An Electric Signal Conduction Characterization Model (ESCCM) for Establishing an Effective Poplar Regenerative System
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
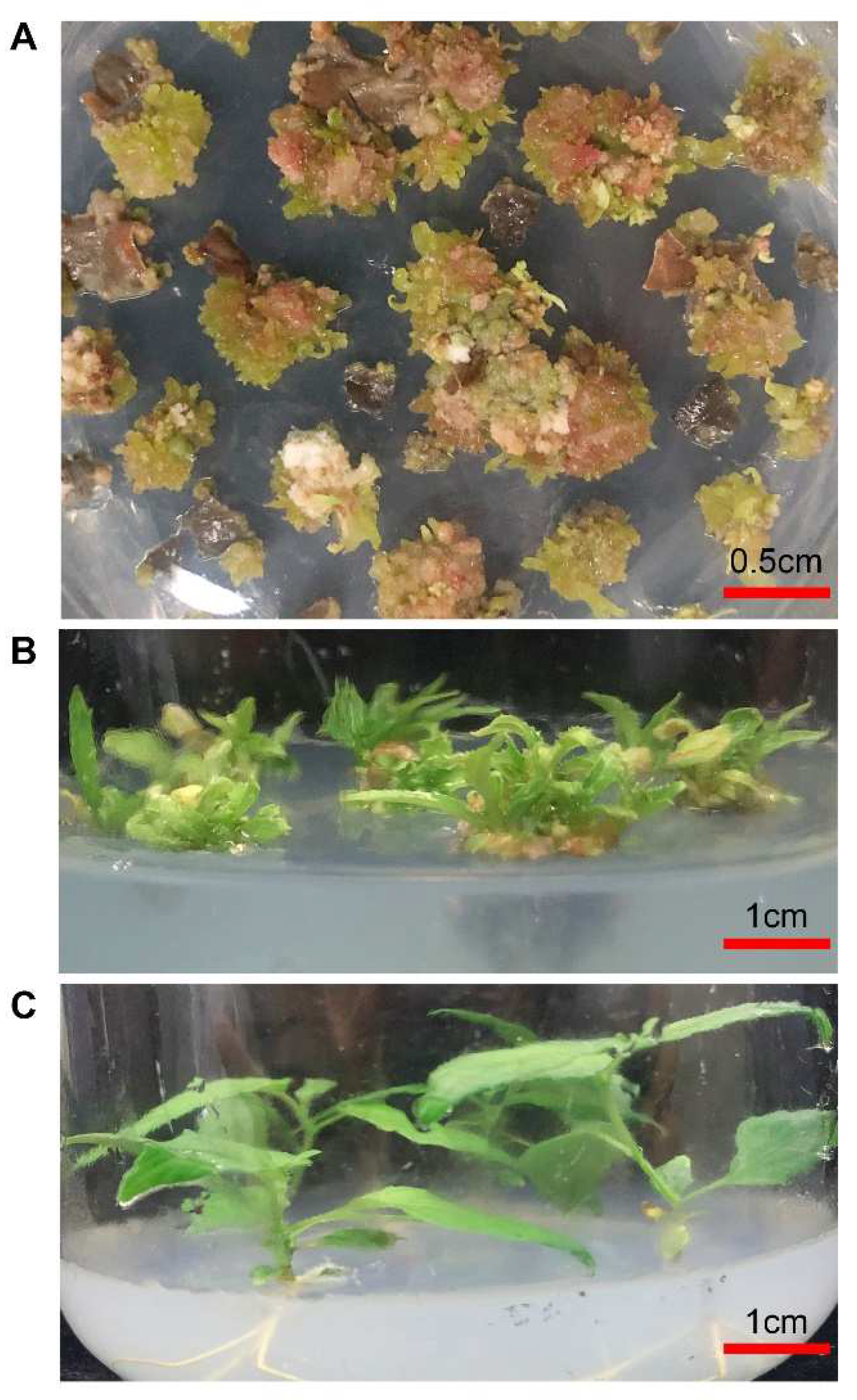
2.2. Explant Disinfection and Tissue Cultrue
2.3. Generation Conditions of Different Hormone Combinations and Concentration Characteristic Signal
2.4. Data Analysis
3. Results
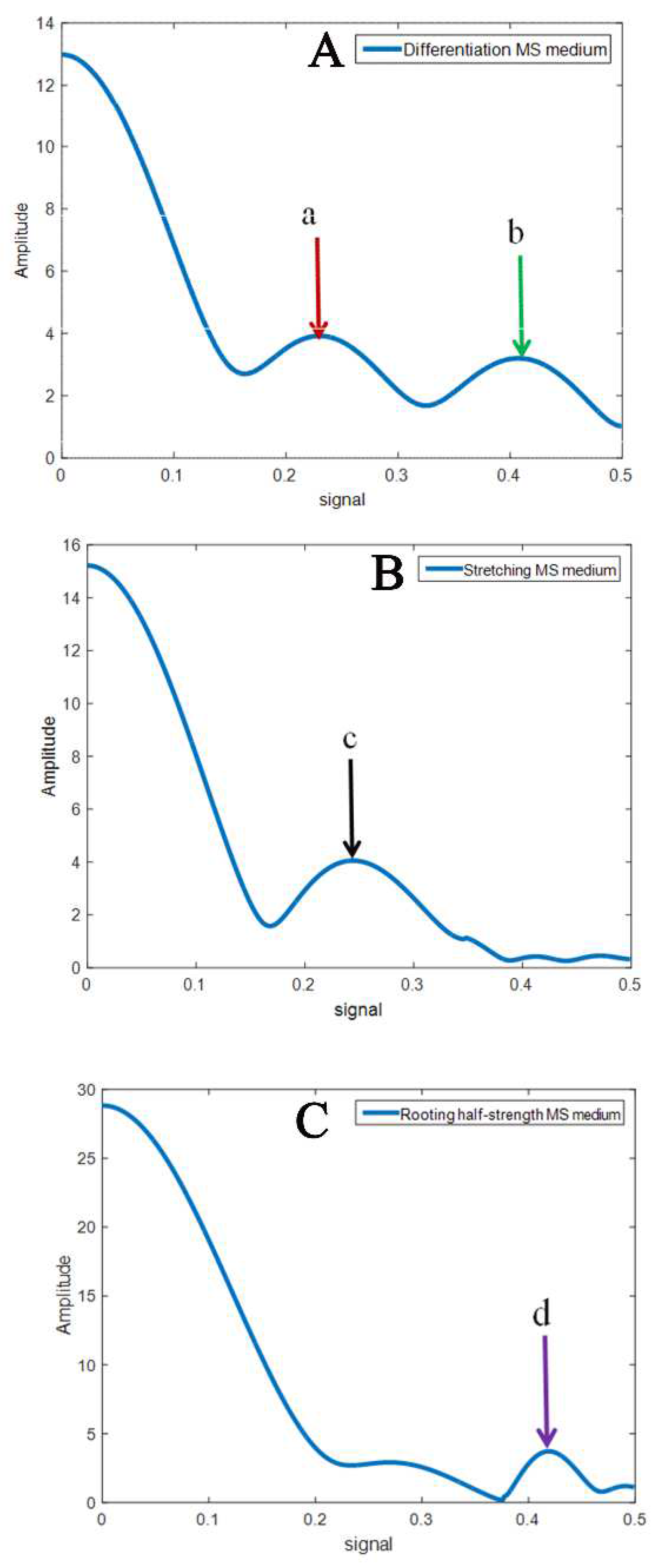
3.1. The Electric Signal Conduction Characterization Model (ESCCM)
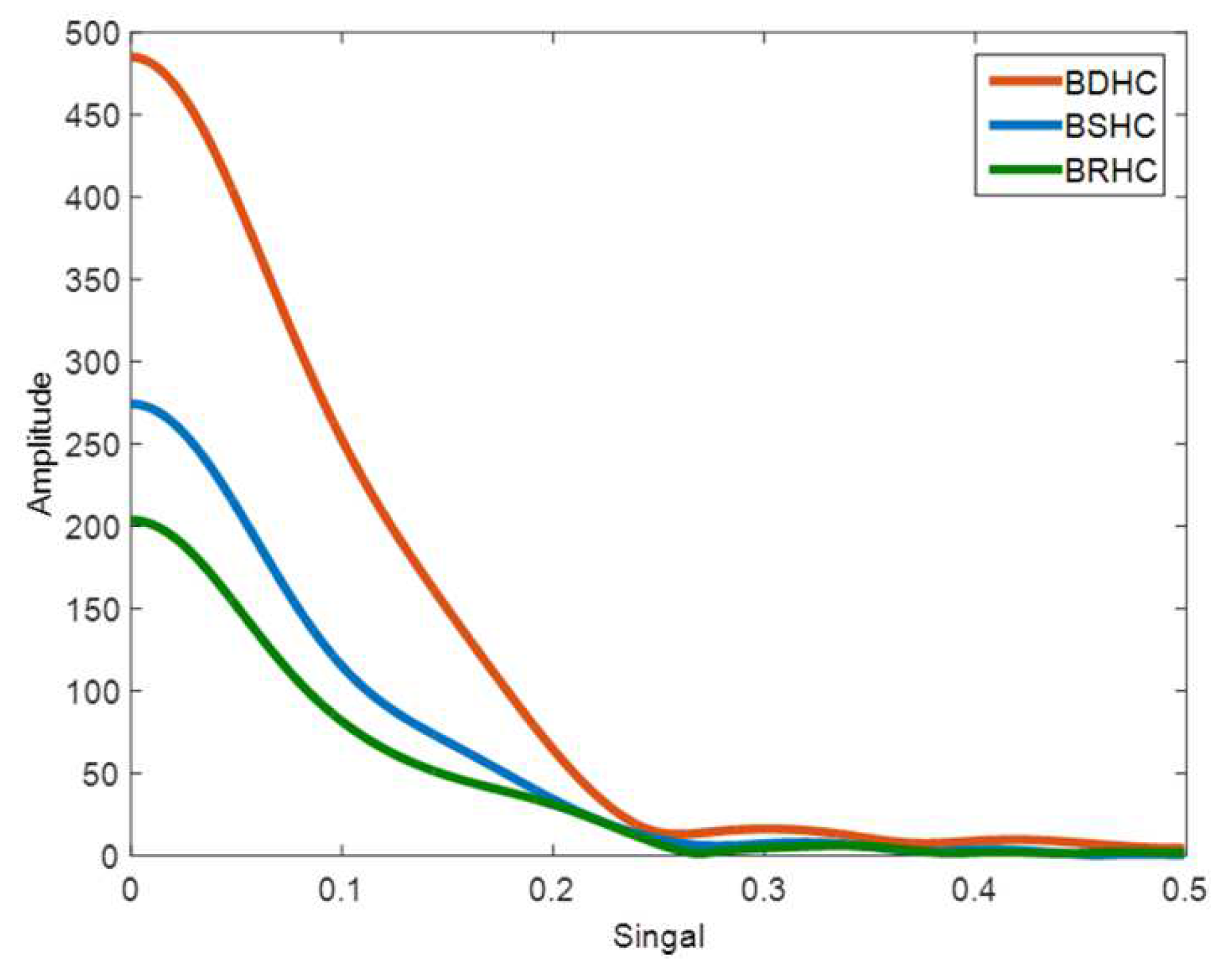
3.2. Hormone Combination Characteristic Signals
3.3. Characteristic Signal of the Medium under Different Hormone Concentrations
4. Discussion
4.1. Characteristic Signals under Hormone Combinations
4.2. Characteristic Signals under Different Hormone Combinations and Concentrations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MS | Murashige and Skoog |
| NAA | 1-naphthaleneacetic acid |
| BA | 6-Benzylaminopurine |
| Ec | Electrical conductivity |
| DS | Differentiation signal |
| SS | Stretching signal |
| RS | Rooting signal |
| BDHC | Best differentiation hormone concentration |
| LDHC | Low differentiation hormone concentration |
| HDHC | High differentiation hormone concentration |
| BSHC | Best stretching hormone concentration |
| LSHC | Low stretching hormone concentration |
| HSHC | High stretching hormone concentration |
| BRHC | Best rooting hormone concentration |
| LRHC | Low rooting hormone concentration |
| HRHC | High rooting hormone concentration |
| PRS | Poplar regeneration systems |
| ESCCM | Electrical Signal Conduction Characterization Model |
| GR | Growth rate |
| DR | Differentiation rate |
| RR | Rooting rate |
| SR | Stretching rate |
References
- Hu, L.; Lu, H.; Liu, Q.; Chen, X.; Jiang, X. Overexpression of mtld gene in transgenic populus tomentosa improves salt tolerance through accumulation of mannitol. Tree Physiol. 2005, 25, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Delledonne, M.; Allegro, G.; Belenghi, B.; Balestrazzi, A.; Franco Picco, F.; Alex Levine, A.; Samantha Zelasco, S.; Paolo Calligari, P.; Massimo Confalonieri, M. Transformation of white poplar (Populus alba L.) with a novel Arabidopsis thaliana cysteine proteinase inhibitor and analysis of insect pest resistance. Mol. Breed. 2001, 7, 35–42. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Murashige, T. Plant propagation through tissue cultures. Annu. Rev. Plant Physiol. 1974, 25, 135–166. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. Manipulation of organ initiation in plant tissue cultures. Bot. Bull. Acad. Sin. 1977, 18, 1–24. [Google Scholar]
- Edwards, S.F. A new method for the evaluation of electric conductivity in metals. Philos. Mag. 1958, 3, 1020–1031. [Google Scholar] [CrossRef]
- Kadomtsev, B.B.; Pogutse, O.P. Electric Conductivity of a Plasma in a Strong Magnetic Field. J. Exp. Theor. Phys. 1968, 26, 1146. [Google Scholar]
- Mateeva, N.; Niculescu, H.; Schlenoff, J.; Testardi, L.R. Correlation of seebeck coefficient and electric conductivity in polyaniline and polypyrrole. J. Appl. Phys. 1998, 83, 3111–3117. [Google Scholar] [CrossRef]
- Dhar, S.; Dash, T.; Palei, B.B.; Rout, T.K.; Biswal, S.K.; Mitra, A.; Sahu, A.K.; Biswal, S.K. Silicon-graphene composite synthesis: Microstructural, spectroscopic and electrical conductivity characterizations. Mater. Today Proc. 2020, 33, 5136–5142. [Google Scholar] [CrossRef]
- Ranjbarfordoei, A. Chlorophyll fluorescence performance of sweet almond [Prunus dulcis (Miller) D. Webb] in response to salinity stress induced by NaCl. Photosynthetica 2006, 44, 513–522. [Google Scholar] [CrossRef]
- Ghanem, M.E.; Van Elteren, J.; Albacete, A.; Quinet, M.; Martínez-Andújar, C.; Kinet, J.M.; Pérez-Alfocea, F.; Lutts, S. Impact of salinity on early reproductive physiology of tomato (Solanum lycopersicum) in relation to a heterogeneous distribution of toxic ions in flower organs. Funct. Plant Biol. 2009, 36, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Rouphael, Y.; Rea, E.; Cardarelli, M. Grafting cucumber plants enhance tolerance to sodium chloride and sulfate salinization. Sci. Hortic. 2012, 135, 177–185. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Panda, S.K.; Dutta, B.K. Growth, Chlorophyll and Electric Conductivity responses of rice cultivars to different levels of Submergence and Post-submergence Stress. J. Phytol. 2009, 1, 425–432. [Google Scholar]
- Katsuyuki, Y.; Kiyoshi, M. A simple method of evaluating the freezing resistance of tea plants (camellia sinensis (l.) kuntze) by measuring electrolyte leakage from low-temperature-treated overwintering buds and leaves. Chagyo Kenkyu Hokoku (Tea Res. J.) 2012, 113, 63–69. [Google Scholar] [CrossRef][Green Version]
- Borchert, R. Induction of rehydration and bud break by irrigation or rain in decidous trees of a tropical dry forest in Costa Rica. Trees 1994, 8, 198–204. [Google Scholar] [CrossRef]
- Hsu, F.H.; Lin, J.B.; Chang, S.R. Effects of waterlogging on seed germination, electric conductivity of seed leakage and developments of hypocotyl and radicle in sudangrass. Bot. Bull. Acad. Sin. 2000, 41, 267–273. [Google Scholar]
- Abou-Hadid, A.F.; Abd-Elmoniem, E.M.; El-Shinawy, M.Z.; Abou-Elsoud, M. Electrical conductivity effect on growth and mineral composition of lettuce plants in hydroponic system. Acta Hortic. 1996, 434, 59–66. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Mason, T.J.; Paniwnyk, L.; Lelas, V. Ultrasonic Effect on pH, Electric Conductivity, and Tissue Surface of Button Mushrooms, Brussels Sprouts and Cauliflower. Czech J. Food Sci. 2007, 25, 90–100. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, J.R.; Plant, R.E.; Lambert, J.J.; Smart, D.R. Using apparent soil electrical conductivity (eca) to characterize vineyard soils of high clay content. Precis. Agric. 2011, 12, 775–794. [Google Scholar] [CrossRef]
- Dorai, M.; Papadopoulos, A.P.; Gosselin, A. Influence of electric conductivity management on greenhouse tomato yield and fruit quality. Agronomie 2001, 21, 367–383. [Google Scholar] [CrossRef]
- Benedetto, J.J.; Frazier, M.W.; Torrésani, B. Wavelets: Mathematics and applications. Phys. Today 1994, 47, 90. [Google Scholar] [CrossRef]
- Dremin, I.M. Wavelets: Mathematics and applications. Phys. At. Nucl. 2005, 68, 508–520. [Google Scholar] [CrossRef][Green Version]
- Daubechies, I.; Sweldens, W. Factoring wavelet transforms into lifting steps. J. Fourier Anal. Appl. 1998, 4, 247–269. [Google Scholar] [CrossRef]
- Zhu, Z.T.; Kang, X.Y.; Zhang, Z.Y. Studies on selection of natural triploids of Populus tomentosa. Sci. Silvae Sin. 1998, 34, 22–31. [Google Scholar]
- Li, D.; Song, S.; Xia, X.; Yin, W. Two CBLgenes from Populus euphratica confer multiple stress tolerance in transgenic triploid white poplar. Plant Cell Tissue Organ Cult. 2012, 109, 477–489. [Google Scholar] [CrossRef]
- Torrence, C.; Compo, G.P. A Practical Guide to Wavelet Analysis. Bull. Am. Meteorol. Soc. 1998, 79, 61–78. [Google Scholar] [CrossRef]
- Cui, Z.Y.; Li, X.P.; Hu, J.X.; Xi, R.C. Application of Conductivity Method in Botanical Research. J. Anhui Agri. Sci. 2014, 42, 5358–5359, 5366. [Google Scholar]
- Xiang, Y.; He, Q.; Liu, K.Q.; Gao, T.; Wen, L. Effects of Plant Growth Regulator on SOD Activity and Electrical conductivity(Ⅱ). J. Chang. Univ. Electr. Power (Nat. Sci.) 1996, 11, 408–411. [Google Scholar]
- Meyer, Y. Wavelets—Algorithms and Applications; Society for Industrial & Applied Mathematics: Philadelphia, PA, USA, 1993. [Google Scholar]
- Pintér, B.; Jain, R.; Tripathi, D.; Isobe, H. Prominence Seismology: Wavelet Analysis of Filament Oscillations. Astrophys. J. 2008, 680, 1560–1568. [Google Scholar] [CrossRef]
- Anfang, M.; Shani, E. Transport mechanisms of plant hormones. Curr. Opin. Plant Biol. 2021, 63, 102055. [Google Scholar] [CrossRef]
- Feuillet, C.; Lauvergeat, V.; Deswarte, C.; Pilate, G.; Grima-Pettenati, J. Tissue- and cell-specific expression of a cinnamyl alcohol dehydrogenase promoter in transgenic poplar plants. Plant Mol. Biol. 1995, 27, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Bondt, A.D.; Eggermont, K.; Druart, P.; Vil, M.D.; Broekaert, W.F. Agrobacterium-mediated transformation of apple (malus x domestica borkh.): An assessment of factors affecting gene transfer efficiency during early transformation steps. Plant Cell Rep. 1994, 13, 587–593. [Google Scholar] [CrossRef] [PubMed]

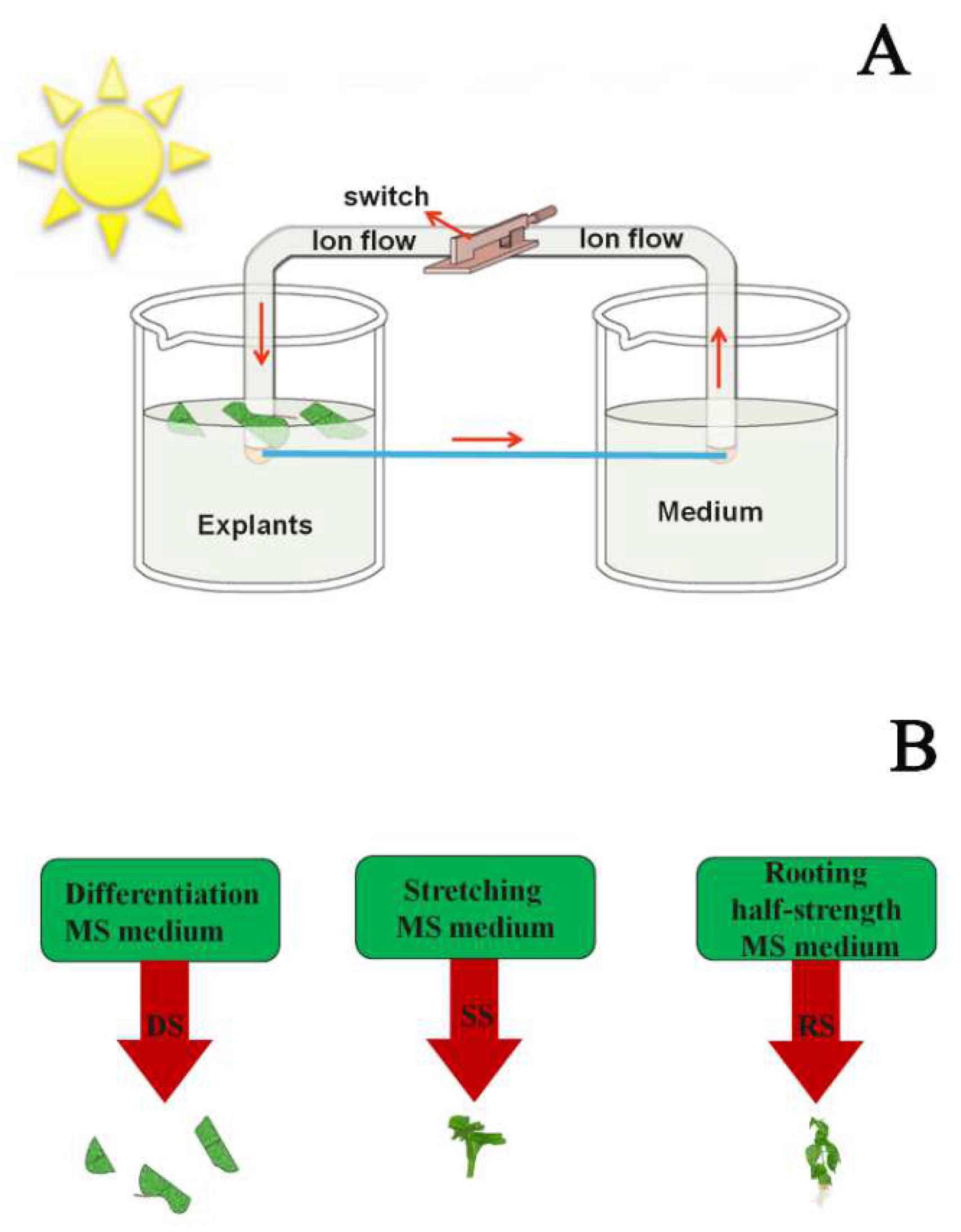
 : The Ec was measured using a DDS-307 conductivity meter daily for five days. (B) Q, R, and S are the generation conditions of the hormone combination characteristic signal; Q+, R+, and S+ are the generation conditions of the hormone concentration characteristic signal; Q: The wounded leaf was placed on a differentiation MS medium with 3% sucrose and 0.6% agar; R: Buds were placed on a stretching MS medium with 3% sucrose and 0.6% agar; S: Stretching buds were placed on a half-strength MS medium with 3% sucrose and 0.6% agar; Q+: Containing various combinations and concentrations of hormone based on Q; R+: Containing various combinations and concentrations of hormone based on R; S+: Containing various combinations and concentrations of hormone based on S;
: The Ec was measured using a DDS-307 conductivity meter daily for five days. (B) Q, R, and S are the generation conditions of the hormone combination characteristic signal; Q+, R+, and S+ are the generation conditions of the hormone concentration characteristic signal; Q: The wounded leaf was placed on a differentiation MS medium with 3% sucrose and 0.6% agar; R: Buds were placed on a stretching MS medium with 3% sucrose and 0.6% agar; S: Stretching buds were placed on a half-strength MS medium with 3% sucrose and 0.6% agar; Q+: Containing various combinations and concentrations of hormone based on Q; R+: Containing various combinations and concentrations of hormone based on R; S+: Containing various combinations and concentrations of hormone based on S;  : The Ec was measured using a DDS-307 conductivity meter daily for five days.
: The Ec was measured using a DDS-307 conductivity meter daily for five days.
 : The Ec was measured using a DDS-307 conductivity meter daily for five days. (B) Q, R, and S are the generation conditions of the hormone combination characteristic signal; Q+, R+, and S+ are the generation conditions of the hormone concentration characteristic signal; Q: The wounded leaf was placed on a differentiation MS medium with 3% sucrose and 0.6% agar; R: Buds were placed on a stretching MS medium with 3% sucrose and 0.6% agar; S: Stretching buds were placed on a half-strength MS medium with 3% sucrose and 0.6% agar; Q+: Containing various combinations and concentrations of hormone based on Q; R+: Containing various combinations and concentrations of hormone based on R; S+: Containing various combinations and concentrations of hormone based on S;
: The Ec was measured using a DDS-307 conductivity meter daily for five days. (B) Q, R, and S are the generation conditions of the hormone combination characteristic signal; Q+, R+, and S+ are the generation conditions of the hormone concentration characteristic signal; Q: The wounded leaf was placed on a differentiation MS medium with 3% sucrose and 0.6% agar; R: Buds were placed on a stretching MS medium with 3% sucrose and 0.6% agar; S: Stretching buds were placed on a half-strength MS medium with 3% sucrose and 0.6% agar; Q+: Containing various combinations and concentrations of hormone based on Q; R+: Containing various combinations and concentrations of hormone based on R; S+: Containing various combinations and concentrations of hormone based on S;  : The Ec was measured using a DDS-307 conductivity meter daily for five days.
: The Ec was measured using a DDS-307 conductivity meter daily for five days.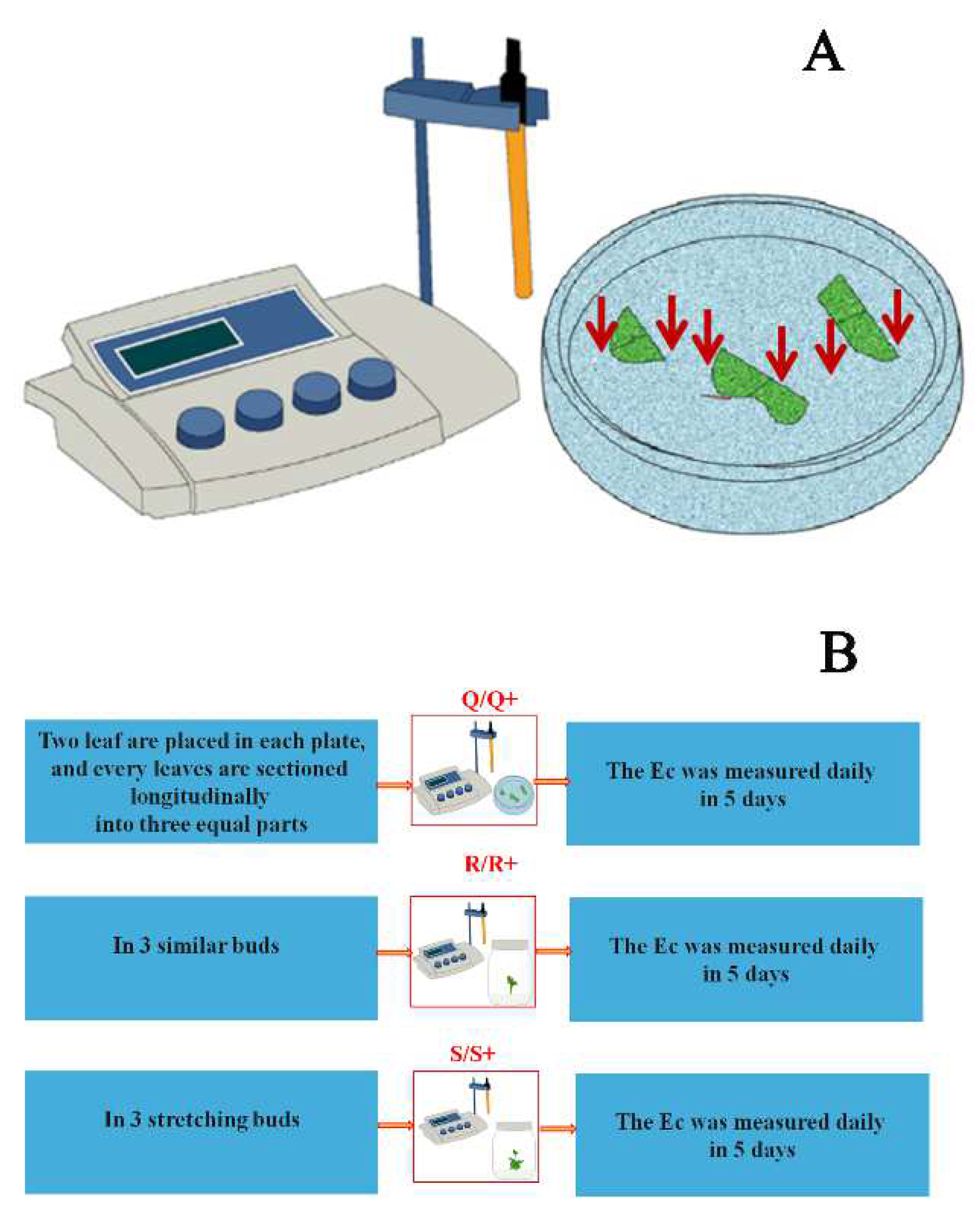



| Treatment | NAA (mg·L−1) | BA (mg·L−1) | Basal Medium |
|---|---|---|---|
| LDHC | 0.01 | 0.01 | MS |
| BDHC | 0.1 | 0.02 | MS |
| HDHC | 1.0 | 0.2 | MS |
| LSHC | - | 0.05 | MS |
| BSHC | - | 0.5 | MS |
| HSHC | - | 5.0 | MS |
| LRHC | 0.005 | - | 1/2 MS |
| BRHC | 0.05 | - | 1/2 MS |
| HRHC | 0.5 | - | 1/2 MS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, Q.; Sen, M.; Han, X.; Wang, X.; Zhou, Y. An Electric Signal Conduction Characterization Model (ESCCM) for Establishing an Effective Poplar Regenerative System. Forests 2022, 13, 835. https://doi.org/10.3390/f13060835
Zhang Y, Li Q, Sen M, Han X, Wang X, Zhou Y. An Electric Signal Conduction Characterization Model (ESCCM) for Establishing an Effective Poplar Regenerative System. Forests. 2022; 13(6):835. https://doi.org/10.3390/f13060835
Chicago/Turabian StyleZhang, Yue, Qing Li, Meng Sen, Xiao Han, Xiaoling Wang, and Yangyan Zhou. 2022. "An Electric Signal Conduction Characterization Model (ESCCM) for Establishing an Effective Poplar Regenerative System" Forests 13, no. 6: 835. https://doi.org/10.3390/f13060835
APA StyleZhang, Y., Li, Q., Sen, M., Han, X., Wang, X., & Zhou, Y. (2022). An Electric Signal Conduction Characterization Model (ESCCM) for Establishing an Effective Poplar Regenerative System. Forests, 13(6), 835. https://doi.org/10.3390/f13060835







