Sex-Specific Physiological Responses of Populus cathayana to Uranium Stress
Abstract
:1. Introduction
2. Methods
2.1. Planting of Populus cathayana and Experimental Treatments
2.2. Measurement of Gas Exchange Parameters
2.3. Measurement of Chlorophyll Fluorescence
2.4. Measurement of Soluble Proteins, Soluble Sugars, and Proline for P. cathayana
2.5. Measurement of Hydrogen Peroxide (H2O2), Malondialdehyde (MDA), Superoxide Dismutase (SOD), and Plant Peroxidase (POD)
2.6. Measurement Growth and Determination of U Enrichment in Leaves and Roots of P. cathayana
2.7. Statistical Analysis
3. Results
3.1. Distribution and Content of U for Female and Male of P. cathayana
3.2. Effects of U on the Growth of Female and Male Trees of P. cathayana
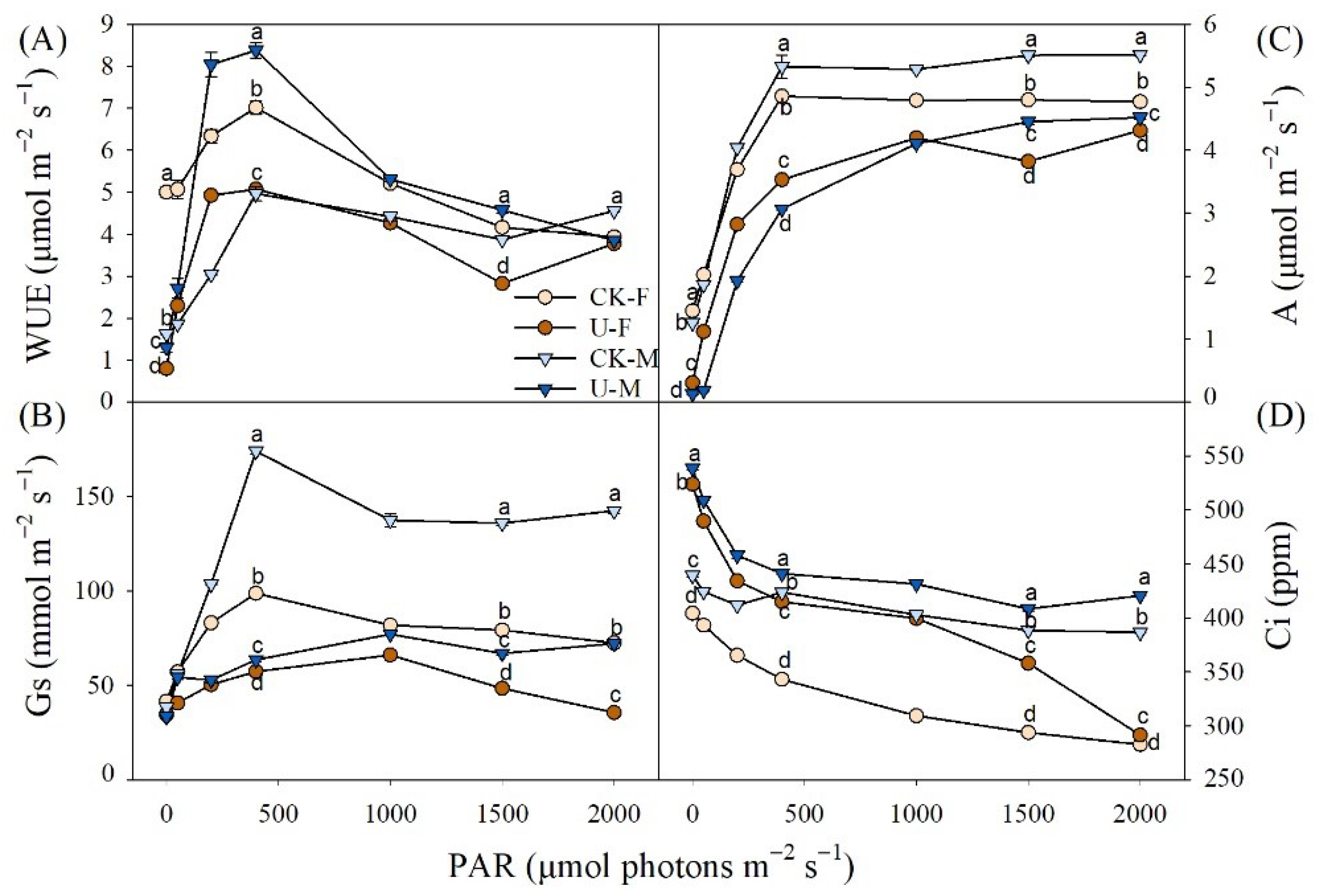
3.3. Effects of U on Gas Exchange between Male and Female of P. cathayana
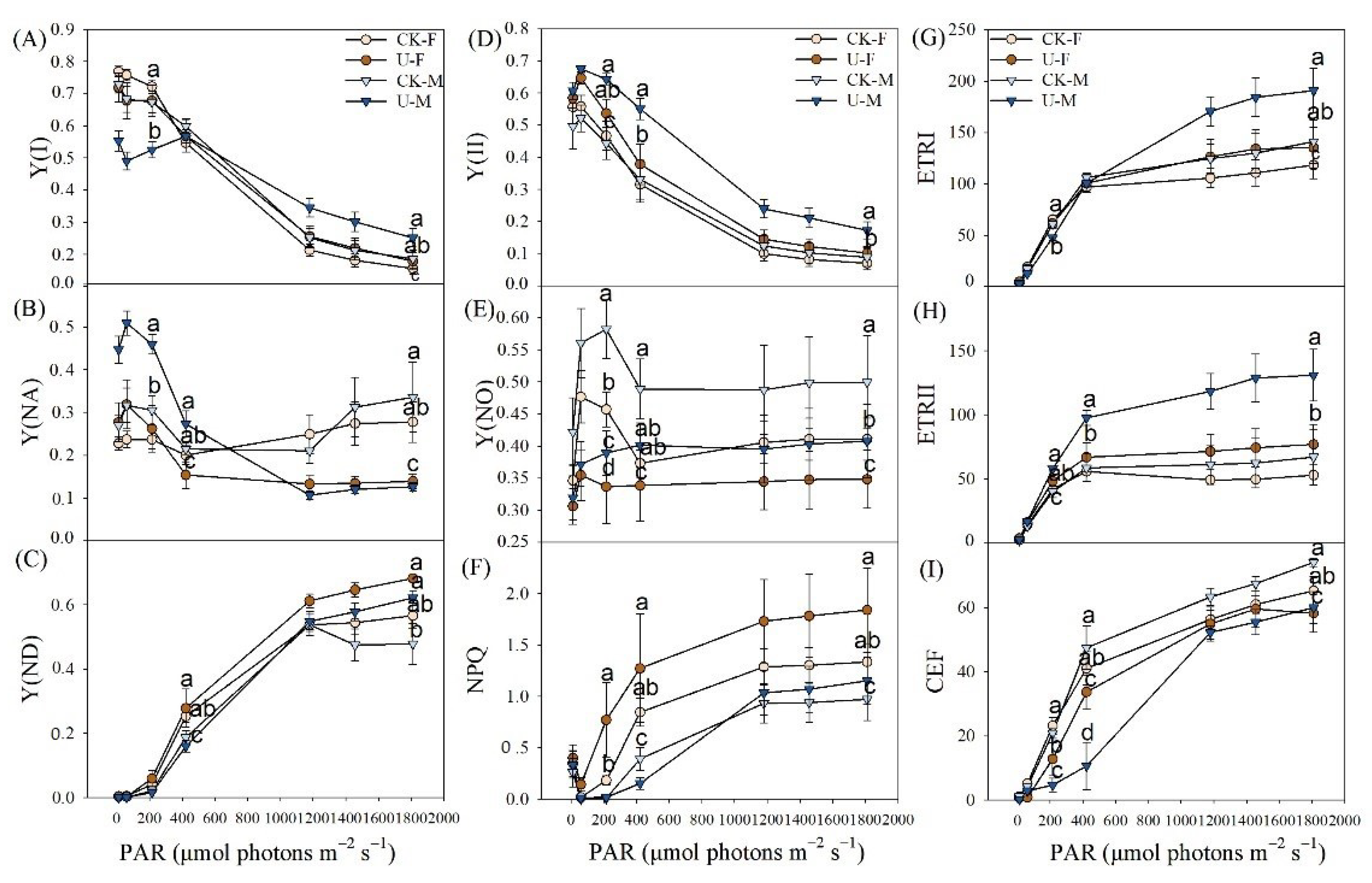
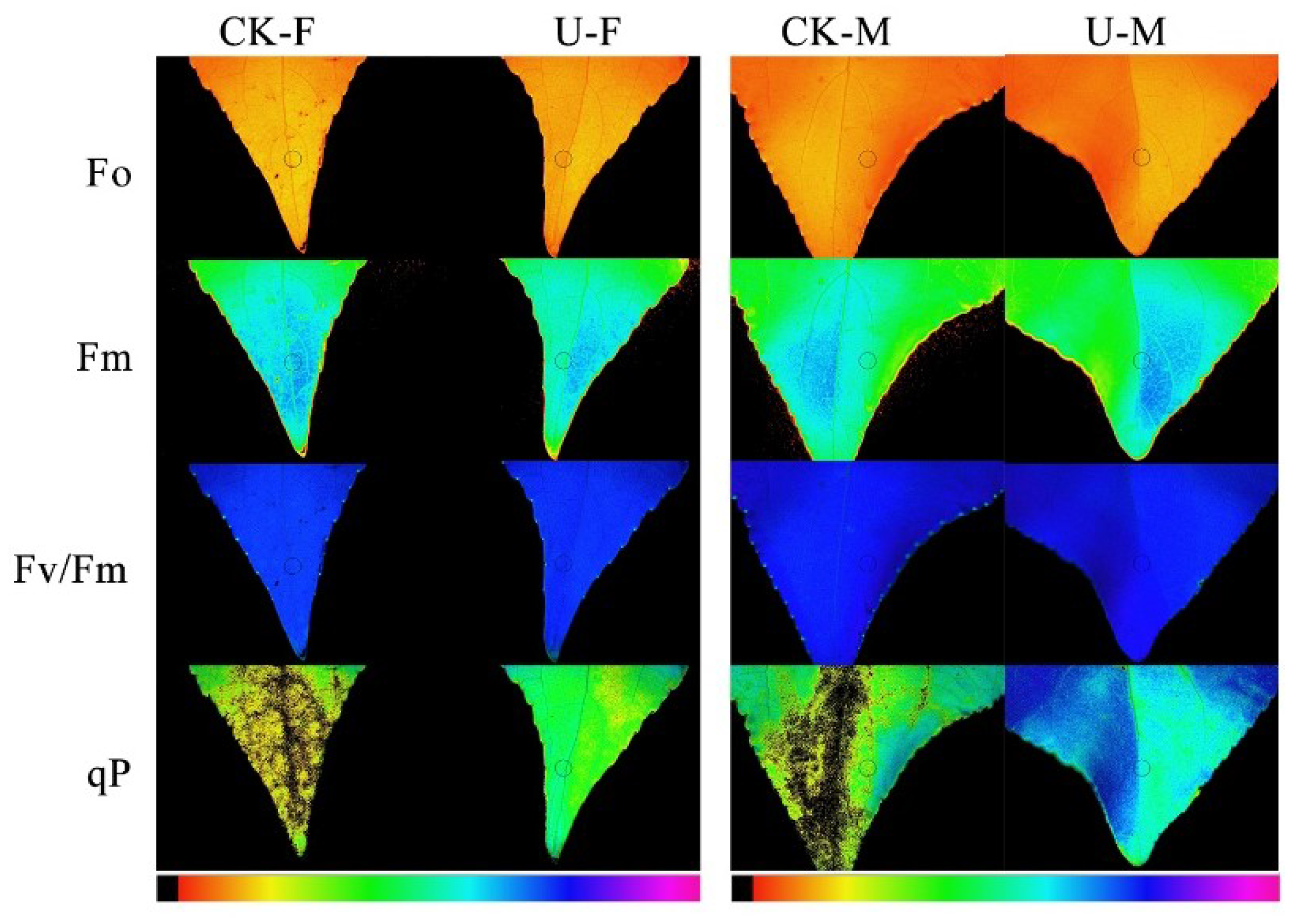
3.4. Effect of U on Chlorophyll Fluorescence for Female and Male of P. cathayana
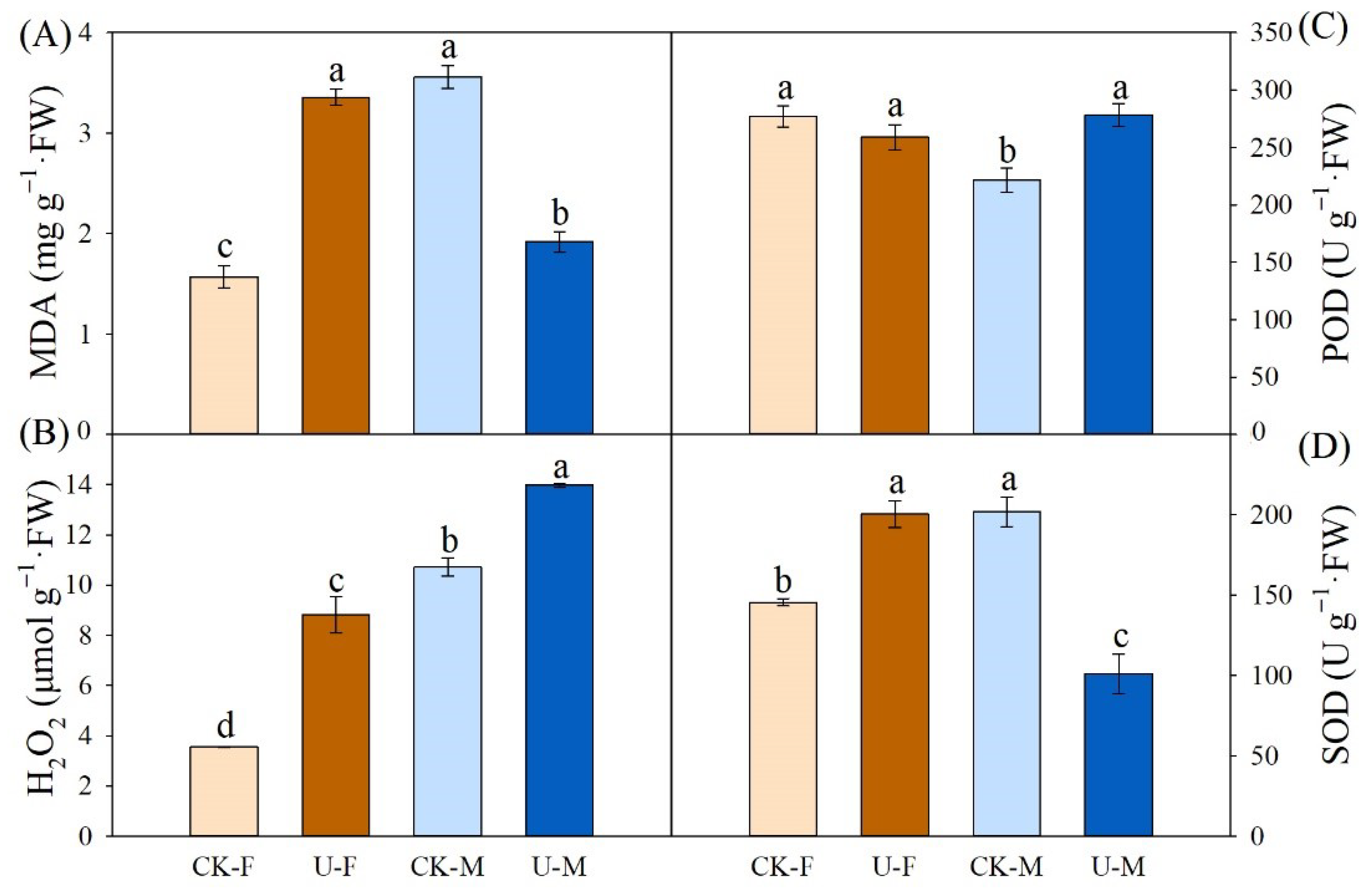
3.5. Effects of U on Contents of H2O2 and MDA and Activities of Antioxidant Enzymes in Leaves of P. cathayana
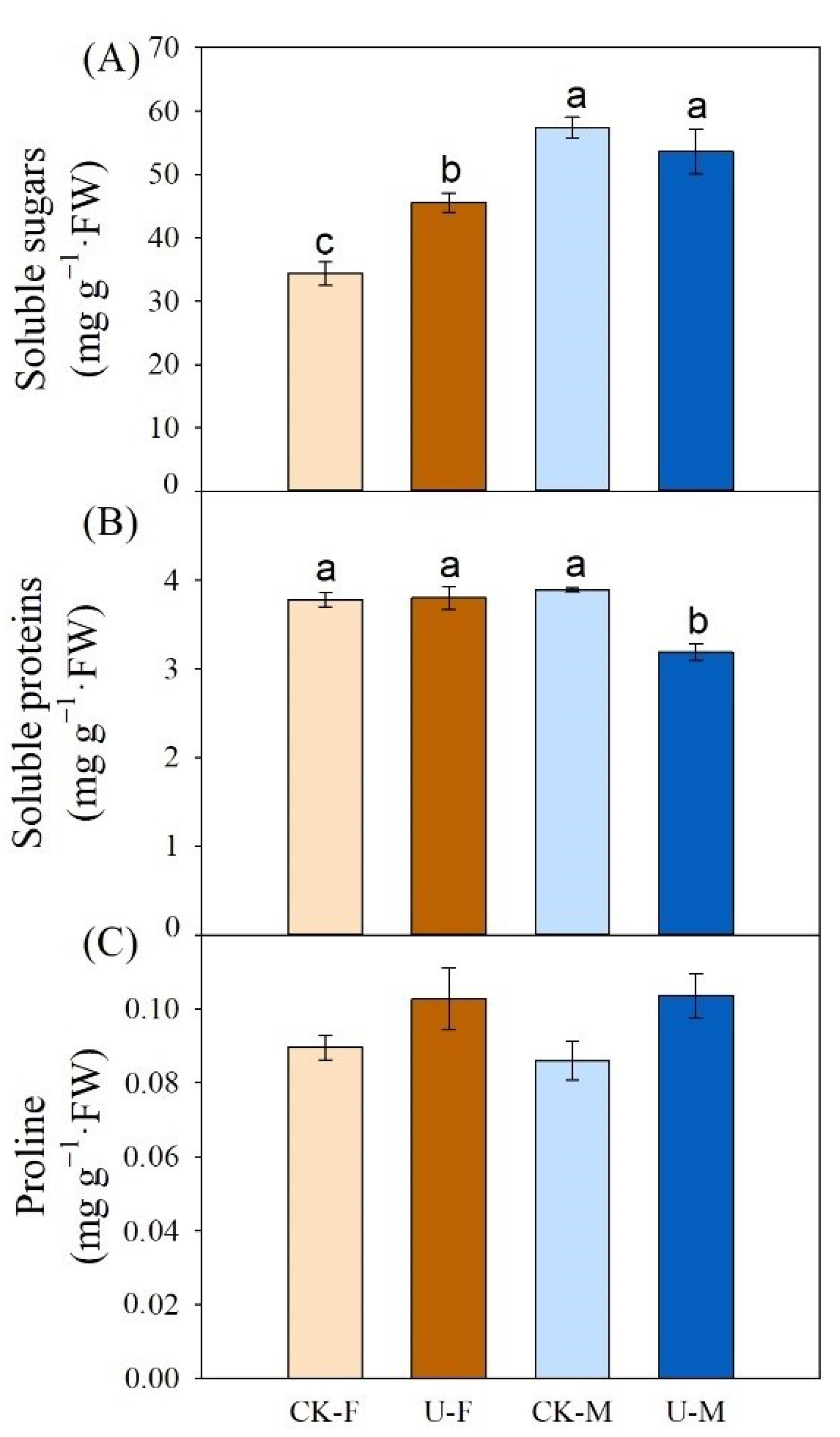
3.6. Effects of U on Soluble Sugars, Soluble Proteins, and Proline in Leaves of P. cathayana
4. Discussion
4.1. Sex Differences in Growth, U Enrichment, and Distribution
4.2. Sex Differences in Gas Exchange and Chlorophyll Fluorescence
4.3. Effects of U on ROS and Antioxidant Enzymes in Leaves of P. cathayana
4.4. Effects of U on Soluble Proteins, Soluble Sugars, and Proline
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mihucz, V.G.; Varga, Z.; Tatár, E.; Virág, I.; van Grieken, R.; Koleszár, Z.; Záray, G. Redistribution of uranium and thorium by soil/plant interaction in a recultivated mining area. Microchem. J. 2008, 90, 44–49. [Google Scholar] [CrossRef]
- Viehweger, K.; Geipel, G. Uranium accumulation and tolerance in Arabidopsis halleri under native versus hydroponic conditions. Environ. Exp. Bot. 2010, 69, 39–46. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.R.; Zhang, W.X.; Zhou, J.Q.; Luo, D.Q.; Li, Z.M. Uranium (U) source, speciation, uptake, toxicity and bioremediation strategies in soil-plant system: A review. J. Hazard. Mater. 2021, 413, 125319. [Google Scholar] [CrossRef] [PubMed]
- Thakare, M.; Sarma, H.; Datar, S.; Roy, A.; Pawar, P.; Gupta, K.; Pandit, S.; Prasad, R. Understanding the holistic approach to plant-microbe remediation technologies for removing heavy metals and radionuclides from soil. Curr. Res. Biotechnol. 2021, 3, 84–98. [Google Scholar] [CrossRef]
- Lomaglio, T.; Rocco, M.; Trupiano, D.; De Zio, E.; Grosso, A.; Marra, M.; Delfine, S.; Chiatante, D.; Morabito, D.; Scippa, G.S. Effect of short-term cadmium stress on Populus nigra L. detached leaves. J. Plant Physiol. 2015, 182, 40–48. [Google Scholar] [CrossRef]
- Redovnikovic, I.R.; De Marco, A.; Proietti, C.; Hanousek, K.; Sedak, M.; Bilandzic, N.; Jakovljevic, T. Poplar response to cadmium and lead soil contamination. Ecotoxicol. Environ. Saf. 2017, 144, 482–489. [Google Scholar] [CrossRef]
- Mishra, A. Phytoremediation of heavy metal-contaminated soils: Recent advances, challenges, and future prospects. In Bioremediation for Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2021; pp. 29–51. [Google Scholar]
- Straczek, A.; Duquene, L.; Wegrzynek, D.; Chinea-Cano, E.; Wannijn, J.; Navez, J.; Vandenhove, H. Differences in U root-to-shoot translocation between plant species explained by U distribution in roots. J. Environ. Radioact. 2010, 101, 258–266. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Gładysz, O.; Szentner, K.; Goliński, P. Role of glutathione in abiotic stress tolerance. In Oxidative Damage to Plants; Academic Press: Cambridge, MA, USA, 2014; pp. 149–181. [Google Scholar]
- Shalmani, A.; Ullah, U.; Muhammad, I.; Zhang, D.; Sharif, R.; Jia, P.; Saleem, N.; Gul, N.; Rakhmanova, A.; Tahir, M.M.; et al. The TAZ domain-containing proteins play important role in the heavy metals stress biology in plants. Environ. Res. 2021, 197, 1110–1130. [Google Scholar] [CrossRef]
- Li, F.L.; Bao, W.K. Responses of the morphological and anatomical structure of the plant leaf to environmental change. Chin. Bull. Bot. 2005, 22, 118–127. [Google Scholar]
- Sun, L.; Ji, N.N.; Mu, L.Q.; Xu, W.Y. Effect of heavy metal stress on leaf anatomical structure of Syringa microphylla. J. Northeast For. Univ. 2012, 40, 1–4+10. [Google Scholar]
- Bhusal, N.; Bhuasl, S.J.; Yoon, T.M. Comparisons of physiological and anatomical charaterstics between two cultivars in bi-leader apple trees (Malus × domestica Borkh.). Sci. Hortic. 2018, 231, 73–81. [Google Scholar] [CrossRef]
- Liao, J.; Cai, Z.Y.; Song, H.F.; Zhang, S. Poplar males and willow females exhibit superior adaptation to nocturnal warming than the opposite sex. Sci. Total Environ. 2020, 717, 137–179. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.T.; Tang, J.Y.; He, F.; Chen, G.; Shi, Y.J.; Wang, X.G.; Han, S.; Li, S.; Zhu, T.H.; Chen, L.H. Sexual differences in above- and belowground herbivore resistance between male and female poplars as affected by soil cadmium stress. Sci. Total Environ. 2022, 803, 150081. [Google Scholar] [CrossRef] [PubMed]
- Muyle, A.; Martin, H.; Zemp, N.; Mollion, M.; Gallina, S.; Tavares, R.; Silva, A.; Bataillon, T.; Widmer, A.; Glemin, S.; et al. Dioecy is associated with high genetic diversity and adaptation rates in the plant genus silene. Mol. Biol. Evol. 2021, 38, 805–818. [Google Scholar] [CrossRef]
- Juvany, M.; Munné-Bosch, S. Sex-related differences in stress tolerance in dioecious plants: A critical appraisal in a physiological context. J. Exp. Bot. 2015, 66, 6083–6092. [Google Scholar] [CrossRef] [Green Version]
- Leigh, A.; Nicotra, A.B. Sexual dimorphism in reproductive allocation and water use efficiency in Maireana pyramidata (Chenopodiaceae), a dioecious, semi-arid shrub. Aust. J. Bot. 2003, 51, 509–514. [Google Scholar] [CrossRef]
- Pickering, C.M.; Arthur, J.M. Patterns of resource allocation in the dioecious alpine herb Aciphylla simplicifolia (Apiaceae). Austral. Ecol. 2003, 28, 566–574. [Google Scholar] [CrossRef]
- Liu, M.; Liu, X.X.; Kang, J.Y.; Korpelainen, H.; Li, C.Y. Are males and females of Populus cathayana differentially sensitive to Cd stress? J. Hazard. Mater. 2020, 393, 122–411. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.T.; Liu, X.C.; Korpelainen, H.; Li, C.Y. Intra- and intersexual interactions shape microbial community dynamics in the rhizosphere of Populus cathayana females and males exposed to excess Zn. J. Hazard. Mater. 2021, 402, 123783. [Google Scholar] [CrossRef]
- Peng, S.M.; Wu, L.R.; Seyler, B.C.; Pei, X.J.; Li, S.X.; Huang, Y. The combined effects of Cu and Pb on the sex-specific growth and physiology of the dioecious Populus yunnanensis. Environ. Res. 2020, 184, 109276. [Google Scholar] [CrossRef]
- Huang, W.; Yang, S.J.; Zhang, S.B.; Zhang, J.L.; Cao, K.F. Cyclic electron flow plays an important role in photoprotection for the resurrection plant Paraboea rufescens under drought stress. Planta 2012, 235, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Palta, J.P.; Mori, M.; Kasuga, J. Difference in freezing tolerance between young potato plants derived from tissue culture plantlets and seed tubers. CryoLetters 2020, 41, 317–322. [Google Scholar] [PubMed]
- Wu, S.; Yao, X.H.; Wang, K.L.; Yang, S.P.; Ren, H.D.; Huang, M.; Chang, J. Quality analysis and comprehensive evaluation of fruits from different cultivars of pecan (Carya illinoinensis (Wangenheim) K. Koch). Forests 2022, 13, 746. [Google Scholar] [CrossRef]
- Shekhawat, G.S.; Mahawar, L.; Rajput, P.; Rajput, V.D.; Minkina, T.; Singh, R.K. Role of engineered carbon nanoparticles (CNPs) in promoting growth and metabolism of Vigna radiata (L.) Wilczek: Insights into the biochemical and physiological responses. Forests 2021, 10, 1317. [Google Scholar] [CrossRef]
- Wan, H.X.; Du, J.Y.; He, J.L.; Lyu, D.G.; Li, H.F. Copper accumulation, subcellular partitioning and physiological and molecular responses in relation to different copper tolerance in apple rootstocks. Tree Physiol. 2019, 39, 1215–1234. [Google Scholar] [CrossRef]
- Rong, L.S.; Liang, Y.; Liu, Y.J.; Yang, R.L.; Liu, Q.; Xie, S.B. Comparative study of uranium accumulation abilities of five herbs. Environ. Sci. Technol. 2015, 38, 33–36+56. [Google Scholar]
- Hao, L.T.; Chen, L.H.; Zhu, P.; Zhang, J.; Zhang, D.J.; Xiao, J.J.; Xu, Z.F.; Zhang, L.; Liu, Y.; Li, H.; et al. Sex-specific responses of Populus deltoides to interaction of cadmium and salinity in root systems. Ecotoxicol. Environ. Saf. 2020, 195, 110437. [Google Scholar] [CrossRef]
- Stojanović, M.D.; Stevanović, D.R.; Milojković, J.V.; Grubišić, M.S.; Ileš, D.A. Phytotoxic effect of the uranium on the growing up and development the plant of corn. Water Air Soil Pollut. 2009, 209, 401–410. [Google Scholar] [CrossRef]
- Saini, S.; Kaur, N.; Pati, P.K. Phytohormones: Key players in the modulation of heavy metal stress tolerance in plants. Ecotoxicol. Environ. Saf. 2021, 223, 1125–1178. [Google Scholar] [CrossRef]
- Jiang, H.; Korpelainen, H.; Li, C.Y. Populus yunnanensis males adopt more efficient protective strategies than females to cope with excess zinc and acid rain. Chemosphere 2013, 91, 1213–1220. [Google Scholar] [CrossRef]
- Tauqeer, H.M.; Ali, S.; Rizwan, M.; Ali, Q.; Saeed, R.; Iftikhar, U.; Ahmad, R.; Farid, M.; Abbasi, G. Phytoremediation of heavy metals by Alternanthera bettzickiana: Growth and physiological response. Ecotoxicol. Environ. Saf. 2016, 126, 138–146. [Google Scholar] [CrossRef]
- Mihalík, J.; Henner, P.; Frelon, S.; Camilleri, V.; Février, L. Citrate assisted phytoextraction of uranium by sunflowers: Study of fluxes in soils and plants and resulting intra-planta distribution of Fe and U. Environ. Exp. Bot. 2012, 77, 249–258. [Google Scholar] [CrossRef]
- Maršík, P.; Zunová, T.; Vaněk, T.; Podlipná, R. Metazachlor effect on poplar—Pioneer plant species for riparian buffers. Chemosphere 2021, 274, 129711. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, A.; Seth, C.S. Does jasmonic acid regulate photosynthesis, clastogenecity, and phytochelatins in Brassica juncea L. in response to Pb-subcellular distribution? Chemosphere 2020, 243, 125361. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, A.B.; Gallagher, F.J.; Caplan, J.S.; Grabosky, J.C. Maintenance of photosynthesis by Betula populifolia in metal contaminated soils. Sci. Total Environ. 2018, 625, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.L.; Arce Cubas, L.; Gray, J.E.; Hepworth, C. The influence of stomatal morphology and distribution on photosynthetic gas exchange. Plant J. 2020, 101, 768–779. [Google Scholar] [CrossRef] [Green Version]
- Bhusal, N.; Kim, H.S.; Han, S.G.; Yoon, T.M. Photosynthetic traits and plant-water relation of two apple cultives grown as bi-leader trees under long-term waterlogging conditions. Environ. Exp. Bot. 2020, 176, 104111. [Google Scholar] [CrossRef]
- Begovic, L.; Mlinaric, S.; Antunovic Dunic, J.; Katanic, Z.; Loncaric, Z.; Lepedus, H.; Cesar, V. Response of Lemna minor L. to short-term cobalt exposure: The effect on photosynthetic electron transport chain and induction of oxidative damage. Aquat. Toxicol. 2016, 175, 117–126. [Google Scholar] [CrossRef]
- Solti, Á.; Sárvári, É.; Tóth, B.; Mészáros, I.; Fodor, F. Incorporation of iron into chloroplasts triggers the restoration of cadmium induced inhibition of photosynthesis. J. Plant Physiol. 2016, 202, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Fernàndez-Martínez, J.; Zacchini, M.; Fernández-Marín, B.; García-Plazaola, J.I.; Fleck, I. Gas-exchange, photo- and antioxidant protection, and metal accumulation in I-214 and Eridano Populus sp. clones subjected to elevated zinc concentrations. Environ. Exp. Bot. 2014, 107, 144–153. [Google Scholar] [CrossRef]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.; Salami, S.; Babalar, M. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci. Rep. 2019, 9, 19250. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, N.; Lee, M.; Lee, H.; Adhikari, A.; Han, A.R.; Han, A.; Kim, H.S. Evaluation of morphological, physiological, and biochemical traits for assessing drought resistance in eleven tree species. Sci. Total Environ. 2021, 77, 146466. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Long, C.; Wang, D.; Yang, J. Phytoremediation of cadmium (Cd) and uranium (U) contaminated soils by Brassica juncea L. enhanced with exogenous application of plant growth regulators. Chemosphere 2020, 242, 125112. [Google Scholar] [CrossRef]
- Stobrawa, K.; Lorenc-Plucinska, G. Changes in carbohydrate metabolism in fine roots of the native European black poplar (Populus nigra L.) in a heavy-metal-polluted environment. Sci. Total Environ. 2007, 373, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.F.; Luo, X.G.; Jia, Y.; Li, B.; Jin, X.; Han, X. Effects of uranium stress on physiological and biochemical index of Amaranthus retroflexus L. Environ. Sci. Technol. 2016, 39, 31–35. [Google Scholar]

| Female Trees | Male Trees | p-Value | |||
|---|---|---|---|---|---|
| Root | Leaf | Root | Leaf | ||
| U content (mg kg−1 DW) | 4.462 ± 0.747b | 0.241 ± 0.150c | 13.058 ± 2.870a | N/A | <0.001 |
| Factor | Treatment | Plant Height (cm) | Root Length (cm) |
|---|---|---|---|
| Female | CK-F | 71.0 ± 1.88a | 30.9 ± 4.98a |
| U-F | 44.2 ± 4.87b | 31.8 ± 2.99a | |
| Male | CK-M | 82.9 ± 3.29a | 30.4 ± 3.51a |
| U-M | 74.6 ± 5.51a | 37.1 ± 2.57a | |
| Psex | <0.001 | 0.519 | |
| PU | 0.003 | 0.323 | |
| Psex × U | 0.056 | 0.456 |
| Factor | Treatment | Fo | Fm | Fv/Fm | qP |
|---|---|---|---|---|---|
| Female | CK-F | 0.475 ± 0.021ab | 1.151 ± 0.057b | 0.579 ± 0.035ab | 0.528 ± 0.063b |
| U-F | 0.386 ± 0.051b | 1.130 ± 0.211b | 0.637 ± 0.027ab | 0.666 ± 0.069ab | |
| Male | CK-M | 0.515 ± 0.026a | 1.223 ± 0.127b | 0.551 ± 0.058b | 0.578 ± 0.050b |
| U-M | 0.441 ± 0.090a | 1.449 ± 0.308a | 0.575 ± 0.124a | 0.681 ± 0.135a | |
| Psex | 0.022 | 0.047 | 0.818 | 0.111 | |
| PU | 0.241 | 0.148 | 0.030 | 0.004 | |
| PSex × U | 0.198 | 0.116 | 0.364 | 0.449 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, H.; Cheng, X.; Zheng, L.; Ren, H.; Li, W.; Lei, Y.; Plenković-Moraj, A.; Chen, K. Sex-Specific Physiological Responses of Populus cathayana to Uranium Stress. Forests 2022, 13, 1123. https://doi.org/10.3390/f13071123
Xia H, Cheng X, Zheng L, Ren H, Li W, Lei Y, Plenković-Moraj A, Chen K. Sex-Specific Physiological Responses of Populus cathayana to Uranium Stress. Forests. 2022; 13(7):1123. https://doi.org/10.3390/f13071123
Chicago/Turabian StyleXia, Hongxia, Xinyan Cheng, Liuliu Zheng, Hui Ren, Wanting Li, Yanbao Lei, Anđelka Plenković-Moraj, and Ke Chen. 2022. "Sex-Specific Physiological Responses of Populus cathayana to Uranium Stress" Forests 13, no. 7: 1123. https://doi.org/10.3390/f13071123
APA StyleXia, H., Cheng, X., Zheng, L., Ren, H., Li, W., Lei, Y., Plenković-Moraj, A., & Chen, K. (2022). Sex-Specific Physiological Responses of Populus cathayana to Uranium Stress. Forests, 13(7), 1123. https://doi.org/10.3390/f13071123






