Significant Loss of Ecosystem Services by Environmental Changes in the Mediterranean Coastal Area
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. The AIRTREE Model
2.2.1. Acquisition of Meteorological Variables
2.2.2. Acquisition of Vegetation Map
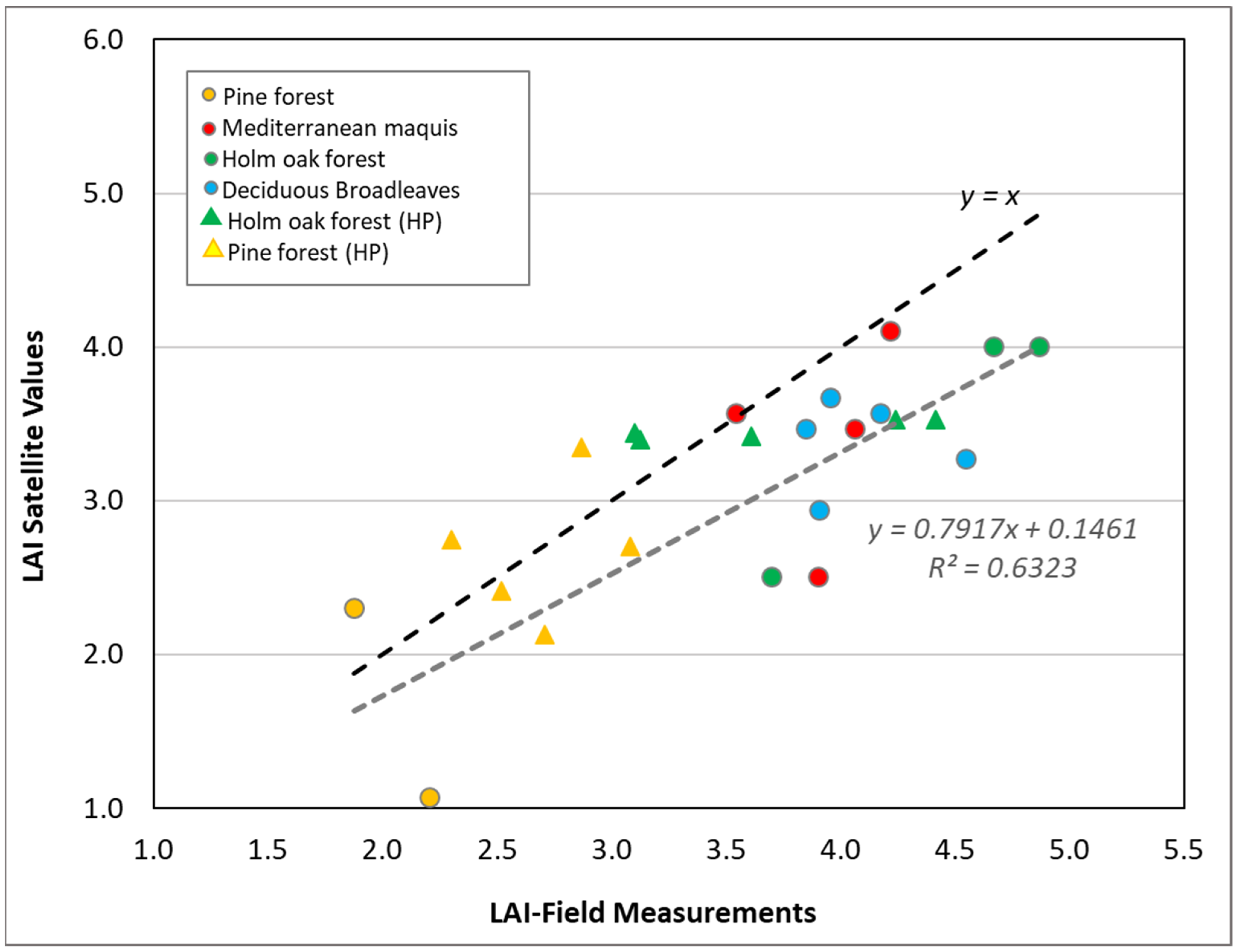
2.2.3. Retrieval of Biometric Vegetation Data
3. Results and Discussions
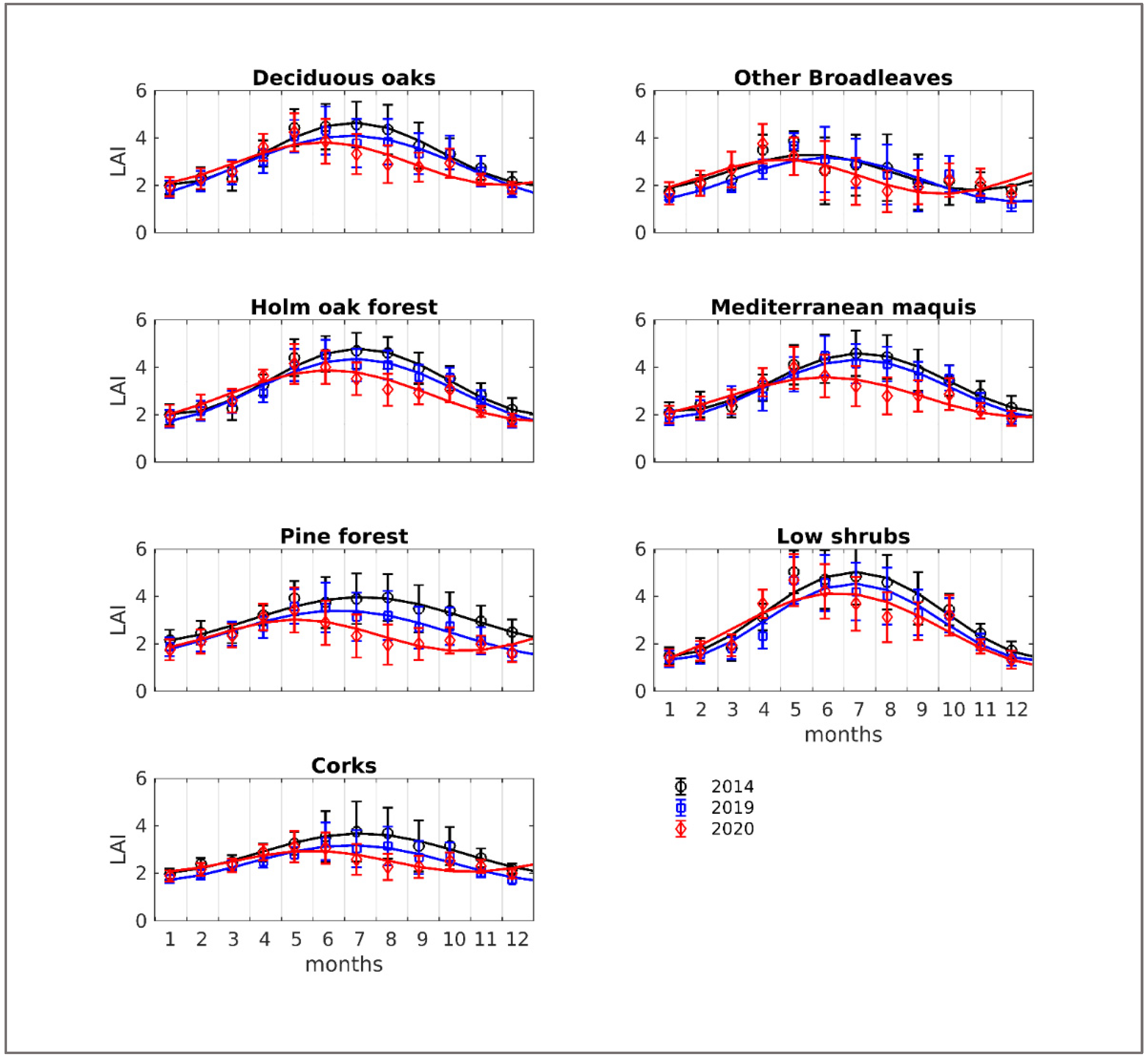
3.1. LAI and Canopy Cover Dynamics
3.2. Sequestration of Carbon Dioxide
3.3. Sequestration of Pollutants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Reid, W.V.; Mooney, H.A.; Cropper, A.; Capistrano, D.; Carpenter, S.R.; Chopra, K.; Dasgupta, P.; Dietz, T.; Duraiappah, A.K.; Hassan, R.; et al. Millennium ecosystem assessment. In Ecosystems and Human Well-Being; Island Press United States of America: Washington, DC, USA, 2005; Volume 5. [Google Scholar]
- Bäckstrand, K.; Lövbrand, E. Planting Trees to Mitigate Climate Change: Contested Discourses of Ecological Modernization, Green Governmentality and Civic Environmentalism. Glob. Environ. Politics 2006, 6, 50–75. [Google Scholar] [CrossRef]
- Mengist, W.; Soromessa, T.; Feyisa, G.L. A global view of regulatory ecosystem services: Existed knowledge, trends, and research gaps. Ecol. Process. 2020, 9, 40. [Google Scholar] [CrossRef]
- Villamagna, M.; Angermeier, P.L.; Bennett, E.M. Capacity, pressure, demand, and flow: A conceptual framework for analyzing ecosystem service provision and delivery. Ecol. Complex. 2013, 15, 114–121. [Google Scholar] [CrossRef]
- Bernstein, L.; Bosch, P.; Canziani, O.; Chen, Z.; Christ, R.; Riahi, K. IPCC, 2007: Climate Change 2007: Synthesis Report; IPCC: Geneva, Switzerland, 2008. [Google Scholar]
- Otu-Larbi, F.; Conte, A.; Fares, S.; Wild, O.; Ashworth, K. Current and future impacts of drought and ozone stress on Northern Hemisphere forests. Glob. Chang. Biol. 2020, 26, 6218–6234. [Google Scholar] [CrossRef]
- Wang, Y.; Frankenberg, C. On the impact of canopy model complexity on simulated carbon, water, and solar-induced chlorophyll fluorescence fluxes. Biogeosciences 2022, 19, 29–45. [Google Scholar] [CrossRef]
- ARPA Lazio, Air Quality Documental Section. Traffic Stations Description. 2016. Available online: http://www.arpalazio.net/main/aria/doc/RQA/inqRQA.php (accessed on 24 March 2016).
- Deserti, M.; Savoia, E.; Cacciamani, C.; Golinelli, M.; Kerschbaumer, A.; Leoncini, G.; Selvini, A.; Paccagnella, T.; Tibaldi, S. Operational meteorological pre-processing at Emilia-Romagna ARPA meteorological service as a part of a decision support system for air quality management. Int. J. Environ. Pollut. 2001, 16, 571–582. [Google Scholar] [CrossRef]
- Fares, S.; Conte, A.; Alivernini, A.; Chianucci, F.; Grotti, M.; Zappitelli, I.; Petrella, F.; Corona, P. Testing Removal of Carbon Dioxide, Ozone, and Atmospheric Particles by Urban Parks in Italy. Environ. Sci. Technol. 2020, 54, 14910–14922. [Google Scholar] [CrossRef]
- Haylock, M.R.; Hofstra, N.; Tank, A.M.G.K.; Klok, E.J.; Jones, P.D.; New, M. A European daily high-resolution gridded data set of surface temperature and precipitation for 1950–2006. J. Geophys. Res. Atmos. 2008, 113, 20119. [Google Scholar] [CrossRef]
- Holloway, T.; Miller, D.; Anenberg, S.; Diao, M.; Duncan, B.; Fiore, A.M.; Henze, D.K.; Hess, J.; Kinney, P.L.; Liu, Y.; et al. Satellite Monitoring for Air Quality and Health. Annu. Rev. Biomed. Data Sci. 2021, 4, 417–447. [Google Scholar] [CrossRef]
- Bonan, G.B.; Patton, E.G.; Finnigan, J.J.; Baldocchi, D.D.; Harman, I.N. Moving beyond the incorrect but useful paradigm: Reevaluating big-leaf and multilayer plant canopies to model biosphere-atmosphere fluxes—A review. Agric. For. Meteorol. 2021, 306, 108435. [Google Scholar] [CrossRef]
- Fares, S.; Alivernini, A.; Conte, A.; Maggi, F. Ozone and particle fluxes in a Mediterranean forest predicted by the AIRTREE model. Sci. Total Environ. 2019, 682, 494–504. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Air Quality Guidelines for Europe. 2000. Available online: https://apps.who.int/iris/handle/10665/107335 (accessed on 1 March 2022).
- Delaria, E.R.; Place, B.K.; Liu, A.X.; Cohen, R.C. Laboratory measurements of stomatal NO2 deposition to native California trees and the role of forests in the NOx cycle. Atmos. Chem. Phys. 2020, 20, 14023–14041. [Google Scholar] [CrossRef]
- Paoletti, E. Impact of ozone on Mediterranean forests: A review. Environ. Pollut. 2006, 144, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, L.; Mereu, S.; Salvatori, E.; Agliari, E.; Fares, S.; Manes, F. Modeling ozone uptake by urban and peri-urban forest: A case study in the Metropolitan City of Rome. Environ. Sci. Pollut. Res. 2018, 25, 8190–8205. [Google Scholar] [CrossRef] [PubMed]
- Hoshika, Y.; Paoletti, E.; Centritto, M.; Gomes, M.T.G.; Puértolas, J.; Haworth, M. Species-specific variation of photosynthesis and mesophyll conductance to ozone and drought in three Mediterranean oaks. Physiol. Plant. 2022, 174, e13639. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.T.; Woodrow, I.E.; Berry, J.A. A Model Predicting Stomatal Conductance and its Contribution to the Control of Photosynthesis under Different Environmental Conditions. In Progress in Photosynthesis Research; Springer: Dordrecht, The Netherlands, 1987; pp. 221–224. [Google Scholar] [CrossRef]
- Medlyn, B.E.; Duursma, R.A.; Eamus, D.; Ellsworth, D.S.; Prentice, I.C.; Barton, C.V.M.; Crous, K.Y.; DE Angelis, P.; Freeman, M.; Wingate, L. Reconciling the optimal and empirical approaches to modelling stomatal conductance. Glob. Chang. Biol. 2011, 17, 2134–2144. [Google Scholar] [CrossRef]
- Katul, G.G.; Palmroth, S.; Oren, R. Leaf stomatal responses to vapour pressure deficit under current and CO2-enriched atmosphere explained by the economics of gas exchange. Plant Cell Environ. 2009, 32, 968–979. [Google Scholar] [CrossRef]
- Fares, S.; Conte, A.; Chabbi, A. Ozone flux in plant ecosystems: New opportunities for long-term monitoring networks to deliver ozone-risk assessments. Environ. Sci. Pollut. Res. 2018, 25, 8240–8248. [Google Scholar] [CrossRef]
- Fuster, B.; Sánchez-Zapero, J.; Camacho, F.; García-Santos, V.; Verger, A.; Lacaze, R.; Weiss, M.; Baret, F.; Smets, B. Quality Assessment of PROBA-V LAI, fAPAR and fCOVER Collection 300 m Products of Copernicus Global Land Service. Remote Sens. 2020, 12, 1017. [Google Scholar] [CrossRef]
- Kamenova, I.; Dimitrov, P. Evaluation of Sentinel-2 vegetation indices for prediction of LAI, fAPAR and fCover of winter wheat in Bulgaria. Eur. J. Remote Sens. 2020, 54, 89–108. [Google Scholar] [CrossRef]
- Conte, A.; Otu-Larbi, F.; Alivernini, A.; Hoshika, Y.; Paoletti, E.; Ashworth, K.; Fares, S. Exploring new strategies for ozone-risk assessment: A dynamic-threshold case study. Environ. Pollut. 2021, 287, 117620. [Google Scholar] [CrossRef] [PubMed]
- Otu-Larbi, F.; Conte, A.; Fares, S.; Wild, O.; Ashworth, K. FORCAsT-gs: Importance of Stomatal Conductance Parameterization to Estimated Ozone Deposition Velocity. J. Adv. Modeling Earth Syst. 2021, 13, e2021MS002581. [Google Scholar] [CrossRef]
- Ratna, S.B.; Ratnam, J.V.; Behera, S.K.; Cherchi, A.; Wang, W.; Yamagata, T. The unusual wet summer (July) of 2014 in Southern Europe. Atmos. Res. 2017, 189, 61–68. [Google Scholar] [CrossRef][Green Version]
- Nimbus Web Climatologia Locale. Available online: http://www.nimbus.it/clima/2015/150114clima2014.htm (accessed on 24 March 2022).
- Conte, A.; Fares, S.; Salvati, L.; Savi, F.; Matteucci, G.; Mazzenga, F.; Spano, D.; Sirca, C.; Marras, S.; Galvagno, M.; et al. Ecophysiological Responses to Rainfall Variability in Grassland and Forests Along a Latitudinal Gradient in Italy. Front. For. Glob. Chang. 2019, 2, 16. [Google Scholar] [CrossRef]
- Donzelli, G.; Cioni, L.; Cancellieri, M.; Morales, A.L.; Suárez-Varela, M.M.M. The Effect of the Covid-19 Lockdown on Air Quality in Three Italian Medium-Sized Cities. Atmosphere 2020, 11, 1118. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Bertanza, G.; Pedrazzani, R.; Ricciardi, P.; Miino, M.C. Lockdown for CoViD-2019 in Milan: What are the effects on air quality? Sci. Total Environ. 2020, 732, 139280. [Google Scholar] [CrossRef]
- Cameletti, M. The Effect of Corona Virus Lockdown on Air Pollution: Evidence from the City of Brescia in Lombardia Region (Italy). Atmos. Environ. 2020, 239, 117794. [Google Scholar] [CrossRef]
- Gualtieri, G.; Brilli, L.; Carotenuto, F.; Vagnoli, C.; Zaldei, A.; Gioli, B. Quantifying road traffic impact on air quality in urban areas: A Covid19-induced lockdown analysis in Italy. Environ. Pollut. 2020, 267, 115682. [Google Scholar] [CrossRef]
- Balasubramaniam, D.; Kanmanipappa, C.; Shankarlal, B.; Saravanan, M. Assessing the impact of lockdown in US, Italy and France–What are the changes in air quality? Energy Sources Part A Recovery Util. Environ. Eff. 2020. [Google Scholar] [CrossRef]
- Cucca, B.; Recanatesi, F.; Ripa, M.N. Evaluating the Potential of Vegetation Indices in Detecting Drought Impact Using Remote Sensing Data in a Mediterranean Pinewood. Lect. Notes Comput. Sci. 2020, 12253, 50–62. [Google Scholar] [CrossRef]
- Recanatesi, F.; Giuliani, C.; Rossi, C.M.; Ripa, M.N. A Remote Sensing-Assisted Risk Rating Study to Monitor Pinewood Forest Decline: The Study Case of the Castelporziano State Nature Reserve (Rome). Smart Innov. Syst. Technol. 2019, 100, 68–75. [Google Scholar] [CrossRef]
- della Rocca, B.; Pignatti, S.; Mugnoli, S.; Bianco, P.M. La carta della vegetazione della Tenuta di Castelporziano. Accad. Naz. Delle Sci. Detta Dei XL Scr. E Doc. 2001, 26, 709–748. [Google Scholar]
- Davison, B.; Taipale, R.; Langford, B.; Misztal, P.; Fares, S.; Matteucci, G.; Loreto, F.; Cape, J.N.; Rinne, J.; Hewitt, C.N. Concentrations and fluxes of biogenic volatile organic compounds above a Mediterranean macchia ecosystem in western Italy. Biogeosciences 2009, 6, 1655–1670. [Google Scholar] [CrossRef]
- Giordano, E.; Recanatesi, F.; Scrinzi, G. La Biodiversit Forestale Della Tenuta Presidenziale di Castelporziano. 2017. Available online: https://dspace.unitus.it/handle/2067/35638 (accessed on 1 March 2022).
- Manes, F.; Grignetti, A.; Tinelli, A.; Lenz, R.; Ciccioli, P. General features of the Castelporziano test site. Atmos. Environ. 1997, 31, 19–25. Available online: https://www.researchgate.net/profile/Aleandro-Tinelli/publication/354031145_General_features_of_the_castelporziano_test_site_1997/links/611fba3d169a1a01031631f2/General-features-of-the-castelporziano-test-site-1997.pdf (accessed on 17 March 2022). [CrossRef]
- Fares, S.; Mereu, S.; Mugnozza, G.S.; Vitale, M.; Manes, F.; Frattoni, M.; Ciccioli, P.; Gerosa, G.; Loreto, F. The ACCENT-VOCBAS field campaign on biosphere-atmosphere interactions in a Mediterranean ecosystem of Castelporziano (Rome): Site characteristics, climatic and meteorological conditions, and eco-physiology of vegetation. Biogeosci. Discuss. 2009, 6, 1185–1227. [Google Scholar] [CrossRef]
- Fusaro, L.; Salvatori, E.; Mereu, S.; Silli, V.; Bernardini, A.; Tinelli, A.; Manes, F. Researches in Castelporziano test site: Ecophysiological studies on Mediterranean vegetation in a changing environment. Rend. Lincei 2015, 26, 473–481. [Google Scholar] [CrossRef]
- Fares, S.; Savi, F.; Muller, J.; Matteucci, G.; Paoletti, E. Simultaneous measurements of above and below canopy ozone fluxes help partitioning ozone deposition between its various sinks in a Mediterranean Oak Forest. Agric. For. Meteorol. 2014, 198, 181–191. [Google Scholar] [CrossRef]
- Fischer, G.; Nachtergaele, F.; Prieler, S.; van Velthuizen, H.T.; Verelst, L.; Wiberg, D. Global Agro-Ecological Zones Assessment for Agriculture (GAEZ 2008); IIASA: Laxenburg, Austria; FAO: Rome, Italy, 2008; Volume 10. [Google Scholar]
- Baldocchi, D. An analytical solution for coupled leaf photosynthesis and stomatal conductance models. Tree Physiol. 1994, 14, 1069–1079. [Google Scholar] [CrossRef]
- Keenan, T.; Sabate, S.; Gracia, C. Soil water stress and coupled photosynthesis–conductance models: Bridging the gap between conflicting reports on the relative roles of stomatal, mesophyll conductance and biochemical limitations to photosynthesis. Agric. For. Meteorol. 2010, 150, 443–453. [Google Scholar] [CrossRef]
- Lombardozzi, D.; Sparks, J.P.; Bonan, G. Integrating O3 influences on terrestrial processes: Photosynthetic and stomatal response data available for regional and global modeling. Biogeosci. Discuss. 2013, 10, 6973–7012. [Google Scholar] [CrossRef]
- Lombardozzi, D.; Levis, S.; Bonan, G.; Sparks, J.P. Predicting photosynthesis and transpiration responses to ozone: Decoupling modeled photosynthesis and stomatal conductance. Biogeosciences 2012, 9, 3113–3130. [Google Scholar] [CrossRef]
- Lombardozzi, D.L.; Levis, S.; Bonan, G.; Hess, P.G.; Sparks, J.P. The Influence of Chronic Ozone Exposure on Global Carbon and Water Cycles. J. Clim. 2015, 28, 292–305. [Google Scholar] [CrossRef]
- Zhang, L.; Brook, J.R.; Vet, R. On ozone dry deposition—with emphasis on non-stomatal uptake and wet canopies. Atmos. Environ. 2002, 36, 4787–4799. [Google Scholar] [CrossRef]
- Baldocchi, D.D.; Hicks, B.B.; Camara, P. A canopy stomatal resistance model for gaseous deposition to vegetated surfaces. Atmos. Environ. 1987, 21, 91–101. [Google Scholar] [CrossRef]
- Pederson, J.; Massman, W.; Mahrt, L.; Delany, A.; Oncley, S.; Hartog, G.; Neumann, H.; Mickle, R.; Shaw, R.; Paw, K.T.; et al. California ozone deposition experiment: Methods, results, and opportunities. Atmos. Environ. 1995, 29, 3115–3132. [Google Scholar] [CrossRef]
- Bidwell, R.G.S.; Fraser, D.E. Carbon monoxide uptake and metabolism by leaves. Can. J. Bot. 2011, 50, 1435–1439. [Google Scholar] [CrossRef]
- Wesely, M.L. Parameterization of surface resistances to gaseous dry deposition in regional-scale numerical models. Atmos. Environ. 2007, 41, 52–63. [Google Scholar] [CrossRef]
- Lovett, G.M. Atmospheric Deposition of Nutrients and Pollutants in North America: An Ecological Perspective. Ecol. Appl. 1994, 4, 629–650. [Google Scholar] [CrossRef]
- Aromolo, R.; Moretti, V.; Sorgi, T. Setting Up Optimal Meteorological Networks: An Example From Italy. Strateg. Plan. Energy Environ. 2021, 40, 39–54. [Google Scholar] [CrossRef]
- Recanatesi, F. Variations in land-use/land-cover changes (LULCCs) in a peri-urban Mediterranean nature reserve: The estate of Castelporziano (Central Italy). Rend. Lincei 2015, 3, 517–526. [Google Scholar] [CrossRef]
- European Commission Directorate-General Joint Research Centre. Leaf Area Index: Version 1333 m Resolution, Globe, 10-Daily. 2017. Available online: http://land.copernicus.vgt.vito.be/geonetwork/srv/api/records/urn:cgls:global:lai300_v1_333m (accessed on 27 March 2022).
- Gielen, B.; de Beeck, M.; Michilsens, M.; Papale, D. ICOS Ecosystem Instructions for Ancillary Vegetation Measurements in Forest (Version 20200330); ICOS Ecosystem Thematic Centre: Viterbo, Italy, 2017. [Google Scholar] [CrossRef]
- Potapov, P.; Li, X.; Hernandez-Serna, A.; Tyukavina, A.; Hansen, M.C.; Kommareddy, A.; Pickens, A.; Turubanova, S.; Tang, H.; Silva, C.E.; et al. Mapping global forest canopy height through integration of GEDI and Landsat data. Remote Sens. Environ. 2020, 253, 112165. [Google Scholar] [CrossRef]
- At Risk Mediterranean Forests Make Vital Contributions to Development UN News. Available online: https://news.un.org/en/story/2018/11/1026761 (accessed on 24 March 2022).
- Penuelas, J.; Gracia, C.; Jump, I.F.A.; Carnicer, J.; Coll, M.; Lloret, F.; Yuste, J.C.; Estiarte, M.; Rutishauser, T.; Ogaya, R. Introducing the climate change effects on Mediterranean forest ecosystems: Observation, experimentation, simulation and management. In Forêt Méditerranéenne t. XXXI, n 4, 4th ed.; Association Forêt Méditerranéenne: Marseille, France, 2010; pp. 357–362. Available online: http://hdl.handle.net/2042/39215 (accessed on 23 March 2022).
- Sardans, J.; Peñuelas, J. Plant-soil interactions in Mediterranean forest and shrublands: Impacts of climatic change. Plant Soil 2013, 365, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Misson, L.; Degueldre, D.; Collin, C.; Rodriguez, R.; Rocheteau, A.; Ourcival, J.-M.; Rambal, S. Phenological responses to extreme droughts in a Mediterranean forest. Glob. Chang. Biol. 2011, 17, 1036–1048. [Google Scholar] [CrossRef]
- Bongers, F.J.; Olmo, M.; Lopez-Iglesias, B.; Anten, N.P.R.; Villar, R. Drought responses, phenotypic plasticity and survival of Mediterranean species in two different microclimatic sites. Plant Biol. 2017, 19, 386–395. [Google Scholar] [CrossRef]
- Castagneri, D.; Regev, L.; Boaretto, E.; Carrer, M. Xylem anatomical traits reveal different strategies of two Mediterranean oaks to cope with drought and warming. Environ. Exp. Bot. 2017, 133, 128–138. [Google Scholar] [CrossRef]
- Recanatesi, F.; Giuliani, C.; Ripa, M.N. Monitoring Mediterranean Oak Decline in a Peri-Urban Protected Area Using the NDVI and Sentinel-2 Images: The Case Study of Castelporziano State Natural Reserve. Sustainability 2018, 10, 3308. [Google Scholar] [CrossRef]
- Ogaya, R.; Peñuelas, J. Tree growth, mortality, and above-ground biomass accumulation in a holm oak forest under a five-year experimental field drought. Plant Ecol. 2006, 189, 291–299. [Google Scholar] [CrossRef]
- Barbeta, A.; Peñuelas, J. Sequence of plant responses to droughts of different timescales: Lessons from holm oak (Quercus ilex) forests. Plant Ecol. Divers. 2016, 9, 321–338. [Google Scholar] [CrossRef]
- Barbeta, A.; Mejía-Chang, M.; Ogaya, R.; Voltas, J.; Dawson, T.E.; Peñuelas, J. The combined effects of a long-term experimental drought and an extreme drought on the use of plant-water sources in a Mediterranean forest. Glob. Chang. Biol. 2015, 21, 1213–1225. [Google Scholar] [CrossRef]
- Medrano, H.; Flexas, J.; Galmés, J. Variability in water use efficiency at the leaf level among Mediterranean plants with different growth forms. Plant Soil 2009, 317, 17–29. [Google Scholar] [CrossRef]
- Sperlich, D.; Chang, C.T.; Peñuelas, J.; Gracia, C.; Sabaté, S. Seasonal variability of foliar photosynthetic and morphological traits and drought impacts in a Mediterranean mixed forest. Tree Physiol. 2015, 35, 501–520. [Google Scholar] [CrossRef] [PubMed]
- Lloret, F.; Siscart, D.; Dalmases, C. Canopy recovery after drought dieback in holm-oak Mediterranean forests of Catalonia (NE Spain). Glob. Chang. Biol. 2004, 10, 2092–2099. [Google Scholar] [CrossRef]
- Rosas, T.; Galiano, L.; Ogaya, R.; Peñuelas, J.; Martínez-Vilalta, J. Dynamics of non-structural carbohydrates in three mediterranean woody species following long-term experimental drought. Front. Plant Sci. 2013, 4, 400. [Google Scholar] [CrossRef] [PubMed]
- Sarris, D.; Koutsias, N. Ecological adaptations of plants to drought influencing the recent fire regime in the Mediterranean. Agric. For. Meteorol. 2014, 184, 158–169. [Google Scholar] [CrossRef]
- Lempereur, M.; Limousin, J.-M.; Guibal, F.; Ourcival, J.-M.; Rambal, S.; Ruffault, J.; Mouillot, F. Recent climate hiatus revealed dual control by temperature and drought on the stem growth of Mediterranean Quercus ilex. Glob. Chang. Biol. 2017, 23, 42–55. [Google Scholar] [CrossRef]
- Cutini, A.; Matteucci, G.; Mugnozza, G.S. Estimation of leaf area index with the Li-Cor LAI 2000 in deciduous forests. For. Ecol. Manag. 1998, 105, 55–65. [Google Scholar] [CrossRef]
- Manes, F.; Anselmi, S.; Giannini, M.; Melini, S. Relationships between leaf area index (LAI) and vegetation indices to analyze and monitor Mediterranean ecosystems. Int. Soc. Opt. Photonics 2001, 4171, 328–335. [Google Scholar] [CrossRef]
- Gratani, L.; Crescente, M.F. Map-Making of Plant Biomass and Leaf Area Index for Management of Protected Areas. Aliso A J. Syst. Florist. Bot. 2000, 19, 1–12. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Camarero, J.J.; Grau, J.M.; De La Cruz, A.C.; Gil, P.M.; Minaya, M.; Cancio, F. Analysing Atmospheric Processes and Climatic Drivers of Tree Defoliation to Determine Forest Vulnerability to Climate Warming. Forests 2016, 8, 13. [Google Scholar] [CrossRef]
- Peña-Gallardo, M.; Vicente-Serrano, S.M.; Camarero, J.J.; Gazol, A.; Sánchez-Salguero, R.; Domínguez-Castro, F.; El Kenawy, A.; Beguería-Portugés, S.; Gutiérrez, E.; de Luis, M.; et al. Drought Sensitiveness on Forest Growth in Peninsular Spain and the Balearic Islands. Forests 2018, 9, 524. [Google Scholar] [CrossRef]
- Gea-Izquierdo, G.; Viguera, B.; Cabrera, M.; Cañellas, I. Drought induced decline could portend widespread pine mortality at the xeric ecotone in managed mediterranean pine-oak woodlands. For. Ecol. Manag. 2014, 320, 70–82. [Google Scholar] [CrossRef]
- Braisier, M. Phytophthora cinnamomi and oak decline in southern Europe. Environmental constraints including climate change. Ann. Des Sci. For. 1996, 53, 347–358. [Google Scholar] [CrossRef]
- Corcobado, T.; Cubera, E.; Juárez, E.; Moreno, G.; Solla, A. Drought events determine performance of Quercus ilex seedlings and increase their susceptibility to Phytophthora cinnamomi. Agric. For. Meteorol. 2014, 192–193, 1–8. [Google Scholar] [CrossRef]
- Camarero, J.J.; Álvarez-Taboada, F.; Hevia, A.; Castedo-Dorado, F. Radial growth and wood density reflect the impacts and susceptibility to defoliation by gypsy moth and climate in radiata pine. Front. Plant Sci. 2018, 871, 1582. [Google Scholar] [CrossRef]
- Mecheri, H.; Kouidri, M.; Boukheroufa-Sakraoui, F.; Adamou, A.E. Variation du taux d’infestation par Thaumetopoea pityocampa du pin d’Alep: Effet sur les paramètres dendrométriques dans les forêts de la région de Djelfa (Atlas saharien, Algérie). Comptes Rendus Biol. 2018, 341, 380–386. [Google Scholar] [CrossRef]
- Avila, J.M.; Gallardo, A.; Ibáñez, B.; Gómez-Aparicio, L. Quercus suber dieback alters soil respiration and nutrient availability in Mediterranean forests. J. Ecol. 2016, 104, 1441–1452. [Google Scholar] [CrossRef]
- Erkan, N. Impact of pine processionary moth (Thaumetopoea wilkinsoni Tams) on growth of Turkish red pine (Pinus brutia Ten.). Afr. J. Agric. Res. 2011, 6, 4983–4988. [Google Scholar] [CrossRef]
- Savi, F.; Savi, F.; Fares, S. Ozone dynamics in a Mediterranean Holm oak forest: Comparison among transition periods characterized by different amounts of precipitation. Ann. Silvic. Res. 2014, 38, 1–6. [Google Scholar] [CrossRef]
- Peñuelas, J.; Sardans, J. Global Change and Forest Disturbances in the Mediterranean Basin: Breakthroughs, Knowledge Gaps, and Recommendations. Forests 2021, 12, 603. [Google Scholar] [CrossRef]

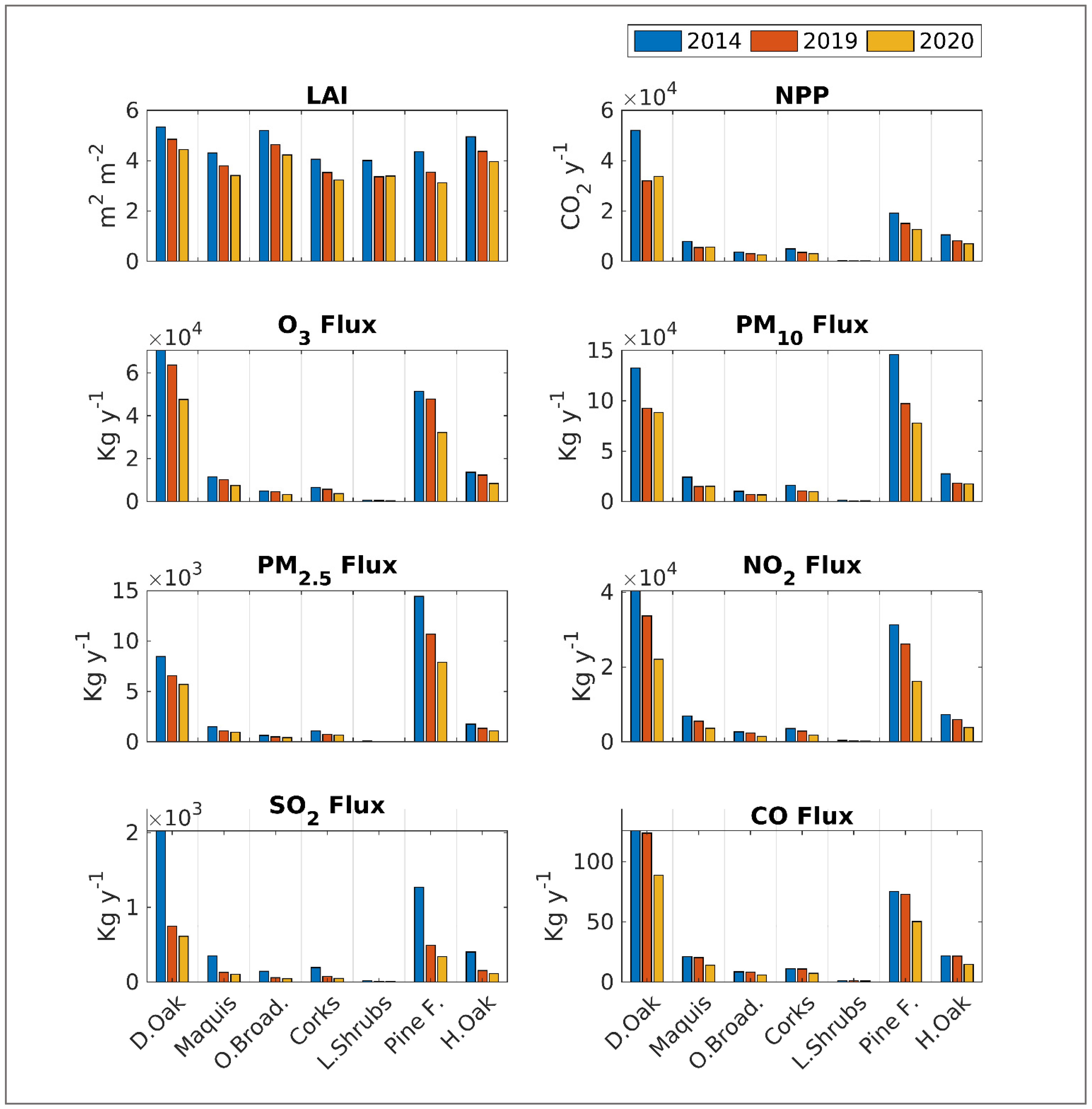

| LCT | Year | NPP | O3 Flux | PM10 Flux | PM2.5 Flux | NO2 Flux | SO2 Flux | CO Flux |
|---|---|---|---|---|---|---|---|---|
| (Kg CO2 m2 y−1) | (g m2 y−1) | (g m2 y−1) | (g m2 y−1) | (g m2 y−1) | (g m2 y−1) | (g m2 y−1) | ||
| Low shrubs | 2014 | 1.53 ± 0.44 | 2.52 ± 0.30 | 5.78 ± 0.27 | 0.36 ± 0.02 | 1.60 ± 0.24 | 0.08 ± 0.013 | 0.005 ± 0.0003 |
| 2019 | 0.74 ± 0.26 | 2.22 ± 0.07 | 3.26 ± 0.40 | 0.23 ± 0.06 | 1.22 ± 0.02 | 0.03 ± 0.003 | 0.005 ± 0.0003 | |
| 2020 | 0.94 ± 0.09 | 1.65 ± 0.12 | 3.97 ± 0.64 | 0.25 ± 0.04 | 0.84 ± 0.05 | 0.02 ± 0.003 | 0.004 ± 0.0002 | |
| Other broadleaves | 2014 | 2.46 ± 0.31 | 3.23 ± 0.19 | 6.69 ± 0.58 | 0.42 ± 0.04 | 1.79 ± 0.11 | 0.10 ± 0.006 | 0.006 ± 0.0002 |
| 2019 | 2.10 ± 0.38 | 3.14 ± 0.13 | 4.72 ± 0.59 | 0.34 ± 0.04 | 1.60 ± 0.05 | 0.04 ± 0.003 | 0.006 ± 0.0002 | |
| 2020 | 1.81 ± 0.20 | 2.32 ± 0.12 | 4.63 ± 0.61 | 0.29 ± 0.04 | 1.05 ± 0.04 | 0.03 ± 0.002 | 0.004 ± 0.0001 | |
| Holm oak forest | 2014 | 2.45 ± 0.43 | 3.17 ± 0.32 | 6.35 ± 0.96 | 0.40 ± 0.06 | 1.69 ± 0.18 | 0.09 ± 0.012 | 0.005 ± 0.0002 |
| 2019 | 1.91 ± 0.54 | 2.91 ± 0.32 | 4.27 ± 0.80 | 0.31 ± 0.07 | 1.41 ± 0.11 | 0.04 ± 0.005 | 0.005 ± 0.0002 | |
| 2020 | 1.75 ± 0.38 | 2.11 ± 0.30 | 4.28 ± 0.83 | 0.27 ± 0.05 | 0.95 ± 0.11 | 0.03 ± 0.005 | 0.004 ± 0.0002 | |
| Mediterranean maquis | 2014 | 1.86 ± 0.65 | 2.74 ± 0.34 | 5.61 ± 1.72 | 0.35 ± 0.11 | 1.66 ± 0.21 | 0.08 ± 0.015 | 0.005 ± 0.0003 |
| 2019 | 1.32 ± 0.57 | 2.56 ± 0.26 | 3.57 ± 1.31 | 0.26 ± 0.09 | 1.38 ± 0.10 | 0.03 ± 0.005 | 0.005 ± 0.0003 | |
| 2020 | 1.43 ± 0.36 | 1.95 ± 0.17 | 3.78 ± 1.20 | 0.23 ± 0.07 | 0.96 ± 0.07 | 0.03 ± 0.004 | 0.004 ± 0.0002 | |
| Pine forest | 2014 | 2.09 ± 0.38 | 5.62 ± 0.35 | 15.78 ± 2.72 | 1.56 ± 0.27 | 3.42 ± 0.20 | 0.14 ± 0.016 | 0.008 ± 0.0001 |
| 2019 | 1.69 ± 0.49 | 5.43 ± 0.43 | 10.89 ± 2.45 | 1.20 ± 0.27 | 2.96 ± 0.15 | 0.06 ± 0.008 | 0.008 ± 0.0001 | |
| 2020 | 1.54 ± 0.39 | 4.02 ± 0.41 | 9.52 ± 2.35 | 0.96 ± 0.24 | 2.03 ± 0.16 | 0.04 ± 0.008 | 0.006 ± 0.0001 | |
| Deciduous oaks | 2014 | 2.36 ± 0.61 | 3.20 ± 0.42 | 5.95 ± 0.90 | 0.38 ± 0.06 | 1.83 ± 0.22 | 0.09 ± 0.014 | 0.006 ± 0.0002 |
| 2019 | 1.43 ± 0.68 | 2.92 ± 0.26 | 4.21 ± 0.84 | 0.30 ± 0.06 | 1.55 ± 0.08 | 0.03 ± 0.005 | 0.006 ± 0.0002 | |
| 2020 | 1.55 ± 0.36 | 2.21 ± 0.23 | 4.05 ± 0.79 | 0.26 ± 0.05 | 1.03 ± 0.07 | 0.03 ± 0.005 | 0.004 ± 0.0002 | |
| Corks | 2014 | 2.03 ± 0.50 | 2.66 ± 0.30 | 6.60 ± 1.41 | 0.45 ± 0.10 | 1.47 ± 0.17 | 0.08 ± 0.012 | 0.005 ± 0.0004 |
| 2019 | 1.52 ± 0.32 | 2.42 ± 0.25 | 4.33 ± 0.82 | 0.31 ± 0.07 | 1.21 ± 0.10 | 0.03 ± 0.004 | 0.005 ± 0.0004 | |
| 2020 | 1.41 ± 0.37 | 1.70 ± 0.28 | 4.48 ± 0.81 | 0.31 ± 0.06 | 0.81 ± 0.11 | 0.02 ± 0.005 | 0.003 ± 0.0003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conte, A.; Zappitelli, I.; Fusaro, L.; Alivernini, A.; Moretti, V.; Sorgi, T.; Recanatesi, F.; Fares, S. Significant Loss of Ecosystem Services by Environmental Changes in the Mediterranean Coastal Area. Forests 2022, 13, 689. https://doi.org/10.3390/f13050689
Conte A, Zappitelli I, Fusaro L, Alivernini A, Moretti V, Sorgi T, Recanatesi F, Fares S. Significant Loss of Ecosystem Services by Environmental Changes in the Mediterranean Coastal Area. Forests. 2022; 13(5):689. https://doi.org/10.3390/f13050689
Chicago/Turabian StyleConte, Adriano, Ilaria Zappitelli, Lina Fusaro, Alessandro Alivernini, Valerio Moretti, Tiziano Sorgi, Fabio Recanatesi, and Silvano Fares. 2022. "Significant Loss of Ecosystem Services by Environmental Changes in the Mediterranean Coastal Area" Forests 13, no. 5: 689. https://doi.org/10.3390/f13050689
APA StyleConte, A., Zappitelli, I., Fusaro, L., Alivernini, A., Moretti, V., Sorgi, T., Recanatesi, F., & Fares, S. (2022). Significant Loss of Ecosystem Services by Environmental Changes in the Mediterranean Coastal Area. Forests, 13(5), 689. https://doi.org/10.3390/f13050689








