Abstract
Postemergence application of herbicides can cause phytotoxicity problems in eucalyptus seedlings. Foliar fertilization can improve seedling development and mitigate the effects of herbicides on eucalyptus. Thus, the objective of this work was to evaluate the sensitivity of eucalyptus clones subjected to herbicides applied postemergence and associated with the application of foliar fertilizer. For this, a field experiment was carried out with the application of the products indaziflam, clomazone, glyphosate + S-metolachlor, sulfentrazone, and diuron + sulfentrazone, plus the application of an additional foliar fertilizer (composition in g/L of 78, 13, 40.3, 1.17, 0.78, 16.9, 13, 14.3, 0.52, and 29.9, respectively, for C, N, S, B, Co, Fe, Cu, Mn, Mo, and Zn). Height, stem diameter, shoot dry mass, chlorophyll content, and visual intoxication were the analyzed variables. The herbicides indaziflam and glyphosate + S-metolachlor were the most harmful to the tested eucalyptus clones, interfering with the growth variables. Among the evaluated clones, Clone AEC 144 had more significant changes in the analyzed variables in treatments with herbicides and foliar fertilizer application. The eucalyptus seedlings were generally more sensitive to indaziflam and glyphosate + S-metolachlor herbicides. Foliar fertilization reduced the intoxication caused by indaziflam in Clone AEC 056. The fertilizer intensified treatment symptoms with clomazone and diuron + sulfentrazone in Clone AEC 144 and with sulfentrazone and diuron + sulfentrazone in Clone AEC 2034.
1. Introduction
Eucalyptus is the most cultivated tree in Brazil and is used to produce charcoal, cellulose, paper, and wood, among other products [1]. Eucalyptus plantation areas have expanded recently, surpassing the 5.7 million hectares planted in 2019 [2,3]. Brazil has one of the highest average productions globally, with about 36 m3 ha−1 per year and crops with shorter rotations than other world producers [3].
Although genetic improvement programs for this crop are in their advanced stages, other factors contribute to maintaining high productivity, such as pest, disease, and weed control [4]. Weed control is a determining factor for eucalyptus development and productivity, as it is susceptible to competition for resources such as water, nutrients, and space [5,6,7]. Grasses are the most problematic weeds for this tree, as they are highly competitive, have a high growth rate, and have high propagule production [8,9].
Weeds in forest crops are mostly controlled by herbicides. Herbicides with long residual effects are an alternative for controlling grasses, as they affect the seed bank, preventing germination. There are 187 herbicides registered for eucalyptus in Brazil, 32 of which are indicated to control grasses [10]. However, most registered herbicides are not selective for eucalyptus, and when applied postemergence, even in targeted applications, they can cause phytotoxicity and growth alterations to plants [11].
In the early years of development, eucalyptus was more sensitive to changes caused by herbicides [12]. Toxicity is mainly due to application failure and drift [13]. The drift of glyphosate + carfentrazone-ethyl, non-culture-selective herbicides, can cause anatomical changes, cell degeneration, chlorosis, necrosis, and wilting [14]. Tembotrione causes a reduction in the height of eucalyptus clones, nicosulfuron decreases the increase in stem diameter, and fluazifop-p-butyl + fomesafen influences the accumulation of dry matter [15].
Foliar fertilization contributes to better eucalyptus development [16], making the plants healthier and less susceptible to the action of herbicides that may affect it by drift [17]. In this sense, foliar fertilizers can be used as crop protectors and aid in weed management in forest areas [17].
Thus, the objective of this work was to evaluate the sensitivity of eucalyptus clones subjected to herbicides applied postemergence and associated with the application of foliar fertilizer.
2. Materials and Methods
The work was carried out at the Federal Institute of Northern Minas Gerais in São João Evangelista–MG. The experiments were set up in a randomized block design, with five replications and treatments arranged in a 5 × 2 factorial scheme. The first factor consisted of the control treatment (without herbicide) plus the herbicides indaziflam (Esplanade®), clomazone (Gamit®), glyphosate + S-metolachlor (Sequence®), sulfentrazone (Solara®) and diuron + sulfentrazone (Stone®) at doses 150 mL/ha, 2 L/ha, 6 L/ha, 1 L/ha, 2.5 L/ha, respectively. The second factor was composed of the application or not of supplementary foliar fertilization [composition in g/L of 78; 13; 40.3; 1.17; 0.78; 16.9; 13; 14.3; 0.52 and 29.9, respectively, for carbon (C), nitrogen (N), sulfur (S), boron (B), cobalt (Co), iron (Fe), copper (Cu), manganese (Mn), molybdenum (Mo), and zinc (Zn)].
Three experiments were carried out for each commercial eucalyptus clone: Clone AEC 056, Clone AEC 144, and Clone AEC 2034. In these experiments, the 45-day-old eucalyptus seedlings were transplanted into 15 L pots with soil samples previously fertilized as recommended for the crop (Table 1).

Table 1.
Physicochemical characteristics of the soil samples used in the experiment.
The application of herbicides was carried out on the seedlings with a coastal electric spray (Yamaho FT5®, with 5 L) 30 days after transplanting. For the phytotoxicity tests, doses corresponding to 0, ¼, ½ and 1 times the recommended commercial dose for the crop were applied [10]. Foliar fertilization was also performed with a coastal electric spray. The product at a dose of 500 mL/ha was applied to the seedlings one hour before using herbicides.
The height and stem diameter variables were measured on the day of herbicide application, and after 120 days, increments were calculated based on the difference between final and initial measurements. The increment measures (between the final and initial) were used in the analyses and tables. Height was measured with a ruler, and stem diameter with a digital caliper. The aerial part dry mass was collected 120 days after applying the herbicides and placed in a forced circulation oven at 60 °C until at constant weight. Chlorophyll content (SPAD index) was measured with a chlorophyll meter (SPAD–502 PLUS). For the height, stem diameter, shoot dry mass, and chlorophyll content variables, data referring to the application of commercial doses of each herbicide and the control treatment were analyzed. Data referring to doses of 0, ¼, ½ and 1 times the commercial dose were used to assess intoxication. Intoxication was measured by visual evaluation, attributing scores from 0 (no symptoms of intoxication) to 100% (plant death) performed at 7, 14, 21, and 28 days after the application of the herbicides [18].
Data were subjected to analysis of variance and differentiated by Scott-Knott grouping for the herbicide factor and F-test for the foliar fertilization factor for the variables height, stem diameter, shoot dry mass, and chlorophyll content. For intoxication, analyses of multiple linear regressions were performed, and 3D response surfaces were made. All statistical analyzes were performed at 5% significance.
3. Results
The clones evaluated in this study behaved differently regarding changes in the analyzed variables. Clone AEC 056 had only height and intoxication influenced by foliar fertilization. On the other hand, Clone AEC 144 had its height, chlorophyll content, and intoxication influenced by foliar fertilizer, while Clone AEC 2034 only had stem diameter and intoxication influenced.
3.1. Effects on Clone AEC 056
The increase in the height of eucalyptus seedlings of clone AEC 056 differed between treatments with herbicides and foliar fertilizer application. Without fertilizer application, minor changes in plant height were in the control treatments and with the herbicides indaziflam and glyphosate + S-metolachlor. With fertilizer application, the lowest average height was for plants subjected to indaziflam. In the comparison made among plants treated with herbicides, foliar fertilization provided greater growth of seedlings with the herbicides glyphosate + S-metolachlor and diuron + sulfentrazone; with indaziflam and sulfentrazone the fertilization reduced the increase in height (Table 2).

Table 2.
Height (cm), stem diameter (cm), dry mass of the aerial part (g) and chlorophyll content (SPAD) of commercial eucalyptus clone AEC 056, submitted to the application of the herbicides indaziflam, clomazone, glyphosate + S-metolachlor, sulfentrazone, diuron + sulfentrazone, and control treatment, with and without foliar fertilization.
There was no significant interaction between foliar fertilization and herbicide application to increase seedling stem diameter. With or without foliar fertilization, the largest diameter of the plants among the herbicides applied was only in the treatment with sulfentrazone (Table 2).
There was also no significant interaction between foliar fertilization and herbicide application for shoot dry mass accumulation. Without foliar fertilization, lower dry mass was seen in the treatment with glyphosate + S-metolachlor, and with fertilization in the control treatments, with glyphosate + S-metolachlor, and with diuron + sulfentrazone (Table 2).
There was no significant difference between treatments with herbicide applications and foliar fertilizer for chlorophyll content measured in the eucalyptus seedlings (Table 2).
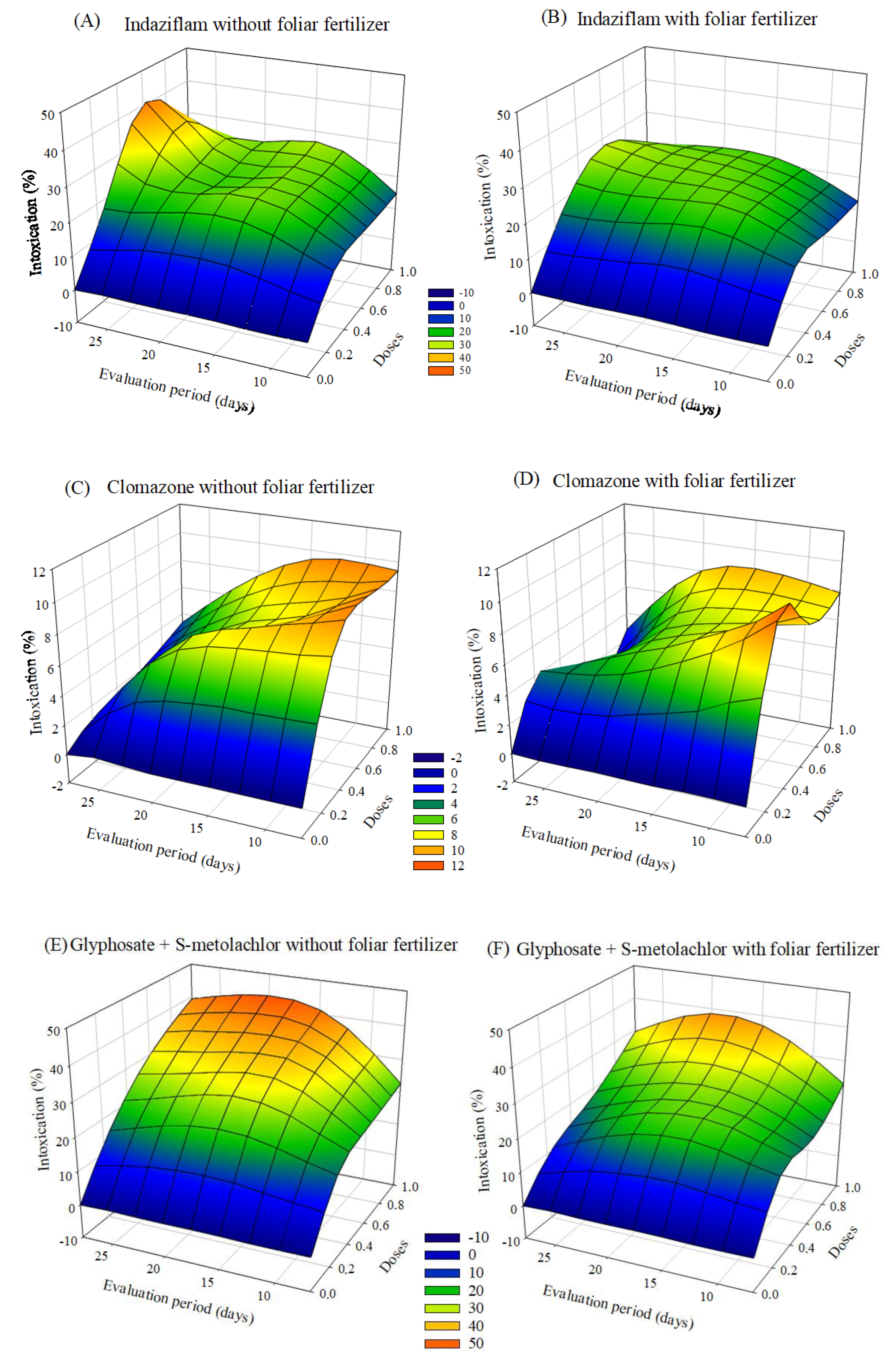
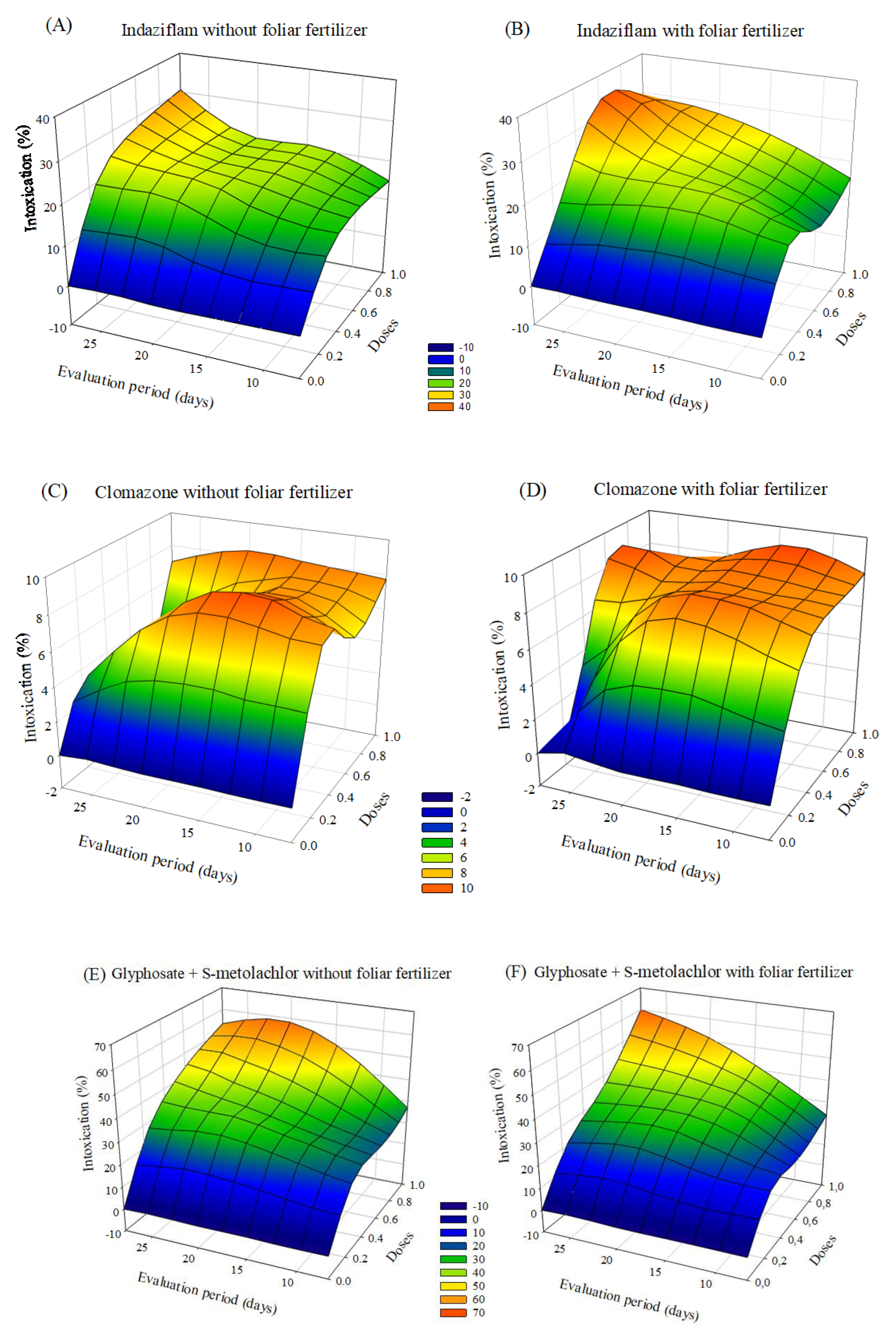
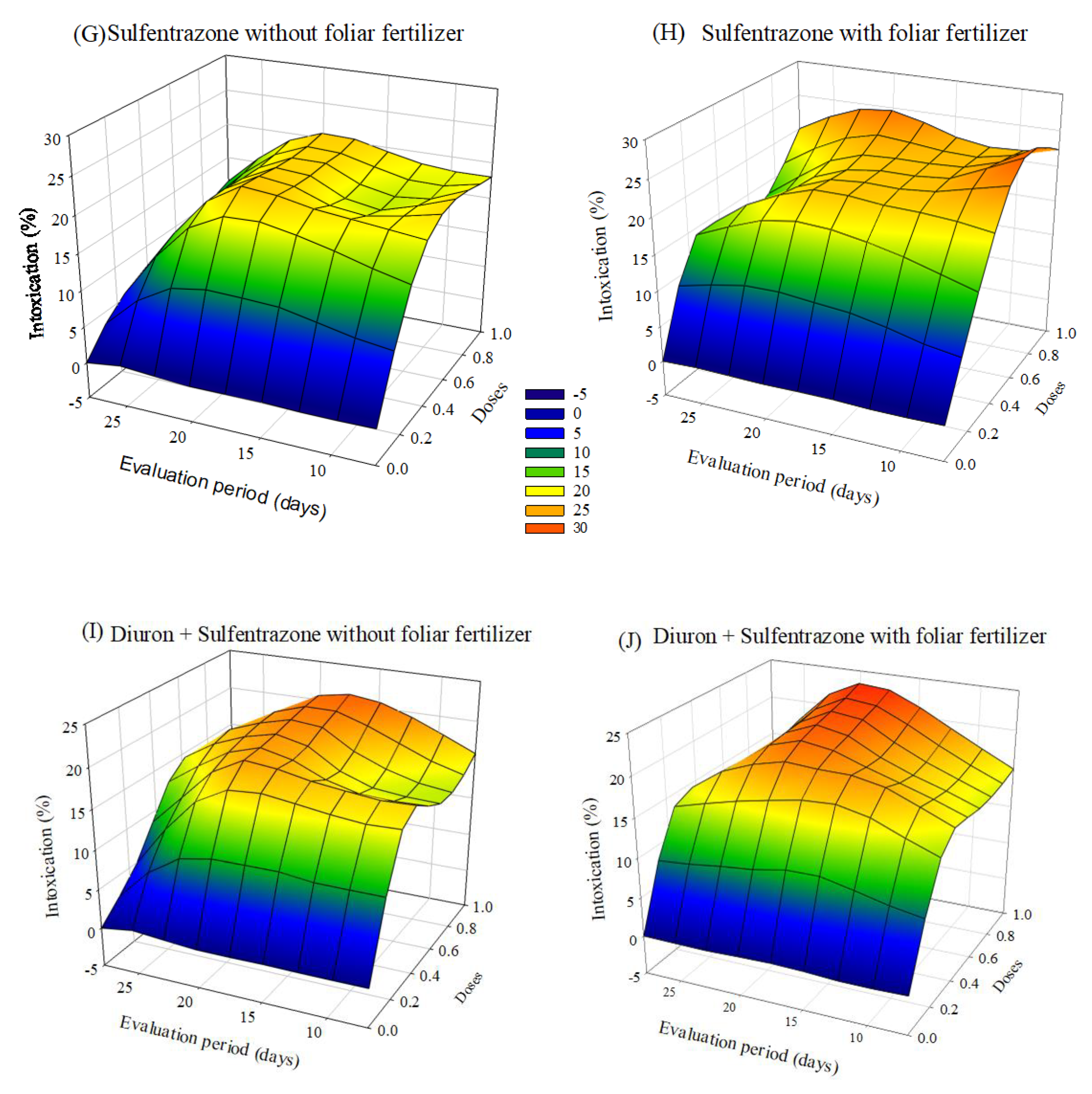
The herbicides that caused more significant plant intoxication were indaziflam and glyphosate + S-metolachlor. The symptoms of intoxication observed in eucalyptus seedlings after applying herbicides with or without foliar fertilization varied depending on the dose and the evaluation period. After 20 days of application, there was a tendency to a decrease in intoxication, mainly at the lowest doses. In the treatments with indaziflam and glyphosate + S-metolachlor with fertilizer application, the intoxication values were lower than those without foliar fertilization (Figure 1).
Figure 1.
Intoxication of eucalyptus clone AEC 056 subjected to herbicides indaziflam (A,B), clomazone (C,D), glyphosate + S-metolachlor (E,F), sulfentrazone (G,H), and diuron + sulfentrazone (I,J), with and without foliar fertilizer application. (A): z = −11.76 + 77.29x + 1.15y − 55.01x2 − 0.02y2; R2 = 0.81. (B): z = −10.99 + 72.26x + 1.33y − 54.06x2 − 0.03y2; R2 = 0.93. (C): z = 1.34 + 19.59x + 0.23y − 12.78x2 − 0.01y2; R2 = 0.86. (D): z = 2.70 + 19.03x + 0.04y − 13.19x2 − 0.007y2; R2 = 0.77. (E): z = −19.23 + 74.01x + 2.43y − 38.43x2 − 0.06y2; R2 = 0.83. (F): z = −14.42 + 52.88x + 2.22y − 22.92x2 − 0.06y2; R2 = 0.92. (G): z = −3.24 + 52.83x + 0.99y − 38.25x2 − 0.04y2; R2 = 0.92. (H): z = 26.49 × exp(0.5 × (((x − 0.74)/0.42)2 + ((y − 14.30)/10.18)2)); R2 = 0.77. (I): z = −4.82 + 49.95x + 1.37y − 32.60x2 − 0.05y2; R2 = 0.91. (J): z = −5.07 + 47.67x + 1.39y − 34.84x2 − 0.05y2; R2 = 0.87.
3.2. Effects on Clone AEC 144
The increase in the height of eucalyptus seedlings of clone AEC 144 was significant between treatments with herbicides and foliar fertilizer application. Without fertilizer application, the lowest seedling heights were in the treatments with indaziflam and glyphosate + S-metolachlor. With fertilizer application, the herbicides indaziflam and sulfentrazone provided lower plant growth. The application of foliar fertilization provided lower growth of plants with the treatment containing sulfentrazone compared to the same without fertilization; and a more significant increase of glyphosate + S-metolachlor (Table 3).

Table 3.
Height (cm), stem diameter (cm), dry mass of the aerial part (g) and chlorophyll content (SPAD) of commercial eucalyptus clone AEC 144, subjected to the application of the herbicides indaziflam, clomazone, glyphosate + S-metolachlor, sulfentrazone, diuron + sulfentrazone, and control treatment, with and without foliar fertilization.
There was no statistical difference between treatments for the stem diameter variable (Table 3).
There was no significant interaction between foliar fertilization and herbicide application for shoot dry mass accumulation. Without foliar fertilization, lower dry mass was seen in treatments with indaziflam and with glyphosate + S-metolachlor; and with fertilization, in the control treatments, with indaziflam, and with glyphosate + S-metolachlor (Table 3).
Treatments with herbicides and foliar fertilization influenced the chlorophyll content measured in the eucalyptus seedlings. The lowest chlorophyll content was measured without fertilizer application in seedlings treated with clomazone. With fertilizer application, there was no significant difference among treatments with herbicides. The application of foliar fertilization provided lower levels of chlorophyll in treatments containing indaziflam and glyphosate + S-metolachlor (Table 3).
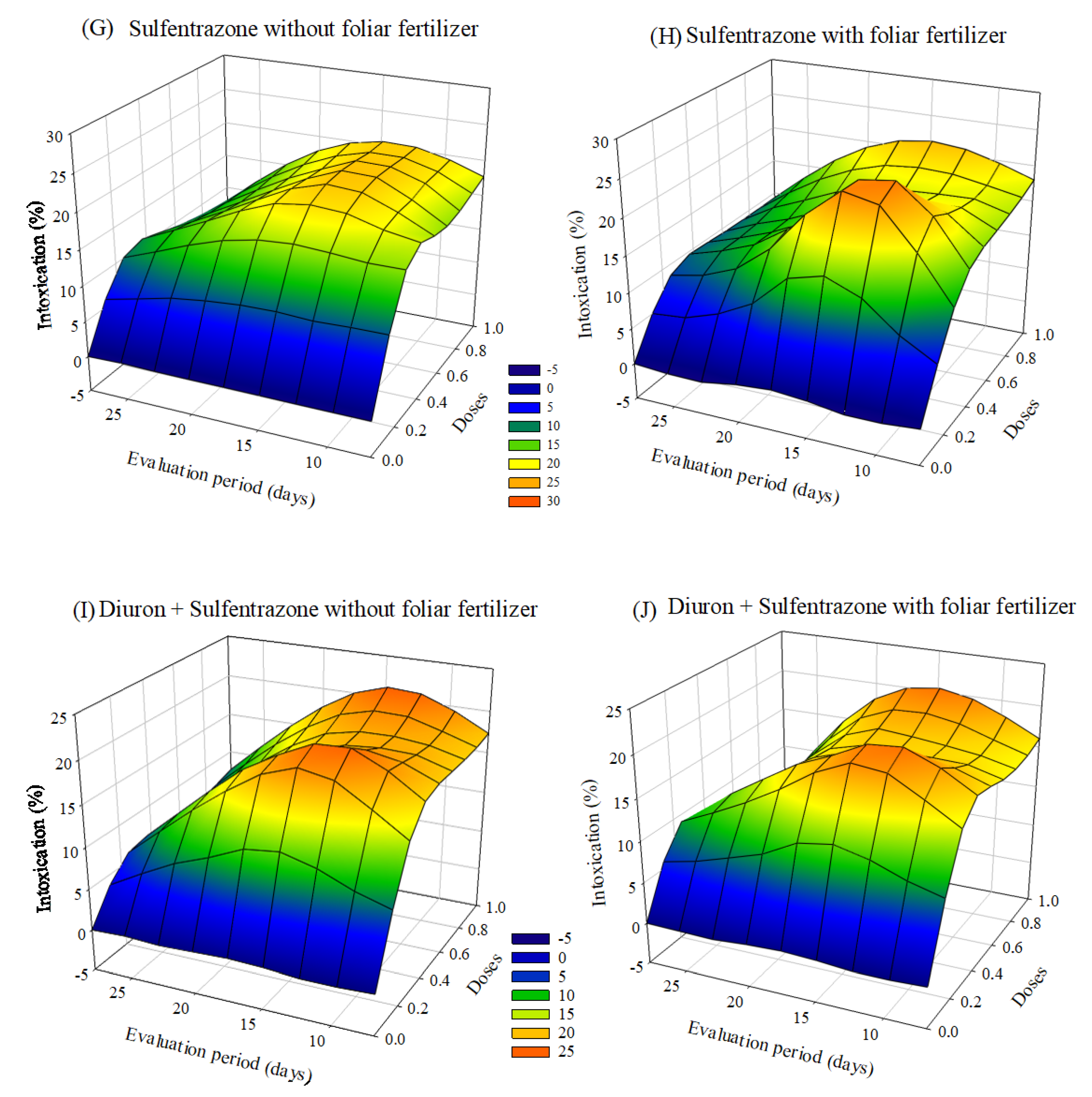
Visual intoxication of Clone AEC 144 eucalyptus seedlings was higher in the treatment containing the ready-mix glyphosate + S-metolachlor. The symptoms of intoxication observed in the eucalyptus seedlings after applying herbicides with or without foliar fertilization varied depending on the dose and the evaluation period. After 20 days of application, there was a tendency of a decrease in the intoxication at lower doses. In the case of treatments with clomazone or diuron + sulfentrazone, the intoxication values were higher with fertilizer application than without foliar fertilization (Figure 2).
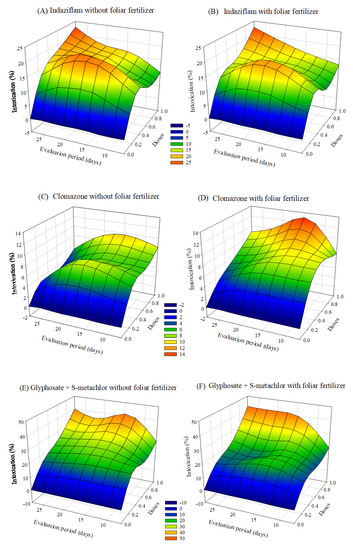
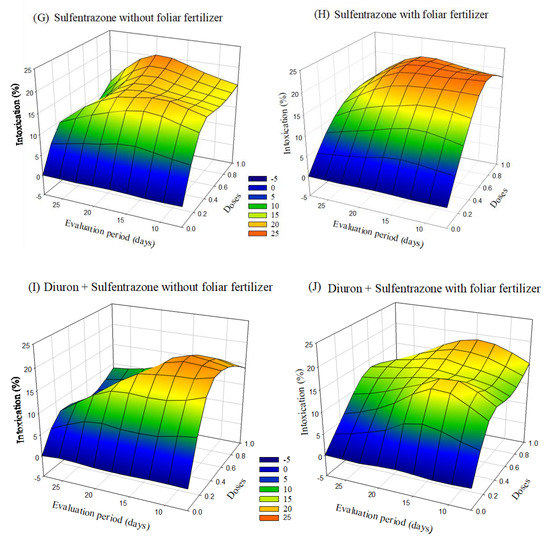
Figure 2.
Intoxication of eucalyptus clone AEC 144 subjected to herbicides indaziflam (A,B), clomazone (C,D), glyphosate + S-metolachlor (E,F), sulfentrazone (G,H), and diuron + sulfentrazone (I,J), with and without foliar fertilizer application. (A): z = −9.06 + 52.73x + 1.15y − 37.84x2 − 0.03y2; R2 = 0.85. (B): z = −6.71 + 44.67x + 0.93y−29.21x2 − 0.02y2; R2 = 0.82. (C): z = −2.54 + 17.86x + 0.60y−10.72x2 −0.02y2; R2 = 0.81. (D): z= −2.54 + 20.71x + 0.51y − 11.28x2 − 0.02y2; R2 = 0.86. (E): z = −9.38 + 59.51x + 1.30y − 26.09x2 − 0.03y2; R2 = 0.91. (F): z = −14.33 + 41.90x + 1.83y − 8.77x2 − 0.04y2; R2 = 0.80. (G): Z = −4.85 + 45.14x + 1.08y − 30.10x2 − 0.03y2; R2 = 0.88. (H): z = −2.74 + 49.41x + 0.63y − 31.56x2 − 0.02y2; R2 = 0.93. (I): z = 2.76 + 48.34x + 0.23y − 37.41x2 − 0.02y2; R2 = 0.87. (J): z = −4.40 + 41.33x + 0.94y − 27.64x2 − 0.03y2; R2 = 0.81.
3.3. Effects on Clone AEC 2034
There was no significant interaction between foliar fertilization and herbicide application to increase the height of clone AEC 2034. The lowest heights were for seedlings treated with indaziflam and glyphosate + S-metolachlor (Table 4).

Table 4.
Height (cm), stem diameter (cm), dry mass of the aerial part (g), and chlorophyll content (SPAD) of commercial eucalyptus clone AEC 2034, subjected to the application of the herbicides indaziflam, clomazone, glyphosate + S-metolachlor, sulfentrazone, diuron + sulfentrazone and control treatment, with and without foliar fertilization.
The increase in the diameter of eucalyptus seedlings was significant among treatments with herbicides and the application of foliar fertilizer. Without fertilizer application, the minor increase in the diameters of the seedlings was in the treatments with indaziflam and with glyphosate + S-metolachlor. With fertilizer application, the herbicides indaziflam and sulfentrazone retarded plant growth. The application of foliar fertilization provided a minor increase in diameter in the treatment containing sulfentrazone compared to the same one without fertilization (Table 4).
There was no significant interaction between foliar fertilization and herbicide application to accumulate the dry mass of clone AEC 2034. In treatments without fertilizer application and foliar fertilization, the lowest dry mass values were for seedlings treated with indaziflam and glyphosate + S-metolachlor (Table 4).
There was also no significant interaction between foliar fertilization and herbicide application for the chlorophyll content variable. In the treatment without foliar fertilizer application, a higher chlorophyll value was observed only in the treatment with indaziflam. The lowest chlorophyll values were from control and indaziflam-treated plants (Table 4).
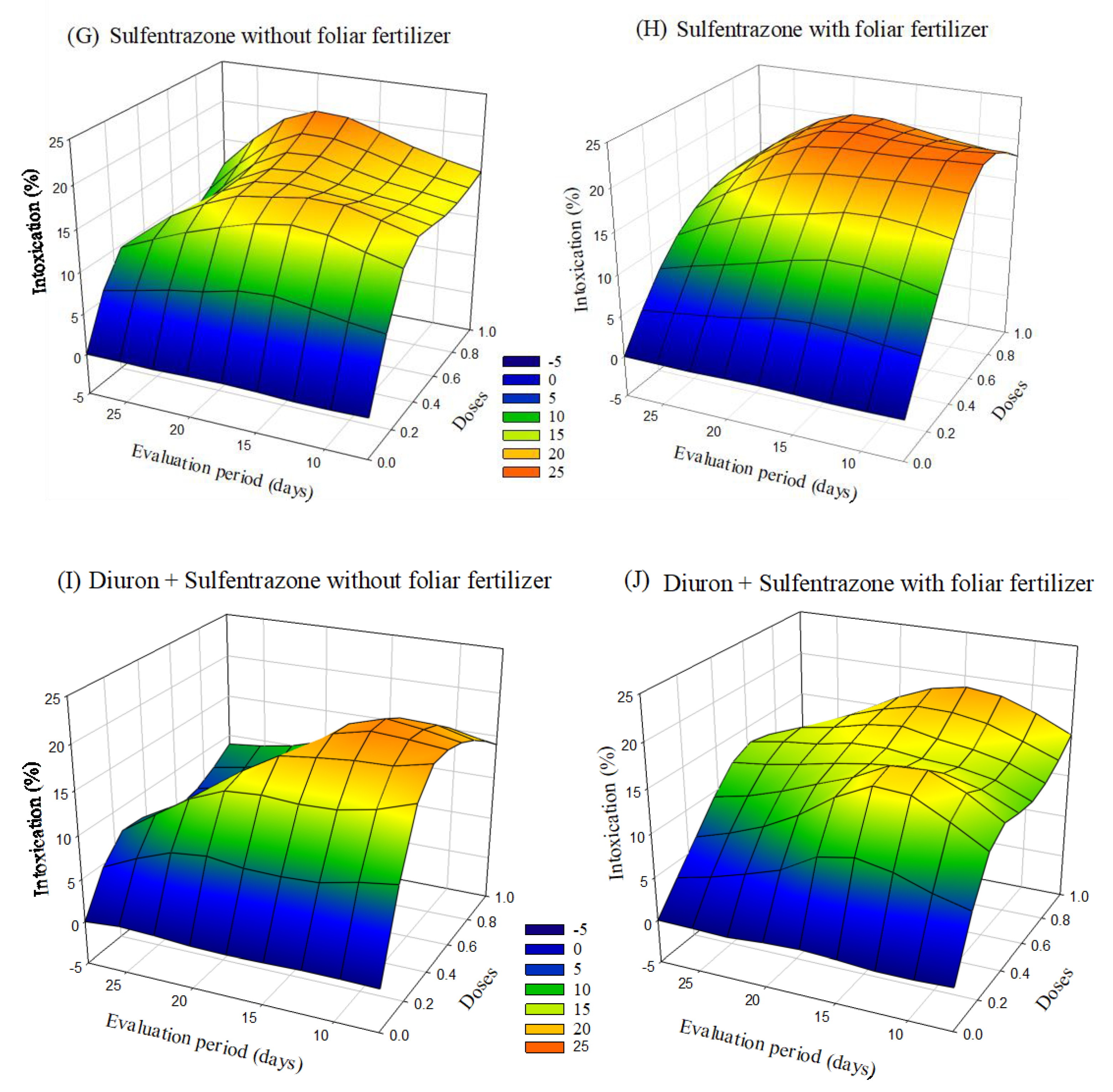
The symptoms of intoxication observed in eucalyptus seedlings after applying herbicides with or without foliar fertilization varied depending on the dose and the evaluation period. The greatest intoxications were observed in seedlings treated with indaziflam and glyphosate + S-metolachlor. After 20 days of application, there was a tendency of a decrease in intoxication at lower doses. In the case of treatments with sulfentrazone and diuron + sulfentrazone, with fertilizer application the intoxication values were higher than without foliar fertilization (Figure 3).
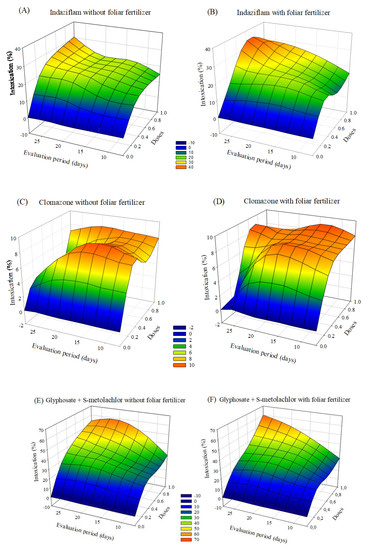
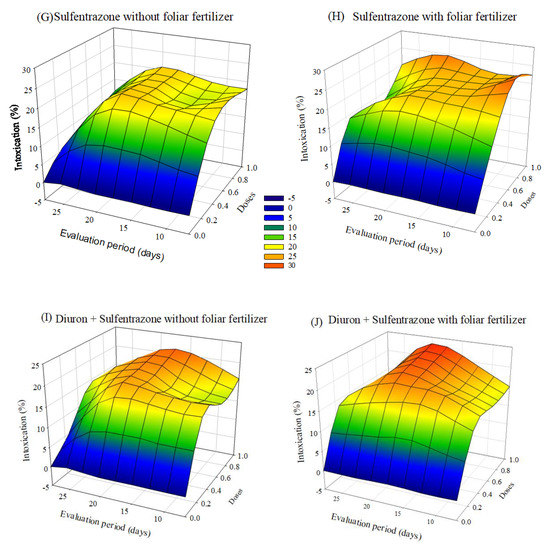
Figure 3.
Intoxication of eucalyptus clone AEC 2034 subjected to herbicides indaziflam (A,B),clomazone (C,D), glyphosate + S-metolachlor (E,F), sulfentrazone (G,H), and diuron + sulfentrazone (I,J), with and without foliar fertilizer application. (A): z = −4.57 + 65.49x + 0.19y − 44.73x2 + 0.005y2; R2 = 0.81. (B): z = −8.72 + 65.97x + 0.83y − 43.23x2 − 0.01y2; R2 = 0.89. (C): z = −1.25 + 17.64x + 0.48y − 10.90x2 − 0.02y2; R2 = 0.72. (D): z = −1.83 + 22.42x + 0.41y − 14.75x2 − 0.01y2; R2 = 0.78. (E): z = −20.22 + 89.42x + 2.22y − 44.81x2 − 0.05y2; R2 = 0.81. (F): z = −18.90 + 74.58x + 1.91y − 31.94x2 − 0.04y2; R2 = 0.87. (G): z = −5.87 + 53.03x + 1.37y − 36.95x2 − 0.05y2; R2 = 0.89. (H): z = −2.32 + 59.58x + 0.65y − 38.58x2 − 0.02y2; R2 = 0.89. (I): z = −6.17 + 49.35x + 1.27y − 33.76x2 − 0.04y2; R2 = 0.88. (J): z = −9.11 + 54.28x + 1.47y − 37.79x2 − 0.04y2; R2 = 0.82.
4. Discussion
4.1. Effects on Clone AEC 056
Herbicides are products used to control weeds in forestry and agronomic crops. However, the occurrence of drift can reduce plant physiological and growth characteristics, which affects productivity [14,19].
For Clone AEC 056, both in the comparison of herbicides with fertilization and in treatments without fertilization, indaziflam was the herbicide that caused the lowest growth averages, which is related to its mechanism of action [20,21]. This herbicide inhibits cellulose biosynthesis in plants [22,23], which is the main supporting structure of the plant cell wall [24]. Loss of function of proteins linked to cellulose synthesis causes complete or partial loss of anisotropic growth in expanding cells, resulting in radial swelling [23]. Although this effect is predominantly observed in grasses [25], perennial dicotyledonous species such as Coffea arabica have already been shown to be sensitive to the presence of indaziflam [26]. Foliar fertilization may have a protective effect on the action of herbicides on plants [13,27], but this action did not have a positive impact on the application of indaziflam and sulfentrazone, which provided lower seedling heights with fertilization, compared to treatments without fertilization. Fertilization improves plant nutrition and vigor. However, there was no effect on the herbicide mechanism of action in this research. Growth was reduced in these cases by inhibiting cellulose synthesis from indaziflam [23] or by the accumulation of protoporphyrinogen IX in the cytoplasm, causing membrane disruption and generating chlorosis followed by sulfentrazone necrosis [28].
Foliar fertilization did not influence the mean values of stem diameter and shoot dry mass. Thus, smaller diameter values seen in the treatment with sulfentrazone and of dry mass with glyphosate + S-metolachlor and diuron + sulfentrazone are related only to the action of herbicides alone or in mixtures. Sulfentrazone causes oxidation of the cellular lipid layer after generating an accumulation of protoporphyrinogen IX in the cytoplasm, causing the membrane to rupture and generating chlorosis followed by necrosis in sensitive plants [28].
Glyphosate inhibits the enzyme 5-enolpyruvylshikimate-3-phosphate synthase, causing a reduction in the levels of aromatic amino acids and consequently reducing the synthesis of cell-wall proteins and secondary plant products [29,30]. The phytotoxic action of the herbicide S-metolachlor occurs through the inhibition of protein synthesis in the apical meristem [31], which together with glyphosate is an effective product in the control of weeds as they inhibit growth. Diuron, in turn, inhibits photosynthesis by blocking the transport of electrons from QA to QB in photosystem II [31]. In this way, these herbicides directly or indirectly inhibit the growth and consequently the increase in diameter of sensitive plants.
The synthesis of chlorophyll is dependent on nitrogen content; the deficiency of this nutrient affects the chlorophyll contents but supplementary fertilization does not guarantee higher values [32]. Furthermore, none of the evaluated herbicides directly influence nitrogen contents and, consequently, chlorophyll synthesis. For these reasons, no significant differences were observed for this variable.
Higher intoxication values observed in treatments with indaziflam and glyphosate + S-metolachlor may be related to the mechanisms of action of these herbicides. Although indaziflam and S-metolachlor are used to control herbaceous plants, phytotoxic effects on seedlings of tree species such as Leucaena leucocephala can happen due to application made postemergence [33], as in pre-emergence it can be selective for crops with deep roots [31], such as eucalyptus. On the other hand, glyphosate is not selective for eucalyptus and causes chlorosis and leaf necrosis [34]. Foliar fertilization, in these cases, attenuated the toxic effects of these herbicides, as foliar fertilization can act as a protector against the damage caused by these products as they balance the nutrients present in the plants [27].
4.2. Effects on Clone AEC 144
As observed in experiment one (AEC144), indaziflam was one of the herbicides that most negatively influenced the growth of eucalyptus seedlings, along with glyphosate + S-metolachlor and sulfentrazone. Foliar fertilization may have a protective effect against the action of herbicides on plants [13,27] and this has already been reported for products with the herbicide glyphosate [27], but it may have a negative effect on other products such as sulfentrazone, intensifying the effects of chlorosis caused by the herbicide.
Foliar fertilization and herbicide treatments did not influence the stem diameter of eucalyptus seedlings, possibly due to this clone’s greater tolerance of the products and the indirect action on this variable. Even with foliar fertilizer application, no difference in dry mass was seen in the interaction. Only the herbicide factor was different, and once again it highlighted indaziflam and glyphosate as the most problematic, decreasing the mean values of dry shoot mass.
The herbicide clomazone negatively influenced the chlorophyll content of Clone AEC 144 without fertilizer application. This may be related to the mechanism of action of this herbicide, which promotes the photodegradation of chlorophyll after blocking the synthesis of carotenoids [31]. Clone AEC 144 had the highest plant height of the three, without having greater shoot dry mass or stem diameter. This means that this clone reaches a greater height in less time with greater leaf distribution along the stem. Thus, the illumination of each leaf segment can be increased, requiring more significant protection against light. While for the other clones (shorter at this stage of development) the eventual effect of clomazone did not change chlorophyll synthesis, for clone AEC 144 the greater height requires it to protect its leaves, which is compromised with the application of clomazone. However, foliar fertilization helped to alleviate this lack. Foliar fertilization was harmful to chlorophyll accumulation in plants treated with indaziflam and glyphosate + S-metolachlor due to some unidentified reaction between the compounds of each product.
The phytotoxicity effect of products containing glyphosate on eucalyptus seedlings is already known [34]; adding another active ingredient such as S-metolachlor, also non-selective, may have intensified the symptoms of intoxication, which caused a greater sensitivity of this clone to these herbicides. The same happened for the mixture of diuron + sulfentrazone with the application of foliar fertilizer, which despite being used as a crop protector, can have a negative effect due to the interaction of products, as seen in the treatment containing clomazone.
4.3. Effects on Clone AEC 2034
As in the AEC 056 and AEC 144 clones, for AEC 2034 the height variable was negatively influenced by the herbicides indaziflam and glyphosate + S-metolachlor due to the non-selective action of these products postemergence [31]. Similarly, the diameter increment, shoot dry mass accumulation, and chlorophyll content were also affected by indaziflam and glyphosate + S-metolachlor, with the addition of the negative effect of sulfentrazone for the first two variables and of indaziflam for the chlorophyll content. The negative influence of sulfentrazone was even more significant with foliar fertilization because eucalyptus selectivity of this herbicide depends on the clone’s genetic material [35].
Similar to Clone AEC 144, AEC 2034 was more intoxicated by the herbicides indaziflam and glyphosate + S-metolachlor. Although indaziflam and S-metolachlor are used to control herbaceous plants, postemergence applications can cause poisoning in tree seedlings [33]. In the case of clone AEC 2034, more intense effects of intoxication were observed with fertilizer application on plants later treated with sulfentrazone and diuron + sulfentrazone. Intoxication by sulfentrazone is directly linked to the clone, reaching more than 60%. The addition of diuron, an inhibitor of photosystem II, to sulfentrazone, interacting with the foliar fertilization made previously, may have contributed to the increase in the intoxication averages in these treatments.
5. Conclusions
Responses to foliar fertilization and herbicide application are variable among eucalyptus clones. Among the clones evaluated, AEC 144 had greater changes in the variables analyzed in treatments with herbicides and foliar fertilizer application. Eucalyptus seedlings were generally more sensitive to indaziflam and glyphosate + S-metolachlor herbicides. Foliar fertilization reduced intoxication by indaziflam from Clone AEC 056. In the other clones evaluated, the fertilizer intensified treatment symptoms of clomazone and diuron + sulfentrazone in Clone AEC 144 and of sulfentrazone and diuron + sulfentrazone in Clone AEC 2034.
Author Contributions
Conceptualization, G.M.B. and J.B.D.S.; methodology, A.J.E.D.C.; software, L.P.B.; validation, J.M.C. and B.C.C.F.; formal analysis, G.M.B.; investigation, I.G.C.; resources, D.V.S. and J.B.D.S.; data curation, I.G.C. and T.S.D.; writing—original draft preparation, G.M.B.; writing—review and editing, G.M.B., D.V.S. and J.B.D.S.; visualization, A.J.E.D.C.; supervision, J.B.D.S.; project administration, A.J.E.D.C.; funding acquisition, D.V.S. and J.B.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data are available on request from the corresponding author.
Acknowledgments
To the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)-Código Financeiro 001, Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), and FMC.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Embrapa Florestas. Available online: https://www.embrapa.br/florestas/transferencia-de-tecnologia/eucalipto (accessed on 13 October 2020).
- Da Cunha, T.Q.G.; Santos, A.C.; Novaes, E.; Hansted, A.L.S.; Yamaji, F.M.; Sette, C.R., Jr. Eucalyptus expansion in Brazil: Energy yield in new forest frontiers. Biomass Bioenergy 2021, 144, 105900. [Google Scholar] [CrossRef]
- Industria Brasileira De Árvores. Available online: https://iba.org/datafiles/publicacoes/relatorios/iba-relatorioanual2019.pdf (accessed on 12 February 2021).
- Wilcken, C.F.; Lima, A.C.V.; Dias, T.K.R.; Masson, M.V.; Ferreira Filho, P.J.; Dal Pogetto, M.H.F.A. Guia Prático de Manejo de Plantações De Eucalipto; FEPAF: Botucatu, Brazil, 2008. [Google Scholar]
- De Lima Neto, A.J.; Neves, J.C.L.; Martinez, H.E.P.; Sousa, J.S.; Fernandes, L.V. Nutrient accumulation and nutritional efficiency in eucalyptus. J. Plant. Nutr. 2021, 44, 2421–2434. [Google Scholar] [CrossRef]
- De Souza Kulmann, M.S.; Arruda, W.S.; Vitto, B.B.; de Souza, R.O.S.; Berghetti, Á.L.P.; Tarouco, C.P.; Araújo, M.M.; Nicoloso, F.T.; Schumacher, M.V.; Brunetto, G. Morphological and physiological parameters influence the use efficiency of nitrogen and phosphorus by Eucalyptus seedlings. New For. 2022, 53, 431–448. [Google Scholar] [CrossRef]
- Freitas, C.D.M.; Oliveira, F.S.D.; Mesquita, H.C.D.; Cortez, A.O.; Porto, M.A.F.; Silva, D.V. Efeito da competição na interação entre milho e plantas daninhas expostas à deficiência hídrica. Rev. Caatinga 2019, 32, 719–729. [Google Scholar] [CrossRef]
- Maciel, J.C.; Duque, T.S.; Ferreira, E.A.; Zanuncio, J.C.; Plata-Rueda, A.; Silva, V.P.; Silva, D.V.; Fernandes, B.C.C.; Júnior, A.P.B.; Dos Santos, J.B. Growth, Nutrient Accumulation, and Nutritional Efficiency of a Clonal Eucalyptus Hybrid in Competition with Grasses. Forests 2022, 13, 1157. [Google Scholar] [CrossRef]
- Bacha, A.L.; Pereira, F.C.M.; Pires, R.N.; Nepomuceno, M.P.; Alves, A.L.D.C.A. Interference of seeding and regrowth of signalgrass weed (Urochloa decumbens) during the initial development of Eucalyptus urograndis (E. grandis x E. urophylla). Aust. J. Crop. Sci. 2016, 10, 322. [Google Scholar] [CrossRef]
- Ministério da Agricultura, Pecuária e Abastecimento. Available online: http://extranet.agricultura.gov.br/agrofit_cons/principal_agrofit_cons (accessed on 5 September 2022).
- Minogue, P.J.; Osiecka, A.; Lauer, D.K. Selective herbicides for establishment of Eucalyptus benthamii plantations. New For. 2018, 49, 529–550. [Google Scholar] [CrossRef]
- Santos Junior, A.; Tuffi Santos, L.D.; Ferreira, F.A.; Ferreira, L.R.; Felix, R.C.; Amaral, G.C.; Cruz, L.R. Glyphosate drift in eucalyptus plants. Planta Daninha 2015, 33, 615–621. [Google Scholar] [CrossRef]
- Santos, A., Jr.; Freitas, F.C.L.; Santos, I.T.; Silva, D.C.; Alcantara-De la Cruz, R.; Ferreira, L.R. Use of Fertiactyl Pos® for Protection of Eucalyptus Plants Subjected to Herbicide Drift. Planta Daninha 2020, 38, 1–8. [Google Scholar] [CrossRef]
- Santos, S.A.; Tuffi-Santos, L.D.; Alfenas, A.C.; Faria, A.T.; Sant’anna-Santos, B.F. Differential Tolerance of Clones of Eucalyptus grandis Exposed to Drift of the Herbicides Carfentrazone-Ethyl and Glyphosate. Planta Daninha 2019, 37, 1–10. [Google Scholar] [CrossRef]
- Tiburcio, R.A.S.; Ferreira, F.A.; Paes, F.A.S.V.; Melo, C.A.D.; Medeiros, W.N. Crescimento de mudas de clones de eucalipto submetidos à deriva simulada de diferentes herbicidas. Rev. Árvore 2012, 36, 65–73. [Google Scholar] [CrossRef]
- Carrero, O.; Stape, J.L.; Allen, L.; Arrevillaga, M.C.; Ladeira, M. Productivity gains from weed control and fertilization of short-rotation Eucalyptus plantations in the Venezuelan Western Llanos. For. Ecol. Manag. 2018, 430, 566–575. [Google Scholar] [CrossRef]
- Machado, M.S.; Ferreira, L.R.; Paula, J.L.D.; Pereira, G.A.M.; Gonçalves, V.A. Use of liquid fertilizer to reduce the phytotoxic effects of glyphosate on eucalyptus. Rev. Caatinga 2017, 30, 730–737. [Google Scholar] [CrossRef][Green Version]
- Veline, E.D.; Osipe, R.; Gazziero, D.L.P. Sociedade Brasileira da Ciência das Plantas Daninhas. In Procedimentos Para Instalação, Avaliação e Análise de Experimentos com Herbicidas; SBCPD: London, UK, 1995; 21p. [Google Scholar]
- Haring, S.C.; Ou, J.; Al-Khatib, K.; Hanson, B.D. Grapevine Injury and Fruit Yield Response to Simulated Auxin Herbicide Drift. HortScience 2022, 57, 384–388. [Google Scholar] [CrossRef]
- Gonçalves, V.A.; Ferreira, L.R.; Teixeira, M.F.; Freitas, F.C.L.D.; D’antonino, L. Sorption of indaziflam in brazilian soils with different pH values. Rev. Caatinga 2021, 34, 494–504. [Google Scholar] [CrossRef]
- Silva, F.B.; Costa, A.C.; Müller, C.; Almeida, G.M.; Nascimento, K.J.T.; Batista, P.F.; Domingos, M. Searching for biomarkers of early detection of 2,4-D effects in a native tree species from the Brazilian Cerrado biome. J. Environ. Sci. Health A 2022, 57, 71–80. [Google Scholar] [CrossRef]
- Smith, S.C.; Jennings, K.M.; Monks, D.W.; Jordan, D.L.; Reberg-Horton, S.C.; Schwarz, M.R. Sweetpotato tolerance and Palmer amaranth control with indaziflam. Weed Technol. 2022, 36, 202–206. [Google Scholar] [CrossRef]
- Brabham, C.; Lei, L.; Gu, Y.; Stork, J.; Barrett, M.; DeBolt, S. Indaziflam herbicidal action: A potent cellulose biosynthesis inhibitor. Plant. Physiol 2014, 166, 1177–1185. [Google Scholar] [CrossRef]
- Jarvis, M.C. Cellulose biosynthesis: Counting the chains. Plant. Physiol. 2013, 163, 1485–1486. [Google Scholar] [CrossRef]
- Sebastian, D.J.; Sebastian, J.R.; Nissen, S.J.; Beck, K.G. A potential new herbicide for invasive annual grass control on rangeland. Rangel. Ecol. Manag. 2016, 69, 195–198. [Google Scholar] [CrossRef]
- Nascimento, J.L.M.; Pereira, G.A.M.; Adriano, R.C.; Pucci, L.F.; Júnior, L.H.B.; Ferreira, L.R. Tolerância de plantas jovens de café a herbicidas aplicados isoladamente ou em mistura com o fertilizante fertiactyl. Rev. Bras. Herbic. 2020, 18, 681. [Google Scholar] [CrossRef]
- Machado, M.S.; Ferreira, L.R.; Pereira, G.A.M.; Gonçalves, V.A.; Paixão, G.P. Efeito protetor em plantas de eucalipto e controle de capim-braquiária com mistura de glifosato e fertilizante líquido em tanque. Planta Daninha 2017, 35, 1–8. [Google Scholar]
- Dayan, F.E.; Watson, S.B. Plant cell membrane as a marker for light dependent and light independent herbicide mechanisms of action. Pestic. Biochem. Phys. 2011, 101, 182–190. [Google Scholar] [CrossRef]
- Eceiza, M.V.; Gil-Monreal, M.; Barco-Antoñanzas, M.; Zabalza, A.; Royuela, M. The moderate oxidative stress induced by glyphosate is not detected in Amaranthus palmeri plants overexpressing EPSPS. J. Plant. Physiol. 2022, 274, 153720. [Google Scholar] [CrossRef] [PubMed]
- Velini, E.; Duke, S.O.; Trindade, M.L.B.; Meschede, D.K.; Carbonari, C.A. Mode of Action of Glyphosate; USDA: Washington, DC, USA, 2009; pp. 113–134. [Google Scholar]
- Barroso, M.A.A.; Murata, A.T. Matologia: Estudos Sobre Plantas Daninhas; Fábrica da Palavra: Jaboticabal, Brazil, 2021; p. 547. [Google Scholar]
- Wen, B.; Li, C.; Fu, X.; Li, D.; Li, L.; Chen, X.; Gao, D. Effects of nitrate deficiency on nitrate assimilation and chlorophyll synthesis of detached apple leaves. Plant. Physiol. Biochem. 2019, 142, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.C.; Gomes, D.M.; Anunciato, V.M.; Bianchi, L.; Carbonari, C.A.; Velini, E.D. Herbicides selectivity on seedlings of White Leadtree (Leucaena leucocephala). Científica 2020, 48, 56–66. [Google Scholar] [CrossRef][Green Version]
- Tuffi Santos, L.D.; Ferreira, F.A.; Meira, R.M.S.A.; Barros, N.F.; Ferreira, L.R.; Machado, A.F.L. Crescimento e morfoanatomia foliar de eucalipto sob efeito de deriva do glyphosate. Planta Daninha 2005, 23, 133–142. [Google Scholar] [CrossRef]
- Carbonari, C.A.; Velini, E.D.; Gomes, G.L.G.C.; Takahashi, E.N.; Araldi, R. Seletividade e absorção radicular do sulfentrazone em clones de eucalipto. Planta Daninha 2012, 30, 147–153. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).