by joining all trees of the plot (mean fructification scoring, dimensionless); Table S7: Number of drought duration and max cumulative water deficit for (a) Beech and (b) Norway spruce during the long time series (from 1998 to 2019); Figure S1: Scattergram of POD1 and Basal Area increment. The annual points are shown as pie charts of the fructification frequency classes: Frequency of fructification (absent in light green, scarce in dark green, common in orange and abundant in red color highlighted for the years 1998 to 2019; Figure S2: Scattergram of drought duration and Basal Area increment. The annual points are shown as pie charts of the fructification frequency classes: frequency of fructification (absent in light green, scarce in dark green, common in orange and abundant in red color highlighted for the years 1998 to 2019; Figure S3: Mean BHD per tree; and Figure S4: Mean Basal area per tree; Table S8: Results of Random Forest Analysis (RFA) showing the most important predictors in determining fructification; Table S9: Results of Random Forest Analysis (RFA) showing the most important predictors in determining Basal area increment.
Author Contributions
Conceptualization, H.E., W.W. and P.S.; methodology, H.E., W.W. and P.S.; software, H.E, W.W. and P.S.; formal analysis, H.E.; investigation, H.E. and W.W.; writing—original draft preparation, H.E.; writing—review and editing, W.W., A.D.M. and P.S.; visualization, H.E. and W.W.; supervision, W.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The publication was funded by the Open Access Fund of University Trier and the German Research Foundation (DFG) within the Open Access Publishing funding programme.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Joachim Block, Hans-Werner Schröck, and Martin Greve from the Forschungsanstalt für Waldökologie und Forstwirtschaft Rheinland-Pfalz, Germany (FAWF) for providing the data used in this publication, discussions and their patience and ongoing interest in this topic, as well as Philipp Reiter (Klima-Kompetenz-Zentrum Rheinland-Pfalz) for providing meteorological data on Level II stations. The authors also gratefully acknowledge the ICP Vegetation and ICP Forest.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Panek, J.; Saah, D.; Esperanza, A.; Bytnerowicz, A.; Fraczek, W.; Cisneros, R. Ozone distribution in remote ecologically vulnerable terrain of the southern Sierra Nevada, CA. Environ. Pollut. 2013, 182, 343–356. [Google Scholar] [CrossRef]
- Paoletti, E.; De Marco, A.; Anav, A.; Gasparini, P.; Pompei, E. Five-year volume growth of European beech does not respond to ozone pollution in Italy. Environ. Sci. Pollut. Res. 2018, 25, 8233–8239. [Google Scholar] [CrossRef] [PubMed]
- Proietti, C.; Fornasier, M.F.; Sicard, P.; Anav, A.; Paoletti, E.; De Marco, A. Trends in tropospheric ozone concentrations and forest impact metrics in Europe over the time period 2000–2014. J. For. Res. 2021, 32, 543–551. [Google Scholar] [CrossRef]
- De Marco, A.; Anav, A.; Sicard, P.; Feng, Z.; Paoletti, E. High spatial resolution ozone risk-assessment for Asian forests. Environ. Res. Lett. 2020, 15, 104095. [Google Scholar] [CrossRef]
- Anav, A.; De Marco, A.; Proietti, C.; Alessandri, A.; Dell’Aquila, A.; Cionni, I.; Vitale, M. Comparing concentration-based (AOT40) and stomatal uptake (PODY) metrics for ozone risk assessment to European forests. Glob. Chang. Biol. 2016, 22, 1608–1627. [Google Scholar] [CrossRef] [PubMed]
- Sicard, P.; Serra, R.; Rossello, P. Spatiotemporal trends in ground-level ozone concentrations and metrics in France over the time period 1999–2012. Environ. Res. 2016, 149, 122–144. [Google Scholar] [CrossRef] [PubMed]
- Anav, A.; De Marco, A.; Friedlingstein, P.; Savi, F.; Sicard, P.; Sitch, S.; Paoletti, E. Growing season extension affects ozone uptake by European forests. Sci. Total Environ. 2019, 669, 1043–1052. [Google Scholar] [CrossRef]
- Sicard, P.; Anav, A.; De Marco, A.; Paoletti, E. Projected global ground-level ozone impacts on vegetation under different emission and climate scenarios. Atmos. Chem. Phys. 2017, 17, 12177–12196. [Google Scholar] [CrossRef] [Green Version]
- Moura, B.B.; Alves, E.S.; Marabesi, M.A.; de Souza, S.R.; Schaub, M.; Vollenweider, P. Ozone affects leaf physiology and causes injury to foliage of native tree species from the tropical Atlantic Forest of southern Brazil. Sci. Total Environ. 2018, 610, 912–925. [Google Scholar] [CrossRef] [PubMed]
- Sicard, P.; De Marco, A.; Carrari, E.; Dalstein-Richier, L.; Hoshika, Y.; Badea, O.; Paoletti, E. Epidemiological derivation of flux-based critical levels for visible ozone injury in European forests. J. For. Res. 2020, 31, 1509–1519. [Google Scholar] [CrossRef]
- Hoshika, Y.; Fares, S.; Savi, F.; Gruening, C.; Goded, I.; De Marco, A.; Paoletti, E. Stomatal conductance models for ozone risk assessment at canopy level in two Mediterranean evergreen forests. Agric. For. Meteorol. 2017, 234, 212–221. [Google Scholar] [CrossRef]
- Hoshika, Y.; Watanabe, M.; Kitao, M.; Häberle, K.H.; Grams, T.E.; Koike, T.; Matyssek, R. Ozone induces stomatal narrowing in European and Siebold’s beeches: A comparison between two experiments of free-air ozone exposure. Environ. Pollut. 2015, 196, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Proietti, C.; Anav, A.; De Marco, A.; Sicard, P.; Vitale, M. A multi-site analysis on the ozone effects on Gross Primary Production of European forests. Sci. Total Environ. 2016, 556, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cailleret, M.; Ferretti, M.; Gessler, A.; Rigling, A.; Schaub, M. Ozone effects on European forest growth—Towards an integrative approach. J. Ecol. 2018, 106, 1377–1389. [Google Scholar] [CrossRef]
- Chappelka, A.H.; Samuelson, L.J. Ambient ozone effects on forest trees of the eastern United States: A review. New Phytol. 1998, 139, 91–108. [Google Scholar] [CrossRef]
- Skärby, L.; Ro-Poulsen, H.; Wellburn, F.A.; Sheppard, L.J. Impacts of ozone on forests: A European perspective. New Phytol. 1998, 139, 109–122. [Google Scholar] [CrossRef]
- Karnosky, D.F.; Werner, H.; Holopainen, T.; Percy, K.; Oksanen, T.; Oksanen, E.; Matyssek, R. Free-air exposure systems to scale up ozone research to mature trees. Plant Biol. 2007, 9, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Wittig, V.E.; Ainsworth, E.A.; Naidu, S.L.; Karnosky, D.F.; Long, S.P. Quantifying the impact of current and future tropospheric ozone on tree biomass, growth, physiology and biochemistry: A quantitative meta-analysis. Glob. Chang. Biol. 2009, 15, 396–424. [Google Scholar] [CrossRef]
- Matyssek, R.; Bytnerowicz, A.; Karlsson, P.E.; Paoletti, E.; Sanz, M.; Schaub, M.; Wieser, G. Promoting the O3 flux concept for European forest trees. Environ. Pollut. 2007, 146, 587–607. [Google Scholar] [CrossRef]
- Manning, W.J. Establishing a cause-and-effect relationship for ambient ozone exposure and tree growth in the forest: Progress and an experimental approach. Environ. Pollut. 2005, 137, 443–454. [Google Scholar] [CrossRef]
- Braun, S.; Schindler, C.; Rihm, B. Growth trends of beech and Norway spruce in Switzerland: The role of nitrogen deposition, ozone, mineral nutrition and climate. Sci. Total Environ. 2017, 599, 637–646. [Google Scholar] [CrossRef]
- Nabuurs, G.J.; Lindner, M.; Verkerk, P.J.; Gunia, K.; Deda, P.; Michalak, R.; Grassi, G. First signs of carbon sink saturation in European forest biomass. Nat. Clim. Chang. 2013, 3, 792–796. [Google Scholar] [CrossRef]
- Martinez del Castillo, E.; Zang, C.S.; Buras, A.; Hacket-Pain, A.; Esper, J.; Serrano-Notivoli, R.; de Luis, M. Climate-change-driven growth decline of European beech forests. Commun. Biol. 2022, 5, 163. [Google Scholar] [CrossRef]
- Braun, S.; Schindler, C.; Rihm, B. Growth losses in Swiss forests caused by ozone: Epidemiological data analysis of stem increment of Fagus sylvatica L. and Picea abies Karst. Environ. Pollut. 2014, 192, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Charru, M.; Seynave, I.; Morneau, F.; Bontemps, J.D. Recent changes in forest productivity: An analysis of national forest inventory data for common beech (Fagus sylvatica L.) in north-eastern France. For. Ecol. Manag. 2010, 260, 864–874. [Google Scholar] [CrossRef]
- Gschwantner, T. Zuwachsänderungen nach den Daten der Österreichischen Waldinventur und ihre klimatischen Ursachen: Abdruck der im April 2004 approbierten Dissertation, no. 133. Ph.D. Thesis, Bundesforschungs-und Ausbildungszentrum für Wald, Naturgefahren und Landschaft, Wien, Austria, 2006. [Google Scholar]
- Jump, A.S.; Hunt, J.M.; Penuelas, J. Rapid climate change-related growth decline at the southern range edge of Fagus sylvatica. Glob. Change Biol. 2006, 12, 2163–2174. [Google Scholar] [CrossRef] [Green Version]
- Piovesan, G.; Biondi, F.; Filippo, A.D.; Alessandrini, A.; Maugeri, M. Drought-driven growth reduction in old beech (Fagus sylvatica L.) forests of the central Apennines, Italy. Glob. Chang. Biol. 2008, 14, 1265–1281. [Google Scholar] [CrossRef]
- Wipfler, P.; Seifert, T.; Heerdt, C.; Werner, H.; Pretzsch, H. Growth of adult Norway spruce (Picea abies [L.] Karst.) and European beech (Fagus sylvatica L.) under free-air ozone fumigation. Plant Biol. 2005, 7, 611–618. [Google Scholar] [CrossRef] [Green Version]
- Dittmar, C.; Zech, W.; Elling, W. Growth variations of common beech (Fagus sylvatica L.) under different climatic and environmental conditions in Europe—A dendroecological study. For. Ecol. Manag. 2003, 173, 63–78. [Google Scholar] [CrossRef]
- Mäkinen, H.; Nöjd, P.; Kahle, H.P.; Neumann, U.; Tveite, B.; Mielikäinen, K.; Spiecker, H. Radial growth variation of Norway spruce (Picea abies (L.) Karst.) across latitudinal and altitudinal gradients in central and northern Europe. For. Ecol. Manag. 2002, 171, 243–259. [Google Scholar] [CrossRef]
- Angert, A.; Biraud, S.; Bonfils, C.; Henning, C.C.; Buermann, W.; Pinzon, J.; Fung, I. Drier summers cancel out the CO2 uptake enhancement induced by warmer springs. Proc. Natl. Acad. Sci. USA 2005, 102, 10823–10827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hacket-Pain, A.J.; Cavin, L.; Friend, A.D.; Jump, A.S. Consistent limitation of growth by high temperature and low precipitation from range core to southern edge of European beech indicates widespread vulnerability to changing climate. Eur. J. For. Res. 2016, 135, 897–909. [Google Scholar] [CrossRef] [Green Version]
- Obladen, N.; Dechering, P.; Skiadaresis, G.; Tegel, W.; Keßler, J.; Höllerl, S.; Seim, A. Tree mortality of European beech and Norway spruce induced by 2018–2019 hot droughts in central Germany. Agric. For. Meteorol. 2021, 307, 108482. [Google Scholar] [CrossRef]
- Hacket-Pain, A.J.; Friend, A.D.; Lageard, J.G.; Thomas, P.A. The influence of masting phenomenon on growth–climate relationships in trees: Explaining the influence of previous summers’ climate on ring width. Tree Physiol. 2015, 35, 319–330. [Google Scholar] [CrossRef] [PubMed]
- CLRTAP. Mapping Critical Levels for Vegetation, Chapter III of Manual on Methodologies and Criteria for Modelling and Mapping Critical Loads and Levels and Air Pollution Effects, Risks and Trends UNECE Convention on Long-Range Transboundary Air Pollution. 2017. Available online: http://icpvegetation.ceh.ac.uk/ (accessed on 20 May 2022).
- Braun, S.; Rihm, B.; Schindler, C. Epidemiological Estimate of Growth Reduction by Ozone in Fagus sylvatica L. and Picea abies Karst.: Sensitivity Analysis and Comparison with Experimental Results. Plants 2022, 11, 777. [Google Scholar] [CrossRef]
- Büker, P.; Feng, Z.; Uddling, J.; Briolat, A.; Alonso, R.; Braun, S.; Emberson, L.D. New flux based dose–response relationships for ozone for European forest tree species. Environ. Pollut. 2015, 206, 163–174. [Google Scholar] [CrossRef]
- Karlsson, P.E.; Braun, S.; Broadmeadow, M.; Elvira, S.; Emberson, L.; Gimeno, B.S.; Wilkinson, M. Risk assessments for forest trees: The performance of the ozone flux versus the AOT concepts. Environ. Pollut. 2007, 146, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Mills, G.; Pleijel, H.; Braun, S.; Büker, P.; Bermejo, V.; Calvo, E.; Simpson, D. New stomatal flux-based critical levels for ozone effects on vegetation. Atmos. Environ. 2011, 45, 5064–5068. [Google Scholar] [CrossRef]
- Emberson, L.D.; Ashmore, M.R.; Cambridge, H.M.; Simpson, D.; Tuovinen, J.P. Modelling stomatal ozone flux across Europe. Environ. Pollut. 2000, 109, 403–413. [Google Scholar] [CrossRef]
- Paoletti, E.; Alivernini, A.; Anav, A.; Badea, O.; Carrari, E.; Chivulescu, S.; Hoshika, Y. Toward stomatal–flux based forest protection against ozone: The MOTTLES approach. Sci. Total Environ. 2019, 691, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, H.; Grams, T.; Häberle, K.H.; Pritsch, K.; Bauerle, T.; Rötzer, T. Growth and mortality of Norway spruce and European beech in monospecific and mixed-species stands under natural episodic and experimentally extended drought. Results of the KROOF throughfall exclusion experiment. Trees 2020, 34, 957–970. [Google Scholar] [CrossRef]
- ICP Forests Manual 2016—Part I. 2016. Available online: https://www.icp-forests.org/pdf/manual/2016/Manual_Part_I.pdf (accessed on 20 May 2022).
- Rheinlandpfalz Landesamt Für Umwelt. Available online: www.luft-rlp.de (accessed on 20 May 2022).
- Eghdami, H.; Werner, W.; Büker, P. Spatio-Temporal Variation of Ozone Concentrations and Ozone Uptake Conditions in Forests in Western Germany. Atmosphere 2020, 11, 1261. [Google Scholar] [CrossRef]
- Eghdami, H.; Werner, W.; Büker, P.; Sicard, P. Assessment of ozone risk to Central European forests: Time series indicates perennial exceedance of ozone critical levels. Environ. Res. 2022, 203, 111798. [Google Scholar] [CrossRef] [PubMed]
- Dobler, L.; Hinterding, A.; Gerlach, N. INTERMET—Interpolation Stündlicher und Tagesbasierter Meteorologischer Parameter—Gesamtdokumentation; Unveröffentlichter Projektbericht, Institut für Geoinformatik der Universität Münster: Münster, Germany, 2004. [Google Scholar]
- United Nations Economic Commission for Europe. ICP Forest Manual Part IV—Visual Assessment of Crown Condition and Damaging Agents; UNECE: Geneva, Switzerland, 2020. [Google Scholar]
- Lockwood, B.R.; Maxwell, J.T.; Robeson, S.M.; Au, T.F. Assessing bias in diameter at breast height estimated from tree rings and its effects on basal area increment and biomass. Dendrochronologia 2021, 67, 125844. [Google Scholar] [CrossRef]
- Bouriaud, O.; Bréda, N.; Dupouey, J.L.; Granier, A. Is ring width a reliable proxy for stem-biomass increment? A case study in European beech. Can. J. For. Res. 2005, 35, 2920–2933. [Google Scholar] [CrossRef]
- Livingston, W.H.; Pontius, J.; Costanza, K.K.; Trosper, S. Using changes in basal area increments to map relative risk of HWA impacts on hemlock growth across the Northeastern USA. Biol. Invasions 2017, 19, 1577–1595. [Google Scholar] [CrossRef]
- Vospernik, S. Basal area increment models accounting for climate and mixture for Austrian tree species. For. Ecol. Manag. 2021, 480, 118725. [Google Scholar] [CrossRef]
- Begon, M.; Mortimer, M. Population Ecology: A Unified Study of Animals and Plants; Sinauer: Sunderland, MA, USA, 1986. [Google Scholar]
- Büker, P.; Morrissey, T.; Briolat, A.; Falk, R.; Simpson, D.; Tuovinen, J.P.; Alonso, R.; Barth, S.; Baumgarten, M.; Grulke, N.; et al. DO 3 SE modelling of soil moisture to determine ozone flux to forest trees. Atmos. Chem. Phys. 2012, 12, 5537–5562. [Google Scholar] [CrossRef] [Green Version]
- Nunn, A.J.; Kozovits, A.R.; Reiter, I.M.; Heerdt, C.; Leuchner, M.; Lütz, C.; Liu, X.; Löw, M.; Winkler, J.B.; Grams, T.E.E.; et al. Comparison of ozone uptake and sensitivity between a phytotronstudy with young beech and a field experiment with adult beech (Fagus sylvatica). Environ. Pollut. 2005, 137, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Fares, S.; Savi, F.; Muller, J.; Matteucci, G.; Paoletti, E. Simultaneousmeasurements ofabove and below canopy ozone fluxes help partitioning ozone deposition between ist various sinks in a Mediterranean Oak Forest. Agric. For. Meteorol. 2014, 198–199, 181–191. [Google Scholar] [CrossRef]
- Bender, J.; Bergmann, E.; Weigel, H.J.; Grünhage, L.; Schröder, M.; Builtjes, P.; Schaap, M.K.; Wichink Kruit, R.; Stern, R.; Baumgarten, M.; et al. Anwendung und Überprüfung neuer Methoden zur Flächenhaften Bewertung der Auswirkung von Bodennahem Ozon auf die Biodiversität Terrestrischer Ökosysteme. 2015. Available online: https://www.umweltbundesamt.de/publikationen/anwendung-ueberpruefung-neuer-methoden-zur (accessed on 20 May 2022).
- Albert, M.; Nagel, J.; Schmidt, M.; Nagel, R.-V.; Spellmann, H. Neue Ertragstafel für Fichte und Buche. Nordwestdeutsche Forstliche Versuchsanstalt (Hrsg.). 2021. Available online: https://www.nw-fva.de/unterstuetzen/waldpflege-und-nutzung/neue-ertragstafeln (accessed on 20 May 2021).
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Araminienė, V.; Sicard, P.; Anav, A.; Agathokleous, E.; Stakėnas, V.; De Marco, A.; Girgždienė, R. Trends and inter-relationships of ground-level ozone metrics and forest health in Lithuania. Sci. Total Environ. 2019, 658, 1265–1277. [Google Scholar] [CrossRef]
- Sicard, P.; Hoshika, Y.; Carrari, E.; De Marco, A.; Paoletti, E. Testing visible ozone injury within a Light Exposed Sampling Site as a proxy for ozone risk assessment for European forests. J. For. Res. 2021, 32, 1351–1359. [Google Scholar] [CrossRef]
- Dohrenbusch, A.; Jaehne, S.; Bredemeier, M.; Lamersdorf, N. Growth and fructification of a Norway spruce (Picea abies L. Karst) forest ecosystem under changed nutrient and water input. Ann. For. Sci. 2002, 59, 359–368. [Google Scholar] [CrossRef]
- Schäfer, C.; Rötzer, T.; Thurm, E.A.; Biber, P.; Kallenbach, C.; Pretzsch, H. Growth and tree water deficit of mixed Norway spruce and European beech at different heights in a tree and under heavy drought. Forests 2019, 10, 577. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Salguero, R.; Navarro-Cerrillo, R.M.; Camarero, J.J.; Fernández-Cancio, Á. Selective drought-induced decline of pine species in southeastern Spain. Clim. Change 2012, 113, 767–785. [Google Scholar] [CrossRef]
- Adams, H.D.; Kolb, T.E. Tree growth response to drought and temperature in a mountain landscape in northern Arizona, USA. J. Biogeogr. 2005, 32, 1629–1640. [Google Scholar] [CrossRef]
- Granier, A.; Reichstein, M.; Bréda, N.; Janssens, I.A.; Falge, E.; Ciais, P.; Wang, Q. Evidence for soil water control on carbon and water dynamics in European forests during the extremely dry year: 2003. Agric. For. Meteorol. 2007, 143, 123–145. [Google Scholar] [CrossRef]
- Castagneri, D.; Nola, P.; Motta, R.; Carrer, M. Summer climate variability over the last 250 years differently affected tree species radial growth in a mesic Fagus–Abies–Picea old-growth forest. For. Ecol. Manag. 2014, 320, 21–29. [Google Scholar] [CrossRef]
- Agyei, T.; Jurán, S.; Kwakye, K.O.; Šigut, L.; Urban, O.; Marek, M.V. The impact of drought on total ozone flux in a mountain Norway spruce forest. J. For. Sci. 2020, 66, 280–287. [Google Scholar] [CrossRef]
- Juraň, S.; Edwards-Jonašova, M.; Cudlin, P.; Zapletal, M.; Šigut, L.; Grace, J.; Urban, O. Prediction of ozone effects on net ecosystem production of Norway spruce forest. iForest 2018, 11, 743–750. [Google Scholar] [CrossRef] [Green Version]
- Braun, S.; Schindler, C.; Rihm, B.; Flückiger, W. Shoot growth of mature Fagus sylvatica and Picea abies in relation to ozone. Environ. Pollut. 2007, 146, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Seifert, T.; Müller-Starck, G. Impacts of fructification on biomass production and correlated genetic effects in Norway spruce (Picea abies [L.] Karst.). Eur. J. For. Res. 2009, 128, 155–169. [Google Scholar] [CrossRef]
- Kohler, M.; Sohn, J.; Nägele, G.; Bauhus, J. Can drought tolerance of Norway spruce (Picea abies (L.) Karst.) be increased through thinning? Eur. J. For. Res. 2010, 129, 1109–1118. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Schulze, E.D.; Mooney, H.A. The ecology and economics of storage in plants. Annu. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).