Abstract
All North American ash (Fraxinus spp.) species are threatened by the emerald ash borer (EAB; Agrilus planipennis), an exotic beetle which has already destroyed millions of ash trees in the U.S. and Canada. Although both chemical insecticides and biological control can be effective, and host resistance appears possible, the speed of the invasion has defied traditional management approaches. One potential, innovative approach to managing this destructive insect is to develop a host tree-induced gene silencing strategy using RNA interference (RNAi) constructs targeting EAB-specific genes. An important requirement for applying RNAi technology is a reliable transformation/regeneration system for the host tree species. We developed an Agrobacterium-mediated gene transfer system for white ash (F. americana) and green ash (F. pennsylvanica) using the embryogenic cultures of these species as target material. Embryogenic suspension cultures of multiple genotypes of both species were plated and inoculated with A. tumefaciens carrying the pFHI-GUSi expression vector, which carries the nptII selectable marker and intron-GUS reporter genes, followed by selection on a semi-solid medium containing geneticin. Putative transgenic events showed expression of the GUS gene at all tested developmental stages from callus to plantlets, and transgene presence in the leaves of regenerated plants was confirmed using PCR. The overall average transformation efficiency achieved was 14.5 transgenic events per gram of tissue. Transgenic somatic seedlings of two white ash and three green ash genotypes were produced and acclimated to greenhouse conditions.
1. Introduction
Ash (Fraxinus spp.) trees are a common component of many forest ecosystems and residential settings [1], and in the past were one of the more commonly planted trees in urban and suburban landscapes, frequently planted along roadsides and in parks [2]. The trees are prolific seed producers and an important nutritional resource for many birds and mammals [2]. Among the 16 species of ash native to the United States [3], the most important species with regard to native range size and economic significance are white ash (F. americana) and green ash (F. pennsylvanica). White ash is the most abundant of North American ash species, with a range that covers the eastern half of the U.S., extending into the southern Ontario and Quebec provinces in Canada. It is found on moist upland slopes, valleys, coves and dry-to-mesic woods, but does not occupy poorly drained soils [4]. Green ash has the largest range of any North American ash, covering the eastern U.S. and extending onto the former Great Plains as far west as Montana and into southern Canada. It tends to be found in poorly drained bottomlands, riparian zones and swamp edges [4]. White ash is well-known as a preferred wood for baseball bats, hockey sticks, oars and other sports equipment due to its resiliency; however, the wood of both species is used for these purposes [4,5].
Many North American ash species are now listed as endangered due to invasion by the emerald ash borer (EAB), Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) (IUCN 2020). EAB is an exotic phloem-feeding beetle native to Asia that was accidently introduced from China in the 1990s via solid wood packaging material [6]. Larval EAB feed on phloem beneath the bark, creating serpentine galleries and damaging the cambium, cutting off the flow of water and nutrients and affecting the tree’s ability to heal [7]. In its native range in China, EAB attacks weakened trees and is not considered a pest, although reports of EAB outbreaks in the 1960s occurred in North American ash growing in plantations in China [8]. North American ash species are highly susceptible, and EAB can attack and breed in healthy trees, preferring white, green, and black ash (F. nigra). It is also capable of infesting pumpkin and blue ash, F. profunda and F. quadrangulata, respectively [9], although blue ash appears to have some degree of resistance [10]. Since its discovery in 2002, EAB has killed millions of ash trees throughout its invaded range [1,11], devastating urban forests, posing a threat to forest ecosystems, reducing biodiversity, and causing unprecedented economic losses [12]. Areas near the epicenter of the EAB introduction in North America have experienced > 99% mortality [7], requiring extensive, costly efforts to maintain or remove trees infested or at risk [13].
The insecticidal suppression of EAB is effective [14], but classical biological control has been the mainstay of sustainable EAB management attempts in North American forests. Four Asian parasitoids have been screened and released in the US. Spathius agrili (Hymenoptera: Braconidae), Tetrastichus planipennisi (Hymenoptera: Eulophidae), and Oobius agrili (Hymenoptera: Encyrtidae) have been released since 2007 [15], and S. galinae since 2015 [16]. However, biological control is notoriously difficult to implement and slow to establish under the best of conditions. At this stage, the likelihood of its successful application for EAB management remains unknown.
Another approach to EAB management is the genetic resistance/tolerance of the ash hosts. Individual healthy green and white ash trees, known as “lingering ash”, were identified in infested populations, in which over 95% of the ash trees in the stand were killed, and the survival of the remaining trees may be due to natural genetic variation. Some of these lingering ash trees are being employed to breed trees with enhanced resistance to EAB [17].
In spite of some success with conventional approaches, additional innovative strategies are needed. One strategy involves transgenic approaches incorporating genes for resistance, such as the Bacillus thuringiensis endotoxin gene [18]. Another approach, which we are pursuing in a project associated with the work presented here, is the use of gene silencing using RNA interference (RNAi). RNA interference, or double-stranded RNA (dsRNA)-mediated gene silencing, is a cellular antiviral mechanism that evolved prior to the divergence of plants and animals [19]. The RNAi pathway limits the expression of target genes and can be induced by a variety of natural or synthetic genetic sequences, including hairpin RNA or double-stranded RNA (dsRNA) [20]. Once the RNAi pathway is triggered, the dsRNA is processed by the enzyme Dicer into small interfering RNA (siRNA) containing 21 to 23 nucleotides. The siRNA is then loaded into the RISC protein complex (RNA-induced silencing complex) and guides this complex to its complementary sequence in the messenger RNA (mRNA), which is then cleaved, preventing the translation of the gene into a protein, causing gene knockdown [21,22]. When designed appropriately, RNAi silences specific genes, disrupts protein function, and can cause insect mortality. It is an emerging biotechnology for insect pest control that has been a breakthrough tool for crop protection.
Although the efficiency of RNAi varies among insect orders, coleopterans are especially sensitive to dsRNA either by feeding or by topical application [23]. This technology has demonstrated the effectiveness against EAB [24,25,26,27], suggesting that gene silencing using RNAi could be a viable option when implemented as one aspect of an integrated management strategy for EAB. One potential approach for protecting ash trees using RNAi is to introduce the dsRNA to EAB via expression in transgenic trees.
Genetic transformation has been reported for a number of ash species. The Agrobacterium-mediated transformation of hypocotyls of newly germinated seedlings, followed by the regeneration of adventitious shoots under kanamycin selection, was used to produce transgenic green ash, white ash and pumpkin ash (F. profunda) trees expressing the reporter genes β-Glucuronidase (GUS) or enhanced green florescent protein (EGFP) [28,29,30]. Black ash transformation with a Bacillus thuringiensis endotoxin gene (cry8D2) was also achieved using this system, with the goal of producing trees that were resistant to EAB. While transgenic shoots expressing the endotoxin gene were produced, no complete plantlets were obtained for testing due to problems with the regenerated shoots [18].
Embryogenic cultures are an alternative target for transformation that may offer some advantages over adventitious shoot-based systems for some forest tree species. These advantages include scalability using suspension cultures and amenability to cryostorage [31]. Embryogenic cultures have been employed for the transformation of a number of hardwood tree species, including yellow-poplar (Liriodendron tulipifera) [32,33], hybrid sweetgum (Liquidambar styraciflua × L. formosana) [34], chestnut (Castanea) species [35,36,37,38,39] and oak (Quercus) species [40,41]. We have previously reported the production of embryogenic cultures of multiple genotypes of both green ash and white ash with the potential for scaled-up clonal propagation [42,43]. Some of these ash cultures have been maintained for several years by serial transfer without losing the ability to produce plants. We recently optimized a vitrification-based cryostorage protocol for embryogenic cultures of both ash species [44]. Here, we report the production of transgenic plants of multiple genotypes of both white ash and green ash via the Agrobacterium-mediated transformation of the embryogenic cultures of these species. Our long-term goal is to apply the transformation system to produce transgenic ash trees expressing EAB RNAi constructs, so they can be tested for their ability to protect the trees from EAB infestation. The specific aims of the experiments described here were to demonstrate the feasibility of stably transforming multiple ash genotypes using a reporter gene construct and embryogenic cultures as target material, and to determine whether a single protocol could be successfully used to transform different genotypes of both ash species.
2. Materials and Methods
2.1. Embryogenic Culture Establishment and Maintenance
Embryogenic cultures that were employed as target material for transformation included two white ash and six green ash genotypes, although all eight genotypes were not used in each experiment. These culture lines were from a larger set of cultures that we initiated over multiple years from seeds collected from surviving ash trees in different parts of the ranges of the two species, in order to build a broad collection of germplasm [43]. Some of the seeds were collected from source trees that met the definition of “lingering ash” given in the Introduction, but whether any of the cultures harbored genes for resistance to EAB is unknown, especially since all the seeds were open-pollinated. Target genotypes for each experiment were chosen based on which embryogenic suspension cultures were judged to be at the optimal proliferation stage at the time when we were ready to start the particular transformation experiment. Table 1 shows which culture lines were used in each experiment. The cultures represented the progeny of four source trees. Lines LA112-10 and LA115-5 were derived from immature samaras (single-seeded fruit) collected in 2013 from two white ash trees growing in Oakland County, MI. Lines 5-FP-1, 5-FP-2 and 5-FP-4 were derived from immature samaras collected in 2018 from a green ash tree growing in Beaver County, PA. Lines GRBSP01-1, GRBSP01-2 and GRBSP01-3 were derived from immature samaras collected in 2018 from a green ash tree growing in Winona County, MN. Culture initiation and maintenance were accomplished using protocols we previously reported for ash [43]. Samaras were surface-disinfested and dissected to remove the developing zygotic embryos, which were cultured in 60 mm plastic Petri plates containing semisolid induction-maintenance medium (IMM) [45], a modified woody plant medium (WPM) [46] with 30 g/L sucrose, and 0.5 g/L filter-sterilized L-glutamine, as well as either 2 mg/L of 2,4-dichlorophenoxyacetic acid (2,4-D), 4 mg/L of 2,4-dichlorophenoxyacetic acid (2,4-D) or 0.2 mg/L picloram. The semisolid medium was gelled with 3 mg/L Gellex gellan gum (Caisson). Cultures were incubated in the dark at 25 °C. After one month, the cultures were transferred to fresh IMM containing the same concentration of plant growth regulators (PGRs) as that on which the explant was originally cultured. Explants showing evidence of embryogenesis induction after two months in culture (or in some cases, longer) were individually transferred to plates of IMM with the same PGRs and concentrations. Thereafter, cultures were maintained by transfer to fresh medium every three weeks, except for one line originally cultured on 0.1 mg/L picloram and later maintained on 0.2 mg/L picloram to suppress premature somatic embryo development.

Table 1.
Overview of Transformation Experiments 1–4.
2.2. Antibiotic Sensitivity Testing of Target Culture Lines
Embryogenic lingering white ash genotypes, LA112-10 and LA115-5, were the first culture lines selected as potential target material for transformation experiments, so these cultures were tested for their sensitivity to geneticin to determine their efficacy as selection agents. Suspension cultures were initiated by inoculating approximately 0.5 g of proembryogenic masses (PEMs) into 125 mL Erlenmeyer flasks containing 30 mL of liquid IMM with 2 mg/L 2,4-D. The cultures were agitated at 100 rpm on a rotary shaker and maintained at constant temperature of 25 °C in the dark. They were fed every two weeks by pouring off old medium and adding 32 mL of fresh IMM. After 45 days of growth in liquid suspension, cultures were size fractionated using two stacked stainless-steel CELLECTOR® sieves (Bellco Glass), the upper sieve with a mesh size of 860 µm and the lower sieve with a mesh size of 140 µm. Liquid IMM was poured through the sieve to wash all the smaller PEMs down onto the smaller screen. The larger cell clumps remaining on the top sieve were discarded, and PEMs collected on the bottom sieve were resuspended in a new 125 mL Erlenmeyer flask containing 30 mL of liquid IMM. The fractionated PEMs were allowed to settle to the bottom of the flask and then collected using a 10 mL wide-tip pipette (Corning). PEMs were given 5 min to settle to the tip of the pipette. Then, 0.1 mL settled cell volume (SCV), which was approximately 76 mg of PEMs, was resuspended in liquid IMM and plated onto squares of 30 µm pore size nylon mesh (Lamports Filter Media, Cleveland, OH, USA). Excess liquid medium was removed using gentle house vacuum applied to a glass funnel and fritted glass stopper base (Wheaton filtration assembly) connected to a 500 mL sidearm flask. The nylon mesh squares with cell clumps were then placed on semi-solid IMM in 100 mm plastic Petri dishes containing 0, 2.5, 5, 10, or 20 mg/L geneticin. All the semi-solid media were supplemented with 300 mg/L of the antibiotic timentin, which would later be used to eradicate Agrobacterium. The two antibiotics were filter-sterilized and added to an autoclaved medium following cooling to 59 °C. The cultures were maintained in the dark at 25 °C. After six weeks, plates were evaluated for cell growth. Since tissue was observed growing on medium containing 20 mg/L geneticin in the first test, a second test was performed to assess the effect of higher levels of geneticin. PEMs of the same two genotypes used in the first test were plated on IMM supplemented with 0, 20, 30, 40, or 60 mg/L geneticin, in addition to timentin at 300 mg/L. The cultures were maintained and evaluated in the same manner as the first sensitivity experiment.
2.3. Agrobacterium Preparation
The Agrobacterium tumefaciens (Agro) strain AGL1 harboring the pFHI-GUSi binary vector [42] was used for all ash transformation experiments. The pFHI-GUSi binary vector carries the nptII gene and the intron version of the β-glucuronidase gene (intron-GUS), which prevents bacterial expression of the enzyme. Two days prior to co-cultivation with the plant cells, the Agro with pFHI-GUSi plasmid was grown on semi-solid YEP medium supplemented with 50 mg/L rifampicin, 100 mg/L kanamycin, and 100 mg/L carbenicillin. A single colony from the semi-solid medium was chosen and used to inoculate 25 mL of liquid YEP medium using the same antibiotics. The Agro culture was then grown overnight in the dark at 28 °C on a rotary shaker set at 200 rpm. From this overnight Agro culture, a new culture was initiated by adding 50 µL of this culture to a new flask containing 25 mL of liquid YEP supplemented with the aforementioned antibiotics and grown under the same conditions as the previous Agro cultures. On the day of Agro co-cultivation with the plant cells, the desired optical density (OD600) of the Agro culture was between 0.600–0.700. The Agro cells were collected in 50 mL conical tubes and centrifuged at 4100 rpm for 15 min at 25 °C. Then, the supernatant was poured off and the pellet was resuspended in 25 mL of Agro induction medium (AIM), which contained AB medium [47] with 250 mM acetosyringone for induction of the virulence genes. The Agro cells were placed in the dark on a rocker set at 225 rpm for 1–2 h prior to being used for co-cultivation.
2.4. Transformation Experiment 1
Information on all four transformation experiments can be found in Table 1. The two white ash genotypes (LA112-10 and LA115-5) chosen as target material for transformation for Experiment 1 were grown in suspension for 45 days in liquid IMM with 2 mg/L 2,4-D, size fractionated, and the desired fraction was plated onto disks of nylon mesh using the protocol described above. The nylon mesh disks with cell clumps were placed on semi-solid IMM supplemented with 250 µM acetosyringone, and 600 µL of Agro-AIM-aceto solution was dripped onto the cell clumps. An AIM-aceto solution without Agro was used as a control for cell viability and growth. Cultures were incubated in the dark at 25 °C for 3 days. Following co-cultivation, the cells were gently scooped off the nylon mesh with a stainless-steel spatula, transferred into sterile 50 mL conical tubes, and resuspended in wash medium containing liquid IMM with 300 mg/L timentin. The tubes were gently inverted, the wash medium was poured off, and this was repeated until the wash medium was clear. Then, cultures were resuspended in 30 mL of liquid wash medium, and 0.1 mL SCV aliquots were pipetted onto nylon mesh squares, as described above. Nylon mesh squares with cell clumps were placed on semi-solid IMM selection medium, which was IMM supplemented with 40 mg/L geneticin and 300 mg/L timentin, or on semi-solid IMM recovery medium containing only 300 mg/L timentin. After two days, the cultures on the recovery medium were moved to the selection medium, where they remained for the duration of the experiment. Numbers of selection medium plates per genotype depended on how much culture material was available for plating for a given genotype (Table 1). Cultures were grown in the dark at 25 °C. The nylon disks with cell clumps were moved to fresh medium every two weeks for six weeks. Any putatively transformed colonies that grew on the nylon mesh were harvested and moved to fresh selection medium.
2.5. Transformation Experiment 2
This experiment followed the same protocol as for the first experiment, except that we lowered the concentration of geneticin in the IMM selection medium to 35 mg/L, because geneticin-resistant colony growth was slow in the first experiment. Target material included the same two white ash genotypes used in Experiment 1 plus two green ash genotypes (5-FP-2 and 5-FP-4). One other change with this experiment was that the PGRs differed for the white ash lines and green ash lines. Because the white ash lines had been maintained in 2 mg/L 2, 4-D, the IMM selection medium also contained 2 mg/L 2,4-D. However, one green ash line, 5-FP-2, had been maintained in IMM with 4 mg/L 2, 4-D and the other one, 5-FP-4, had been maintained in IMM with 0.2 mg/L picloram. Therefore, these PGRs and concentrations were used in the liquid medium for suspension cultures and in the IMM selection medium for each of these lines, respectively (Table 1). As it became apparent that both green ash cultures were escaping selection on semi-solid medium (see Results), we decided to increase the selection stringency for the green ash material. For this, all of the embryogenic culture material growing on two of the selection plates, one of each green ash genotype, was collected and transferred into 125 mL flasks containing 30 mL of liquid IMM selection medium with 40 mg/L geneticin. The resulting suspension cultures were grown on a gyratory shaker at 100 rpm in the dark at 25 °C for six weeks and fed with a fresh selection medium every two weeks until putatively geneticin-resistant cell clumps appeared in the flasks. The resistant colonies were then harvested from the flasks and individually inoculated into 50 mL flasks containing 20 mL of the same IMM liquid selection medium to eliminate any remaining escape tissue.
2.6. Transformation Experiment 3
Target material for this experiment included the same white ash and green ash genotypes used in Experiment 2 and followed the same protocol as for the previous experiments. However, in this experiment, following co-cultivation and washing, the inoculated ash cultures were plated onto IMM selection media that contained 35 mg/L geneticin with 300 mg/L timentin, 40 mg/L geneticin with 300 mg/L timentin, or 300 mg/L timentin only. Another difference from the previous experiments was that, in this experiment, following cultivation, washing and collection on nylon mesh, none of the cultures were given a recovery period prior to placement on the selection media.
2.7. Transformation Experiment 4
Because of the problem with escapes for green ash material in the second transformation experiment, this experiment was conducted to test if lowering the plating density of inoculated material would help generate more transgenic events while minimizing escapes on the semi-solid selection medium. Six green ash genotypes were used in the experiment: 5-FP-1, 5-FP-2, 5-FP-4, GRBSP01-1, GRBSP01-2 and GRBSP01-3. The PGRs used in liquid medium and selection medium for all lines are listed in Table 1. Target culture preparation, co-cultivation and washing were the same as in the previous experiments, except that aliquots of only 0.05 mL SCV (rather than 0.1 mL SCV, used in the previous experiments) of PEMs were pipetted onto the nylon mesh for selection. Nylon mesh disks with cells were placed on IMM supplemented with 35 mg/L geneticin and 300 mg/L timentin. Cultures were maintained in the same manner as in the previous transformation experiments.
2.8. Somatic Embryo and Somatic Seedling Production from Transgenic Events
Individual transgenic events resulting from different transformation experiments were selected for somatic embryo and somatic seedling production. We did not regenerate transgenic plantlets for each target genotype used in each experiment, although we ultimately did produce transgenic plantlets from five of the eight white ash and green ash genotypes used in the study. The same procedures as those described above for preparing cultures for transformation were used to initiate and grow the embryogenic suspension cultures. However, liquid IMM selection medium (with the appropriate PGRs and concentrations for each target line, 35 mg/L geneticin and 300 mg/L timentin) was used instead of regular liquid IMM. The same size fractionation and plating method used to prepare material for transformation was used to collect PEMs of the desired size range for somatic embryo production on nylon mesh. The PEMs on nylon mesh were cultured in semi-solid embryo development medium (EDM), which was the same as IMM, but lacking PGRs, in 100 mm plastic Petri plates. Selection was eliminated at this point, so the EDM was not supplemented with geneticin or timentin. Plates were incubated in the dark at 25 °C to allow for development of somatic embryos. Once populations of somatic embryos had developed on EDM, cotyledonary-stage embryos at least 2 mm long were harvested and transferred to fresh plates of semisolid EDM to enlarge for 2–3 weeks. Then, the mature embryos, still on the plates of EDM, received an 8-week pre-germination cold treatment at 4 °C in the dark. Following cold treatment, embryos were removed from the EDM plates and “planted” in GA-7 vessels (Magenta Corp.) containing 100 mL of semisolid germination medium (GM). GM was the same as EDM but lacked glutamine and was supplemented with 10 mg/L filter-sterilized gibberellic acid and 0.5 g activated charcoal. GA-7s were incubated in a lighted growth chamber at 25 °C under cool white fluorescent light at 100 µmol·m−2·s−1 with 16 h of light per day. Once somatic seedlings produced multiple leaves, they were removed from in vitro conditions and potted in moistened peat:perlite:vermiculite (1:1:1) mix in 4-inch plastic pots. Pots were placed on top of water-saturated perlite in clear plastic dome-covered trays to maintain humidity and grown under cool white fluorescent lights (80 µmol·m−2·s−1) with 16 h day lengths. Somatic seedlings were watered and fertilized with 10 mL of Miracle-Gro® fertilizer each week. For acclimatization, vents on the domed trays were slowly opened over the following two months, followed by complete removal of the domes. Then, the somatic seedlings were transferred to the greenhouse to continue growth.
2.9. GUS Expression Assays
Transient and stable expression of GUS activity was determined by histochemical staining as described by Jefferson [48]. Clusters of embryogenic cells, individual somatic embryos and leaves of regenerated plantlets were incubated in the substrate solution (X-gluc), which contained 1 mM 5-bromo-4-chloro-3-indolyl-β-D-glucuronide, overnight at 37 °C. GUS expression was assayed by blue staining of tissues. The stained leaf tissues were cleared using 100% ethanol followed by incubation for twelve hours at 37 °C, and clearing treatments were repeated with fresh ethanol until removal of the chlorophyll was complete.
2.10. PCR Assays for Transgene Presence
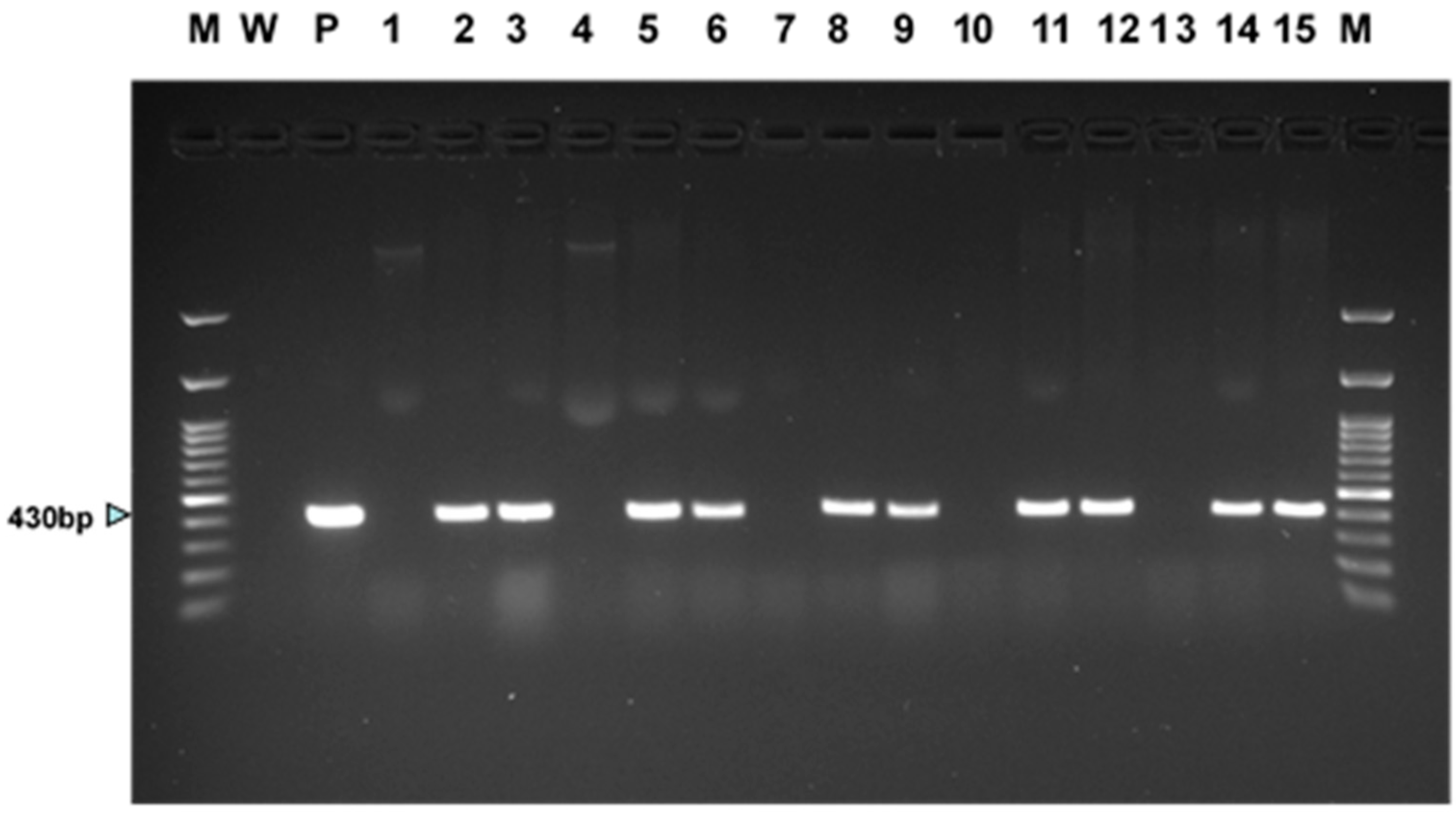
We chose plantlets derived from two putative transgenic events in each ash genotype to assay for transgene presence using PCR. Genomic DNA was isolated from 100 mg of leaf or stem tissue of putatively transgenic white ash and green ash plants and wildtype plants (or, for one wildtype genotype, clusters of embryogenic cells), using the Omega Bio-Tek (Norcross, GA, USA) E.Z.N.A.® HP Plant DNA Mini Kit. The genomic DNA was used as a template for PCR amplification assays. Standard PCR reactions were carried out in 25 µL reactions containing 13 µL ultra-pure water, 190 ng of ash DNA, 1 µL of 1.0 µM of each primer, 1 µL 25 mM MgCl2, and 10 µL of Taq® 5X Master Mix (New England BioLabs, Inc., Ipswich, MA, USA), which is an optimized ready-to-use solution containing Taq DNA polymerase, dNTPs, MgCl2, KCl and stabilizers. The primer set designed to screen for integration of the uidA (GUS) reporter gene yielded a 430 bp PCR product. The PCR forward primer was 5′-ACA CCG ATA CCA TCA GCG AT-3′ and the reverse primer was 5′-TCA CCG AAG TTC ATG CCA GT-3′. The PCR conditions used in the Thermal Cycler PTC 100® (MJ Research, Inc., Watertown, MA, USA) were 94 °C for 2 min, followed by 31 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min. A final extension at 72 °C for 10 min was followed by a 4 °C hold. PCR products were resolved by gel electrophoresis on a 1% agarose gel and visualized by staining using by SYBR® Safe DNA (Invitrogen, Waltham, MA, USA).
3. Results
3.1. Antibiotic Sensitivity of Culture Lines
In the first sensitivity experiment, the growth of neither of the tested culture lines appeared to be inhibited by the lower concentrations (2.5, 5, 10 mg/L) of geneticin. At the highest tested level (20 mg/L), growth of LA112-10 was suppressed, although not completely, and the LA115-5 showed little negative effect. Therefore, higher geneticin levels (20–60 mg/L) were tested in the second sensitivity experiment. In this experiment, growth of both LA112-10 and LA115-5 was inhibited, but again, not completely halted, at 30 mg/L, while 40 mg/L and higher concentrations completely inhibited the growth of both lines, with the tissue turning brown after a few weeks. Based on these results, we chose 40 mg/L geneticin for selection in the first transformation experiment, although we later modulated the level in subsequent experiments.
3.2. Transformation Experiment 1
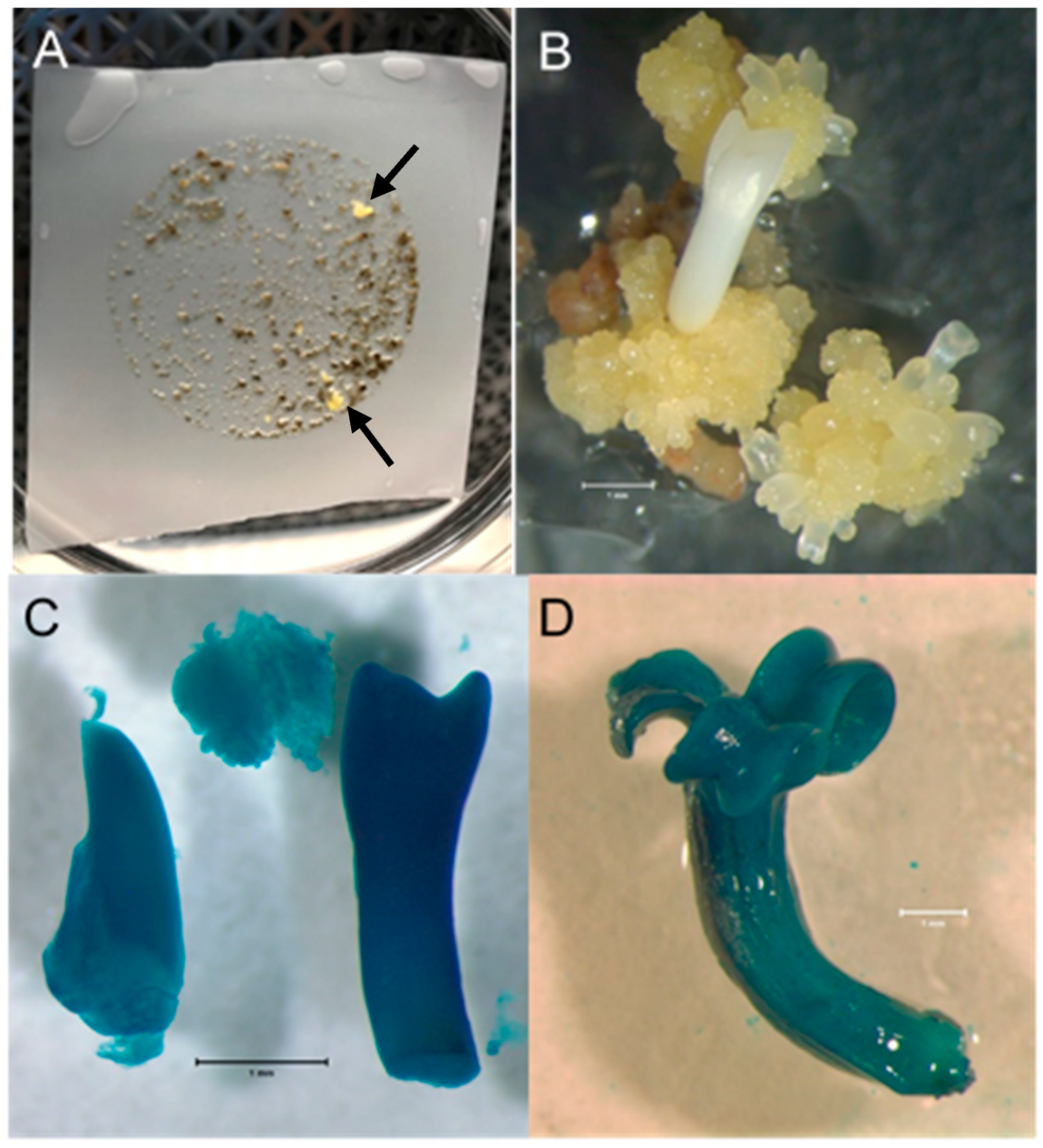
Results from all four transformation experiments are summarized in Table 1. Small colonies of apparently geneticin-resistant ash embryogenic material began to appear in the selection medium approximately three weeks following transfer to the selection medium (Figure 1A). As these colonies appeared on the nylon mesh, they were moved directly onto the surface of the selection medium adjacent to the edge of the mesh. Those colonies that continued to grow directly on the medium after 2–4 weeks were transferred to fresh plates of selection medium with 40 mg/L geneticin. Some of these colonies died on the fresh selection medium, indicating that they may have been escapes. However, eight geneticin-resistant transgenic events were obtained for LA112-10—four from material plated on the selection medium directly following co-cultivation and four from material allowed two days of recovery following co-cultivation before transfer to the selection medium. Even though their growth was initially very slow, the colonies retained a healthy light-yellow color typical of ash embryogenic material growing on IMM and eventually began to more rapidly proliferate (Figure 1B). Embryogenic culture samples derived from the eight putative transgenic events, which continued to proliferate following the move to fresh selection medium, displayed GUS expression in both the callus and somatic embryos, indicating a stable transformation with the GUSi gene (Figure 1C,D). However, the intensity of GUS staining varied among the events. After over two months in the selection medium, a single geneticin-resistant colony was recovered for the LA115-5 genotype. A tissue sample from this colony was positive for GUS expression.
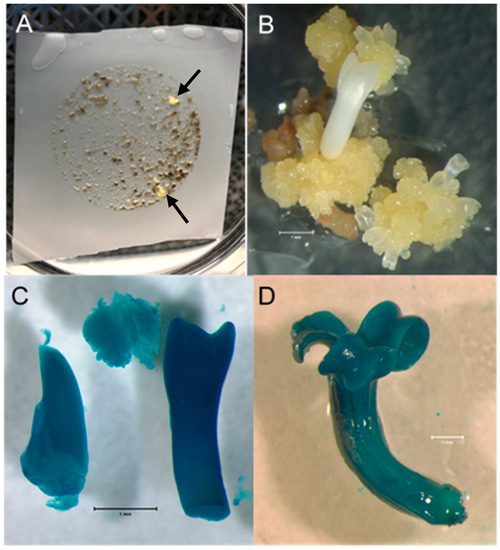
Figure 1.
Ash embryogenic culture transformation. (A). Ash PEMs on nylon mesh following Agrobacterium co-cultivation and approximately six weeks of geneticin selection on semisolid medium. Petri plate is 100 mm in diameter. The first geneticin-resistant transgenic events to grow are marked with arrows. (B). A geneticin-resistant white ash colony continuing proliferation, with somatic embryos developing, 6 weeks after being picked from the nylon mesh and moved directly onto selection medium. Bar = 1 mm. (C). Transgenic white ash somatic embryos and callus displaying GUS expression following staining with X-gluc. Bar = 1 mm. (D). Transgenic green ash somatic embryo displaying GUS expression following staining with X-gluc. Bar = 1 mm.
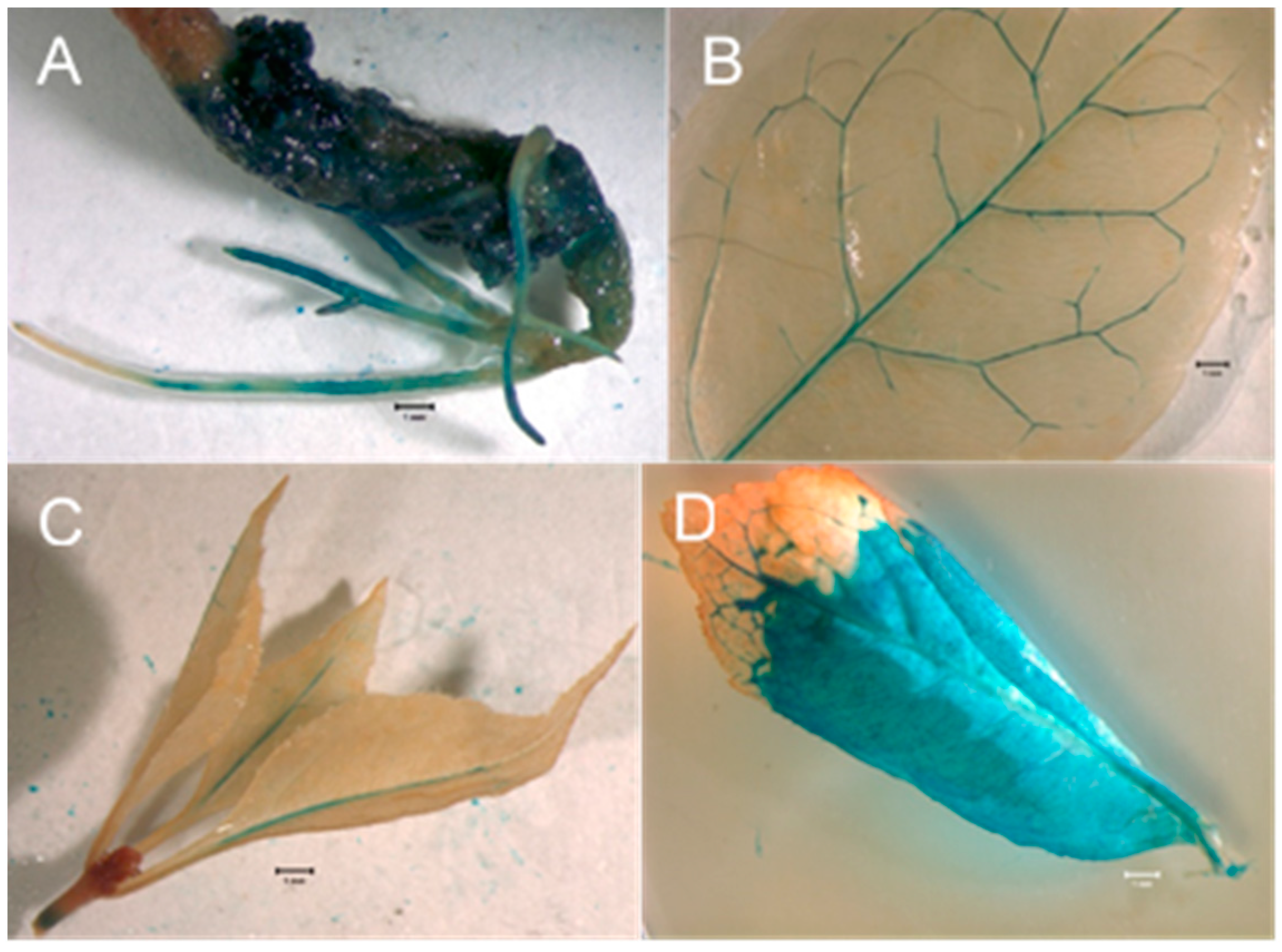
All eight LA-112-10 transgenic events and the single LA-115-5 event were grown in suspension culture in liquid IMM selection medium. Following size fractionation of the suspension cultures (Figure 2A), collection on nylon mesh and plating on semi-solid IMM (without geneticin), all the transgenic cultures produced somatic embryos (Figure 2B) that germinated to produce somatic seedlings. Leaves, roots, and hypocotyls sampled from plantlets at different stages of development and stained with X-gluc showed variable patterns of GUS expression (Figure 3A–D). PCR results for plantlets, representing two independent transgenic events for LA112-10, indicated that the GUSi gene was present in the leaves of the plantlets (Figure 4). A subset of plantlets was transferred to potting mix, acclimatized (Figure 5A) and moved to the greenhouse (Figure 5B).

Figure 2.
Production of transgenic ash somatic seedlings. (A). Suspension culture of ash PEMs from a single transgenic event, following 6 weeks of growth in liquid medium and size fractionation, just prior to collection on nylon mesh for plating on embryo development medium. (B). Transgenic white ash somatic embryos derived from suspension cultures, 5 weeks following fractionation, collection on nylon mesh and plating on embryo development medium. Bar = 1 mm. (C). Transgenic green ash somatic seedlings growing in germination medium with activated charcoal.
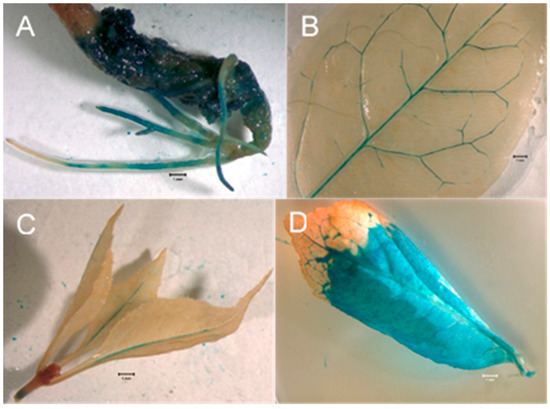
Figure 3.
GUS expression assays in regenerated ash somatic seedlings. (A). Hypocotyl and roots of a germinated transgenic ash somatic embryo showing GUS expression. Bar = 1 mm. (B). Leaf from transgenic ash somatic seedling showing GUS expression in vascular tissues. Bar = 1 mm. (C). Shoot tip with leaves from transgenic ash somatic seedling showing GUS expression in shoot tissue and mid-veins of leaves. Bar = 1 mm. (D). Leaf from transgenic ash somatic seedling showing GUS expression in both veins and interveinal tissue. Bar = 1 mm.
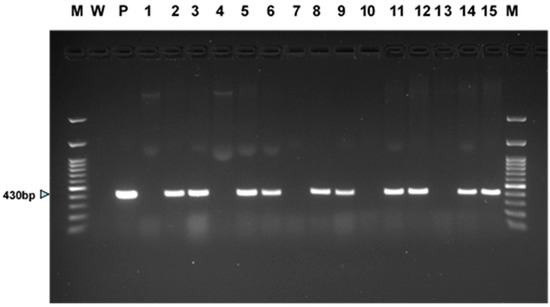
Figure 4.
PCR results for presence of GUSi gene in tissues derived from two independent transgenic events for each of the two white ash genotypes (LA112-10, LA115-5) and three green ash genotypes (5-FP-1, 5-FP-2, 5-FP-4). DNA of transgenic and wildtype control somatic seedlings was extracted from leaf tissue, except for DNA of wildtype 5-FP-2, which was extracted from embryogenic tissue. Lanes are as follows: (M) 100 bp molecular weight ladder, (W) no DNA control, (P) GUSi plasmid, (1) LA112-10 wildtype, (2) LA112-10-8 GUSi, (3) LA112-10-9 GUSi; (4) LA115-5 wildtype, (5) LA115-5-1 GUSi, (6) LA115-5-3 GUSi, (7) 5-FP-1 wildtype, (8) 5-FP-1-1 GUSi, (9) 5-FP-1-3 GUSi, (10) 5-FP-2 wildtype, (11) 5-FP-2-4 GUSi, (12) 5-FP-2-5 GUSi, (13) 5-FP-4 wildtype, (14) 5-FP-4-2 GUSi, (15) 5-FP-4-5 GUSi, and (M) 100 bp molecular weight ladder.

Figure 5.
Transgenic green ash and white ash somatic seedlings following transfer from in vitro conditions. (A). Transgenic ash somatic seedlings following transfer to potting mix in domed trays for acclimatization. (B). Acclimatized transgenic ash somatic seedlings in the greenhouse.
3.3. Transformation Experiment 2
The production of transgenic events from the two tested white ash lines was very low in this experiment, with just two events for LA112-10, both of which were GUS-positive, and no events for LA115-5. By contrast, a great deal of geneticin-resistant material grew on the selection plates for both tested green ash lines, but it became apparent that the green ash material was escaping selection, as the tissue quickly covered the nylon mesh. At this point, as described in the Materials and Methods, we applied enhanced selection stringency by transferring the tissue of both of the green ash target lines from a semi-solid selection medium to liquid selection medium and, subsequently, harvesting geneticin-resistant colonies from the suspension culture flasks. Sixteen geneticin-resistant colonies were obtained from the 5-FP-2 suspension culture, all of which continued to proliferate when individually transferred to 50 mL flasks with 20 mL of liquid selection medium, producing cultures that were GUS-positive. Similarly, ten geneticin-resistant colonies were obtained from the 5-FP-4 suspension culture, all of which were GUS-positive. Five putative transgenic events were selected for each green ash genotype to be grown in 125 mL flasks of IMM selection medium, followed by fractionation and plating to produce somatic embryos. Transgenic somatic embryos and somatic seedlings were produced from multiple events for both green ash genotypes (Figure 2C). PCR results from leaves of plantlets regenerated from two events in each green ash background showed the presence of the GUSi gene in plantlets from all four of the tested events (Figure 4). We did not attempt to produce somatic seedlings from the white ash (LA112-10) transgenic events, since we already had transgenic plantlets of that genotype from Experiment 1.
3.4. Transformation Experiment 3
No obvious differences in the production of transgenic events were seen between the two levels of geneticin used for selection (35 and 40 mg/L) for the four tested white ash and two green ash lines. For 5-FP-2, two geneticin-resistant colonies arose on the nylon mesh on 35 mg/L geneticin, and when these were directly placed on selection medium, both survived and were GUS-positive. Similarly, a single colony of this line that emerged on 40 mg/L geneticin survived the transfer to the selection medium and was GUS-positive. We did not produce plantlets from these events since we already produced transgenic 5-FP-2 plantlets from the previous experiment. Eleven putative events were produced on the nylon mesh on 35 mg/L geneticin for LA115-5, but none of these survived when placed directly on the selection medium. Of the ten LA115-5 geneticin-resistant colonies that arose on the nylon mesh on 40 mg/L geneticin, three survived following placement directly on the selection medium, and all of these were GUS-positive. Plantlets were produced from two of these events. PCR confirmation of GUSi gene presence in plantlets from both events is shown in Figure 4. Neither LA112-10 nor 5-FP-4 produced geneticin-resistant colonies on either of the tested levels of geneticin in this experiment.
3.5. Transformation Experiment 4
In this experiment, we tested the effect of lowering the plating density used in the previous experiments from 0.1 mL SCV to 0.05 SCV for eliminating growth of escape tissue under selection, which had caused problems for some of the green ash cultures. Of the six green ash cultures tested in this experiment, three produced transgenic events (GRBSP01-1, GRBSP01-3 and 5-FP-2) that were confirmed by GUS expression. While the rapid growth of non-transformed tissue was not observed, there was still evidence of escapes from the selection. While two of the tested lines (GRBSP01-2 and 5-FP-1) produced no colonies under selection, about half of the colonies produced from the lines that did make them were not GUS-positive, indicating that they were likely to be escapes. Furthermore, none of the eight GUS-positive events for GRBSP01-1 or GRBSP01-3 survived the culture in a liquid selection medium with 35 mg/L geneticin, to which they had been transferred in preparation for somatic embryo production, so no transgenic plantlets were produced from these events. Although 54 GUS-positive transgenic events were produced for 5-FP-2, we did not attempt to produce plantlets from any of these events, since we already produced transgenic plantlets of this genotype in Experiment 2. We note that, while no transgenic events were obtained for 5-FP-1 in this experiment, we later obtained over 100 GUS-positive events in this background in another experiment (Merkle Lab, unpublished data), and transgenic plantlets were produced from five of these events. PCR results confirmed the presence of the GUSi transgene in 5-FP-1 plantlets from the two events we chose to assay (Figure 4).
4. Discussion
While the results of our transformations varied with respect to transformation efficiency, efficacy of selection and ability to produce transgenic plants, each experiment was foundational and provided information that has helped us move the system toward eventual applications for producing transgenic ash carrying candidate genes, in order to test for their potential to confer resistance to EAB infestation. Of the two white ash genotypes and six green ash genotypes employed as target tissue, we were able to stably transform both of the white ash genotypes and five of the six green ash genotypes, producing multiple transgenic events in each background. Transgenic plants were regenerated for the two white ash genotypes and three of the green ash genotypes. The inability to produce transgenic plantlets from two of the green ash genotypes that were transformed can be related to our problems with identifying an optimal concentration of geneticin that would suppress the growth of non-transformed green ash material while allowing for the subsequent proliferation of transformed material in suspension culture. The transfer to suspension culture is an important step, since we use the suspensions to grow individual transgenic events in preparation for somatic embryo production. Particularly for the green ash genotypes tested, we struggled to identify a concentration of geneticin by which we could obtain more than a few transgenic events per gram of target tissue, while eliminating escapes. We obtained transgenic events, confirmed with GUS staining, for two of the green ash genotypes (GRBSP01-1 and GRBSP01-3) using a selection on a semi-solid medium with 35 mg/L geneticin, but when these colonies were transferred to liquid medium with the same concentration of geneticin to be grown for somatic embryo production, they failed to survive the higher stringency of the liquid selection medium. This may be due to the finer structure (i.e., small cell clump size) of the green ash embryogenic cultures, making them more sensitive to geneticin in liquid culture. Thus, it appears that for green ash, we will need to fine tune the selection for each genotype, including making adjustments depending on whether the selection is applied using a semi-solid medium or liquid medium.
Transformation efficiency varied widely with experiment and genotype, ranging from 0 to 210 events per gram of target material (Table 1). When co-cultivations that resulted in no transgenic events were included in the calculation, the transformation efficiency across all four experiments averaged 14.5 events per gram of tissue. Our transformation efficiencies are difficult to compare to those previously reported for ash, since the transformation efficiencies in those reports were calculated on the basis of the percentage of co-cultivated seedling hypocotyl segments that produced transgenic adventitious shoots in green ash (0.5%) [28], white ash (1.3%) [29] and pumpkin ash (5.4%) [30], rather than transgenic events per gram of co-cultivated embryogenic tissue. Similarly, most transformation efficiencies for other tree species, from which embryogenic cultures have been used as transformation targets, were reported on an events-per-explant basis [41,49,50]. An Agrobacterium-mediated transformation protocol for olive (Olea europaea), which is in the same family as ash (Oleaceae), that used somatic embryos as transformation targets, resulted in an average transformation frequency of 8% based on the number of inoculated embryogenic masses that produced GUS-positive calli [49]. Our average transformation efficiency using embryogenic ash cultures were somewhat lower than those that were reported for embryogenic cultures of other hardwood forest trees on an event-per-unit-weight-of-target-tissue basis. American chestnut (Castanea dentata) and sweetgum embryogenic culture transformation efficiencies were four events per 50 mg (80 events per gram), and 30 events per 0.3 mL SCV (approximately 130 events per gram) of co-cultivated tissue were reported, respectively [34,35].
Patterns of GUS expression shown by X-gluc staining in somatic seedlings regenerated from transgenic embryogenic cultures were inconsistent. Large sectors of some leaves showed GUS expression (Figure 3D), while in others, expression appeared to be limited to vascular tissues (Figure 3B,C). We do not believe these plantlets are chimeras, based on the solid GUS expression in developing and germinated somatic embryos of the same transgenic events. It is possible that the Ubiquitin-11 promoter driving GUS expression is not constitutively expressed in green ash and white ash or that there were tissue penetration issues with the X-gluc. Transgenic olive material derived from embryogenic cultures showed a similar expression pattern in that X-gluc-stained somatic embryos were solidly blue, while regenerated plantlets showed sectored X-gluc staining [49]. In photos showing GUS expression in transgenic green ash adventitious shoots transformed via the Agrobacterium co-cultivation of seedling hypocotyl segments, GUS expression was also not consistent in all leaf tissues, even though the GUS gene was driven by an enhanced 35S promoter [28]. Interestingly, however, in those leaves, expression appeared to be limited to interveinal areas of the leaves, with the veins remaining unstained by X-gluc, which is the opposite of what we observed in leaves of some of our transformed plantlets (Figure 3B,C).
The ash transformation system described here was specifically developed to evaluate the transgenic expression of RNAi constructs designed to protect ash trees from lethal infestation by EAB. RNAi can cause gene knockdown and induce mortality in EAB [24,25,26,27], but the effective delivery of the dsRNA is problematic. Multiple plant protection strategies applying RNAi technology have been evaluated or are under development [51,52], including applications of foliar sprays [53], systemic uptake through plant material [27,54,55], oral administration through baits [56], embedding in nanoparticles [57] and expression in genetically engineered microorganisms [58]. Our findings here demonstrate that expression in transgenic plants [23,59] is possible and holds great promise for tree protection, though further optimization is clearly needed, and additional barriers must be overcome. Increasing transformation efficiency is essential, but more significantly, RNAi constructs have some fundamental differences from the expression vector we used. A stable transformation with an RNAi vector with a scorable marker, such as the Phytoene desaturase (PDS) gene [60], is needed to test the function of hairpin-based gene silencing in transgenic ash plants, before transforming with RNAi constructs specifically designed to suppress the expression of EAB genes. Once this step is completed, the production of green and white ash transformed with RNAi constructs specific to EAB is possible.
5. Conclusions
In this study, transgenic somatic seedlings of two white ash and three green ash genotypes were produced and acclimated to greenhouse conditions. The results of the described experiments demonstrated proof of concept for developing transgenic ash that opens a pathway for engineering EAB resistance using RNAi or other candidate resistance gene constructs. This work is foundational to developing tree protection strategies that can directly contribute to forest health in the face of increasing pest pressures and increasingly catastrophic disturbance events, exacerbated by our changing climate. Here, we focused on protecting ash trees from EAB, but this approach could be more broadly applicable for protecting other woody plant species against xylophagous insects.
Author Contributions
Study concept and design, S.M., H.G., L.R., C.D.N.; methodology and data collection, A.R.T. and H.G.; interpretation of data, A.R.T. and H.G.; writing—original draft preparation, S.M.; writing—review and editing, S.M., H.G., A.R.T., L.R, F.P., C.D.N. All authors have read and agreed to the published version of the manuscript.
Funding
The research described in this paper was supported by the USDA Forest Service Southern Research Station (Agreement 19-JV-11330126-084) through a subaward from the University of Kentucky Research Foundation and by McIntire–Stennis project accession no. 1016473 from the USDA National Institute of Food and Agriculture.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Diane Hartzler (The Ohio State University), Tim Tomon (Pennsylvania Bureau of Forestry) and Jonathan Osthus (Minnesota Department of Agriculture), for collecting ash material from which the cultures used in this study were derived. We also thank Shenghua Fan (University of Kentucky, Department of Horticulture) and Albert Abbott for their technical advice and scientific encouragement.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cappaert, D.; McCullough, D.; Poland, T.; Siegert, N. Emerald ash borer in North America: A research and regulatory challenge. Am. Entomol. 2005, 51, 152–165. [Google Scholar] [CrossRef]
- Schlesinger, R. Fraxinus americana L. White Ash. In Silvics of North America: Hardwoods; US Forest Service: Washington, DC, USA, 1990; Volume 2. [Google Scholar]
- Little, E.L., Jr. Checklist of United States Trees (Native and Naturalized); USDA Forest Service: Washington, DC, USA, 1979. [Google Scholar]
- Hardin, J.W.; Leopold, D.J.; White, F.M. Harlow and Harrar’s Textbook of Dendrology, 9th ed.; McGraw-Hill: Boston, MA, USA, 2001. [Google Scholar]
- Pashin, A.J.; de Zeeuw, C. Texbook of Wood Technology, 3rd ed.; McGraw-Hill: Boston, MA, USA, 1970. [Google Scholar]
- Siegert, N.W.; McCullough, D.G.; Liebhold, A.M.; Telewski, F.W. Dendrochronological reconstruction of the epicentre and early spread of emerald ash borer in North America. Divers. Distrib. 2014, 20, 847–858. [Google Scholar] [CrossRef]
- Herms, D.A.; McCullough, D.G. Emerald Ash Borer Invasion of North America: History, Biology, Ecology, Impacts, and Management. Annu. Rev. Entomol. 2014, 59, 13–30. [Google Scholar] [CrossRef]
- Orlova-Bienkowskaja, M.J.; Volkovitsh, M.G. Are native ranges of the most destructive invasive pests well known? A case study of the native range of the emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae). Biol. Invasions 2018, 20, 1275–1286. [Google Scholar] [CrossRef]
- Haack, R.; Baranchikov, Y.; Bauer, L.; Poland, T. Emerald ash borer biology and invasion history. In Biology and Control of Emerald Ash Borer; Van Driesche, R., Reardon, R., Eds.; USDA Forest Service: Morgantown, WV, USA, 2015; pp. 1–13. [Google Scholar]
- Spei, B.A.; Kashian, D.M. Potential for persistence of blue ash in the presence of emerald ash borer in southeastern Michigan. For. Ecol. Manag. 2017, 392, 137–143. [Google Scholar] [CrossRef]
- Poland, T.M.; McCullough, D.G. Emerald ash borer: Invasion of the urban forest and the threat to North America’s ash resource. J. For. 2006, 104, 118–124. [Google Scholar]
- Aukema, J.E.; Leung, B.; Kovacs, K.; Chivers, C.; Britton, K.O.; Englin, J.; Frankel, S.J.; Haight, R.G.; Holmes, T.P.; Liebhold, A.M.; et al. Economic Impacts of Non-Native Forest Insects in the Continental United States. PLoS ONE 2011, 6, e24587. [Google Scholar] [CrossRef]
- Kovacs, K.F.; Haight, R.G.; McCullough, D.G.; Mercader, R.J.; Siegert, N.W.; Liebhold, A.M. Cost of potential emerald ash borer damage in US communities, 2009–2019. Ecol. Econ. 2010, 69, 569–578. [Google Scholar] [CrossRef]
- McCullough, D.G.; Poland, T.M.; Anulewicz, A.C.; Lewis, P.; Cappaert, D. Evaluation of Agrilus planipennis (Coleoptera: Buprestidae) Control Provided by Emamectin Benzoate and Two Neonicotinoid Insecticides, One and Two Seasons after Treatment. J. Econ. Entomol. 2011, 104, 1599–1612. [Google Scholar] [CrossRef]
- USDA-APHIS. Proposed Release of Three Parasitoids for the Biological Control of the Emerald Ash Borer (Agrilus planipennis) in the Continental United States; USDA-APHIS: Riverdale, MD, USA, 2007.
- USDA-APHIS. Field Release of the Parasitoid Spathius galinae for the Biological Control of the Emerald Ash Borer (Agrilus planipennis) in the Contiguous United States; USDA-APHIS: Riverdale, MD, USA, 2015.
- Koch, J.L.; Carey, D.W.; Mason, M.E.; Poland, T.M.; Knight, K.S. Intraspecific variation in Fraxinus pennsylvanica responses to emerald ash borer (Agrilus planipennis). New For. 2015, 46, 995–1011. [Google Scholar] [CrossRef]
- Lee, J.H.; Pijut, P.M. Optimization of Agrobacterium-mediated genetic transformation of Fraxinus nigra and development of black ash for possible emerald ash borer resistance. Plant Cell Tissue Organ Cult. 2018, 134, 217–229. [Google Scholar] [CrossRef]
- Sharp, P.A. RNA interference—2001. Genes Dev. 2001, 15, 485–490. [Google Scholar] [CrossRef]
- Sen, G.L.; Blau, H.M. A brief history of RNAi: The silence of the genes. FASEB J. 2006, 20, 1293–1299. [Google Scholar] [CrossRef]
- Agrawal, N.; Dasaradhi, P.V.N.; Mohmmed, A.; Malhotra, P.; Bhatnagar, R.K.; Mukherjee, S.K. RNA interference: Biology, mechanism, and applications. Microbiol. Mol. Biol. Rev. 2003, 67, 657–685. [Google Scholar] [CrossRef]
- Meister, G.; Tuschl, T. Mechanisms of gene silencing by double-stranded RNA. Nature 2004, 431, 343–349. [Google Scholar] [CrossRef]
- Baum, J.A.; Bogaert, T.; Clinton, W.; Heck, G.R.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M.; et al. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Gonzales, M.A.A.; Poland, T.M.; Mittapalli, O. Core RNAi machinery and gene knockdown in the emerald ash borer (Agrilus planipennis). J. Insect Physiol. 2015, 72, 70–78. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Rieske, L.K.; Duan, J.J.; Mogilicherla, K.; Palli, S.R. Development of RNAi method for screening candidate genes to control emerald ash borer, Agrilus planipennis. Sci. Rep. 2017, 7, 7379. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Duan, J.J.; Palli, S.R.; Rieske, L.K. Identification of highly effective target genes for RNAi-mediated control of emerald ash borer, Agrilus planipennis. Sci. Rep. 2018, 8, 5020. [Google Scholar] [CrossRef]
- Pampolini, F.; Rodrigues, T.B.; Leelesh, R.S.; Kawashima, T.; Rieske, L.K. Confocal microscopy provides visual evidence and confirms the feasibility of dsRNA delivery to emerald ash borer through plant tissues. J. Pest Sci. 2020, 93, 1143–1153. [Google Scholar] [CrossRef]
- Du, N.X.; Pijut, P.M. Agrobacterium-mediated transformation of Fraxinus pennsylvanica hypocotyls and plant regeneration. Plant Cell Rep. 2009, 28, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Palla, K.J.; Pijut, P.M. Agrobacterium-mediated genetic transformation of Fraxinus americana hypocotyls. Plant Cell Tissue Organ Cult. 2015, 120, 631–641. [Google Scholar] [CrossRef]
- Stevens, M.E.; Pijut, P.M. Agrobacterium-mediated genetic transformation and plant regeneration of the hardwood tree species Fraxinus profunda. Plant Cell Rep. 2014, 33, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Corredoira, E.; Merkle, S.A.; Martinez, M.T.; Toribio, M.; Canhoto, J.M.; Correia, S.I.; Ballester, A.; Vieitez, A.M. Non-Zygotic Embryogenesis in Hardwood Species. Crit. Rev. Plant Sci. 2019, 38, 29–97. [Google Scholar] [CrossRef]
- Wilde, H.D.; Meagher, R.B.; Merkle, S.A. Expression of foreign genes in transgenic yellow-poplar plants. Plant Physiol. 1992, 98, 114–120. [Google Scholar] [CrossRef][Green Version]
- Rugh, C.L.; Senecoff, J.F.; Meagher, R.B.; Merkle, S.A. Development of transgenic yellow poplar for mercury phytoremediation. Nat. Biotechnol. 1998, 16, 925–928. [Google Scholar] [CrossRef]
- Dai, J.L.; Balish, R.; Meagher, R.B.; Merkle, S.A. Development of transgenic hybrid sweetgum (Liquidambar styraciflua × L-formosana) expressing gamma-glutamylcysteine synthetase or mercuric reductase for phytoremediation of mercury pollution. New For. 2009, 38, 35–52. [Google Scholar] [CrossRef]
- Andrade, G.M.; Nairn, C.J.; Le, H.T.; Merkle, S.A. Sexually mature transgenic American chestnut trees via embryogenic suspension-based transformation. Plant Cell Rep. 2009, 28, 1385–1397. [Google Scholar] [CrossRef]
- Polin, L.D.; Liang, H.Y.; Rothrock, R.E.; Nishii, M.; Diehl, D.L.; Newhouse, A.E.; Nairn, C.J.; Powell, W.A.; Maynard, C.A. Agrobacterium-mediated transformation of American chestnut (Castanea dentata (Marsh.) Borkh.) somatic embryos. Plant Cell Tissue Organ Cult. 2006, 84, 69–78. [Google Scholar] [CrossRef]
- Newhouse, A.E.; Polin-McGuigan, L.D.; Baier, K.A.; Valletta, K.E.R.; Rottmann, W.H.; Tschaplinski, T.J.; Maynard, C.A.; Powell, W.A. Transgenic American chestnuts show enhanced blight resistance and transmit the trait to T1 progeny. Plant Sci. 2014, 228, 88–97. [Google Scholar] [CrossRef]
- Corredoira, E. Agrobacterium-mediated transformation of European chestnut embryogenic cultures. Plant Cell Rep. 2004, 23, 311–318. [Google Scholar] [CrossRef]
- Corredoira, E.; Valladares, S.; Allona, I.; Aragoncillo, C.; Vieitez, A.M.; Ballester, A. Genetic transformation of European chestnut somatic embryos with a native thaumatin-like protein (CsTL1) gene isolated from Castanea sativa seeds. Tree Physiol. 2012, 32, 1389–1402. [Google Scholar] [CrossRef][Green Version]
- Alvarez, R.; Alonso, P.; Cortizo, M.; Celestino, C.; Hernandez, I.; Toribio, M.; Ordas, R. Genetic transformation of selected mature cork oak (Quercus suber L.) trees. Plant Cell Rep. 2004, 23, 218–223. [Google Scholar] [CrossRef]
- Vidal, N.; Mallon, R.; Valladares, S.; Meijomin, A.M.; Vieitez, A.M. Regeneration of transgenic plants by Agrobacterium-mediated transformation of somatic embryos of juvenile and mature Quercus robur. Plant Cell Rep. 2010, 29, 1411–1422. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.F.; Merkle, S.A. Induction of green ash embryogenic cultures with potential for scalable somatic embryo production using suspension culture. Trees-Struct. Funct. 2014, 28, 253–262. [Google Scholar] [CrossRef]
- Merkle, S.A.; Koch, J.L.; Tull, A.R.; Dassow, J.E.; Carey, D.W.; Barnes, B.F.; Richins, M.W.M.; Montello, P.M.; Eidle, K.R.; House, L.T.; et al. Application of somatic embryogenesis for development of emerald ash borer-resistant white ash and green ash varietals. New For. 2022. [Google Scholar] [CrossRef]
- Richins, M.W.M. Biotechnology for the Conservation and Restoration of Ash Trees. Master’s Thesis, University of Georgia, Athens, GA, USA, 2022. in preparation. [Google Scholar]
- Andrade, G.M.; Merkle, S.A. Enhancement of American chestnut somatic seedling production. Plant Cell Rep. 2005, 24, 326–334. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot -tip culture. Comb. Proc. Int. Plant Propagators’ Soc. 1980, 30, 421–427. [Google Scholar]
- Cangelosi, G.A.; Best, E.A.; Martinetti, G.; Nester, E.W. Genetic analysis of Agrobacterium. Methods Enzymol. 1991, 204, 384–397. [Google Scholar] [PubMed]
- Jefferson, R.A. Assaying chimeric genes in plants: The GUS gene fusion system. Plant Mol. Biol. Report. 1987, 5, 387–405. [Google Scholar] [CrossRef]
- Torreblanca, R.; Cerezo, S.; Palomo-Rios, E.; Mercado, J.A.; Pliego-Alfaro, F. Development of a high throughput system for genetic transformation of olive (Olea europaea L.) plants. Plant Cell Tissue Organ Cult. 2010, 103, 61–69. [Google Scholar] [CrossRef]
- Alvarez, R.; Ordas, R.J. Improved genetic transformation protocol for cork oak (Quercus suber L.). Plant Cell Tissue Organ Cult. 2007, 91, 45–52. [Google Scholar] [CrossRef]
- Poelchau, M.F.; Coates, B.S.; Childers, C.P.; de Leon, A.A.P.; Evans, J.D.; Hackett, K.; Shoemaker, D. Agricultural applications of insect ecological genomics. Curr. Opin. Insect Sci. 2016, 13, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Vogel, E.; Santos, D.; Mingels, L.; Verdonckt, T.W.; Vanden Broeck, J. RNA Interference in Insects: Protecting Beneficials and Controlling Pests. Front. Physiol. 2019, 9, 1912. [Google Scholar] [CrossRef]
- San Miguel, K.; Scott, J.G. The next generation of insecticides: dsRNA is stable as a foliar-applied insecticide. Pest Manag. Sci. 2016, 72, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.B.; Glick, E.; Paldi, N.; Bextine, B.R. Advances in RNA interference: dsRNA Treatment in Trees and Grapevines for Insect Pest Suppression. Southwest. Entomol. 2012, 37, 85–87. [Google Scholar] [CrossRef]
- Dalakouras, A.; Jarausch, W.; Buchholz, G.; Bassler, A.; Braun, M.; Manthey, T.; Krczal, G.; Wassenegger, M. Delivery of Hairpin RNAs and Small RNAs Into Woody and Herbaceous Plants by Trunk Injection and Petiole Absorption. Front. Plant Sci. 2018, 9, 1253. [Google Scholar] [CrossRef]
- Zhou, X.G.; Wheeler, M.M.; Oi, F.M.; Scharf, M.E. RNA interference in the termite Reticulitermes flavipes through ingestion of double-stranded RNA. Insect Biochem. Mol. Biol. 2008, 38, 805–815. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Zhu, K.Y. Chitosan/double-stranded RNA nanoparticle-mediated RNA interference to silence chitin synthase genes through larval feeding in the African malaria mosquito (Anopheles gambiae). Insect Mol. Biol. 2010, 19, 683–693. [Google Scholar] [CrossRef]
- Zhu, F.; Xu, J.J.; Palli, R.; Ferguson, J.; Palli, S.R. Ingested RNA interference for managing the populations of the Colorado potato beetle, Leptinotarsa decemlineata. Pest Manag. Sci. 2011, 67, 175–182. [Google Scholar] [CrossRef]
- Mao, Y.B.; Cai, W.J.; Wang, J.W.; Hong, G.J.; Tao, X.Y.; Wang, L.J.; Huang, Y.P.; Chen, X.Y. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Patade, V.Y.; Khatri, D.; Grover, A.; Kumari, M.; Gupta, S.M.; Ahmed, Z. Silenced phytoene desaturase gene as a scorable marker for plant genetic transformation. Biotechnology 2014, 13, 80–84. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).