Protocol for In Vitro Propagation of Salix acmophylla (Boiss.). Studies on Three Ecotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Culture Conditions
2.3. Axillary Shoot Proliferation
2.4. Rooting and Acclimatization
2.5. Statistical Analysis
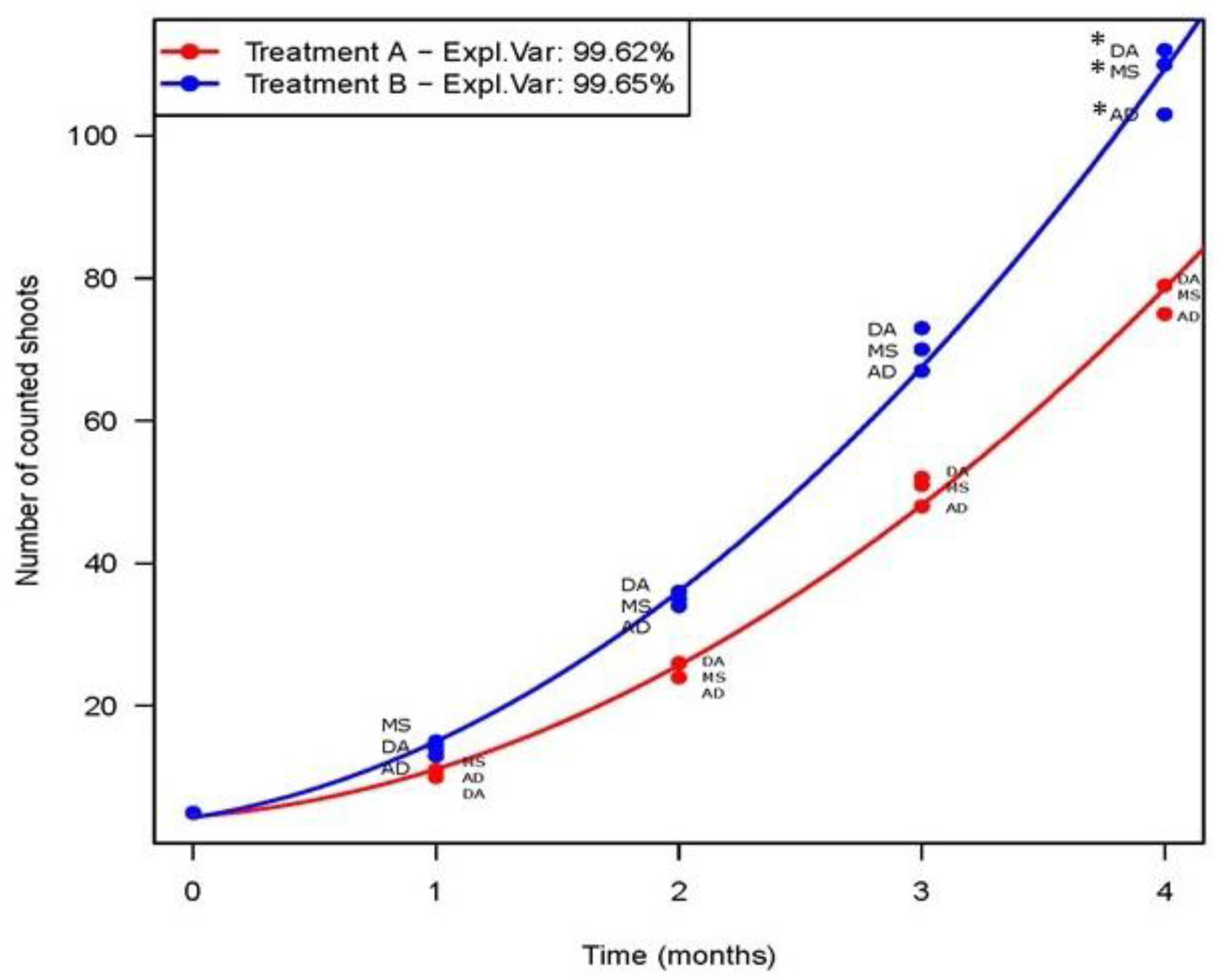
3. Results
3.1. Effect of PGRs on Shoot Proliferation
3.2. Rooting and Acclimatization
4. Discussion
4.1. Moderate Effectiveness of PGR Treatments in the Shoot Proliferation
4.2. An Alternative Procedure Makes the Proliferation Phase More Effective
4.3. Positive Results in the Rooting and Acclimatization Phases
4.4. Differences Found among the Tested Ecotypes
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anstead, L.; Boar, R.R. Willow spiling: Review of streambank stabilisation projects in the UK. Freshw. Rev. 2010, 3, 33–47. [Google Scholar] [CrossRef]
- Foereid, B.; Bro, R.; Overgaard Mogensen, V.; Porter, J.R. Effects of windbreak strips of willow coppice—Modeling and field experiment on barley in Denmark. Agric. Ecosyst. Environ. 2002, 93, 25–32. [Google Scholar] [CrossRef]
- Guidi, W.; Pitre, F.E.; Labrecque, M. Short-rotation coppice of willows for the production of biomass in Eastern Canada. In Biomass Now Sustainable Growth and Use; Matovic, M.D., Ed.; InTech Open Science: Rijeka, Croatia, 2013; pp. 421–448. [Google Scholar] [CrossRef]
- Karp, A. Willows as a source of renewable fuels and diverse products. In Challenges and Opportunities for the World’s Forests in the 21th Century; Fenning, T., Ed.; Springer Science+Business Media: Dordrecht, The Netherlands, 2014; pp. 617–641. [Google Scholar] [CrossRef]
- Kuzovkina, Y.; Quigley, M. Selection of Willows for floral and stem quality and continuous production sequence in temperate North America. HortTechnology 2004, 14, 415–419. [Google Scholar] [CrossRef]
- Rotherham, I.D. Willows in the farming landscape: A forgotten eco-cultural icon. Biodivers. Conserv. 2021. Available online: https://link.springer.com/article/10.1007/s10531-021-02324-2 (accessed on 20 March 2022). [CrossRef]
- Capuana, M. Heavy metals and woody plants—biotechnologies for phytoremediation. iForest 2011, 4, 7–15. [Google Scholar] [CrossRef]
- Guidi, W.; Kadri, H.; Labrecque, M. Establishment techniques to using willow for phytoremediation on a former oil refinery in southern Quebec: Achievements and constraints. Chem. Ecol. 2012, 28, 49–64. [Google Scholar] [CrossRef]
- Mleczek, M.; Rutkowski, P.; Rissmann, I.; Kaczmarek, Z.; Golinski, P.; Szentner, K.; Strazynska, K.; Stachowiak, A. Biomass productivity and phytoremediation potential of Salix alba and Salix viminalis. Biomass Bioenergy 2010, 34, 1410–1418. [Google Scholar] [CrossRef]
- Yang, W.D.; Yang, Y.; Ding, Z.L.; Yang, X.E.; Zhao, F.L.; Zhu, Z.Q. Uptake and accumulation of cadmium in flooded versus non-flooded Salix genotypes: Implications for phytoremediation. Ecol. Eng. 2019, 136, 79–88. [Google Scholar] [CrossRef]
- Bonga, J.M.; von Aderkas, P. (Eds.) In Vitro Culture of Trees; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1992; p. 226. Available online: https://books.google.it/books?hl=it&lr=&id=vjI5KXFZlcoC&oi=fnd&pg=IA9&dq=importance+in+vitro+culture+woody+plants&ots=bCs5h7IYts&sig=K8WQgsEqaa8jnCZiMPVrUzPIceA#v=onepage&q=importance%20in%20vitro%20culture%20woody%20plants&f=false (accessed on 20 March 2022).
- Ahuja, M.R. (Ed.) Micropropagation of Woody Plants; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 2005; p. 501. Available online: https://books.google.it/books?hl=it&lr=&id=vBv_CAAAQBAJ&oi=fnd&pg=PA303&dq=importance+in+vitro+culture+woody+plants&ots=9nvCRgeY8B&sig=ORWlofJflxxTv1JU-b7Sowtx_zA#v=onepage&q=importance%20in%20vitro%20culture%20woody%20plants&f=false (accessed on 20 March 2022).
- Palomo-Ríos, E.; Macalpine, E.; Shield, I.; Amey, J.; Karaoğlu, C.; West, J.; Hanley, S.; Krygier, R.; Karp, A.; Jones, H.D. Efficient method for rapid multiplication of clean and healthy willow clones via in vitro propagation with broad genotype applicability. Can. J. For. Res. 2015, 45, 1662–1667. [Google Scholar] [CrossRef]
- Bhojwani, S.S. Micropropagation method for a hybrid willow (Salix matsudana x alba NZ-1002). N. Z. J. Bot. 1980, 18, 209–214. [Google Scholar] [CrossRef]
- Chalupa, V. In vitro propagation of willows (Salix spp.), European mountain ash (Sorbus aucuparia F.) and black locust (Robinia pseudoacacia F.). Biol. Plant. 1983, 25, 305–307. [Google Scholar] [CrossRef]
- Dhir, K.K.; Angrish, R.M.; Bajaj, M. Micropropagation of Salix babylonica through in vitro shoot proliferation. Proc. Indian Acad. Sci. (Plant Sci.) 1984, 93, 655–660. [Google Scholar] [CrossRef]
- Bergman, L.; von Arnold, S.; Erickson, T. Effect of N6-benzyladenine on shoot of five willow clones (Salix spp.) cultured in vitro. Plant Cell Tiss. Org. 1985, 4, 135–144. [Google Scholar] [CrossRef]
- Stoehr, M.U.; Cai, M.; Zsuffa, L. In vitro plant regeneration via callus culture of mature Salix exigua. Can. J. For. Res. 1989, 19, 1634–1638. [Google Scholar] [CrossRef]
- Neuner, H.; Beiderbeck, R. In vitro propagation of Salix caprea L. by single node explants. Silvae Genet. 1993, 42, 308–310. Available online: https://www.thuenen.de/media/institute/fg/PDF/Silvae_Genetica/1993/Vol._42_Heft_6/42_6_308.pdf (accessed on 20 March 2022).
- Agrawal, D.C.; Gebhardt, K. Rapid micropropagation of hybrid willow (Salix) established by ovary culture. J. Plant Physiol. 1994, 143, 763–765. [Google Scholar] [CrossRef]
- Amo-Marco, J.B.; Lledo, M.D. In vitro propagation of Salix tarraconensis Pau ex Font Quer, an endemic and threatened plant. In Vitro Cell. Dev. Biol. Plant. 1996, 32, 42–46. [Google Scholar] [CrossRef]
- Lyyra, S.; Lima, A.; Merkle, S. In vitro regeneration of Salix nigra from adventitious shoots. Tree Physiol. 2006, 26, 969–975. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y.W.; Moon, H.K.; Murthy, H.N.; Choi, Y.H.; Cho, H.M. Micropropagation of Salix pseudolasiogyne from nodal explants. Plant Cell Tiss. Org. 2008, 93, 341–346. [Google Scholar] [CrossRef]
- Mashkina, O.S.; Tabatskaya, T.M.; Gorobets, A.I.; Shestibratov, K.A. Method of clonal micropropagation of different willow species and hybrids. Appl. Biochem. Microbiol. 2010, 46, 769–775. [Google Scholar] [CrossRef]
- Khan, M.I.; Ahmad, N.; Anis, M. The role of cytokinins on in vitro shoot production in Salix tetrasperma Roxb.: A tree of ecological importance. Trees Struct. Funct. 2011, 25, 577–584. [Google Scholar] [CrossRef]
- Skálová, D.; Navrátilová, B.; Richterová, L.; Knit, M.; Sochor, M.; Vašut, R.J. Biotechnological methods for in vitro propagation in willows (Salix spp.). Cent. Eur. J. Biol. 2012, 7, 931–940. [Google Scholar] [CrossRef]
- Vidal, N.; Correa, B.; Rial, E.; Regueira, M.; Sánchez, C.; Cuenca, B. Comparison of temporary and continuous immersion systems for micropropagation of axillary shoots of chestnut and willow. Acta Hortic. 2015, 1083, 227–233. [Google Scholar] [CrossRef]
- Khan, M.I.; Ahmad, N.; Anis, M.; Alatar, A.A.; Faisal, M. In vitro conservation strategies for the Indian willow (Salix tetrasperma Roxb.), a vulnerable tree species via propagation through synthetic seeds. Biocatal. Agric. Biotechnol. 2018, 16, 17–21. [Google Scholar] [CrossRef]
- Towill, L.E.; Widrlechner, M. Cryopreservation of Salix species using sections from winter vegetative scions. CryoLetters 2004, 25, 71–80. Available online: https://www.ingentaconnect.com/content/cryo/cryo/2004/00000025/00000001/art00009#expand/collapse (accessed on 20 March 2022).
- Vahala, T.; Stabel, P.; Eriksson, T. Genetic transformation of willows Salix spp. by Agrobacterium tumefaciens. Plant Cell Rep. 1989, 8, 55–58. [Google Scholar] [CrossRef]
- Yang, J.L.; Yi, J.S.; Yang, C.P.; Li, C.H. Agrobacterium tumefaciens-mediated genetic transformation of Salix matsudana Koidz. using mature seeds. Tree Physiol. 2013, 33, 628–639. [Google Scholar] [CrossRef]
- Sheikh, M.I. Trees of Pakistan; Pictorial Printing (Pvt.) Ltd.: Islamabad, Pakistan, 1993; p. 142. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.692.295&rep=rep1&type=pdf (accessed on 20 March 2022).
- Rossi Pisa, P.; Bitelli, G.; Bitelli, M.; Speranza, M.; Ferroni, L.; Catizone, P.; Vignudelli, M. Environmental assessment of an archaeological site for the development of an archaeological park. In ARCHAIA, Case Studies on Research Planning, Characterisation, Conservation and Management of Archaeological Sites; Archaeopress: Oxford, UK, 2008; pp. 273–285. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.462.584&rep=rep1&type=pdf (accessed on 20 March 2022).
- Rawat, Y.S.; Vishvakarma, C.R. Contribution of willow in meeting bio-resources needs and land restoration in cold desert of the Lahaul valley, North-Western Himalaya, India. In Proceedings of the International Symposium on Environmental Science and Technology (ISEST 2007), Beijing, China, 13–16 November 2007; Feng, C., Li, S., Eds.; Section one: Ecosystem Restoration. pp. 23–33. Available online: https://www.researchgate.net/profile/Subhash-Cr-Vishvakarma-2/publication/278675368_Contribution_of_willow_in_meeting_bio-resources_needs_and_land_restoration_in_cold_desert_of_the_Lahaul_valley_North-Western_Himalaya_India/links/5582c50708ae1b14a0a2749d/Contribution-of-willow-in-meeting-bio-resources-needs-and-land-restoration-in-cold-desert-of-the-Lahaul-valley-North-Western-Himalaya-India.pdf (accessed on 20 March 2022).
- Ali, M.B.; Vajpayee, P.; Tripathi, R.D.; Rai, U.N.; Singh, S.N.; Singh, S.P. Phytoremediation of lead, nickel, and copper by Salix acmophylla Boiss. Role of antioxidant enzymes and antioxidant substances. Bull. Environ. Contam. Toxicol. 2003, 70, 462–469. [Google Scholar] [CrossRef]
- Hooshmand, S.; Ghamarizare, A.; Shariat, A.; Kiarostami, K.H. The study of phytoremediation potential of 3 species of Salix alba, S. acmophylla and S. fragilis under lead stress. Iran. J. For. Range Prot. Res. 2013, 11, 20. [Google Scholar]
- Palm, E.; Klein, J.D.; Mancuso, S.; Guidi Nissim, W. The physiological response of different brook willow (Salix acmophylla Boiss.) ecotypes to salinity. Plants 2022, 11, 739. [Google Scholar] [CrossRef]
- Muklada, H.; Voet, H.; Deutch, T.; Zachut, M.; Kra, G.; Blum, S.E.; Krifuks, O.; Glasser, T.A.; Klein, J.D.; Davidovich-Rikanati, R.; et al. The effect of willow fodder feeding on immune cell populations in the blood and milk of late-lactating dairy goats. Animal 2020, 14, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Muklada, H.; Davidovich-Rikanati, R.; Wilkerson, D.G.; Klein, J.D.; Deutch-Traubaum, T.; Zou, J.; Awabdeh, S.; Sweidan, R.; Landau, S.Y.; Schwartz, A.; et al. Genotypic diversity in willow (Salix spp.) is associated with chemical and morphological polymorphism, suggesting human-assisted dissemination in the Eastern Mediterranean. Biochem. Syst. Ecol. 2020, 91, 104081. [Google Scholar] [CrossRef]
- Ullah, R.; Hussain, Z.; Iqbal, Z.; Hussain, J.; Khan, U.F.; Khan, N.; Muhammad, Z.; Khattak, S.A.; Ahmad, S.; Ur Rehman, N.; et al. Traditional uses of medicinal plants in Dara Adam Khel NWFP Pakistan. J. Med. Plants Res. 2010, 17, 1815–1821. [Google Scholar] [CrossRef]
- Ullah, M.; Usman, M.; Khan, U.F.; Mahmood, A.; Hussain, M.; Wazir, S.; Daud, M.; Shinwari, Z.K. An ethnobotanical survey of indigenous medicinal plants in Wana district south Waziristan agency, Pakistan. J. Ethnopharmacol. 2013, 150, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Daneshvand, E.; Rahmani, F.; Khodakarimi, A. Genetic diversity among eight species of willow (Salix species) from Iran based on SRAP markers. J Trop. Biol. Conserv. 2015, 12, 75–85. Available online: https://jurcon.ums.edu.my/ojums/index.php/jtbc/article/view/273 (accessed on 20 March 2022).
- Abdullah, M.O. Effect of cutting collection times, lengths and planting methods in propagation and growth of Salix acmophylla Bioss. Mesop. J. Agric. 2009, 37, 158–169. [Google Scholar] [CrossRef][Green Version]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco cultures. Physiol. Plant. 1962, 15, 473–497. Available online: https://florestal81.webnode.com/_files/200000040-03153040fe/07%20Artigo%20MS%201962.pdf (accessed on 15 March 2022). [CrossRef]
- Lloyd, G.; Mc Cown, B. Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot tip culture. Proc. Int. Plant. Propag. Soc. 1981, 30, 421–427. Available online: https://www.cabdirect.org/cabdirect/abstract/19830315515 (accessed on 15 March 2022).
- Liesebach, M.; Naujoks, G. Approaches on vegetative propagation of difficult-to-root Salix caprea. Plant Cell Tiss. Org. 2004, 79, 239–247. [Google Scholar] [CrossRef]
- Iliev, I.A. Factors affecting the axillary and adventitious shoot formation in woody plants in vitro. Acta Hortic. 2015, 1155, 15–28. [Google Scholar] [CrossRef]
- Hung, C.D.; Hong, C.H.; Kim, S.K.; Lee, K.H.; Park, J.Y.; Nam, M.W.; Choi, D.H.; Lee, H.I. LED light for in vitro and ex vitro efficient growth of economically important highbush blueberry (Vaccinium corymbosum L.). Acta Physiol. Plant. 2016, 38, 152. [Google Scholar] [CrossRef]
- Georgieva, M.; Kondakova, V. In vitro propagation of Vaccinium corymbosum L. Bulgarian J. Agric. Sci. 2021, 27, 323–327. [Google Scholar]
- Aftab, F. Progress and prospects for efficient micropropagation of woody plants. In Crop Production for Agricultural Improvement; Ashraf, M., Öztürk, M., Ahmad, M.S.A., Aksoy, A., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 363–377. Available online: https://link.springer.com/content/pdf/10.1007/978-94-007-4116-4_13.pdf (accessed on 15 March 2022).
- del Carmen San José, M.; Tereza Martínez, M.; José Cernadas, M.; Montenegro, R.; Corredoira, E. Application of thidiazuron in the micropropagation of Fagaceae. In Thidiazuron: From Urea Derivative to Plant Growth Regulator; Ahmad, N., Faisal, M., Eds.; Springer: Singapore, 2018; pp. 189–209. [Google Scholar] [CrossRef]
- Vinoth, A.; Ravindhran, R. In vitro morphogenesis of woody plants using thidiazuron. In Thidiazuron: From Urea Derivative to Plant Growth Regulator; Ahmad, N., Faisal, M., Eds.; Springer: Singapore, 2018; pp. 211–230. Available online: https://link.springer.com/content/pdf/10.1007/978-981-10-8004-3_10.pdf (accessed on 15 March 2022).
- Gebhardt, K. Grundlagen und Methoden der Züchtung pharmazeutisch wertvoller Weiden. Die Holzzucht 1992, 46, 9–14. Available online: https://www.cabdirect.org/cabdirect/abstract/19950612506 (accessed on 20 March 2022).
- Regueira, M.; Rial, E.; Blanco, B.; Bogo, B.; Aldrey, A.; Correa, B.; Vidal, N. Micropropagation of axillary shoots of Salix viminalis using a temporary immersion system. Trees 2018, 32, 61–71. [Google Scholar] [CrossRef]
- Khan, M.I.; Anis, M. Modulation of in vitro morphogenesis in nodal segments of Salix tetrasperma Roxb. through the use of TDZ, different media types and culture regimes. Agrofor. Syst. 2012, 86, 95–103. [Google Scholar] [CrossRef]
- Meyer, H.J.; van Staden, J. In vitro multiplication of Ixia flexuosa. Hortscience 1988, 23, 1070–1071. Available online: https://scholar.google.com/scholar?q=Meyer+MM,+Kerns+HR+(1986)+Thidiazuron+and+in+vitro+shoot+proliferation+of+Celtis+occidentalis+L.+Abst.+in+Proceedings+of+the+VI+International+Congress+Plant+Tissue+%26+Cell+Culture,+Minneapolis,+149&hl=it&as_sdt=0,5 (accessed on 20 March 2022).
- Sergeev, R.V.; Zontikov, D.N.; Zontikova, S.A.; Kostromin, D.V.; Lastochkin, D.M.; Medyakov, A.A.; Timakov, A.A.; Alekseev, A.V.; Kamensky, A.D. Influence of cultivation factors on morphogenesis in vitro Salix acutifolia Willd. IOP Conf. Ser. Earth Environ. Sci. 2020, 548, 072065. [Google Scholar] [CrossRef]
- Garton, S.; Read, P.E.; Farnham, R.S. Effect of stock plant nutrition on macro-and micro-propagability of Salix. Acta Hort. 1983, 131, 141–151. [Google Scholar] [CrossRef]
- Whitehead, H.C.M.; Giles, K.L. Rapid propagation of poplars by tissue culture methods. N. Z. J. For. Sci. 1977, 7, 40–43. Available online: https://scion-web.squiz.cloud/__data/assets/pdf_file/0004/58981/NZJFS711977WHITEHEAD40-43.pdf (accessed on 20 March 2022).

| Treatment (μM) | Shoots per Explant | ANOVA p Values | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mea Shearim | Adom | Darom | Treatment Means | ||||||||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Ecotype (E) | Treatment (T) | ExT | |||||
| Control | 0.00 | (0.00) | d | 0.17 | (0.11) | d | 0.08 | (0.08) | d | 0.08 | (0.04) | d | 0.3721 | <0.001 | 1.9867 |
| BA1 | 0.83 | (0.11) | b | 0.58 | (0.15) | b | 0.83 | (0.21) | b | 0.74 | (0.09) | b | |||
| BA5 | 1.25 | (0.13) | a | 1.08 | (0.08) | a | 1.00 | (0.12) | a | 1.12 | (0.06) | a | |||
| ZEA 0.5 | 0.42 | (0.15) | c | 0.25 | (0.13) | c | 0.42 | (0.15) | c | 0.41 | (0.08) | c | |||
| ZEA 1 | 0.50 | (0.15) | c | 0.33 | (0.14) | c | 0.50 | (0.15) | c | 0.44 | (0.09) | c | |||
| TDZ 1 | 0.42 | (0.15) | c | 0.25 | (0.13) | c | 0.33 | (0.14) | c | 0.33 | (0.08) | c | |||
| TDZ 2 | 0.33 | (0.13) | c | 0.36 | (0.15) | c | 0.27 | (0.13) | c | 0.31 | (0.08) | c | |||
| Mean | 0.54 | (0.12) | 0.43 | (0.13) | 0.49 | (0.14) | 0.49 | (0.07) | |||||||
| Ecotype | Treatment | Rooting (%) | Roots (n) | Root Length (mm) | ||
|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |||
| MS | Control | 83.3 a | 3.8 | (0.17) a | 39.7 | (0.36) a |
| IBA | 95.8 a | 3.8 | (0.14) a | 40.4 | (0.34) a | |
| Mean | 89.5 A | 3.8 | (0.16) A | 40.1 | (0.35) A | |
| AD | Control | 58.3 a | 3.1 | (0.14) b | 29.4 | (0.58) a |
| IBA | 87.5 b | 3.8 | (0.13) a | 36.1 | (0.49) b | |
| Mean | 72.9 B | 3.4 | (0.13) B | 32.7 | (0.54) B | |
| DA | Control | 87.5 a | 3.6 | (0.17) a | 38.2 | (0.58) a |
| IBA | 95.8 b | 3.5 | (0.13) a | 40.0 | (0.34) a | |
| Mean | 91.6 A | 3.5 | (0.15) B | 39.1 | (0.42) A | |
| Treatment Means | Control | 76.4 b | 3.5 | (0.16) | 35.8 | (0.51) |
| IBA | 93.0 a | 3.7 | (0.13) | 38.8 | (0.39) | |
| ANOVA | Ecotype (E) | 0.05 | <0.001 | |||
| p-values | Treatment (T) | 0.08 | 0.007 | |||
| ExT | 0.03 | <0.001 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capuana, M.; Nissim, W.G.; Klein, J.D. Protocol for In Vitro Propagation of Salix acmophylla (Boiss.). Studies on Three Ecotypes. Forests 2022, 13, 1124. https://doi.org/10.3390/f13071124
Capuana M, Nissim WG, Klein JD. Protocol for In Vitro Propagation of Salix acmophylla (Boiss.). Studies on Three Ecotypes. Forests. 2022; 13(7):1124. https://doi.org/10.3390/f13071124
Chicago/Turabian StyleCapuana, Maurizio, Werther Guidi Nissim, and Joshua D. Klein. 2022. "Protocol for In Vitro Propagation of Salix acmophylla (Boiss.). Studies on Three Ecotypes" Forests 13, no. 7: 1124. https://doi.org/10.3390/f13071124
APA StyleCapuana, M., Nissim, W. G., & Klein, J. D. (2022). Protocol for In Vitro Propagation of Salix acmophylla (Boiss.). Studies on Three Ecotypes. Forests, 13(7), 1124. https://doi.org/10.3390/f13071124






