Use of Secondary Metabolites of Wood-Decaying Fungi to Reduce Damping off Disease
Abstract
1. Introduction
- (a)
- Fusarium infection and the lesions develop in spite of the application of wood-decay fungi extracts, which may contain both non-volatile compounds (NVOC) and volatile compounds (VOC).
- (b)
- At a certain concentration, extracts from wood-decay fungi effectively restrict Fusarium infection or disease development in Scots pine seedlings.
2. Materials and Methods
2.1. Extracts from Wood Decaying Fungi
2.1.1. Origin of Wood-Decaying Fungi
2.1.2. Preparation of Extracts from Wood Decaying Fungi
2.1.3. Fungal Extracts Analysis by Gas Chromatography-Mass Spectrometry
2.2. Preparation of Pathogens for Testing
2.3. Pinus Sylvestris Seedlings for Use in In Vitro Studies
2.3.1. Pine Seedlings Being Prepared for In Vitro Tests
2.3.2. Use of Extracts for Seedling Protection
2.3.3. Control and Test Treatments
2.3.4. Assessment of Disease Symptoms on Pine Seedlings
2.4. Statistical Analysis
3. Results
3.1. Chemical Composition of Extracts from Wood Decaying Fungi
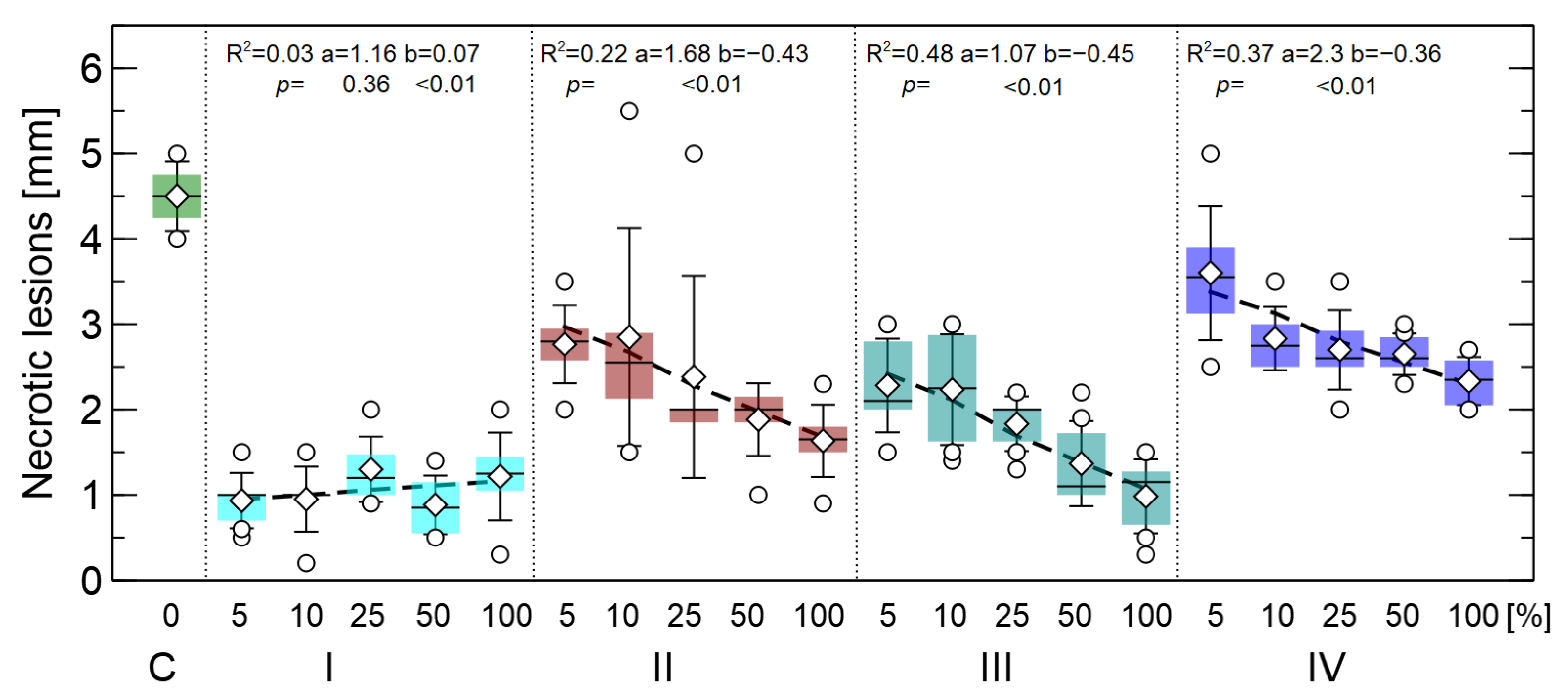
3.2. Effects of Extract Concentrations on the Length of Pine Root Lesion
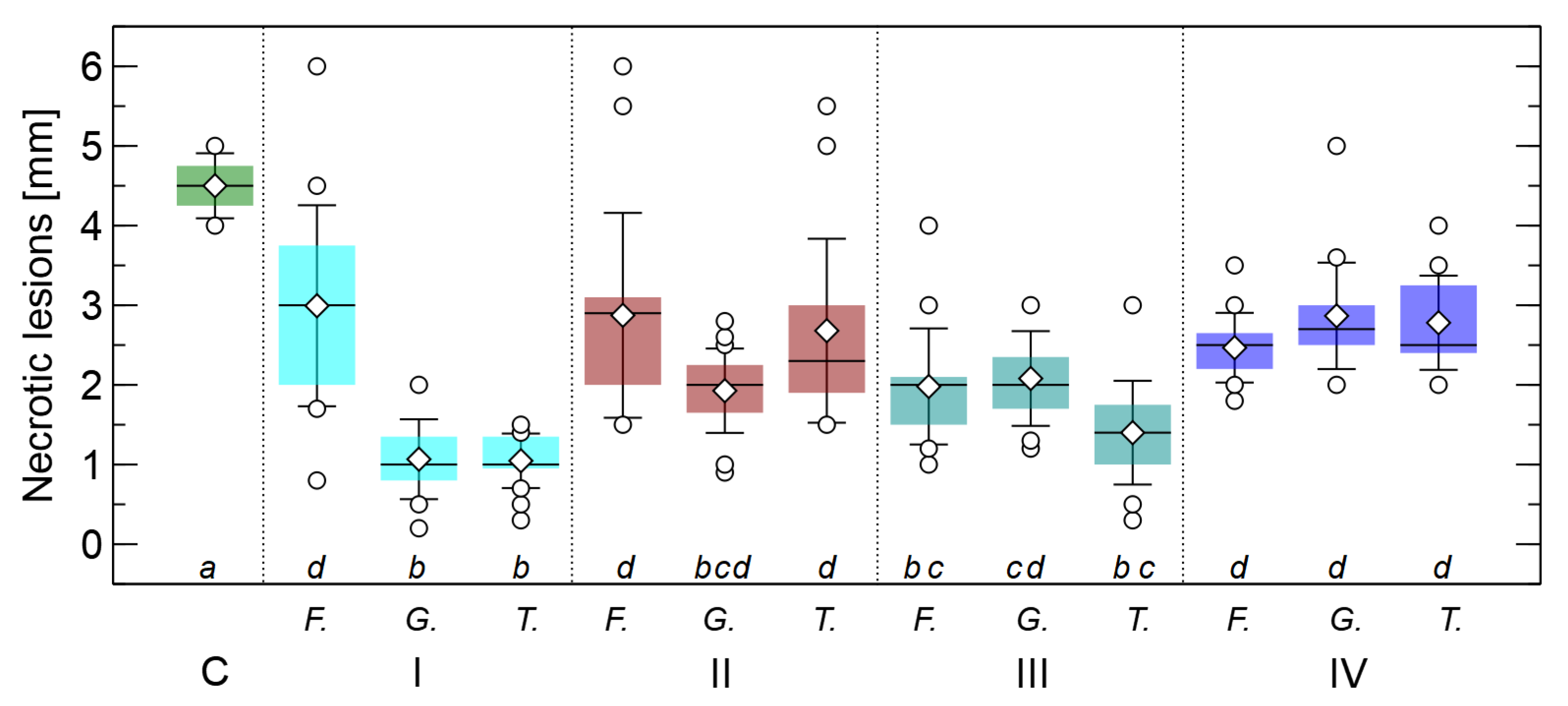
3.3. Influence of Fungal Extracts on Root Lesions Development Caused by F. oxysporum
3.4. Preventive or Curative Effects of Extracts on Disease on P. sylvestris Germinants
4. Discussion
4.1. Choice of Fungal Extracts
4.2. Chemical Composition of Fungal Extracts
4.3. Preventive Measures
4.3.1. Protective Effect of Fungal Extracts
4.3.2. Potential Applications
5. Conclusions
- All three fungal extracts studied showed a curative or preventive effect, resulting in a reduction in the length of root necrosis caused by infection with F. oxysporum.
- The strongest effects were obtained with extracts of G. applanatum and T. versicolor.
- The strongest effect was obtained in treatment I, in which one drop of the extract was applied to the root tip 24 h after infection (curative).
- The full preventive effect of the application of these two extracts in treatment I was achieved at a 5% concentration of the extract—further increasing the concentration did not change the results.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gordon, T.R.; Swett, C.L.; Wingfield, M.J. Management of Fusarium diseases affecting conifers. Crop Prot. 2015, 73, 28–39. [Google Scholar] [CrossRef]
- Brown, B.; Wylie, F. Diseases and pests of Australian forest nurseries: Past and present. In Proceedings of the First Meeting of IUFRO Working Party S. 2.07–09 (Diseases and Insects in Forest Nurseries); Southerland, J.R., Glover, S., Eds.; Forestry Canada, Pacific and Yukon Region, Pacific Forestry Centre: Victoria, BC, Canada, 1991; pp. 3–15. [Google Scholar]
- Smith, R.S., Jr. Charcoal root disease. Macrophomina phaseoli (Maubl) Ashby. In Forest Nursery Diseases in the United States; Agric. Handb; Peterson, G., Smith, R.T.C., Jr., Eds.; USDA Forest Service: Washington, DC, USA, 1975; Volume 470, pp. 11–13. [Google Scholar]
- Wit, M.; Sierota, Z.; Oszako, T.; Mirzwa-Mróz, E.; Wakuliński, W. Fusarium spp. on the above-ground organs of dying oaks-a new threat? Sylwan 2015, 159, 403–410. (In Polish) [Google Scholar] [CrossRef]
- Armstrong, G.; Armstrong, J.K. Formae speciales and races of Fusarium oxysporum causing wilt diseases. In Fusarium: Disease, Biology, and Taxonomy; Nelson, P.E., Toussoun, T.A., Cook, R.J., Eds.; The Pennsylvania State University Press: University Park, PA, USA, 1981; pp. 391–399. [Google Scholar]
- Michielse, C.B.; Rep, M. Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 2009, 10, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.A.; Lichens-Park, A.; Kole, C. Genomics of Plant-Associated Fungi and Oomycetes: Dicot Pathogens; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- de Lamo, F.J.; Takken, F.L. Biocontrol by Fusarium oxysporum using endophyte-mediated resistance. Front. Plant Sci. 2020, 11, 37. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Edel-Hermann, V.; Brenot, S.; Gautheron, N.; Aimé, S.; Alabouvette, C.; Steinberg, C. Ecological fitness of the biocontrol agent Fusarium oxysporum Fo47 in soil and its impact on the soil microbial communities. FEMS Microbiol. Ecol. 2009, 68, 37–45. [Google Scholar] [CrossRef]
- Davydenko, K.; Nowakowska, J.A.; Kaluski, T.; Gawlak, M.; Sadowska, K.; García, J.M.; Diez, J.J.; Okorski, A.; Oszako, T. A Comparative Study of the Pathogenicity of Fusarium circinatum and other Fusarium Species in Polish Provenances of P. sylvestris L. Forests 2018, 9, 560. [Google Scholar] [CrossRef]
- Okorski, A.; Milewska, A.; Pszczółkowska, A.; Karpiesiuk, K.; Kozera, W.; Dąbrowska, J.A.; Radwińska, J. Prevalence of Fusarium fungi and Deoxynivalenol Levels in Winter Wheat Grain in Different Climatic Regions of Poland. Toxins 2022, 14, 102. [Google Scholar] [CrossRef]
- Kulik, T.; Jestoi, M.; Okorski, A. Development of TaqMan assays for the quantitative detection of Fusarium avenaceum/Fusarium tricinctum and Fusarium poae esyn1 genotypes from cereal grain. FEMS Microbiol. Lett. 2011, 314, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Jankowiak, R.; Stępniewska, H.; Bilański, P.; Taerum, S.J. Fungi as potential factors limiting natural regeneration of pedunculate oak (Quercus robur) in mixed-species forest stands in Poland. Plant Pathol. 2022, 71, 805–817. [Google Scholar] [CrossRef]
- Oszako, T.; Sikora, K.; Belbahri, L.; Nowakowska, J.A. Molecular detection of oomycetes species in water courses. Folia For. Pol. 2016, 58, 246–251. [Google Scholar] [CrossRef]
- Orłowski, L.B.; Trzewik, A.; Ptaszek, M.; Orlikowska, T. Relationship between source of water, occurrence, and pathogenicity of Phytophthora plurivora. Acta Mycol. 2012, 47, 1. [Google Scholar] [CrossRef][Green Version]
- Malewski, T.; Brzezińska, B.; Belbahri, L.; Oszako, T. Role of avian vectors in the spread of Phytophthora species in Poland. Eur. J. Plant Pathol. 2019, 155, 1363–1366. [Google Scholar] [CrossRef]
- Stępniewska, H.; Jankowiak, R.; Bilański, P.; Hausner, G. Structure and Abundance of Fusarium Communities Inhabiting the Litter of Beech Forests in Central Europe. Forests 2021, 12, 811. [Google Scholar] [CrossRef]
- Kowalski, T.; Bilański, P. Fungi Detected in the Previous Year’s Leaf Petioles of Fraxinus excelsior and Their Antagonistic Potential against Hymenoscyphus fraxineus. Forests 2021, 12, 1412. [Google Scholar] [CrossRef]
- Okorski, A.; Pszczółkowska, A.; Okorska, S.; Fordoński, G. First Report of Fagus sylvatica Infection by Fusarium avenaceum in Forest Container Nurseries in Northeastern Poland. Plant Dis. 2015, 99, 420. [Google Scholar] [CrossRef] [PubMed]
- James, R.L.; Dumroese, R.K. Investigations of Fusarium diseases within Inland Pacific Northwest forest nurseries. In Proceedings of the 53rd Western International Forest Disease Work Conference, USDA Forest Service, Intermountain Region, Ogden, UT, USA, 15–19 October 2007; pp. 3–11. [Google Scholar]
- Dumroese, R.K.; James, R.L. Root diseases in bareroot and container nurseries of the Pacific Northwest: Epidemiology, management, and effects on outplanting performance. New For. 2005, 30, 185–202. [Google Scholar] [CrossRef]
- Montecchio, L. Damping-off of beech seedlings caused by Fusarium avenaceum in Italy. Plant Dis. 2005, 89, 1014. [Google Scholar] [CrossRef]
- Lilja, A.; Poteri, M.; Petäistö, R.L.; Rikala, R.; Kurkela, T.; Kasanen, R. Fungal diseases in forest nurseries in Finland. Silva Fenn. 2010, 44, 147. [Google Scholar] [CrossRef]
- Peterson, M. Fusarium species-a British Columbia perspective in forest seedling production. In National Proceedings: Forest and Conservation Nursery Associations-2007. Proc. RMRS-P-57; Dumroese, R., Riley, L., Eds.; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2008; Volume 57, pp. 109–125. [Google Scholar]
- Pinto, P.M.; Alonso, J.A.P.; Fernández, V.P.; Casero, J.J.D. Fungi isolated from diseased nursery seedlings in Spain. New For. 2006, 31, 41–56. [Google Scholar] [CrossRef]
- Menkis, A.; Vasiliauskas, R.; Taylor, A.; Stenström, E.; Stenlid, J.; Finlay, R. Fungi in decayed roots of conifer seedlings in forest nurseries, afforested clear-cuts and abandoned farmland. Plant Pathol. 2006, 55, 117–129. [Google Scholar] [CrossRef]
- Zakeri, A.; Hamzeharghani, H.; Banihashemi, Z.; Saadati, S. Pathogenic fungi associated with pre-and post-emergence seedling blight of pine and cypress in Fars Province, Iran. For. Pathol. 2011, 41, 438–443. [Google Scholar] [CrossRef]
- Lazreg, F.; Belabid, L.; Sanchez, J.; Gallego, E.; Bayaa, B. Pathogenicity of Fusarium spp. associated with diseases of Aleppo-pine seedlings in Algerian forest nurseries. J. For. Sci. 2014, 60, 115–120. [Google Scholar] [CrossRef]
- Fajardo, M.A.; León, J.D.; Correa, G.A.; Morales, J.G. The Causal Agent of Damping-off in Pinus patula (Schiede) and Pinus tecunumanii (Schwerdtf.). Floresta E Ambiente 2019, 26. [Google Scholar] [CrossRef]
- Okorski, A.; Oszako, T.; Nowakowska, J.A.; Pszczolkowska, A. The possibilities of biologically protecting plants against diseases in nurseries, with special consideration of Oomycetes and Fusarium fungi. Leśne Pr. Badaw. 2014, 75. [Google Scholar] [CrossRef]
- Klapwijk, M.J.; Bylund, H.; Schroeder, M.; Björkman, C. Forest management and natural biocontrol of insect pests. Forestry 2016, 89, 253–262. [Google Scholar] [CrossRef]
- Balla, A.; Silini, A.; Cherif-Silini, H.; Chenari Bouket, A.; Moser, W.K.; Nowakowska, J.A.; Oszako, T.; Benia, F.; Belbahri, L. The Threat of Pests and Pathogens and the Potential for Biological Control in Forest Ecosystems. Forests 2021, 12, 1579. [Google Scholar] [CrossRef]
- Spremo, N.R.; Tesanović, K.D.; Rakić, M.S.; Janjušević, L.N.; Ignjatov, M.; Bjelić, D.; Karaman, M. Antifungal activity of macrofungi extracts on phytopathogenic fungal strains of genera Fusarium sp. and Alternaria sp. Zb. Matice Srp. Za Prir. Nauk. 2017, 133, 231–240. [Google Scholar] [CrossRef]
- Agrios, G.N. Plant diseases caused by Mollicutes: Phytoplasmas and spiroplasmas. In Plant Pathology; Agrios, G.N., Ed.; Academic Press: New York, NY, USA, 1997; pp. 457–470. [Google Scholar]
- Zjawiony, J.K. Biologically active compounds from Aphyllophorales (polypore) fungi. J. Nat. Prod. 2004, 67, 300–310. [Google Scholar] [CrossRef]
- Alves, M.; Ferreira, I.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A Review on Antifungal Activity of Mushroom (Basidiomycetes) Extracts and Isolated Compounds. Curr. Top. Med. Chem. 2013, 13, 2648–2659. [Google Scholar] [CrossRef]
- Atanasova-Penichon, V.; Barreau, C.; Richard-Forget, F. Antioxidant secondary metabolites in cereals: Potential involvement in resistance to Fusarium and mycotoxin accumulation. Front. Microbiol. 2016, 7, 566. [Google Scholar] [CrossRef]
- Heleno, S.A.; Ferreira, I.C.; Esteves, A.P.; Ćirić, A.; Glamočlija, J.; Martins, A.; Soković, M.; Queiroz, M.J.R. Antimicrobial and demelanizing activity of Ganoderma lucidum extract, p-hydroxybenzoic and cinnamic acids and their synthetic acetylated glucuronide methyl esters. Food Chem. Toxicol. 2013, 58, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Parroni, A.; Bellabarba, A.; Beccaccioli, M.; Scarpari, M.; Reverberi, M.; Infantino, A. Use of the secreted proteome of Trametes versicolor for controlling the cereal pathogen Fusarium langsethiae. Int. J. Mol. Sci. 2019, 20, 4167. [Google Scholar] [CrossRef]
- Schalchli, H.; Hormazabal, E.; Becerra, J.; Birkett, M.; Alvear, M.; Vidal, J.; Quiroz, A. Antifungal activity of volatile metabolites emitted by mycelial cultures of saprophytic fungi. Chem. Ecol. 2011, 27, 503–513. [Google Scholar] [CrossRef]
- Guler, P.; Akata, I.; Kutluer, F. Antifungal activities of Fomitopsis pinicola (Sw.: Fr) Karst and Lactarius vellereus (Pers.) Fr. Afr. J. Biotechnol. 2009, 8, 3811–3813. [Google Scholar]
- Xu, K.; Li, X.Q.; Zhao, D.L.; Zhang, P. Antifungal secondary metabolites produced by the fungal endophytes: Chemical diversity and potential use in the development of biopesticides. Front. Microbiol. 2021, 12, 689527. [Google Scholar] [CrossRef] [PubMed]
- Matuszewska, A.; Jaszek, M.; Stefaniuk, D.; Ciszewski, T.; Matuszewski, Ł. Anticancer, antioxidant, and antibacterial activities of low molecular weight bioactive subfractions isolated from cultures of wood degrading fungus Cerrena unicolor. PLoS ONE 2018, 13, e0197044. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.J.; Xiao, J.H. Secondary metabolites from higher fungi: Discovery, bioactivity, and bioproduction. Biotechnol. China I 2009, 113, 79–150. [Google Scholar] [CrossRef]
- Brizuela, M.; García, L.; Pérez, L.; Mansur, M. Basidiomycetes: A new source of secondary metabolites. Rev. Iberoam. Micol. 1998, 15, 69–74. [Google Scholar] [PubMed]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.; Kannangara, S.D.; Promputtha, I. Fungi vs. fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef] [PubMed]
- Firáková, S.; Šturdíková, M.; Múčková, M. Bioactive secondary metabolites produced by microorganisms associated with plants. Biologia 2007, 62, 251–257. [Google Scholar] [CrossRef]
- Stuper-Szablewska, K.; Przybylska-Balcerek, A.; Rogoziński, T.; Szwajkowska-Michałek, L. Phenolic Compounds in Trees and Shrubs of Central Europe. Appl. Sci. 2020, 10, 6907. [Google Scholar] [CrossRef]
- Tamm, L.; Thürig, B.; Fliessbach, A.; Goltlieb, A.; Karavani, S.; Cohen, Y. Elicitors and soil management to induce resistance against fungal plant diseases. NJAS-Wagening. J. Life Sci. 2011, 58, 131–137. [Google Scholar] [CrossRef]
- Šrobárová, A.; Kogan, G.; Tamas, L.; Machová, E. Protective activity of the fungal polysaccharides against fusariosis. Plant Prot. Sci. 2002, 38, 617–619. [Google Scholar] [CrossRef]
- Roeder, A.; Kirschning, C.J.; Rupec, R.A.; Schaller, M.; Weindl, G.; Korting, H.C. Toll-like receptors as key mediators in innate antifungal immunity. Med. Mycol. 2004, 42, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Havrlentová, M.; Gregusová, V.; Šliková, S.; Nemeček, P.; Hudcovicová, M.; Kuzmová, D. Relationship between the Content of β-D-Glucans and Infection with Fusarium Pathogens in Oat (Avena sativa L.) Plants. Plants 2020, 9, 1776. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Herrera, J.; Ortiz-Castellanos, L. Cell wall glucans of fungi. A review. Cell Surf. 2019, 5, 100022. [Google Scholar] [CrossRef]
- Stadnik, M.J.; Freitas, M.B.d. Algal polysaccharides as source of plant resistance inducers. Trop. Plant Pathol. 2014, 39, 111–118. [Google Scholar] [CrossRef]
- Ryvarden, L.; Melo, I. Poroid fungi of Europe. In Synopsis Fungorum; Fungiflora A/S: Oslo, Norway, 2017; Volume 37. [Google Scholar]
- Karasiński, D.; Wołkowycki, M. An annotated and illustrated catalogue of polypores (Agaricomycetes) of the Białowieża Forest (NE Poland). Pol. Bot. J. 2015, 60, 217–292. [Google Scholar] [CrossRef][Green Version]
- Sadowska, A.; Zapora, E.; Sawicka, D.; Niemirowicz-Laskowska, K.; Surażyński, A.; Sułkowska-Ziaja, K.; Kała, K.; Stocki, M.; Wołkowycki, M.; Bakier, S.; et al. Heterobasidion annosum induces apoptosis in DLD-1 cells and decreases colon cancer growth in in vivo model. Int. J. Mol. Sci. 2020, 21, 3447. [Google Scholar] [CrossRef] [PubMed]
- Cody, R. SAS Statistics by Example; SAS Press: Cary, NC, USA, 2011. [Google Scholar]
- Tsukagoshi, S.; Hashimoto, Y.; Fujii, G.; Kobayashi, H.; Nomoto, K.; Orita, K. Krestin (PSK). Cancer Treat. Rev. 1984, 11, 131–155. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Muszyńska, B.; Sałaciak, K.; Gawalska, A. Trametes versicolor (L.) Lloyd as a source of biologically active compounds with a wide spectrum of action and application. Postępy Fitoter. 2016, 17, 274–281. (In Polish) [Google Scholar]
- Lohmeyer, T.R.; Künkele, U. Grzyby. Rozpoznawanie i Zbieranie; Parragon: Warszawa, Poland, 2006. (In Polish) [Google Scholar]
- Liu, S.; Han, M.L.; Xu, T.M.; Wang, Y.; Wu, D.M.; Cui, B.K. Taxonomy and phylogeny of the Fomitopsis pinicola complex with descriptions of six new species from East Asia. Front. Microbiol. 2021, 12, 644979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Tang, Q.; Zhou, S.; Feng, J.; Chen, H. Polysaccharide of Ganoderma and its bioactivities. In Ganoderma and Health. Advances in Experimental Medicine and Biology; Lin, Z., Yang, B., Eds.; Springer: Singapore, 2019; Volume 1181, pp. 107–134. [Google Scholar] [CrossRef]
- Leong, Y.K.; Yang, F.C.; Chang, J.S. Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohydr. Polym. 2021, 251, 117006. [Google Scholar] [CrossRef]
- Ferreira, I.C.; Heleno, S.A.; Reis, F.S.; Stojkovic, D.; Queiroz, M.J.R.; Vasconcelos, M.H.; Sokovic, M. Chemical features of Ganoderma polysaccharides with antioxidant, antitumor and antimicrobial activities. Phytochemistry 2015, 114, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Yamaç, M.; Bilgili, F. Antimicrobial activities of fruit bodies and/or mycelial cultures of some mushroom isolates. Pharm. Biol. 2006, 44, 660–667. [Google Scholar] [CrossRef]
- Hao, L.; Sheng, Z.; Lu, J.; Tao, R.; Jia, S. Characterization and antioxidant activities of extracellular and intracellular polysaccharides from Fomitopsis pinicola. Carbohydr. Polym. 2016, 141, 54–59. [Google Scholar] [CrossRef]
- Altieri, C.; Bevilacqua, A.; Cardillo, D.; Sinigaglia, M. Antifungal activity of fatty acids and their monoglycerides against Fusarium spp. in a laboratory medium. Int. J. Food Sci. Technol. 2009, 44, 242–245. [Google Scholar] [CrossRef]
- Fischer, C.L.; Blanchette, D.R.; Brogden, K.A.; Dawson, D.V.; Drake, D.R.; Hill, J.R.; Wertz, P.W. The roles of cutaneous lipids in host defense. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2014, 1841, 319–322. [Google Scholar] [CrossRef]
- Frank, C.L.; Ingala, M.R.; Ravenelle, R.E.; Dougherty-Howard, K.; Wicks, S.O.; Herzog, C.; Rudd, R.J. The effects of cutaneous fatty acids on the growth of Pseudogymnoascus destructans, the etiological agent of white-nose syndrome (WNS). PLoS ONE 2016, 11, e0153535. [Google Scholar] [CrossRef] [PubMed]
- Gołębiowski, M.; Urbanek, A.; Oleszczak, A.; Dawgul, M.; Kamysz, W.; Boguś, M.I.; Stepnowski, P. The antifungal activity of fatty acids of all stages of Sarcophaga carnaria L. (Diptera: Sarcophagidae). Microbiol. Res. 2014, 169, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, H.; Zuo, J.; Gong, X.; Yi, F.; Zhu, W.; Li, L. Advances in research on the active constituents and physiological effects of Ganoderma lucidum. Biomed. Dermatol. 2019, 3, 6. [Google Scholar] [CrossRef]
- Cör, D.; Knez, Ž.; Knez Hrnčič, M. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma lucidum terpenoids and polysaccharides: A review. Molecules 2018, 23, 649. [Google Scholar] [CrossRef] [PubMed]
- Skalicka-Wozniak, K.; Szypowski, J.; Los, R.; Siwulski, M.; Sobieralski, K.; Glowniak, K.; Malm, A. Evaluation of polysaccharides content in fruit bodies and their antimicrobial activity of four Ganoderma lucidum (W Curt.: Fr.) P. Karst. strains cultivated on different wood type substrates. Acta Soc. Bot. Pol. 2012, 81, 17–21. [Google Scholar] [CrossRef]
- Zhang, Z.; Diao, H.; Wang, H.; Wang, K.; Zhao, M. Use of Ganoderma Lucidum polysaccharide to control cotton fusarium wilt, and the mechanism involved. Pestic. Biochem. Physiol. 2019, 158, 149–155. [Google Scholar] [CrossRef]
- Oviasogie, F.; Akpaja, E.; Gbona, K.; Akonoafua, E. Antimicrobial properties of Ganoderma applanatum (Pers.) Pat. from Benin city, Nigeria. Niger. J. Agric. Food Environ. 2015, 11, 65–69. [Google Scholar]
- Kim, Y.S.; Rym, K.H.; Lee, C.K.; Han, S.S. Antimicrobial activity of Elfvingia applanata extract alone and in combination with some antibiotics. Yakhak Hoeji 1994, 38, 742–748. [Google Scholar]
- Boh, B.; Hodžar, D.; Dolničar, D.; Berovič, M.; Pohleven, F. Isolation and quantification of triterpenoid acids from Ganoderma applanatum of Istrian origin. Food Technol. Biotechnol. 2000, 38, 11–18. [Google Scholar]
- Nagaraj, K.; Mallikarjun, N.; Naika, R.; Venugopal, T. Phytochemical analysis and in vitro antimicrobial potential of Ganoderma applanatum (Pers.) Pat. of Shivamogga district-Karnataka, India. Int. J. Pharm. Sci. Rev. Res 2013, 23, 36–41. [Google Scholar]
- Jonathan, S.; Awotona, F. Studies on antimicrobial potentials of three Ganoderma species. Afr. J. Biomed. Res. 2010, 13, 131–139. [Google Scholar]
- Mendieta, E.H.; Ramos, M.A.; Miranda, A.S.; Granados, C.R. Evaluation of Crude Extracts of Trametes versicolor (L.: Fr.) Pilát to Control of Phytopathogenic Fungi. Int. J. Plant Res. 2019, 9, 9–13. [Google Scholar] [CrossRef]
- Waithaka, P.N.; Gathuru, E.M.; Githaiga, B.M.; Onkoba, K.M. Antimicrobial Activity of Mushroom (Agaricus bisporus) and Fungal (Trametes gibbosa) Extracts from Mushrooms and Fungi of Egerton Main Campus, Njoro Kenya. J. Biomed. Sci. 2017, 6, 3. [Google Scholar] [CrossRef]
- Kawagishi, H.; Nomura, A.; Yumen, T.; Mizuno, T.; Hagiwara, T.; Nakamura, T. Isolation and properties of a lectin from the fruiting bodies of Agaricus blazei. Carbohydr. Res. 1988, 183, 150–154. [Google Scholar] [CrossRef]
- Fujita, R.; Liu, J.; Shimizu, K.; Konishi, F.; Noda, K.; Kumamoto, S.; Ueda, C.; Tajiri, H.; Kaneko, S.; Suimi, Y.; et al. Anti-androgenic activities of Ganoderma lucidum. J. Ethnopharmacol. 2005, 102, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Dresch, P.; Rosam, K.; Grienke, U.; Rollinger, J.M.; Peintner, U. Fungal strain matters: Colony growth and bioactivity of the European medicinal polypores Fomes fomentarius, Fomitopsis pinicola and Piptoporus betulinus. Amb Express 2015, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Sivanandhan, S.; Khusro, A.; Paulraj, M.G.; Ignacimuthu, S.; Al-Dhabi, N.A. Biocontrol properties of basidiomycetes: An overview. J. Fungi 2017, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Redman, R.S.; Sheehan, K.B.; Stout, R.G.; Rodriguez, R.J.; Henson, J.M. Thermotolerance generated by plant/fungal symbiosis. Science 2002, 298, 1581. [Google Scholar] [CrossRef]
- Waller, F.; Achatz, B.; Baltruschat, H.; Fodor, J.; Becker, K.; Fischer, M.; Heier, T.; Hückelhoven, R.; Neumann, C.; von Wettstein, D.; et al. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc. Natl. Acad. Sci. USA 2005, 102, 13386–13391. [Google Scholar] [CrossRef]
- Bilal, L.; Asaf, S.; Hamayun, M.; Gul, H.; Iqbal, A.; Ullah, I.; Lee, I.J.; Hussain, A. Plant growth promoting endophytic fungi Asprgillus fumigatus TS1 and Fusarium proliferatum BRL1 produce gibberellins and regulates plant endogenous hormones. Symbiosis 2018, 76, 117–127. [Google Scholar] [CrossRef]
- Hill, R.; Llewellyn, T.; Downes, E.; Oddy, J.; MacIntosh, C.; Kallow, S.; Panis, B.; Dickie, J.B.; Gaya, E. Seed Banks as Incidental Fungi Banks: Fungal Endophyte Diversity in Stored Seeds of Banana Wild Relatives. Front. Microbiol. 2021, 12, 508. [Google Scholar] [CrossRef] [PubMed]
- Houterman, P.M.; Speijer, D.; Dekker, H.L.; de Koster, C.G.; Cornelissen, B.J.; Rep, M. The mixed xylem sap proteome of Fusarium oxysporum-infected tomato plants. Mol. Plant Pathol. 2007, 8, 215–221. [Google Scholar] [CrossRef]
- Ma, L.J.; Van Der Does, H.C.; Borkovich, K.A.; Coleman, J.J.; Daboussi, M.J.; Di Pietro, A.; Dufresne, M.; Freitag, M.; Grabherr, M.; Henrissat, B.; et al. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 2010, 464, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.M.; Houterman, P.M.; Schreiver, I.; Ma, L.; Amyotte, S.; Chellappan, B.; Boeren, S.; Takken, F.L.; Rep, M. MITEs in the promoters of effector genes allow prediction of novel virulence genes in Fusarium oxysporum. BMC Genom. 2013, 14, 119. [Google Scholar] [CrossRef] [PubMed]
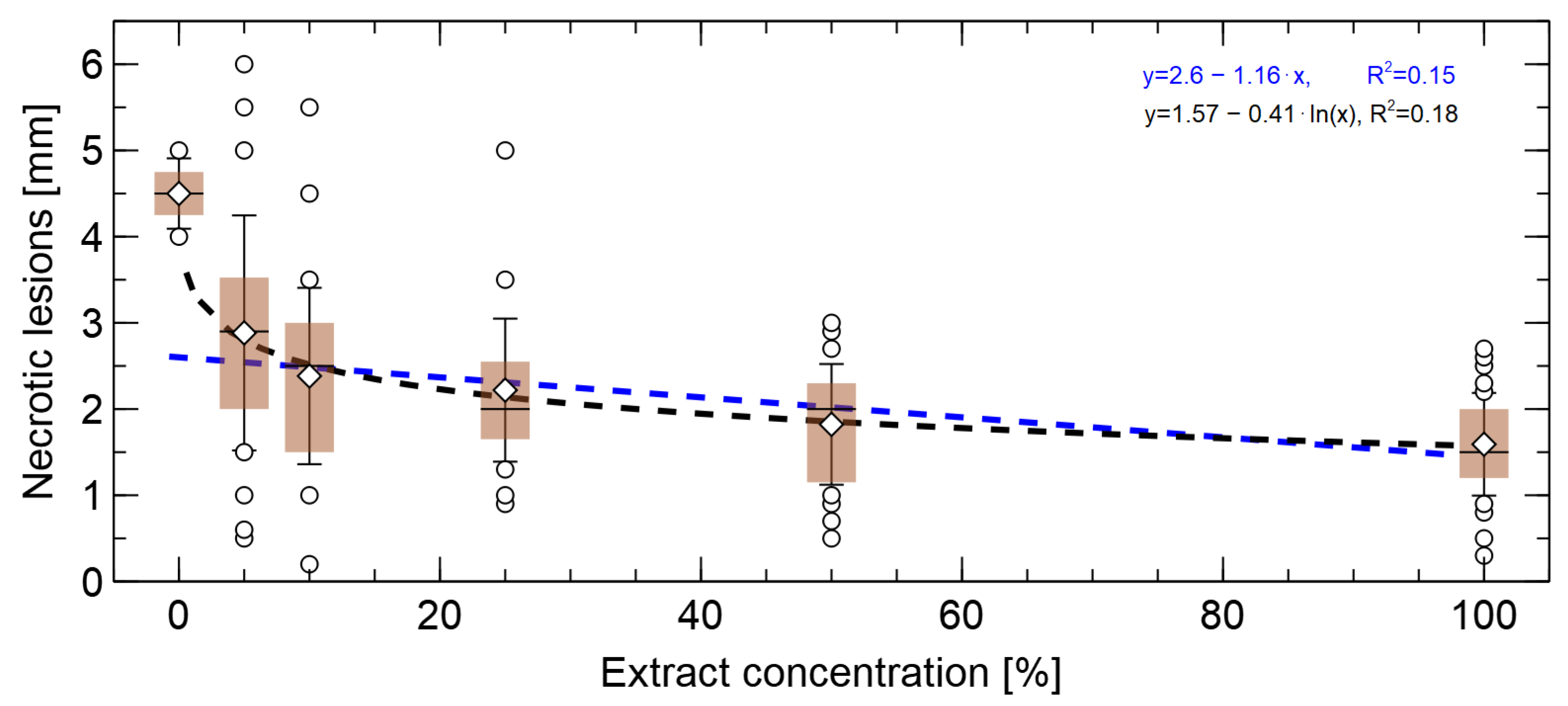
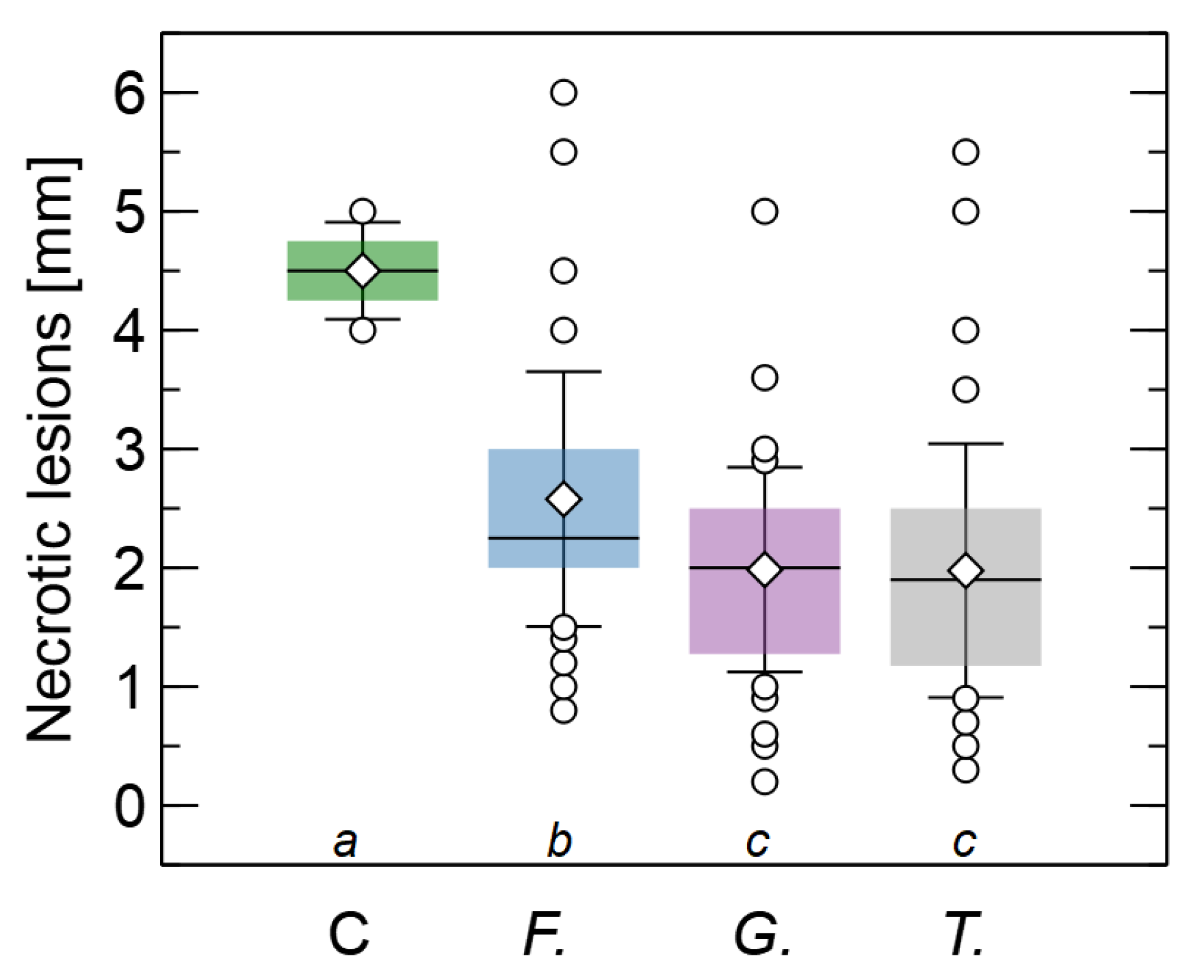
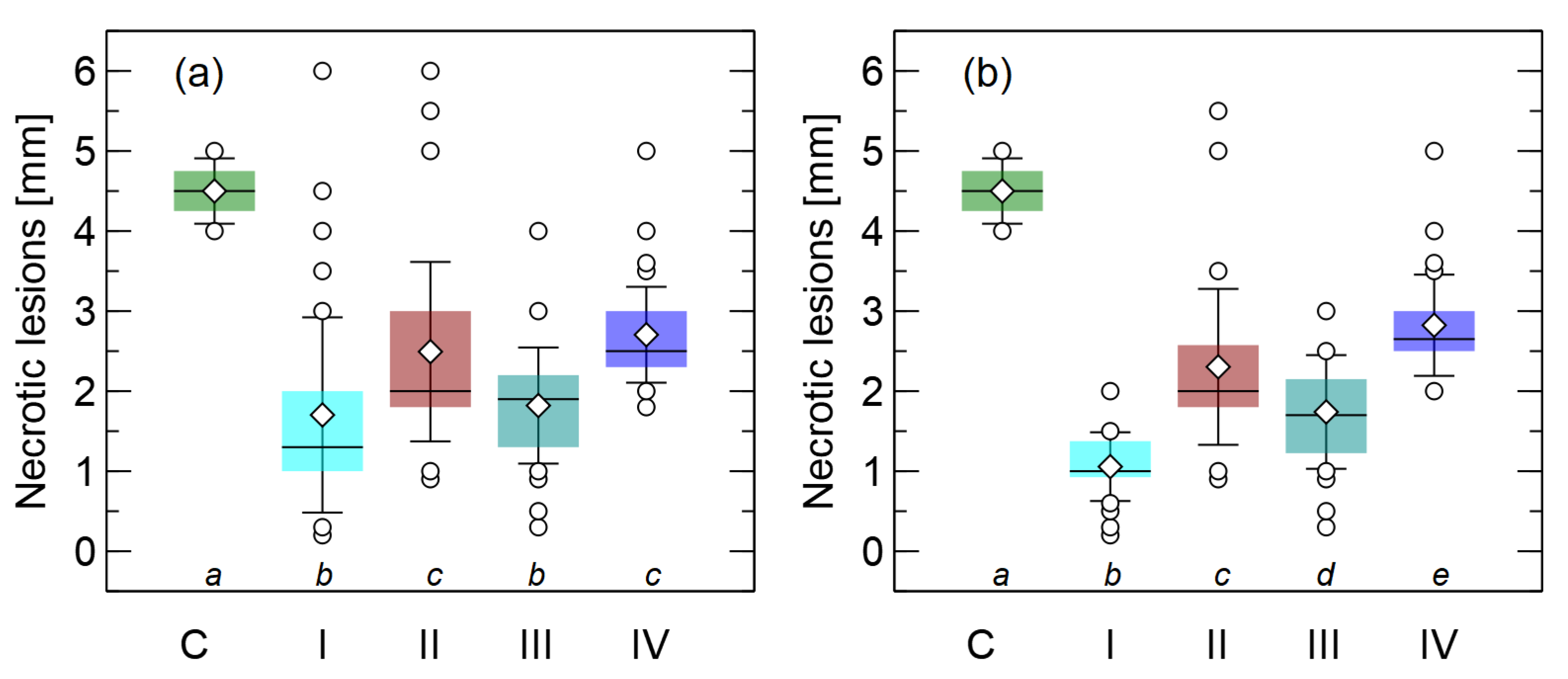
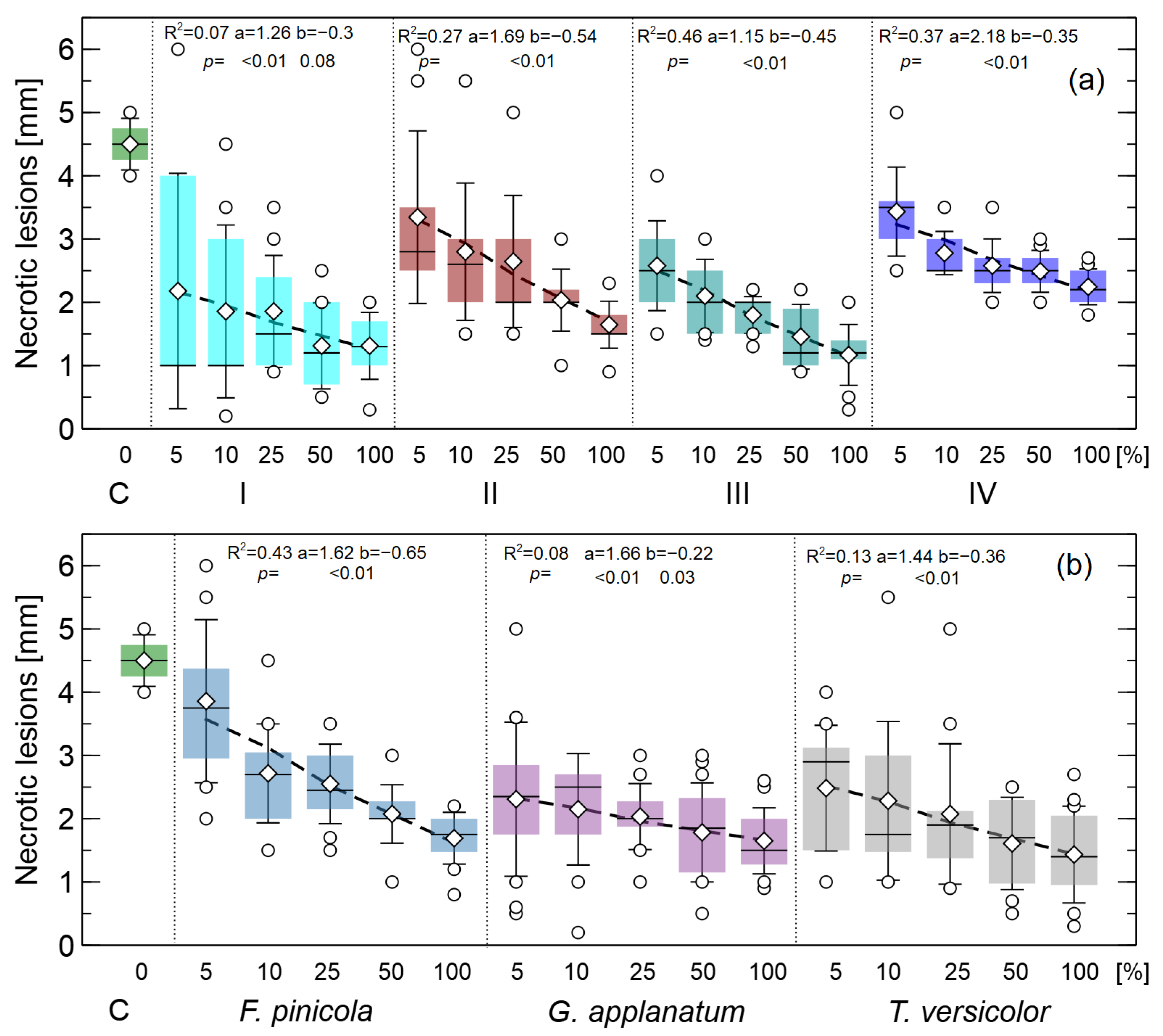
| Treatment | Description |
|---|---|
| I | One drop of the extract was applied to the root tip 24 h after the seedling was inoculated with a 1 cm sized mycelial plug of F. oxysporum. |
| II | One drop of the extract was applied to the root tip 24 h before each seedling was inoculated with a 1 cm sized mycelial plug of F. oxysporum. |
| III | Seedling roots were dipped in the extract for 1 min and inoculated with a 1 cm sized mycelial plug of F. oxysporum immediately after removal from the extract. |
| IV | Seedling roots were dipped in the extract for 1 min and inoculated with a 1 cm mycelial plug of F. oxysporum 24 h after that. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waszczuk, U.; Zapora, E.; Berezovska, D.; Stocki, M.; Wołkowycki, M.; Malewski, T.; Hsiang, T.; Oszako, T.; Borowik, P. Use of Secondary Metabolites of Wood-Decaying Fungi to Reduce Damping off Disease. Forests 2022, 13, 1208. https://doi.org/10.3390/f13081208
Waszczuk U, Zapora E, Berezovska D, Stocki M, Wołkowycki M, Malewski T, Hsiang T, Oszako T, Borowik P. Use of Secondary Metabolites of Wood-Decaying Fungi to Reduce Damping off Disease. Forests. 2022; 13(8):1208. https://doi.org/10.3390/f13081208
Chicago/Turabian StyleWaszczuk, Urszula, Ewa Zapora, Daria Berezovska, Marcin Stocki, Marek Wołkowycki, Tadeusz Malewski, Tom Hsiang, Tomasz Oszako, and Piotr Borowik. 2022. "Use of Secondary Metabolites of Wood-Decaying Fungi to Reduce Damping off Disease" Forests 13, no. 8: 1208. https://doi.org/10.3390/f13081208
APA StyleWaszczuk, U., Zapora, E., Berezovska, D., Stocki, M., Wołkowycki, M., Malewski, T., Hsiang, T., Oszako, T., & Borowik, P. (2022). Use of Secondary Metabolites of Wood-Decaying Fungi to Reduce Damping off Disease. Forests, 13(8), 1208. https://doi.org/10.3390/f13081208










