Tree Fresh Leaf- and Twig-Leached Dissolved Organic Matter Quantity and Biodegradability in Subtropical Plantations in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Plant Sampling and Measurement
2.3. Statistical Analyses
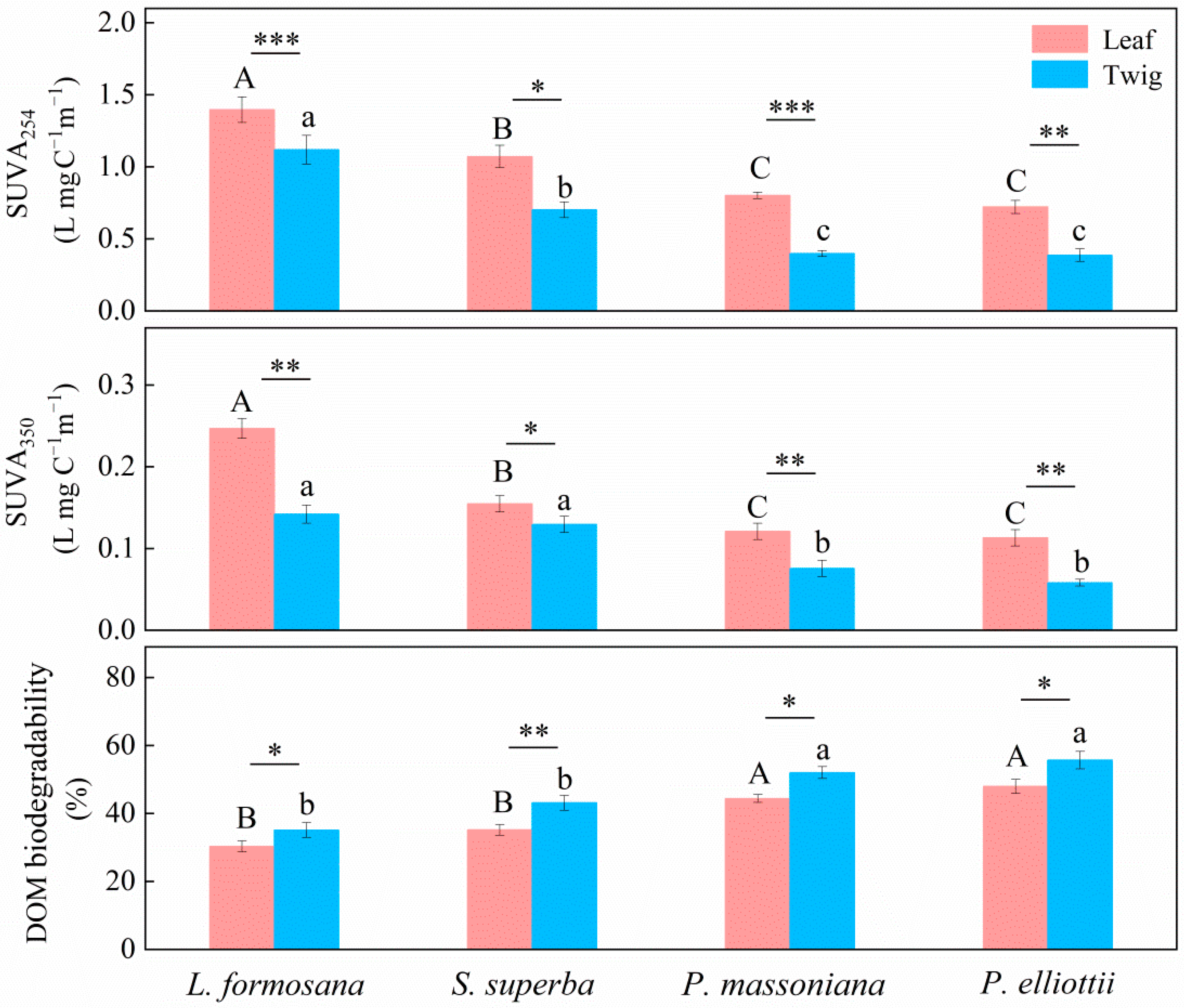
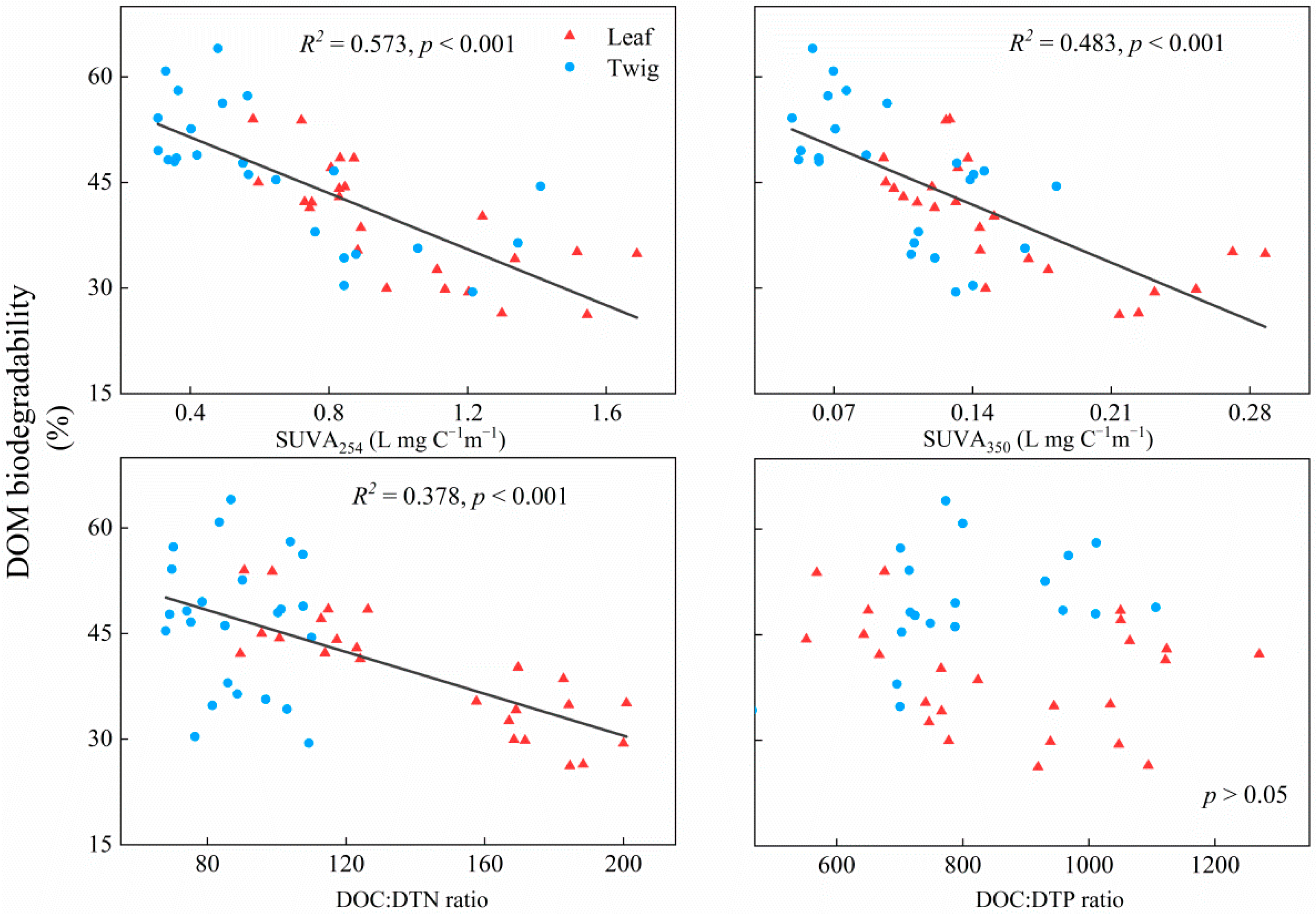
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| C:N Ratio | C:P Ratio | Polyphenols | Soluble Sugars | Starch | |
|---|---|---|---|---|---|
| DOC production | –0.891 *** | –0.701 *** | 0.641 *** | 0.746 *** | 0.538 *** |
| DTN production | –0.870 *** | –0.716 *** | 0.626 *** | 0.709 *** | 0.529 *** |
| DTP production | –0.845 *** | –0.627 *** | 0.779 *** | 0.870 *** | 0.699 *** |
| SUVA254 value | –0.543 *** | –0.475 ** | 0.705 *** | 0.751 *** | 0.758 *** |
| SUVA350 value | –0.481 ** | –0.483 ** | 0.781 *** | 0.775 *** | 0.849 *** |
References
- Kalbitz, K.; Sollinger, S.; Park, J.H.; Michalzik, B.; Matzner, E. Controls on the dynamics of dissolved organic matter in soils: A review. Soil Sci. 2000, 165, 277–304. [Google Scholar] [CrossRef]
- Kalbitz, K.; Kaiser, K. Contribution of dissolved organic matter to carbon storage in forest mineral soils. J. Plant Nutr. Soil Sci. 2008, 171, 52–60. [Google Scholar] [CrossRef]
- Marschner, B.; Kalbitz, K. Controls of bioavailability and biodegradability of dissolved organic matter in soils. Geoderma 2003, 113, 211–235. [Google Scholar] [CrossRef]
- Joly, F.X.; Fromin, N.; Kiikkilä, O.; Hättenschwiler, S. Diversity of leaf litter leachates from temperate forest trees and its consequences for soil microbial activity. Biogeochemistry 2016, 129, 373–388. [Google Scholar] [CrossRef]
- Jílková, V.; Jandová, K.; Cajthaml, T.; Kukla, J.; Jansa, J. Differences in the flow of spruce-derived needle leachates and root exudates through a temperate coniferous forest mineral topsoil. Geoderma 2022, 405, 115442. [Google Scholar] [CrossRef]
- De Marco, A.; Spaccini, R.; Virzo De Santo, A. Differences in nutrients, organic components and decomposition pattern of Phillyrea angustifolia leaf litter across a low maquis. Plant Soil 2021, 464, 559–578. [Google Scholar] [CrossRef]
- Schwesig, D.; Kalbitz, K.; Matzner, E. Mineralization of dissolved organic carbon in mineral soil solution of two forest soils. J. Plant Nutr. Soil Sci. 2003, 166, 585–593. [Google Scholar] [CrossRef]
- Sarker, T.C.; Maisto, G.; De Marco, A.; Esposito, F.; Panico, S.C.; Alam, M.F.; Mazzoleni, S.; Bonanomi, G. Explaining trajectories of chemical changes during decomposition of tropical litter by 13C-CPMAS NMR, proximate and nutrients analysis. Plant Soil 2018, 436, 13–28. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Soong, J.L.; Horton, A.J.; Campbell, E.E.; Haddix, M.L.; Wall, D.H.; Parton, W.J. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 2015, 8, 776–779. [Google Scholar] [CrossRef]
- Neff, J.C.; Asner, G.P. Dissolved organic carbon in terrestrial ecosystems: Synthesis and a model. Ecosystems 2001, 4, 29–48. [Google Scholar] [CrossRef] [Green Version]
- Jansen, B.; Kalbitz, K.; McDowell, W.H. Dissolved organic matter: Linking soils and aquatic systems. Vadose Zone J. 2014, 13, vzj2014.05.0051. [Google Scholar] [CrossRef] [Green Version]
- Cleveland, C.C.; Neff, J.C.; Townsend, A.R.; Hood, E. Composition, dynamics, and fate of leached dissolved organic matter in terrestrial ecosystems: Results from a decomposition experiment. Ecosystems 2004, 7, 175–285. [Google Scholar] [CrossRef]
- Soong, J.L.; Parton, W.J.; Calderon, F.; Campbell, E.E.; Cotrufo, M.F. A new conceptual model on the fate and controls of fresh and pyrolized plant litter decomposition. Biogeochemistry 2015, 124, 27–44. [Google Scholar] [CrossRef]
- Xu, J.W.; Yang, N.; Shi, F.X.; Zhang, Y.; Wan, S.Z.; Mao, R. Bark controls tree branch-leached dissolved organic matter production and bioavailability in a subtropical forest. Biogeochemistry 2022, 158, 345–355. [Google Scholar] [CrossRef]
- Hagedorn, F.; Machwitz, M. Controls on dissolved organic matter leaching from forest litter grown under elevated atmospheric CO2. Soil Biol. Biochem. 2007, 39, 1759–1769. [Google Scholar] [CrossRef]
- Don, A.; Kalbitz, K. Amounts and degradability of dissolved organic carbon from foliar litter at different decomposition stages. Soil Biol. Biochem. 2005, 37, 2171–2179. [Google Scholar] [CrossRef]
- Pinsonneault, A.J.; Moore, T.R.; Roulet, N.T.; Lapierre, J.F. Biodegradability of vegetation-derived dissolved organic carbon in a cool temperate ombrotrophic bog. Ecosystems 2016, 19, 1023–1036. [Google Scholar] [CrossRef]
- Ding, Y.D.; Xie, X.Y.; Ji, J.H.; Li, Q.Q.; Xu, J.W.; Mao, R. Tree mycorrhizal effect on litter-leached DOC amounts and biodegradation is highly dependent on leaf habits in subtropical forests of southern China. J. Soils Sediment 2021, 21, 3572–3579. [Google Scholar] [CrossRef]
- Wu, P.P.; Ding, Y.D.; Li, S.L.; Sun, X.X.; Zhang, Y.; Mao, R. Carbon, nitrogen and phosphorus stoichiometry controls interspecific patterns of leaf litter-derived dissolved organic matter biodegradation in subtropical plantations of China. iForest 2021, 14, 80–85. [Google Scholar] [CrossRef]
- Memoli, V.; De Marco, A.; Esposito, F.; Panico, S.C.; Barile, R.; Maisto, G. Seasonality, altitude and human activities control soil quality in a national park surrounded by an urban area. Geoderma 2019, 337, 1–10. [Google Scholar] [CrossRef]
- De Marco, A.; Fioretto, A.; Giordano, M.; Innangi, M.; Menta, C.; Papa, S.; Virzo De Santo, A. C stocks in forest floor and mineral soil of two Mediterranean beech forests. Forests 2016, 7, 181. [Google Scholar] [CrossRef] [Green Version]
- Chomel, M.; Baldy, V.; Guittonny, M.; Greff, S.; DesRochers, A. Litter leachates have stronger impact than leaf litter on Folsomia candida fitness. Soil Biol. Biochem. 2020, 147, 107850. [Google Scholar] [CrossRef]
- Dale, V.H.; Joyce, L.A.; McNulty, S.; Neilson, R.P.; Ayres, M.P.; Flannigan, M.D.; Hanson, P.J.; Irland, L.C.; Lugo, A.E.; Peterson, C.J.; et al. Climate change and forest disturbances: Climate change can affect forests by altering the frequency, intensity, duration, and timing of fire, drought, introduced species, insect and pathogen outbreaks, hurricanes, windstorms, ice storms, or landslides. BioScience 2001, 51, 723–734. [Google Scholar] [CrossRef] [Green Version]
- Xu, X. Nutrient dynamics in decomposing needles of Pinus luchuensis after typhoon disturbance in a subtropical environment. Ann. For. Sci. 2006, 63, 707–713. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.P.; Xu, M.; Chi, Y.G.; Zheng, Y.P.; Shen, R.C.; Wang, S.L. Effects of freeze damage on litter production, quality and decomposition in a loblolly pine forest in central China. Plant Soil 2014, 374, 449–458. [Google Scholar] [CrossRef]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef]
- Hansen, A.M.; Kraus, T.E.C.; Pellerin, B.A.; Fleck, J.A.; Downing, B.D.; Bergamaschi, B.A. Optical properties of dissolved organic matter (DOM): Effects of biological and photolytic degradation. Limnol. Oceanogr. 2016, 71, 1015–1032. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.W.; Ding, Y.D.; Li, S.L.; Mao, R. Amount and biodegradation of dissolved organic matter leached from tree branches and roots in subtropical plantations of China. For. Ecol. Manag. 2021, 484, 118944. [Google Scholar] [CrossRef]
- Yu, Z.; Dahlgren, R.A. Evaluation of methods for measuring polyphenols in conifer foliage. J. Chem. Ecol. 2000, 26, 2119–2140. [Google Scholar] [CrossRef]
- Wang, F.C.; Chen, F.S.; Wang, G.G.; Mao, R.; Fang, X.M.; Wang, H.M.; Bu, W.S. Effects of experimental nitrogen addition on nutrients and nonstructural carbohydrates of dominant understory plants in a Chinese fir plantation. Forests 2019, 10, 155. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Schreeg, L.A.; Mack, M.C.; Turner, B.L. Nutrient‐specific solubility patterns of leaf litter across 41 lowland tropical woody species. Ecology 2013, 94, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Ebina, J.; Tsutsui, T.; Shirai, T. Simultaneous determination of total nitrogen and total phosphorus in water using peroxodisulfate oxidation. Water Res. 1983, 17, 1721–1726. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Hensgens, G.; Lechtenfeld, O.J.; Guillemette, F.; Laudon, H.; Berggren, M. Impacts of litter decay on organic leachate composition and reactivity. Biogeochemistry 2020, 154, 99–117. [Google Scholar] [CrossRef]
- Chen, H.; Liu, X.J.; Blosser, G.D.; Rücker, A.M.; Conner, W.H.; Chow, A.T. Molecular dynamics of foliar litter and dissolved organic matter during the decomposition process. Biogeochemistry 2020, 150, 17–30. [Google Scholar] [CrossRef]
- Li, S.L.; Xu, J.W.; Ding, Y.D.; Mao, R. Litter water-holding and water-loss characteristics of trees and ferns in the water conservation forests at the middle reaches of the Gan River. J. Soil Water Conserv. 2021, 35, 170–176. [Google Scholar]
- Liang, C. Soil microbial carbon pump: Mechanism and appraisal. Soil Ecol. Lett. 2020, 2, 241–254. [Google Scholar] [CrossRef]
- Bantle, A.; Borken, W.; Ellerbrock, R.H.; Schulze, E.D.; Weisser, W.W.; Matzner, E. Quantity and quality of dissolved organic carbon released from coarse woody debris of different tree species in the early phase of decomposition. For. Ecol. Manag. 2014, 329, 287–294. [Google Scholar] [CrossRef]
- Tang, Z.Y.; Xu, W.T.; Zhou, G.Y.; Bai, Y.F.; Li, J.X.; Tang, X.L.; Chen, D.M.; Liu, Q.; Ma, W.D.; Xiong, G.M.; et al. Patterns of plant carbon, nitrogen, and phosphorus concentration in relation to productivity in China’s terrestrial ecosystems. Proc. Nat. Acad. Sci. USA 2018, 115, 4033–4038. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.Z.; Shi, F.X.; Chen, H.M.; Zhang, Y.; Guo, Y.D.; Mao, R. Relationship between relative growth rate and C:N:P stoichiometry for the marsh herbaceous plants under water-level stress conditions. Glob. Ecol. Conserv. 2021, 25, e01416. [Google Scholar] [CrossRef]
- Kiikkilä, O.; Kitunen, V.; Spetz, P.; Smolander, A. Characterization of dissolved organic matter in decomposing Norway spruce and silver birch litter. Eur. J. Soil Sci. 2012, 63, 476–486. [Google Scholar] [CrossRef]
- Mao, R.; Li, S. Temporal controls on dissolved organic carbon biodegradation in subtropical rivers: Initial chemical composition versus stoichiometry. Sci. Total Environ. 2019, 651, 3064–3069. [Google Scholar] [CrossRef] [PubMed]
- Kammer, A.; Hagedorn, F. Mineralisation, leaching and stabilisation of 13 C-labelled leaf and twig litter in a beech forest soil. Biogeosciences 2011, 8, 2195–2208. [Google Scholar] [CrossRef] [Green Version]
- Gatehouse, J.A. Plant resistance towards insect herbivores: A dynamic interaction. New Phytol. 2002, 156, 145–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.M.; Liu, J.; Zou, B.; Neher, D.A.; Zhu, W.; Li, Z.A. Species-dependent responses of soil microbial properties to fresh leaf inputs in a subtropical forest soil in South China. J. Plant Ecol. 2014, 7, 86–96. [Google Scholar] [CrossRef] [Green Version]
| Species | Organ Type | Organic C | Total N | Total P | C:N Ratio | C:P Ratio | Polyphenols | Soluble Sugars | Starch | Tissue Density |
|---|---|---|---|---|---|---|---|---|---|---|
| mg g−1 | mg g−1 | μg g−1 | mg g−1 | mg g−1 | mg g−1 | g cm−3 | ||||
| L. formosana | Leaf | 465 (3) B | 15.7 (0.29) A | 1338 (28) A | 29.7 (0.4) C | 348 (7) C | 128.2 (1.9) A | 66.5 (1.1) A | 29.9 (1.1) A | 0.782 (0.01) A |
| Twig | 551 (4) a | 5.84 (0.07) a | 704 (11) b | 94.4 (1.6) b | 784 (11) b | 40.5 (0.7) b | 37.5 (1.1) a | 22.0 (0.9) a | 0.567 (0.01) a | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.020 | <0.001 | |
| S. superba | Leaf | 482 (4) A | 14.2 (0.17) B | 1142 (25) B | 34.1 (0.6) B | 424 (12) B | 120.1 (1.6) B | 60.1 (1.6) B | 26.7 (0.6) A | 0.468 (0.01) C |
| Twig | 460 (4) d | 4.12 (0.06) c | 676 (7) b | 111.8(2.0)a | 681 (6) c | 76.8 (0.7) a | 34.9 (0.7) a | 19.8 (1.1) ab | 0.605 (0.02) a | |
| p-value | 0.01 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | |
| P. massoniana | Leaf | 475 (3) AB | 13.5 (0.17) B | 1377 (18) A | 35.4 (0.6) B | 345 (6) C | 61.6 (1.0) D | 52.1 (1.3) C | 21.0 (0.7) B | 0.693 (0.002) B |
| Twig | 516 (4) b | 5.77 (0.06) a | 583 (8) c | 89.4 (0.9) b | 885 (11) a | 23.2 (0.5) c | 29.1 (1.1) b | 18.1 (0.8) b | 0.456 (0.001) b | |
| p-value | 0.01 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.016 | <0.001 | |
| P. elliottii | Leaf | 484 (3) A | 8.8 (0.15) C | 918 (22) C | 55.3 (1.2) A | 528 (13) A | 84.7 (0.9) C | 49.4 (1.4) C | 20.0 (0.8) B | 0.676 (0.002) B |
| Twig | 498 (5) c | 5.27 (0.07) b | 745 (9) a | 94.7 (1.4) b | 669 (10) c | 21.3 (0.7) c | 29.5 (1.1) b | 16.4 (0.8) b | 0.471 (0.001) b | |
| p-value | 0.05 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.042 | <0.001 |
| Sources of Variation | DOC Production | DTN Production | DTP Production | DOC:DTN Ratio | DOC:DTP Ratio | SUVA254 | SUVA350 | DOM Biodegradability |
|---|---|---|---|---|---|---|---|---|
| S | 527 *** | 158 *** | 426 *** | 85 *** | 120 *** | 53 *** | 87 *** | 41 *** |
| O | 2697 *** | 1450 *** | 1783 *** | 481 *** | 89 *** | 60 *** | 119 *** | 27 *** |
| S × O | 707 *** | 417 *** | 329 *** | 67 *** | 85 *** | 0.35 | 10 *** | 0.31 |
| Species | Organ Type | DOC Production | DTN Production | DTP Production | DOC:DTN Ratio | DOC:DTP Ratio |
|---|---|---|---|---|---|---|
| mg g−1 | μg g−1 | μg g−1 | ||||
| L. formosana | Leaf | 60.9 (1.4) A | 323 (4) A | 61.2 (1.5) A | 188 (5) A | 997 (29) B |
| Twig | 7.7 (0.4) b | 80 (4) c | 17.1 (0.6) a | 97 (5) a | 449 (7) c | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| S. superba | Leaf | 35.7 (0.3) B | 212 (4) B | 46.4 (0.8) B | 169 (3) B | 770 (12) C |
| Twig | 8.3 (0.4) b | 107 (4) b | 11.5 (0.7) c | 77 (3) b | 726 (15) b | |
| p-value | <0.001 | 0.001 | <0.001 | <0.001 | 0.055 | |
| P. massoniana | Leaf | 19.2 (0.3) C | 161 (4) C | 17.3 (0.6) C | 120 (2) C | 1114 (34) A |
| Twig | 14.0 (0.6) a | 138 (6) a | 14.0 (0.5) b | 102 (3) a | 998 (25) a | |
| p-value | 0.001 | <0.001 | 0.005 | 0.006 | 0.003 | |
| P. elliottii | Leaf | 13.1 (0.3) D | 134 (5) D | 21.1 (0.8) C | 98 (4) D | 626 (22) D |
| Twig | 8.8 (0.6) b | 114 (4) b | 11.8 (0.6) c | 77 (3) b | 749 (18) b | |
| p-value | 0.002 | 0.003 | 0.001 | 0.014 | 0.010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.-W.; Ji, J.-H.; Hu, D.-N.; Zheng, Z.; Mao, R. Tree Fresh Leaf- and Twig-Leached Dissolved Organic Matter Quantity and Biodegradability in Subtropical Plantations in China. Forests 2022, 13, 833. https://doi.org/10.3390/f13060833
Xu J-W, Ji J-H, Hu D-N, Zheng Z, Mao R. Tree Fresh Leaf- and Twig-Leached Dissolved Organic Matter Quantity and Biodegradability in Subtropical Plantations in China. Forests. 2022; 13(6):833. https://doi.org/10.3390/f13060833
Chicago/Turabian StyleXu, Jia-Wen, Jing-Hao Ji, Dong-Nan Hu, Zhi Zheng, and Rong Mao. 2022. "Tree Fresh Leaf- and Twig-Leached Dissolved Organic Matter Quantity and Biodegradability in Subtropical Plantations in China" Forests 13, no. 6: 833. https://doi.org/10.3390/f13060833
APA StyleXu, J.-W., Ji, J.-H., Hu, D.-N., Zheng, Z., & Mao, R. (2022). Tree Fresh Leaf- and Twig-Leached Dissolved Organic Matter Quantity and Biodegradability in Subtropical Plantations in China. Forests, 13(6), 833. https://doi.org/10.3390/f13060833






