Abstract
This study determined the rate of decomposition of fine roots and leaf litter from birch, larch, and pine, and compared the impact of fine root decomposition and leaf litter on carbon, nitrogen, and phosphorus accumulation in various regenerated and reconstructed forest ecosystems. The control plots were located on podzol soils in managed forest non-degraded habitats. Over a one-year experimental season, the decomposition of birch and larch fine roots released less carbon in comparison to leaf litter. The carbon mass-loss rates were 16% for birch roots and 15% for larch roots, while for birch and larch litter, the rates were 36% and 27%, respectively. For nitrogen, mass-loss rates were 48% for birch fine roots and 60% for larch and pine fine roots, whereas for pine and birch litter the rates were 14%, and 33% for larch litter. The results of our study prove the important role of fine root input to the soil’s carbon, nitrogen, and phosphorus pool and additionally their significance for CO2 sequestration within the studied regenerated terrestrial ecosystems.
1. Introduction
There are many studies on the rate of decomposition of fine roots and litter and their share of the pool of accumulated carbon and nutrients at different climatic zones. However, few research results have been published on the rate of decomposition of fine roots and litter (as well as the accumulation of soil organic matter) in conditions of ecosystem regeneration following large-scale decay or fire phenomena [1,2,3,4] or reclamation of post-mining areas [5,6]. The decomposition of below-ground and above-ground plant biomass is an important factor influencing carbon and nutrient cycling rates in forest ecosystems [7,8,9]. It is estimated that about half of the net primary biomass production of forest ecosystems returns to the soil with litterfall [10]. As a result of the litter decomposition, soil organic matter (SOM) is formed, which contributes to the nutrient cycle and increases soil fertility [11]. Numerous studies have shown that the nutrients released as a result of the litterfall account for 70% to 90% of the total nutrient requirements of forest plants [12]. Leaf litter decomposition is intensified by biotic and abiotic factors [13,14]. The main factor influencing the rate of litter decomposition on a global scale is the climate [15]. However, on a local scale, the influence of climate on litter decomposition is of marginal importance, giving way to litter chemistry and soil chemical properties [16].
Fine roots play an important role, not only in the nutrient and water uptake by plants, but in the circulation of nutrients in the ecosystem [17]. Organic matter derived from fine root decomposition can lead to greater carbon stabilization and the release of a larger pool of nutrients than leaf litter [18]. Although the proportion of fine roots is comparable to that of leaf litter [19], few studies on biomass decomposition have focused on fine roots [20,21]. The main drivers of fine root decomposition are the macronutrient content, especially carbon, nitrogen and phosphorus, and their relationships (C:N and N:P ratios). Some information about the role of calcium content in this context was also given [22,23].
Thus, the annual decay of fine roots is an important link in the circulation of matter and energy in the ecosystem, and moreover, it can compensate for nutrient deficiencies in forest soils [24,25]. Larger particles with a lower lignin content undergo faster decomposition [26], while decreased soil moisture caused by increased air temperature has been shown to contribute to a reduction in the biomass of detritus microorganisms [27].
The distribution of fine roots and leaf litter is responsible for the accumulation and stabilization of soil organic matter, which is crucial for the effective restoration of soil in the initial stages of regeneration and reconstruction of forest ecosystems. The process of soil regeneration in post-fire areas results from changes in the accumulation of soil organic matter (SOM) [28,29,30] as well as alterations in the biomass and activity of soil bacteria [31,32,33]. In areas where fires occur, as a result of high temperature, the soil chemistry changes due to a sudden increase in pH and a jump in the availability of nutrients [34,35,36]. These changes are short-lived, mainly due to erosion losses caused by wind and surface runoff [37,38]. On the other hand, areas where soil reconstruction processes have taken place after opencast mining of deposits differ significantly from naturally formed soils. Soils resulting from mining activities are characterized by a lower content of soil organic matter, nitrogen and phosphorus deficiency, lower microbial activity, and, frequently, high toxicity [39,40].
Knowledge of the rate of fine root decomposition and litter can significantly accelerate the efficient circulation of nutrients in restored and regenerated ecosystems. Thus, the present study determined the rate of decomposition of fine root and litter in stands of different species, composed of Scots pine, common birch, and European larch. Moreover, the effects of fine root and litter decomposition on the accumulation of carbon, nitrogen, and phosphorus in various scenarios of regeneration and reconstruction of forest ecosystems were compared.
2. Materials and Methods
2.1. Study Sites and Field Study
The research was carried out on permanent research plots occurring in areas of large-scale disturbances such as fires (PF) and reclaimed post-mining areas (PM). The plots were located in solid single-species stands: birch stands, pine stands, and larch stands. The control plots were located on Podzolic soils in the managed forests of the Olkusz Forest District (CS) (Figure 1).
Figure 1.
Localization.
Following the fire of 1992, the 1200 ha PF area in the Olkusz Forest District is currently covered by 28-year-old tree stands as a result of artificial regeneration. Average annual air temperature in this region is approximately 7.6 °C, and average annual rainfall is around 737 mm.
In the PM area, the Szczakowa mine sands excavation area is currently covered in 30-year-old stands of common birch, Scots pine, and European larch, occurring in 30 m-wide strips. Average annual air temperature in this region is approximately 8.9 °C, and average annual rainfall is around 731 mm.
In the designated research plots and on the control plot, 27 permanent single-area research plots (3 species × 3 terrain categories × 3 repetitions) were established for single-species stands. The litterbag method was used to study the decomposition rate of dead organic matter. For this purpose, 5 g portions of dried local leaf litter and fine roots were inserted into litterbags (15 × 15 cm) made of nylon mesh with a mesh diameter of 1 × 1 mm. The prepared litterbags were laid out at the beginning of the year in the organic horizon (leaf litter: six litterbags on the surface) or the mineral soil layer from 0 to 10 cm (fine roots: six bag on the surface). One litterbag was collected after four months, two litterbags after eight months, and three litterbags after twelve months. After being transported to the laboratory, the collected litterbags were analyzed for their chemical composition. The total biomass of fine roots was determined by the root ingrowth cores method [41]. Litter biomass measurements were made from a 1 × 1 m frame.
Before laboratory analyses were performed, leaf litter and fine root samples were extracted, cleaned, and dried to constant weight at 65 °C. The remaining small roots and leaf litter were extracted by hand using tweezers.
For root samples and leaf litter, nitrogen (N) and carbon (C) were determined using the TruMac® CNS analyzer (LECO Corporation, St. Joseph, MI, USA); after digestion in a mixture of HNO3 and HClO4 acids, the levels of phosphorus (P) in the extract were analyzed on the ICP-OES iCAP™ 6000 Series system (Thermo scientific, Waltham, MA, USA).
On each research plot, mixed soil samples were collected at depths of 0 to 10 cm and 10 to 30 cm from five points arrayed in an envelope arrangement; the samples collected in this way were used to create aggregate samples representative of each research plot. The collected soil samples were then transported to the laboratory.
Analysis of soil samples: soil grain size was determined by static laser diffraction using the Fritsch ANALYSETTE 22 apparatus (Fritsch GmbH, Idar-Oberstein, Germany); pH was analyzed by potentiometry in 1 M KCl; total C and N content was determined using the TruMac® CNS apparatus, as above; and content of total elemental magnesium (Mg), potassium (K), and P was determined after digestion in a mixture of HNO3 and HClO4, using the ICP-OES iCAP™ 6000 Series system.
2.2. Statistical Analysis
Statistical analysis was performed using Statistica 13.3 software (StatSoft, Inc., Tulsa, OK, USA, 2017). Two-way ANOVA was carried out, followed by the Tukey RIR test (at p < 0.05). The analysis included determination of the differences in the nutrient pool supplied to the soil by fine roots and leaves (C, N, P). Before starting the statistical analyses, the data were checked for normal distribution using the Shapiro–Wilk test followed by logarithmic transformation. Moreover, the data were tested for homogeneity of variance using the Brown–Forsythe test. Principal components analysis was performed in order to determine the factors influencing the distribution of the biomass of fine roots and leaf litter. The analyzed soil properties were pH, grain size composition, and content of C, N, Mg, K, and P.
3. Study Results and Discussion
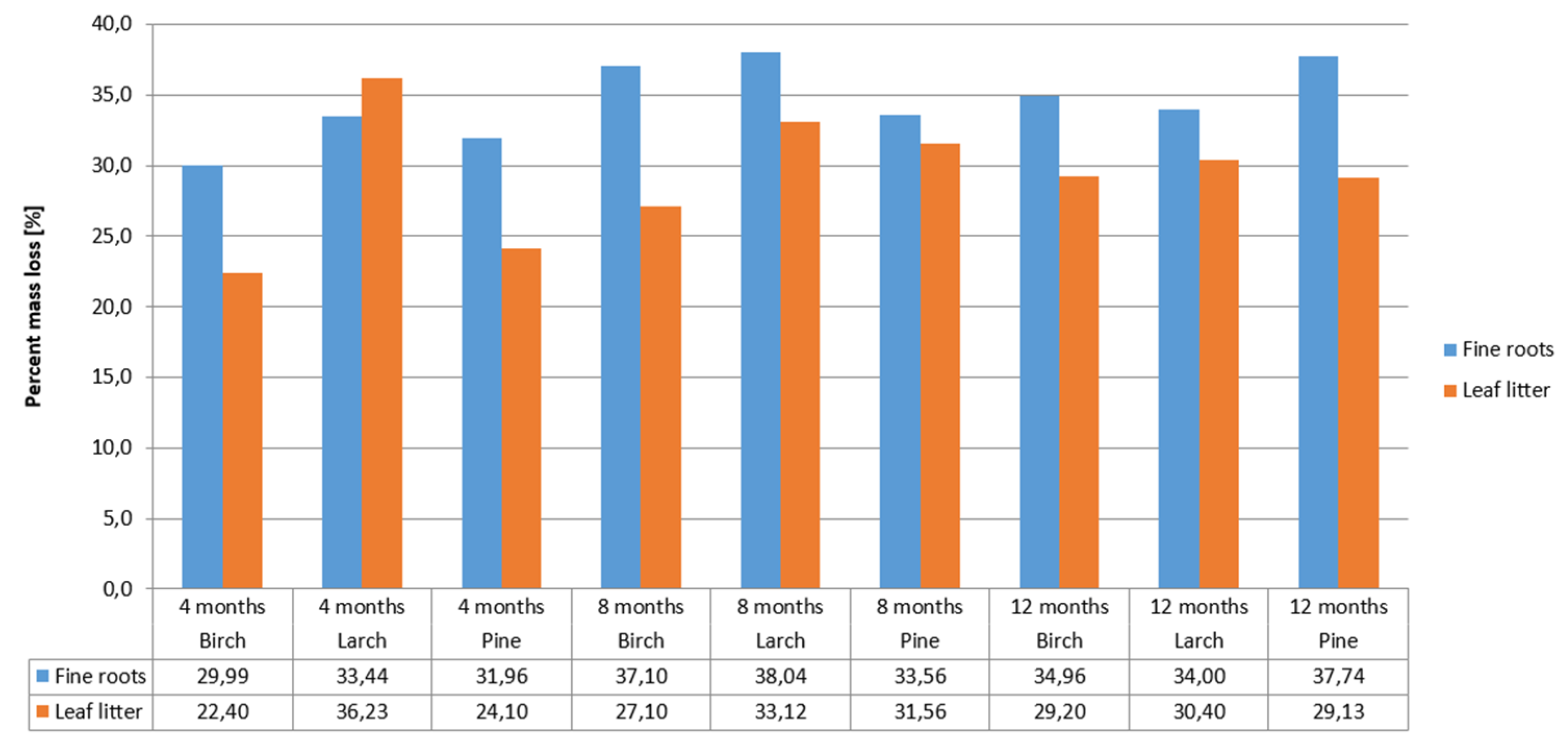
The rate of decomposition of leaf litter and fine roots depends on many biotic and abiotic factors [42]. In our research, the rate of decomposition of fine roots and leaf litter from birch, larch, and pine did not differ. The greatest loss of mass of fine roots and leaf litter occurred in the first four months of decomposition (March to June). The mass loss of fine roots was approximately 31% after 4 months of decomposition, and 34% after 12 months of decomposition. Leaf litter mass loss was 27% after 4 months of decomposition, and 30% after 12 months (Figure 2).
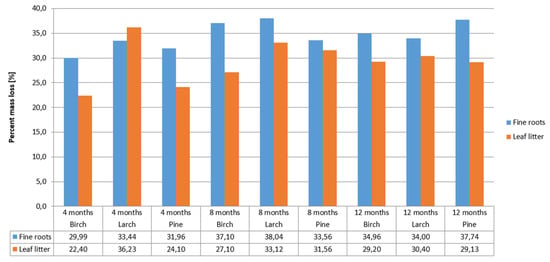
Figure 2.
Decomposition rate of leaf litter and fine roots of particular tree species.
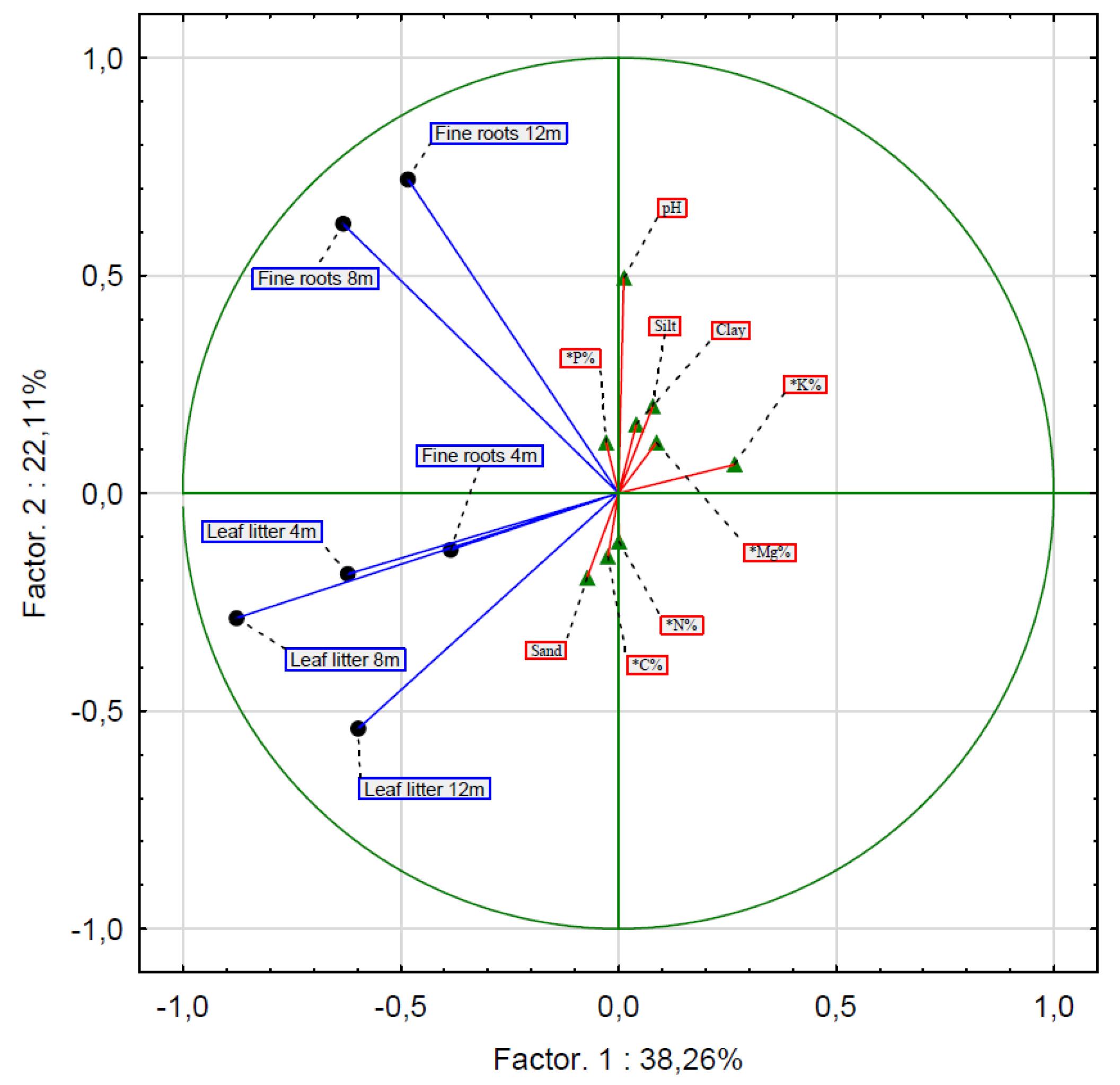
Moreover, none of the analyzed soil properties (C, N, Mg, K, P, pH, and grain size composition) affected the decomposition rate of fine roots and leaf litter. The rate of decomposition of fine roots after 8 and 12 months was influenced by factors other than the rate of leaf litter decomposition after 4, 8, and 12 months (Table 1; Figure 3).

Table 1.
Basic soil properties under birch, larch, and pine growing on reconstructed forest ecosystem (PM), regenerated forest ecosystem (PF), and control plots (CS) (mean ± SE) [43].
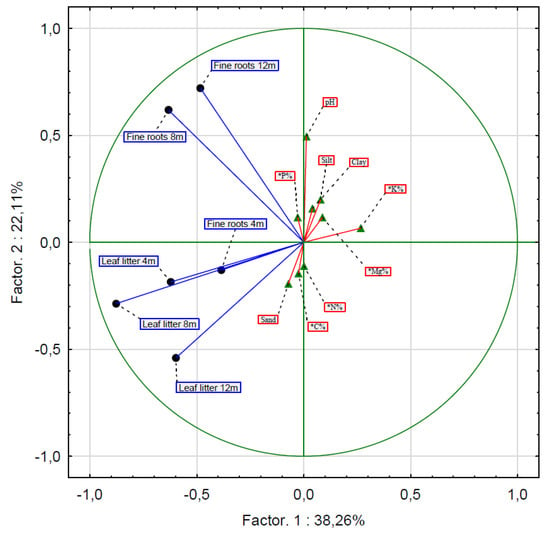
Figure 3.
Principal components analysis to determine the factors influencing the distribution of the biomass of fine roots and leaf litter (* Chemical properties of soils).
It is noted in the literature that the mesh size of the litterbags used in such experiments could be an important factor influencing the pace of fine root decomposition. In some experiments, the mesh size was up to 1 cm, which allowed macro fauna to crush and fragment the litter [44,45,46]. Moreover, the rate of litter decomposition is influenced by soil microorganisms [47]. In reconstructed and regenerated forest ecosystems, the litter decomposition process may be slower as a result of insufficiently well-developed communities of soil microorganisms and mesofauna [48] and low biological activity in the soil [49].
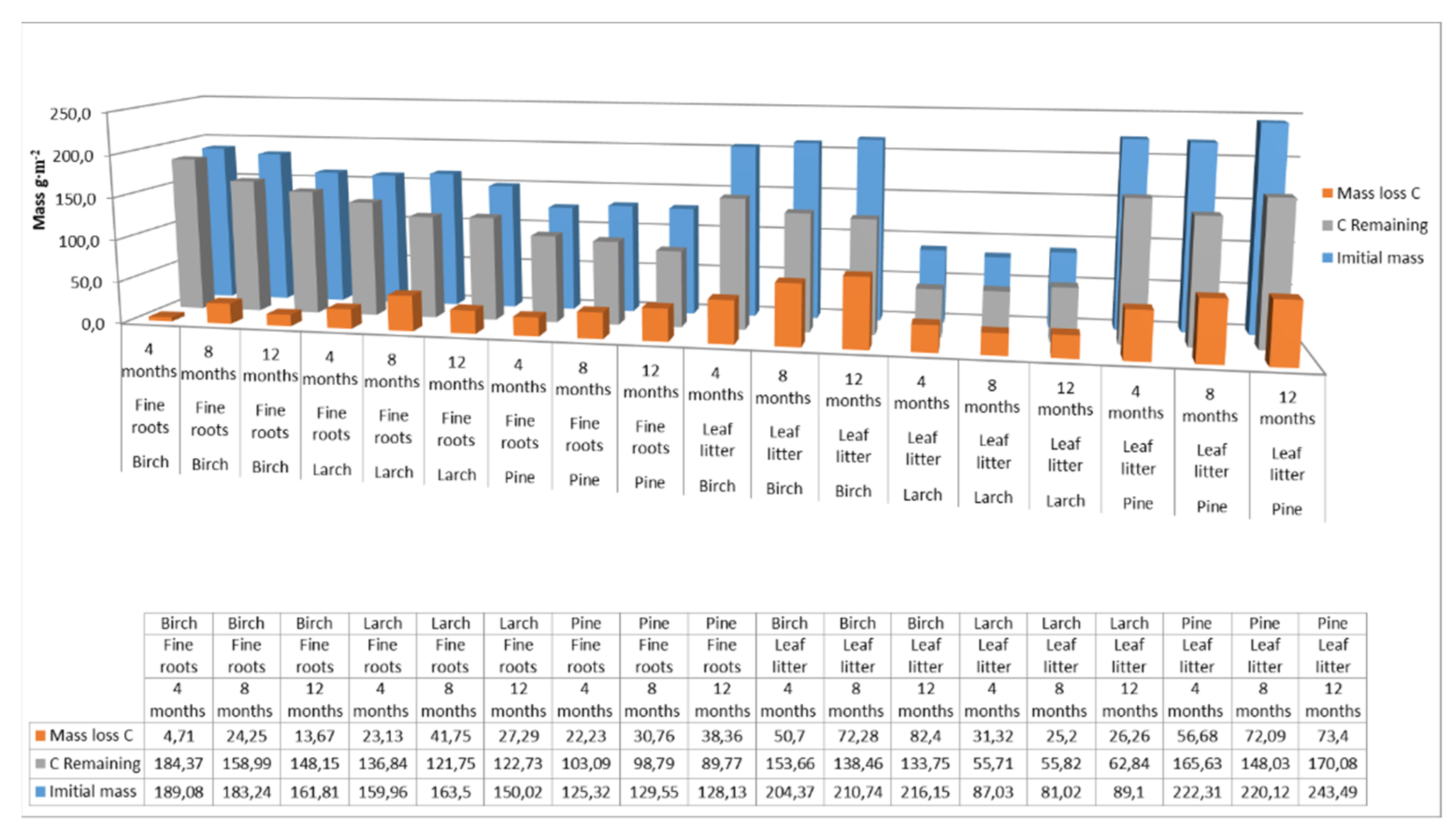
The carbon pool supplied to the soil by the fine roots was 177 g m−2 for birch, 144 g m−2 for larch, and 127 g m−2 for pine. The initial biomass of the fine roots was 556 g m−2 for birch, 437 g m−2 for larch, and 340 g m−2 for pine. After a year of decomposition, the amount of carbon remaining in the fine roots was 148 g m−2 for birch, 122 g m−2 for larch, and 89 g m−2 for pine. For leaf litter, the carbon pool supplied to the soil was 210 g m−2 for birch, 86 g m−2 for larch, and 228 g m−2 for pine. The initial biomass of the litter was 470 g m−2 for pine, 192 g m−2 for larch, and 445 for birch g m−2. After one year of decomposition, the amount of carbon remaining in the leaf litter was 133 g m−2 for birch, 62 g m−2 for larch, and 170 g m−2 for pine. Over this one-year decomposition period, the fine roots of birch and larch therefore released less carbon than leaf litter. Carbon mass-loss rates were 16% for fine birch roots and 15% for larch roots, while for leaf litter the rates were 36% for birch and 27% for larch. Carbon mass-loss rates for leaf litter and fine pine roots were comparable, at around 25% (Figure 4).
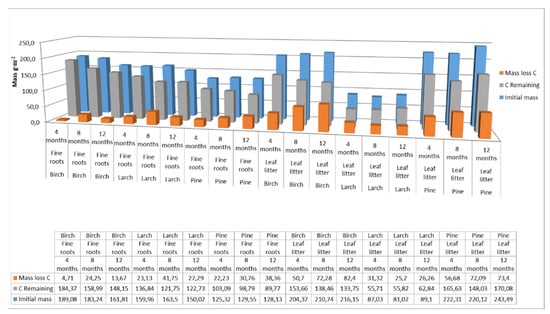
Figure 4.
Carbon pool supplied to soils with fine roots and leaf litter at different decomposition times for different species. (Initial mass—the initial mass of carbon accumulated in fine roots or leaf litter at the end of the growing season; C Remaining—carbon accumulated in fine roots or leaf litter after 4, 8 or 12 months of decomposition; Mass loss C—loss of carbon from the fine roots or leaf litter after a specified decomposition time).
The obtained research results prove that small tree roots can act as carbon reservoirs in regenerated and reconstructed forest ecosystems [50]. Knowledge about the pool of carbon released into the soil during the decomposition of fine roots and leaves in regenerated and reconstructed forest ecosystems over short time intervals remains incomplete [16]. However, there are currently many results from long-term experiments on the slow loss of carbon mass. Data from an experiment in the northern forests of Canada showed that carbon loss is exponential. After two years of decomposition, approximately 40% of carbon remains in birch litter, and around 60% remains in pine litter [16]. For most tree species, after losing about 70%–80% of the original carbon mass, further decomposition of leaf litter becomes very slow. On this basis, it can be concluded that approximately 20%–30% of the carbon pool accumulated in biomass is the soil organic matter pool [16]. A similar phenomenon of carbon release from forest ecosystems has also been described for Scandinavian forests [51]. Carbon loss curves determined on the basis of many years of research have been shown to converge to one point after several years of decomposition, despite significant differences in the initial rate of decomposition of different types of leaf litter [16]. Faster carbon loss from leaf litter in natural forest ecosystems may result from the fact that the most stable carbon-containing compounds are formed as a result of biological processes and chemical reactions, resulting in partial decomposition, followed by the synthesis of secondary compounds that are difficult to decompose; soil biological activity in regenerated and reconstructed forest soils is much lower than in natural soils, therefore resulting in the slower decomposition of leaf litter and leading to slower carbon loss from leaf litter [9,49,52].
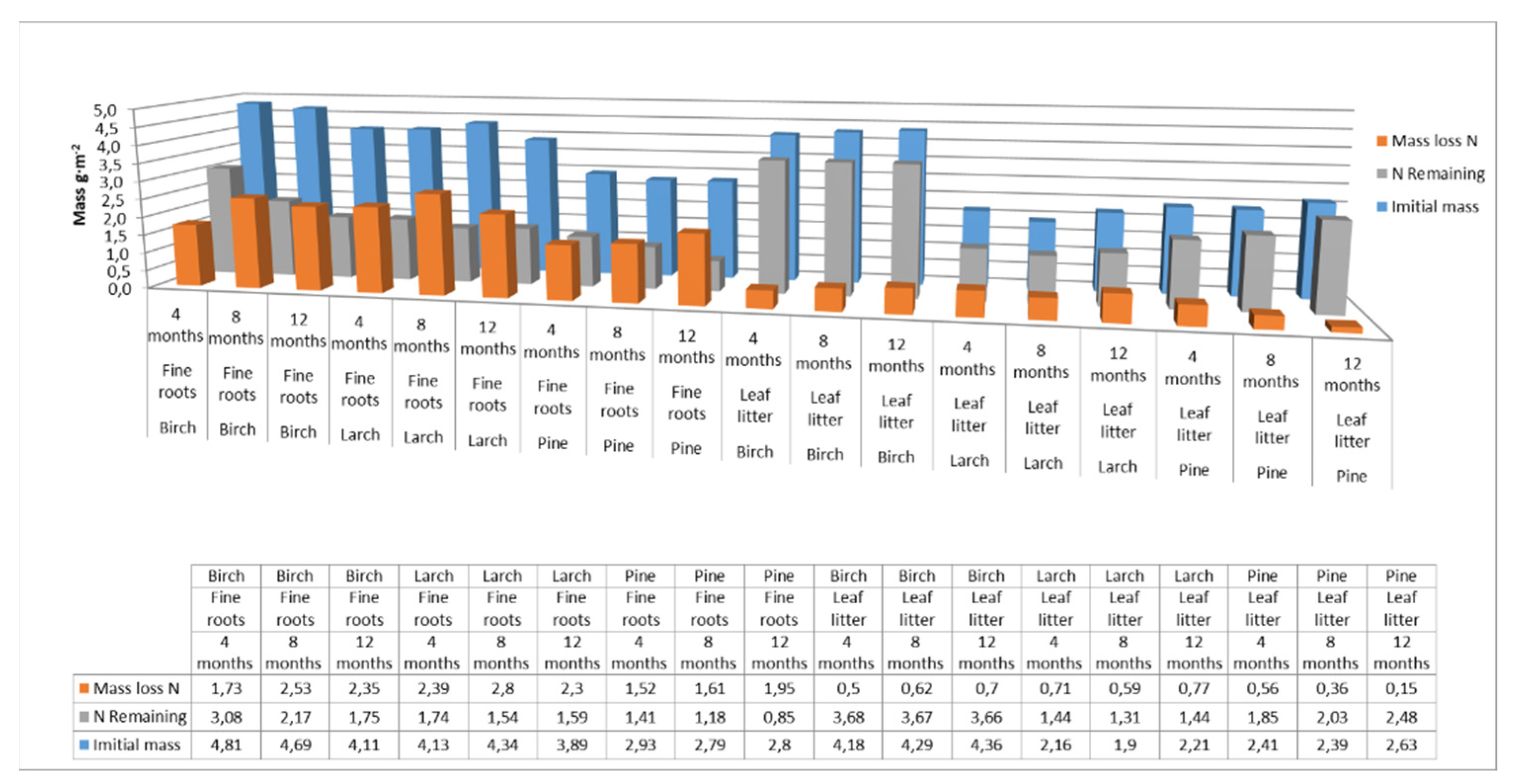
The nitrogen pools supplied to soils by fine roots and leaf litter were comparable, amounting to approximately 4 g m−2 for birch, 3 g m−2 for larch, and 2.5 g m−2 for pine. However, larch, pine, and birch fine roots released more nitrogen than leaf litter over the course of the year. Nitrogen mass-loss rates from the decomposition of fine roots were 48% for birch and 60% for larch and pine over the one-year study period. For the decomposition of leaf litter, nitrogen mass-loss rates for soils were 14% for pine and birch and 33% for larch, over the same period (Figure 5).
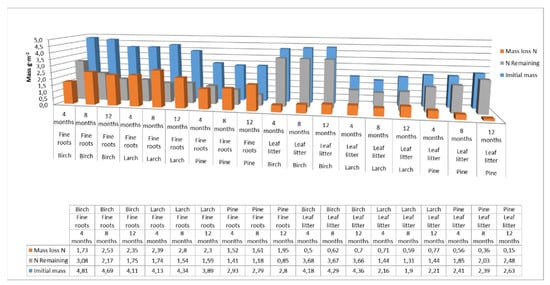
Figure 5.
Nitrogen pool supplied to soils with fine roots and leaf litter at different decomposition times for different species. (Initial mass—the initial mass of nitrogen accumulated in fine roots or leaf litter at the end of the growing season; N Remaining—nitrogen accumulated in fine roots or leaf litter after 4, 8 or 12 months of decomposition; Mass loss N—loss of nitrogen from the fine roots or leaf litter after a specified decomposition time).
Fine roots affect the activity of extracellular enzymes, and may therefore be responsible for the rapid circulation of nitrogen in the soil, as well as the rapid turnover of nitrogen-containing organic compounds, thus increasing nitrogen availability [53,54]. The decomposition of organic matter by extracellular enzymes in combination with symbiotic mycorrhizae increases the rate of nitrogen circulation and turnover in forest ecosystems, while an increase in the decomposition of organic matter may increase nitrogen mineralization [55]. In the forest soils of the temperate climate zone, the activity of fine roots may account for up to 30% of total nitrogen mineralization [56].
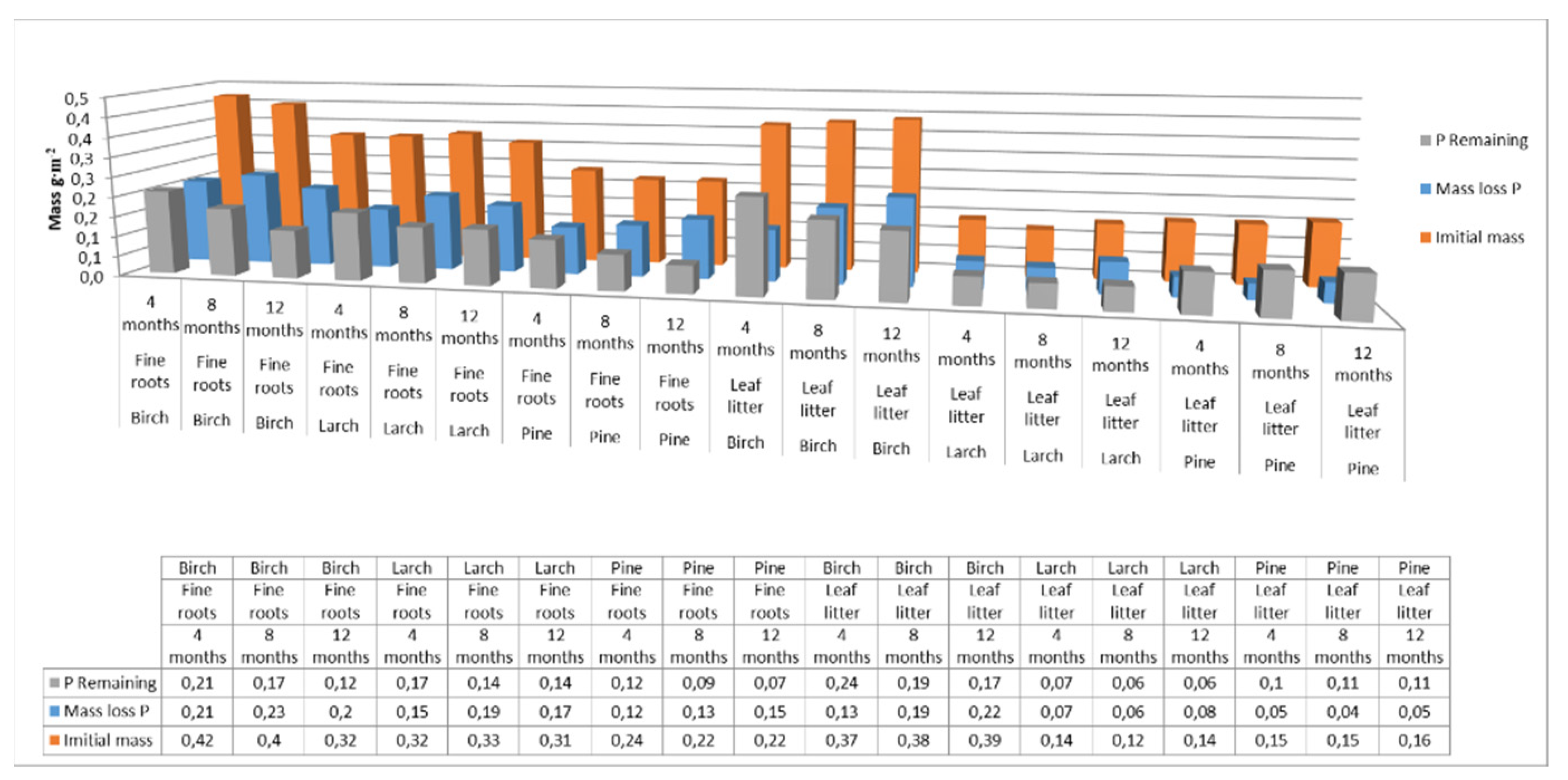
For phosphorus, the mass-loss rates for fine roots were 44% for birch, 50% for larch, and 40% for pine, while for leaf litter the rates were 52% for birch, 46% for larch, and 73% for pine, over the one-year study period (Figure 6).
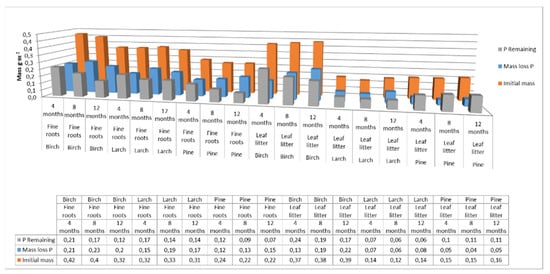
Figure 6.
Phosphorus pool supplied to soils with fine roots and leaf litter at different decomposition times for different species. (Initial mass—the initial mass of phosphorus accumulated in fine roots or leaf litter at the end of the growing season; P Remaining—phosphorus accumulated in fine roots or leaf litter after 4, 8 or 12 months of decomposition; Mass loss C—loss of phosphorus from the fine roots or leaf litter after a specified decomposition time).
Similar to nitrogen, the concentration in leaf litter of phosphorus increases during the decomposition of leaf litter [57]. The low initial concentration of phosphorus in fine roots and litter indicates that this element is highly susceptible to leaching [58]. In addition, phosphorus mineralizes very quickly in leaf litter, creating organic complexes characterized by a much slower decomposition rate [59]. Studies have shown that the rate of phosphorus release from litter depends on the initial phosphorus concentration [57]; in litter with a phosphorus concentration of 0.127%, the loss of phosphorus was found to be comparable to the loss of carbon [57]. Gusewell and Verhoeven [60] suggested that the lower limit below which all phosphorus is bound in structured material is 0.022%.
4. Conclusions
Compared with leaf litter, birch and larch fine roots showed lower rates of carbon mass loss over a one-year period of decomposition. The highest carbon mass-loss rates for fine roots were observed in regenerated forest ecosystems, while for leaf litter, the highest rates were seen under control conditions. These research results prove the important role of fine roots in enriching soils with nitrogen and phosphorus. The nitrogen pools supplied to soils by fine roots and leaf litter were comparable for birch, larch, and pine. Comparing the various scenarios of regeneration and reconstruction of forest ecosystems over the study period, nitrogen mass-loss rates from fine roots were about half that of the initial nitrogen mass, while for leaf litter the rates were clearly less. The phosphorus mass-loss rates for fine roots were comparable to leaf litter. The phosphorus mass-loss rates were the highest for pine leaf litter.
Author Contributions
Conceptualization, B.Ś. and M.P.; methodology, B.Ś. and M.P.; software, B.Ś. and M.P.; validation, B.Ś. and M.P.; formal analysis, B.Ś. and M.P.; investigation, B.Ś. and M.P.; resources, B.Ś. and M.P.; data curation, B.Ś. and M.P.; writing—original draft preparation, B.Ś. and M.P.; writing—review and editing, B.Ś. and M.P.; visualization, B.Ś. and M.P.; supervision, B.Ś. and M.P.; project administration, B.Ś. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed by the National Science Centre, Poland, grant no. 2019/33/N/ST10/02509, and the University of Agriculture in Kraków—founds of activating the scientific achievements A 465.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Angst, G.; Mueller, K.E.; Eissenstat, D.M.; Trumbore, S.; Freeman, K.H.; Hobbie, S.E.; Chorover, J.; Oleksyn, J.; Reich, P.B.; Mueller, C.W. Soil organic carbon stability in forests: Distinct effects of tree species identity and traits. Glob. Chang. Biol. 2019, 25, 1529–1546. [Google Scholar] [CrossRef]
- Binkley, D.; Giardina, C.; Bashkin, M. Soil phosphorus pools and supply under the influence of Eucalyptus saligna and nitrogen-fixing Albizia facaltaria. For. Ecol. Manag. 2000, 128, 241–247. [Google Scholar] [CrossRef]
- Vesterdal, L.; Schmidt, I.K.; Callesen, I.; Nilsson, L.O.; Gundersen, P. Carbon and nitrogen in forest floor and mineral soil under six common European tree species. For. Ecol. Manag. 2008, 255, 35–48. [Google Scholar] [CrossRef]
- Woś, B.; Józefowska, A.; Likus-Cieślik, J.; Chodak, M.; Pietrzykowski, M. Effect of tree species and soil texture on the carbon stock, macronutrient content, and physicochemical properties of regenerated postfire forest soils. Land Degrad. Dev. 2021, 32, 5227–5240. [Google Scholar] [CrossRef]
- Frouz, J. The effect of litter type and macrofauna community on litter decomposition and organic matter accumulation in post-mining sites. Biologia 2008, 63, 249–253. [Google Scholar] [CrossRef]
- Misebo, A.M.; Pietrzykowski, M.; Woś, B. Soil Carbon Sequestration in Novel Ecosystems at Post-Mine Sites—A New Insight into the Determination of Key Factors in the Restoration of Terrestrial Ecosystems. Forests 2022, 13, 63. [Google Scholar] [CrossRef]
- Aerts, R. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: A triangular relationship. Oikos 1997, 79, 439–449. [Google Scholar] [CrossRef]
- Parton, W.; Silver, W.L.; Burke, I.C.; Grassens, L.; Harmon, M.E.; Currie, W.S.; King, J.Y.; Adair, E.C.; Brandt, L.A.; Hart, S.C.; et al. Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 2007, 315, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Bird, J.A.; Kleber, M.; Torn, M.S. 13C and 15N stabilization dynamics in soil organic matter fractions during needle and fine root decomposition. Org. Geochem. 2008, 39, 465–477. [Google Scholar] [CrossRef]
- Baishya, R.; Barik, S.K. Estimation of tree biomass, carbon pool and net primary production of an old-growth Pinus kesiya Royle ex. Gordon forest in north-eastern India. Ann. For. Sci. 2011, 68, 727–736. [Google Scholar] [CrossRef] [Green Version]
- Koehler, B.; Tranvik, L.J. Reactivity continuum modeling of leaf, root, and wood decomposition across biomes. J. Geophys. Res. Biogeosciences 2015, 120, 1196–1214. [Google Scholar] [CrossRef] [Green Version]
- Waring, R.H.; Schlesinger, W.H. Forest ecosystems. In Analysis at Multiples Scales; Elsevier: Amsterdam, The Netherlands, 1985; p. 55. [Google Scholar]
- Chen, Y.; Ma, S.; Jiang, H.; Yangzom, D.; Cheng, G.; Lu, X. Decomposition time, chemical traits and climatic factors determine litter-mixing effects on decomposition in an alpine steppe ecosystem in Northern Tibet. Plant Soil 2019, 459, 23–35. [Google Scholar] [CrossRef]
- Moinet, G.Y.K.; Moinet, M.; Hunt, J.E.; Rumpel, C.; Chabbi, A.; Millard, P. Temperature sensitivity of decomposition decreases with increasing soil organic matter stability. Sci. Total. Environ. 2020, 704, 135460. [Google Scholar] [CrossRef] [PubMed]
- Wall, D.H.; Bradford, M.A.; John, M.G.S.; Behan-Pelletier, V.; Bignell, D.E.; Dangerfield, J.M.; Parton, W.J.; Rusek, J.; Voigt, W.; Wolters, V.; et al. Global decomposition experiment shows soil animal impacts on decomposition are climate dependent. Glob. Chang. Biol. 2008, 14, 2661–2677. [Google Scholar] [CrossRef] [Green Version]
- Prescott, C.E. Litter decomposition: What controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 2010, 101, 133–149. [Google Scholar] [CrossRef]
- Röderstein, M.; Hertel, D.; Leuschner, C. Above- and below-ground litter production in three tropical montane forests in southern Ecuador. J. Trop. Ecol. 2005, 21, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Vogt, K.A.; Grier, C.C.; Vogt, D.J. Production, turnover, and nutrient dynamics of above- and belowground detritus of world forests. Adv. Ecol. Res. 1986, 15, 303–377. [Google Scholar] [CrossRef]
- Gill, R.A.; Jackson, R.B. Global patterns of root turnover for terrestrial ecosystems. New Phytol. 2000, 147, 13–31. [Google Scholar] [CrossRef]
- Goebel, M.; Hobbie, S.E.; Bulaj, B.; Zadworny, M.; Archibald, D.D.; Oleksyn, J.; Reich, P.B.; Eissenstat, D.M. Decomposition of the finest root branching orders: Linking carbon and nutrient dynamics belowground to fine root function and structure. Ecol. Monogr. 2011, 81, 89–102. [Google Scholar] [CrossRef]
- Freschet, G.T.; Cornwell, W.K.; Wardle, D.A.; Elumeeva, T.G.; Liu, W.; Jackson, B.G.; Onipchenko, V.G.; Soudzilovskaia, N.A.; Tao, J.; Cornelissen, J.H. Linking litter decomposition of above- and below-ground organs to plant-soil feedbacks worldwide. J. Ecol. 2013, 101, 943–952. [Google Scholar] [CrossRef]
- Silver, W.L.; Miya, R.K. Global patterns in root decomposition: Comparisons of climate and litter quality effects. Oecologia 2001, 129, 407–419. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Walker, L.R.; Bonner, K.I. Among- and within-species variation in litter decomposition in contrasting long-term chronosequences. Funct. Ecol. 2009, 23, 442–453. [Google Scholar] [CrossRef]
- Brunner, I.; Godbold, D.L. Tree roots in a changing world. J. For. Res. 2007, 12, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Harmon, M.E.; Sexton, J.; Fasth, B. Fine root decomposition and N dynamics in coniferous forests of the Pacific Northwest, USA. J. For. Res. 2002, 32, 320–331. [Google Scholar] [CrossRef]
- Berbeco, M.R.; Melillo, J.M.; Orians, C.M. Soil warming accelerates decomposition of fine woody debris. Plant Soil 2012, 356, 405–417. [Google Scholar] [CrossRef]
- Allison, S.D.; Treseder, K.K. Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Glob. Chang. Biol. 2008, 14, 2898–2909. [Google Scholar] [CrossRef] [Green Version]
- Francos, M.; Úbeda, X.; Pereira, P.; Alcaniz, M. Long-term impact of wild fire on soils exposed to different fire severities. A case study in Cadiretes Massif (NE Iberian Peninsula). Sci. Total Environ. 2018, 615, 664–671. [Google Scholar] [CrossRef]
- Kong, J.J.; Yang, J.; Bai, E. Long-term effects of wild fire on available soil nutrient composition and stoichiometry in a Chinese boreal forest. Sci. Total Environ. 2018, 642, 1353–1361. [Google Scholar] [CrossRef]
- Simard, D.G.; Fyles, J.W.; Pare, D.; Nguyen, T. Impacts of clear cut harvesting and wild fire on soil nutrient status in the Quebec boreal forest. Can. J. Soil. Sci. 2001, 81, 229–237. [Google Scholar] [CrossRef] [Green Version]
- De Long, J.R.; Dorrepaal, E.; Kardol, P.; Nilsson, M.-C.; Teuber, L.M.; Wardle, D.A. Understory plant functional groups and litter species identity are stronger drivers of litter decomposition than warming along a boreal forest post fire successional gradient. Soil Biol. Biochem. 2016, 98, 159–170. [Google Scholar] [CrossRef]
- Lavoie, M.; Mack, M.C. Spatial heterogeneity of understory vegetation and soil in an Alaskan upland boreal forest fire chronosequence. Biogeochemistry 2012, 107, 227–239. [Google Scholar] [CrossRef]
- Longo, M.S.; Urcelay, C.; Nouhra, E. Long term effects of fire on ectomycorrhizas and soil properties in Nothofaguspumilio forests in Argentina. For. Ecol. Manag. 2011, 262, 348–354. [Google Scholar] [CrossRef]
- Brais, S.; David, P.; Ouimet, R. Impact of wildfire severity and salvage harvesting onthe nutrient balance of jack pine and black spruce boreal stands. For. Ecol. Manag. 2000, 137, 231–243. [Google Scholar] [CrossRef]
- Neary, D.G.; Klopatek, C.C.; DeBano, L.F.; Ffolliott, P.F. Fire effects on belowground sustainability: A review and synthesis. For. Ecol. Manag. 1999, 122, 51–71. [Google Scholar] [CrossRef]
- Turner, M.G.; Smithwick, E.A.; Metzger, K.L.; Tinker, D.B.; Romme, W.H. Inorganic nitrogen availability after severe stand-replacing fire in the Greater Yellowstone eco-system. Proc. Natl. Acad. Sci. USA 2007, 104, 4782–4789. [Google Scholar] [CrossRef] [Green Version]
- Fox, D.M.; Darboux, F.; Carrega, P. Topographic controls on soil properties affectingpost-fire erosion and sediment redistribution in a mixed forested-agricultural Medi-terranean catchment. Geophys. Res. Abstr. 2006, 8, 3–4. [Google Scholar]
- Müller, K.; Mason, K.; Strozzi, A.G.; Simpson, R.; Komatsu, T.; Kawamoto, K.; Kawamoto, K.; Clothier, B. Run off and nutrient loss from a water-repellent soil. Geoderma 2018, 322, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, R.K.; Lal, R. Changes in physical and chemical properties of soil after surface mining and reclamation. Geoderma 2011, 161, 168–176. [Google Scholar] [CrossRef]
- Macdonald, S.E.; Landhausser, S.M.; Skousen, J.; Franklin, J.; Frouz, J.; Hall, S.; Jacobs, D.F.; Quideau, S. Forest restoration following surface mining disturbance: Challenges and solutions. New For. 2015, 46, 703–732. [Google Scholar] [CrossRef] [Green Version]
- Świątek, B.; Chodak, M.; Pietrzykowski, M. Estimation of Fine Root Biomass of Alders Growing on Technosols Using Two Different Methods. Commun. Soil Sci. Plant Anal. 2019, 50, 474–481. [Google Scholar] [CrossRef]
- Horodecki, P.; Jagodziński, A.M. Tree species effects on litter decomposition in pure stands on afforested post-mining sites. For. Ecol. Manag. 2017, 406, 23–35. [Google Scholar] [CrossRef]
- Świątek, B.; Pietrzykowski, M. Soil factors determining the fine-root biomass in soil regeneration after a post-fire and soil reconstruction in reclaimed post-mining sites under different tree species. Catena 2021, 204, 105449. [Google Scholar] [CrossRef]
- Wang, S.J.; Ruan, H.H.; Han, Y. Effects of microclimate, litter type, and mesh size on leaf litter decomposition along an elevation gradient in the Wuyi Mountains, China. Ecol. Res. 2010, 25, 1113–1120. [Google Scholar] [CrossRef]
- Slade, E.M.; Riutta, T. Interacting effects of leaf litter species and macrofauna on decomposition in different litter environments. Basic Appl. Ecol. 2012, 13, 423–431. [Google Scholar] [CrossRef]
- Bokhorst, S.; Wardle, D.A. Microclimate within litter bags of different mesh size: Implications for the ‘arthropod effect’ on litter decomposition. Soil Biol. Biochem. 2013, 58, 147–152. [Google Scholar] [CrossRef]
- Mo, J.; Brown, S.; Xue, J.; Fang, Y.; Li, Z. Response of litter decomposition to simulated N deposition in disturbed, rehabilitated and mature forests in subtropical China. Plant Soil 2006, 282, 135–151. [Google Scholar] [CrossRef]
- Chodak, M.; Pietrzykowski, M.; Niklińska, M. Development of microbial properties in a chronosequence of sandy mine soils. Appl. Soil Ecol. 2009, 41, 259–268. [Google Scholar] [CrossRef]
- Frouz, J.; Roubíčková, A.; Heděnec, P.; Tajovský, K. Do soil fauna really hasten litter decomposition? A meta-analysis of enclosure studies. Eur. J. Soil Biol. 2015, 68, 18–24. [Google Scholar] [CrossRef]
- Świątek, B.; Woś, B.; Chodak, M.; Maiti, S.K.; Józefowska, A.; Pietrzykowski, M. Fine root biomass and the associated C and nutrient pool under the alder (Alnus spp.) plantings on reclaimed technosols. Geoderma 2019, 337, 1021–1027. [Google Scholar] [CrossRef]
- Berg, B.; Johansson, M.-B.; Nilsson, A.; Gundersen, P.; Norell, L. Sequestration of carbon in the humus layer of Swedish forests—direct measurements. Can. J. For. Res. 2009, 39, 962–975. [Google Scholar] [CrossRef]
- Grandy, A.S.; Neff, J.C. Molecular C dynamics downstream: The biochemical decomposition sequence and its impact on soil organic matter structure and function. Sci. Total Environ. 2008, 404, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Gutknecht, J.L.M.; Herman, D.J.; Keck, D.C.; Firestone, M.K.; Cheng, W. Rhizosphere priming effects on soil carbon and nitrogen mineralization. Soil Biol. Biochem. 2014, 76, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Meier, I.C.; Pritchard, S.G.; Brzostek, E.R.; Mccormack, M.L.; Phillips, R.P. The rhizosphere and hyphosphere differ in their impacts on carbon and nitrogen cycling in forests exposed to elevated CO2. New Phytol. 2015, 205, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, F.A.; Bader, N.E.; Johnson, D.W.; Cheng, W. Does accelerated soil organic matter decomposition in the presence of plants increase plant N availability? Soil Biol. Biochem. 2009, 41, 1080–1087. [Google Scholar] [CrossRef]
- Finzi, A.C.; Abramoff, R.Z.; Spiller, K.S.; Brzostek, E.R.; Darby, B.A.; Kramer, M.A.; Phillips, R.P. Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Global Chang. Biol. 2015, 21, 2082–2094. [Google Scholar] [CrossRef]
- Moore, T.R.; Trofymow, J.A.; Prescott, C.E.; Titus, B.D. Nature and nurture in the dynamics of C, N and P during litter decomposition in Canadian forests. Plant Soil 2011, 339, 163–175. [Google Scholar] [CrossRef]
- Ahtiainen, M.; Huttunen, P. Long-term effects of forestry managements on water quality and loading in brooks. Boreal Environ. Res. 1999, 4, 101–114. [Google Scholar]
- Devi, A.S.; Yadava, P.S. Wood and leaf litter decomposition of Dipterocarpus tuberculatus Roxb. In a tropical deciduous forest of Manipur. North East India. Curr. Sci. 2007, 93, 243–246. [Google Scholar]
- Güsewell, S.; Verhoeven, J.T.A. Litter N:P ratios indicate whether N or P limits the decomposability of graminoid leaf litter. Plant Soil 2006, 287, 131–143. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).