Differences in Environmental and Hormonal Regulation of Growth Responses in Two Highly Productive Hybrid Populus Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Standard Culture Conditions
2.2. Experimental Design
2.3. Processing of Results
3. Results
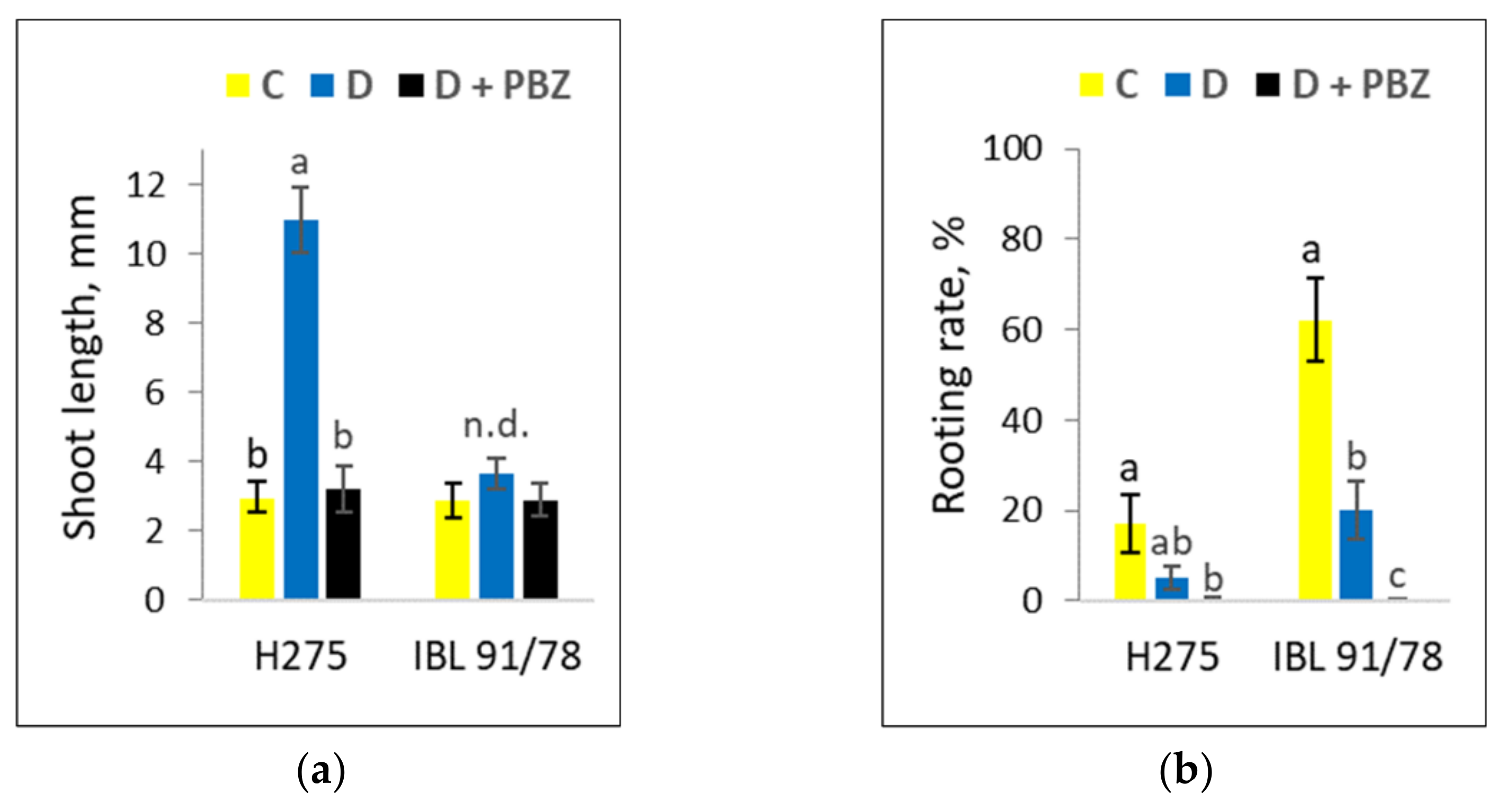
3.1. Populus Shoot Culture Responses to Darkness and the Response Alterations by PBZ
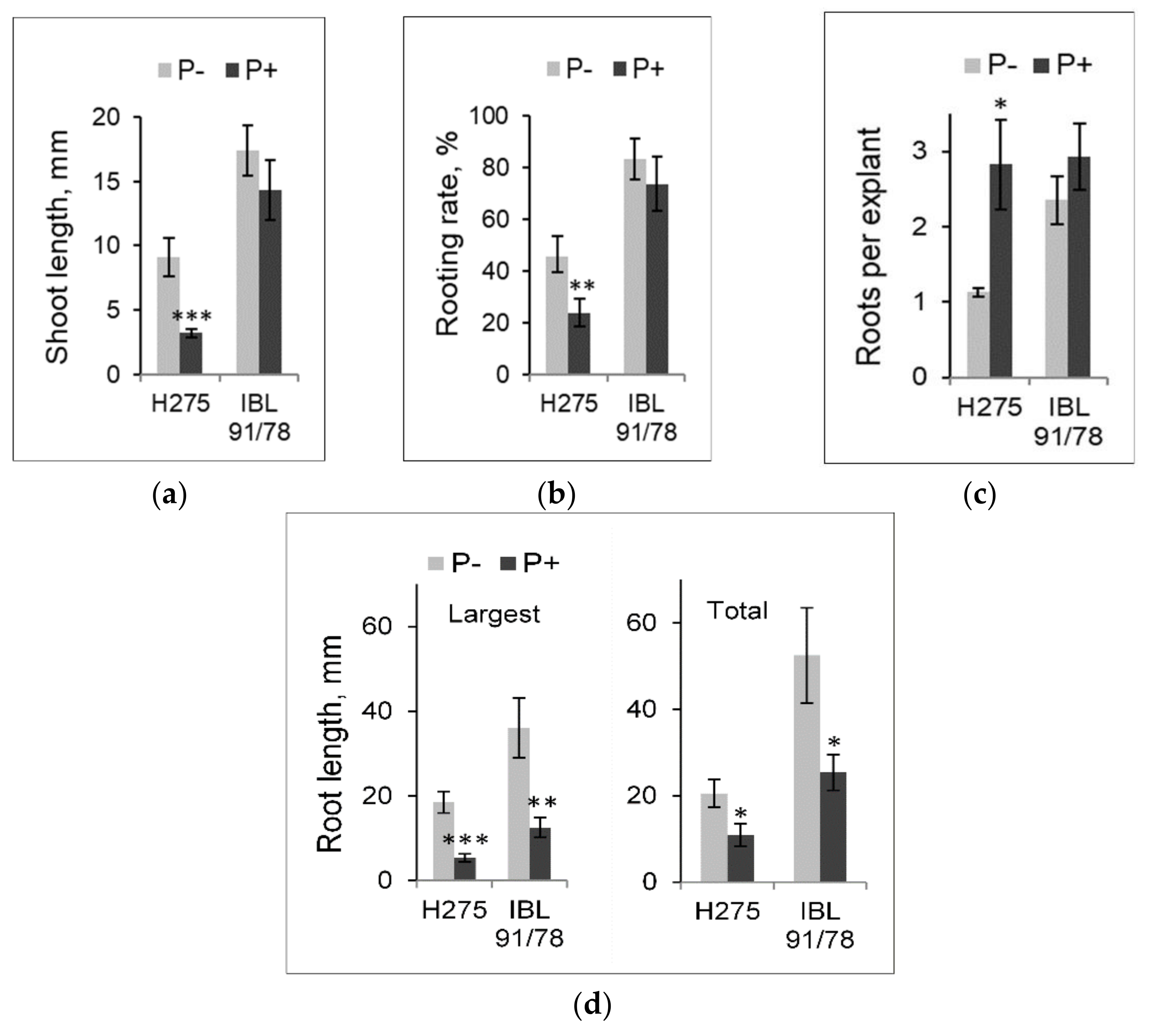
3.2. Alterations in Populus Shoot and Root Development Induced by PBZ in Light
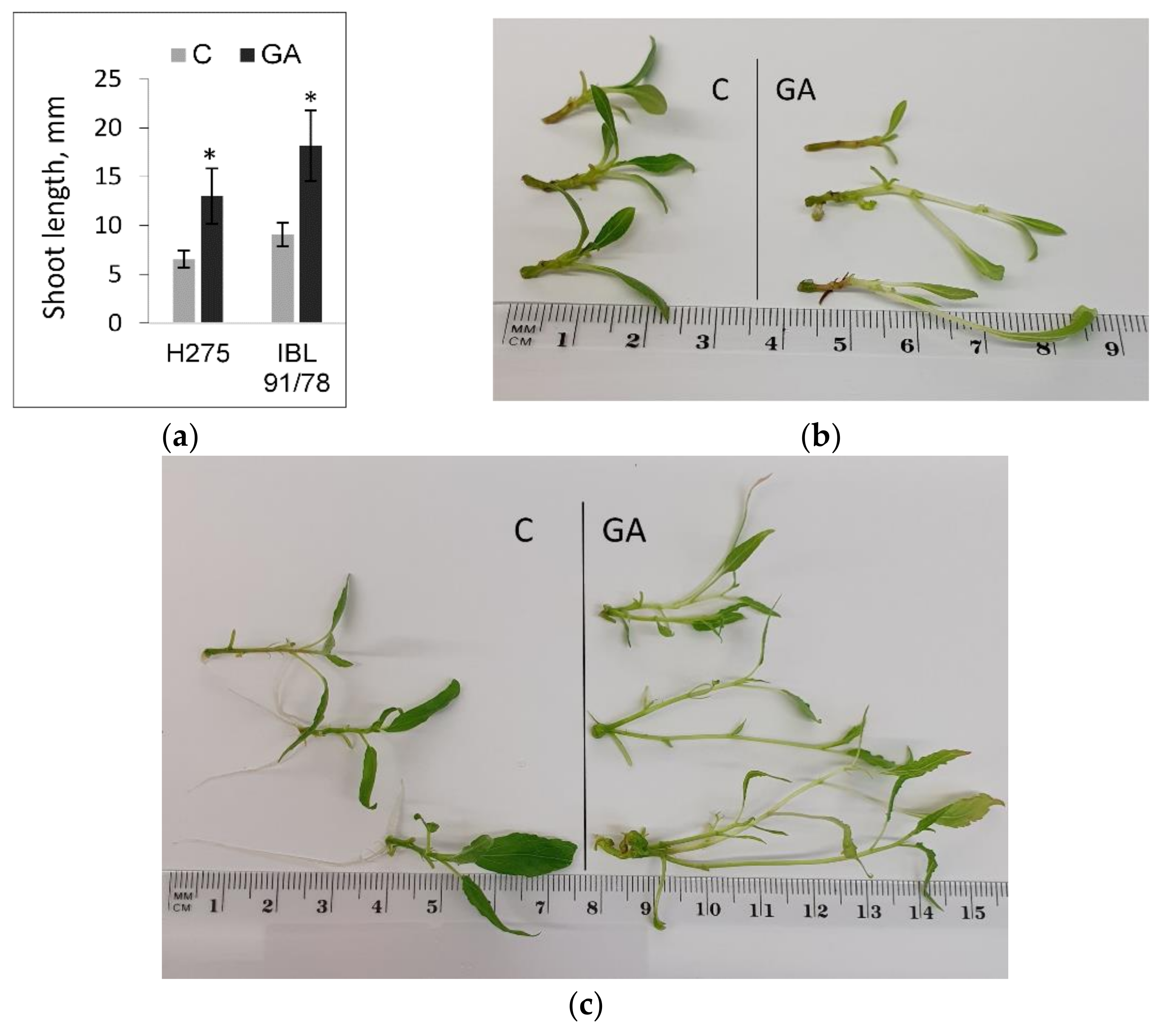
3.3. Populus Shoot Culture Responses to Exogenous GA
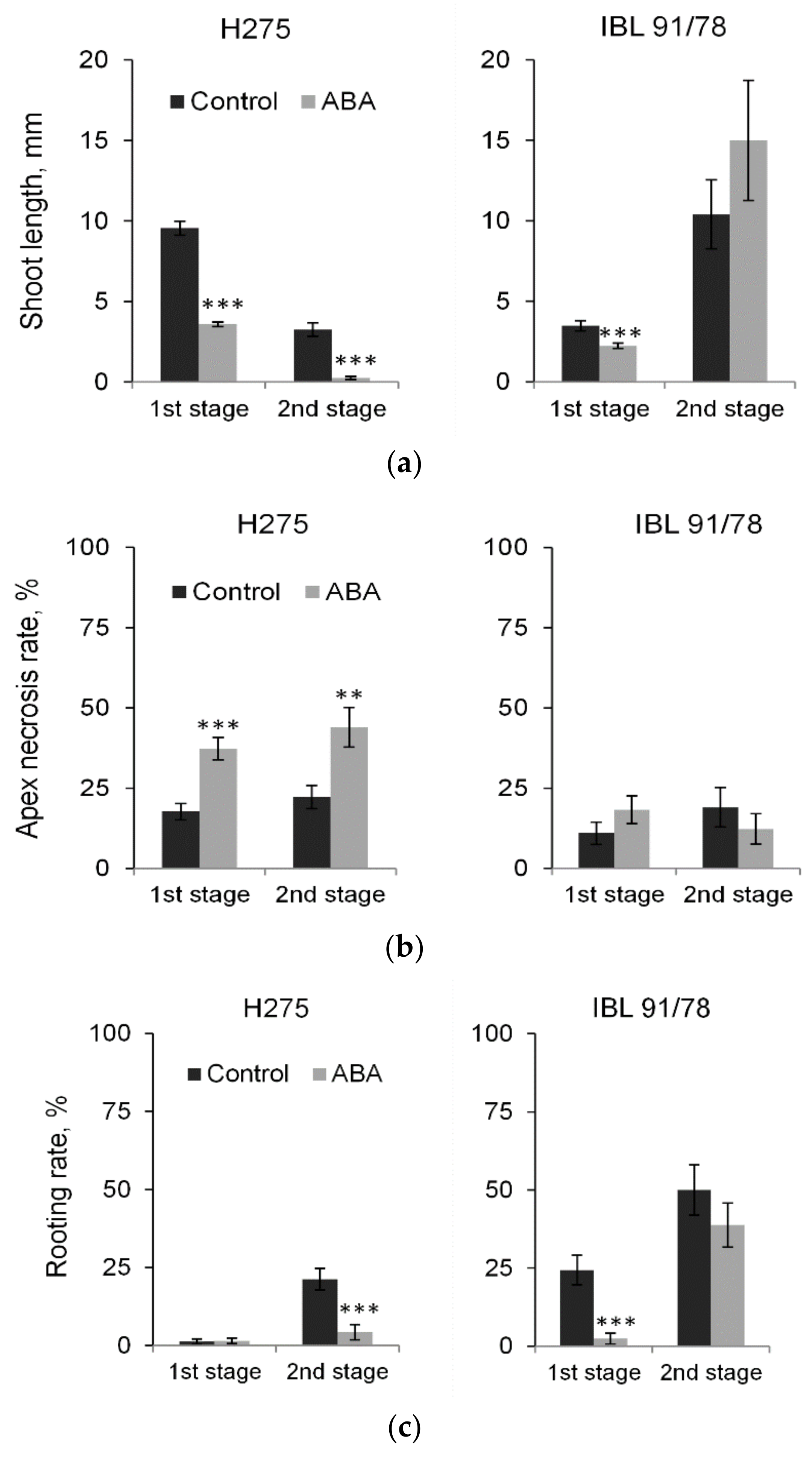
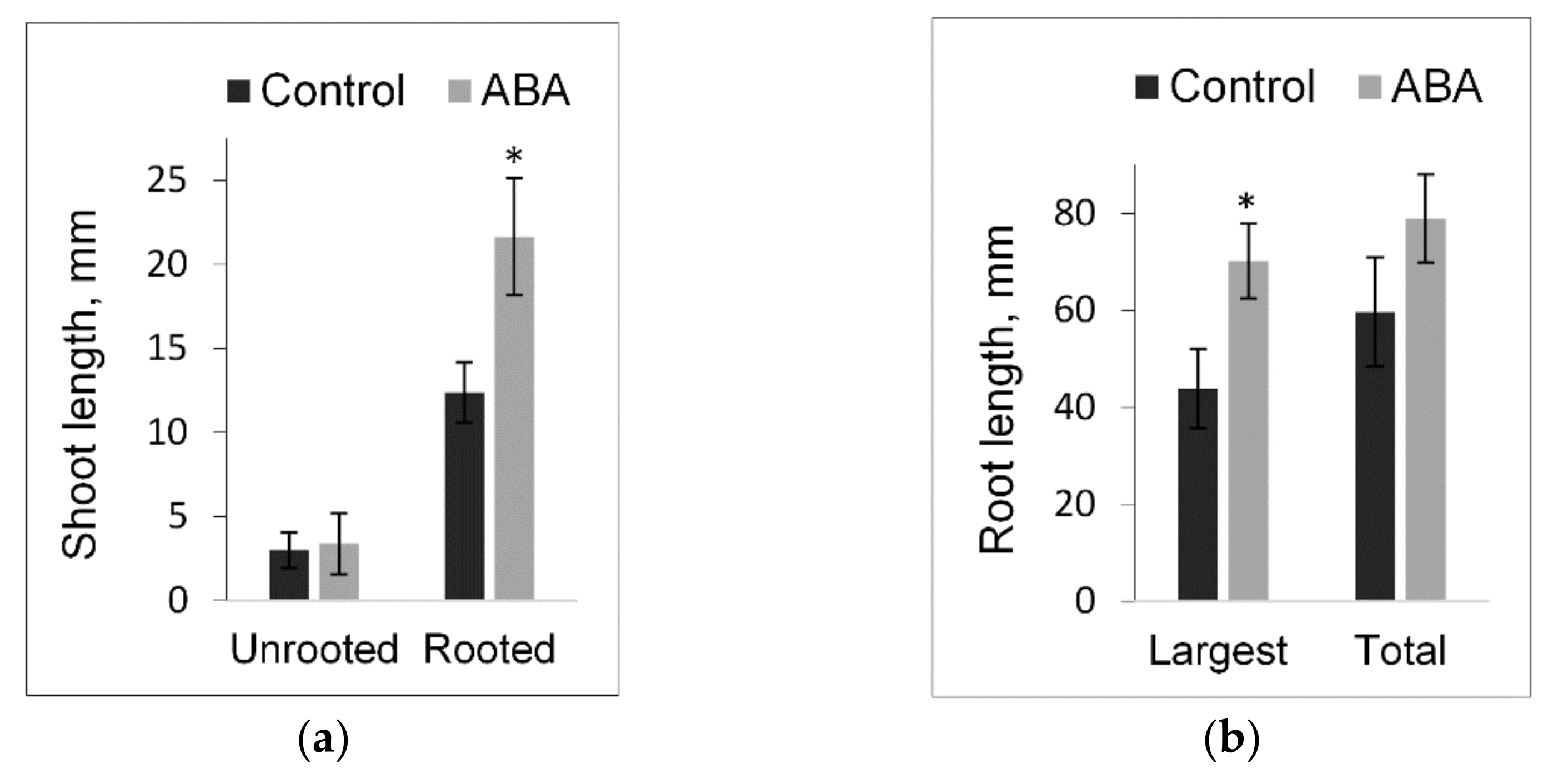
3.4. Short-Term and Long-Term Responses to Exogenous ABA in Populus Shoot Cultures
4. Discussion
4.1. Analysis of Populus Genotype Responses to the Darkness and GA-Related Growth Regulators
4.2. Analysis of Populus Genotype Responses to the ABA Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bradshaw, H.D.; Ceulemans, R.; Davis, J.; Stettler, R. Emerging model systems in plant biology: Poplar (Populus) as a model forest tree. J. Plant Growth Regul. 2000, 19, 306–313. [Google Scholar] [CrossRef]
- Bradshaw, H.D., Jr.; Strauss, S.H. Breeding strategies for the 21st century: Domestication of poplar. Poplar Cult. N. Am. 2001, 14, 383–394. [Google Scholar]
- Isebrands, J.G.; Richardson, J. Poplars and Willows: Trees for Society and the Environment; CABI: Wallingford, UK, 2014; ISBN 9781780641089. [Google Scholar]
- Balatinecz, J.J.; Kretschmann, D.E.; Leclercq, A. Achievements in the utilization of poplar wood—Guideposts for the future. For. Chron. 2001, 77, 265–269. [Google Scholar] [CrossRef]
- Zalesny, R.S.; Stanturf, J.A.; Gardiner, E.S.; Perdue, J.H.; Young, T.M.; Coyle, D.R.; Headlee, W.L.; Bañuelos, G.S.; Hass, A. Ecosystem Services of Woody Crop Production Systems. Bioenergy Res. 2016, 9, 465–491. [Google Scholar] [CrossRef]
- Ghezehei, S.B.; Wright, J.; Zalesny, R.S.; Nichols, E.G.; Hazel, D.W. Matching site-suitable poplars to rotation length for optimized productivity. For. Ecol. Manag. 2020, 457, 117670. [Google Scholar] [CrossRef]
- Jhariya, M.K.; Banerjee, A.; Swaroop, R.; Dhiraj, M.; Yadav, K. Agriculture, Forest and Environmental Management; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 9789811368295. [Google Scholar]
- Stanton, B.J.; Haiby, K.; Gantz, C.; Espinoza, J.; Shuren, R.A. The economics of rapid multiplication of hybrid poplar biomass varieties. Forests 2019, 10, 446. [Google Scholar] [CrossRef]
- Kijowska-Oberc, J.; Staszak, A.M.; Kamiński, J.; Ratajczak, E. Adaptation of forest trees to rapidly changing climate. Forests 2020, 11, 123. [Google Scholar] [CrossRef]
- Zawaski, C.; Busov, V.B. Roles of gibberellin catabolism and signaling in growth and physiological response to drought and short-day photoperiods in Populus trees. PLoS ONE 2014, 9, e86217. [Google Scholar] [CrossRef]
- Vaughton, G.; Ramsey, M. Variation in summer dormancy in the lilioid geophyte Burchardia umbellata (Colchicaceae). Am. J. Bot. 2001, 88, 1223–1229. [Google Scholar] [CrossRef]
- Bradshaw, A.D.; Hardwick, K. Evolution and stress—Genotypic and phenotypic components. Biol. J. Linn. Soc. 1989, 37, 137–155. [Google Scholar] [CrossRef]
- Alabadí, D.; Gil, J.; Blázquez, M.A.; García-Martínez, J.L. Gibberellins repress photomorphogenesis in darkness. Plant Physiol. 2004, 134, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Lorrai, R.; Boccaccini, A.; Ruta, V.; Possenti, M.; Costantino, P.; Paola, V. ABA inhibits hypocotyl elongation acting on gibberellins, DELLA proteins and auxin. AoB Plants 2018, 10, ply061. [Google Scholar] [CrossRef] [PubMed]
- Ait-Ali, T.; Frances, S.; Weller, J.L.; Reid, J.B.; Kendrick, R.E.; Kamiya, Y. Regulation of Gibberellin 20-Oxidase and Gibberellin 3β-Hydroxylase Transcript Accumulation during De-Etiolation of Pea Seedlings. Plant Physiol. 1999, 121, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.L.; Hecht, V.; Vander Schoor, J.K.; Davidson, S.E.; Ross, J.J. Light Regulation of Gibberellin Biosynthesis in Pea Is Mediated through the COP1/HY5 Pathway. Plant Cell 2009, 21, 800–813. [Google Scholar] [CrossRef]
- Humplík, J.F.; Bergougnoux, V.; Jandová, M.; Šimura, J.; Pěnčík, A.; Tomanec, O.; Rolčík, J.; Novák, O.; Fellner, M. Endogenous Abscisic Acid Promotes Hypocotyl Growth and Affects Endoreduplication during Dark-Induced Growth in Tomato (Solanum lycopersicum L.). PLoS ONE 2015, 10, e0117793. [Google Scholar] [CrossRef]
- Ranade, S.S.; Delhomme, N.; García-Gil, M.R. Global gene expression analysis in etiolated and de-etiolated seedlings in conifers. PLoS ONE 2019, 14, e0219272. [Google Scholar] [CrossRef]
- Toh, S.; Imamura, A.; Watanabe, A.; Nakabayashi, K.; Okamoto, M.; Jikumaru, Y.; Hanada, A.; Aso, Y.; Ishiyama, K.; Tamura, N.; et al. High Temperature-Induced Abscisic Acid Biosynthesis and Its Role in the Inhibition of Gibberellin Action in Arabidopsis Seeds. Plant Physiol. 2008, 146, 1368–1385. [Google Scholar] [CrossRef]
- Thomas, S.G.; Sun, T. Update on Gibberellin Signaling. A Tale of the Tall and the Short. Plant Physiol. 2004, 135, 668–676. [Google Scholar] [CrossRef]
- Vera-Sirera, F.; Gomez, M.D.; Perez-Amador, M.A. DELLA Proteins, a Group of GRAS Transcription Regulators That Mediate Gibberellin Signaling. In Plant Transcription Factors; Elsevier: Amsterdam, The Netherlands, 2016; pp. 313–328. [Google Scholar]
- Zhang, Y.; Liu, Z.; Wang, L.; Zheng, S.; Xie, J.; Bi, Y. Sucrose-induced hypocotyl elongation of Arabidopsis seedlings in darkness depends on the presence of gibberellins. J. Plant Physiol. 2010, 167, 1130–1136. [Google Scholar] [CrossRef]
- Nanda, K.K.; Jain, M.K. Utilization of sugars and starch as carbon sources in the rooting of etiolated stem segments of Populus nigra. New Phytol. 1972, 71, 825–828. [Google Scholar] [CrossRef]
- Lu, N.; Dai, L.; Luo, Z.; Wang, S.; Wen, Y.; Duan, H.; Hou, R.; Sun, Y.; Li, Y. Characterization of the Transcriptome and Gene Expression of Tetraploid Black Locust Cuttings in Response to Etiolation. Genes 2017, 8, 345. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G. Populus: Arabidopsis for Forestry. Do We Need a Model Tree? Ann. Bot. 2002, 90, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Stiles, K.A. Light-regulated leaf expansion in two Populus species: Dependence on developmentally controlled ion transport. J. Exp. Bot. 2002, 53, 1651–1657. [Google Scholar] [CrossRef]
- DiFazio, S.P.; Slavov, G.T.; Joshi, C.P. Populus: A Premier Pioneer System for Plant Genomics. In Genetics, Genomics and Breeding of Poplar; Joshi, C., DiFazio, S.P., Kole, C., Eds.; Science Publishers: Enfield, NH, USA, 2011; pp. 1–28. [Google Scholar]
- Peternel, Š.; Gabrovšek, K.; Gogala, N.; Regvar, M. In vitro propagation of European aspen (Populus tremula L.) from axillary buds via organogenesis. Sci. Hortic. 2009, 121, 109–112. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, Z.; Zheng, Q. Direct Regeneration of Plants Derived from in vitro Cultured Shoot Tips and Leaves of Poplar (Populus × euramericana ‘Neva’). J. Life Sci. 2015, 9, 366–372. [Google Scholar] [CrossRef][Green Version]
- García-Angulo, P.; Villar, I.; Giner-Robles, L.; Centeno, M.L. In vitro regeneration of two Populus hybrid clones. The role of pectin domains in cell processes underlying shoot organogenesis induction. Biol. Plant. 2018, 62, 763–774. [Google Scholar] [CrossRef]
- Rademacher, W. Chemical Regulators of Gibberellin Status and Their Application in Plant Production. In Annual Plant Reviews Online; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 359–403. [Google Scholar]
- Vaičiukynė, M.; Žiauka, J.; Žūkienė, R.; Vertelkaitė, L.; Kuusienė, S. Abscisic acid promotes root system development in birch tissue culture: A comparison to aspen culture and conventional rooting-related growth regulators. Physiol. Plant. 2019, 165, 114–122. [Google Scholar] [CrossRef]
- Euring, D.; Ayegbeni, S.; Jansen, M.; Tu, J.; Gomes Da Silva, C.; Polle, A. Growth performance and nitrogen use efficiency of two Populus hybrid clones (P. nigra × P. maximowiczii and P. trichocarpa × P. maximowiczii) in relation to soil depth in a young plantation. iFor.-Biogeosci. For. 2016, 9, 847–854. [Google Scholar] [CrossRef]
- Niemczyk, M.; Kaliszewski, A.; Jewiarz, M.; Wróbel, M.; Mudryk, K. Productivity and biomass characteristics of selected poplar (Populus spp.) cultivars under the climatic conditions of northern Poland. Biomass Bioenergy 2018, 111, 46–51. [Google Scholar] [CrossRef]
- Niemczyk, M.; Przybysz, P.; Przybysz, K.; Karwański, M.; Kaliszewski, A.; Wojda, T.; Liesebach, M. Productivity, growth patterns, and cellulosic pulp properties of hybrid aspen clones. Forests 2019, 10, 450. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Žiauka, J.; Kuusienė, S.; Šilininkas, M. Fast growing aspens in the development of a plant micropropagation system based on plant-produced ethylene action. Biomass Bioenergy 2013, 53, 20–28. [Google Scholar] [CrossRef]
- Cowling, R.J.; Harberd, N.P. Gibberellins control Arabidopsis hypocotyl growth via regulation of cellular elongation. J. Exp. Bot. 1999, 50, 1351–1357. [Google Scholar] [CrossRef]
- Weston, D.E.; Elliott, R.C.; Lester, D.R.; Rameau, C.; Reid, J.B.; Murfet, I.C.; Ross, J.J. The Pea DELLA Proteins LA and CRY Are Important Regulators of Gibberellin Synthesis and Root Growth. Plant Physiol. 2008, 147, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Harberd, N.P.; Belfield, E.; Yasumura, Y. The Angiosperm Gibberellin-GID1-DELLA Growth Regulatory Mechanism: How an “Inhibitor of an Inhibitor” Enables Flexible Response to Fluctuating Environments. Plant Cell 2009, 21, 1328–1339. [Google Scholar] [CrossRef]
- Liu, S.; Xuan, L.; Xu, L.-A.; Huang, M.; Xu, M. Molecular cloning, expression analysis and subcellular localization of four DELLA genes from hybrid poplar. SpringerPlus 2016, 5, 1129. [Google Scholar] [CrossRef]
- King, K.E.; Moritz, T.; Harberd, N.P. Gibberellins are not required for normal stem growth in Arabidopsis thaliana in the absence of GAI and RGA. Genetics 2001, 159, 767–776. [Google Scholar] [CrossRef]
- Zhao, X.; Zheng, H.; Li, S.; Yang, C.; Jiang, J.; Liu, G. The rooting of poplar cuttings: A review. New For. 2014, 45, 21–34. [Google Scholar] [CrossRef]
- San José, M.; Romero, L.; Janeiro, L. Effect of indole-3-butyric acid on root formation in Alnus glutinosa microcuttings. Silva Fenn. 2012, 46, 916. [Google Scholar] [CrossRef][Green Version]
- Wei, K.; Ruan, L.; Wang, L.; Cheng, H. Auxin-Induced Adventitious Root Formation in Nodal Cuttings of Camellia sinensis. Int. J. Mol. Sci. 2019, 20, 4817. [Google Scholar] [CrossRef]
- Gray, W.M.; Ostin, A.; Sandberg, G.; Romano, C.P.; Estelle, M. High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc. Natl. Acad. Sci. USA 1998, 95, 7197–7202. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Wang, Z.; Ji, C.Y.; Jeong, J.C.; Lee, H.-S.; Li, H.; Xu, B.; Deng, X.; Kwak, S.-S. Transgenic poplar expressing Arabidopsis YUCCA6 exhibits auxin-overproduction phenotypes and increased tolerance to abiotic stress. Plant Physiol. Biochem. 2015, 94, 19–27. [Google Scholar] [CrossRef]
- Jensen, P.J.; Hangarter, R.P.; Estelle, M. Auxin Transport Is Required for Hypocotyl Elongation in Light-Grown but Not Dark-Grown Arabidopsis. Plant Physiol. 1998, 116, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Halliday, K.J.; Martinez-Garcia, J.F.; Josse, E.-M. Integration of Light and Auxin Signaling. Cold Spring Harb. Perspect. Biol. 2009, 1, a001586. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.J.; O’Neill, D.P.; Wolbang, C.M.; Symons, G.M.; Reid, J.B. Auxin-Gibberellin Interactions and Their Role in Plant Growth. J. Plant Growth Regul. 2001, 20, 346–353. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, D.P.; Davidson, S.E.; Clarke, V.C.; Yamauchi, Y.; Yamaguchi, S.; Kamiya, Y.; Reid, J.B.; Ross, J.J. Regulation of the gibberellin pathway by auxin and DELLA proteins. Planta 2010, 232, 1141–1149. [Google Scholar] [CrossRef]
- Reid, J.B.; Davidson, S.E.; Ross, J.J. Auxin acts independently of DELLA proteins in regulating gibberellin levels. Plant Signal. Behav. 2011, 6, 406–408. [Google Scholar] [CrossRef]
- Fu, X.; Harberd, N.P. Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 2003, 421, 740–743. [Google Scholar] [CrossRef]
- Chapman, E.J.; Greenham, K.; Castillejo, C.; Sartor, R.; Bialy, A.; Sun, T.; Estelle, M. Hypocotyl Transcriptome Reveals Auxin Regulation of Growth-Promoting Genes through GA-Dependent and -Independent Pathways. PLoS ONE 2012, 7, e36210. [Google Scholar] [CrossRef]
- Liu, X.; Hou, X. Antagonistic Regulation of ABA and GA in Metabolism and Signaling Pathways. Front. Plant Sci. 2018, 9, 251. [Google Scholar] [CrossRef]
- Shu, K.; Zhou, W.; Chen, F.; Luo, X.; Yang, W. Abscisic Acid and Gibberellins Antagonistically Mediate Plant Development and Abiotic Stress Responses. Front. Plant Sci. 2018, 9, 416. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Wang, X.; Feng, J.; Hong, S.; Liang, Y.; Ren, B.; Zuo, J. Cytokinin Antagonizes Abscisic Acid-Mediated Inhibition of Cotyledon Greening by Promoting the Degradation of ABSCISIC ACID INSENSITIVE5 Protein in Arabidopsis. Plant Physiol. 2014, 164, 1515–1526. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Finlayson, S.A. Abscisic Acid Is a General Negative Regulator of Arabidopsis Axillary Bud Growth. Plant Physiol. 2015, 169, 611–626. [Google Scholar] [CrossRef] [PubMed]
- Arend, M.; Schnitzler, J.-P.; Ehlting, B.; Hänsch, R.; Lange, T.; Rennenberg, H.; Himmelbach, A.; Grill, E.; Fromm, J. Expression of the Arabidopsis Mutant abi1 Gene Alters Abscisic Acid Sensitivity, Stomatal Development, and Growth Morphology in Gray Poplars. Plant Physiol. 2009, 151, 2110–2119. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Zhu, J.-K. Regulation of Abscisic Acid Biosynthesis. Plant Physiol. 2003, 133, 29–36. [Google Scholar] [CrossRef]
- Staswick, P.E.; Serban, B.; Rowe, M.; Tiryaki, I.; Maldonado, M.T.; Maldonado, M.C.; Suza, W. Characterization of an Arabidopsis Enzyme Family That Conjugates Amino Acids to Indole-3-Acetic Acid. Plant Cell 2005, 17, 616–627. [Google Scholar] [CrossRef]
- Zhang, L.; Lan, Q.; Han, S.; Qi, L. A GH3-like gene, LaGH3, isolated from hybrid larch (Larix leptolepis × Larix olgensis) is regulated by auxin and abscisic acid during somatic embryogenesis. Trees 2019, 33, 1723–1732. [Google Scholar] [CrossRef]
- Rasmussen, S.; Andersen, A.S. Water stress and root formation in pea cuttings. II. Effect of abscisic acid treatment of cuttings from stock plants grown under two levels of irradiance. Physiol. Plant. 1980, 48, 150–154. [Google Scholar] [CrossRef]
- Blake, T.J.; Atkinson, S.M. The physiological role of abscisic acid in the rooting of poplar and aspen stump sprouts. Physiol. Plant. 1986, 67, 638–643. [Google Scholar] [CrossRef]
- Tartoura, K.A.H. Effect of abscisic acid on endogenous IAA, auxin protector levels and peroxidase activity during adventitious root initiation in Vigna radiata cuttings. Acta Physiol. Plant. 2001, 23, 149–156. [Google Scholar] [CrossRef]
- Chen, C.-W.; Yang, Y.-W.; Lur, H.-S.; Tsai, Y.-G.; Chang, M.-C. A Novel Function of Abscisic Acid in the Regulation of Rice (Oryza sativa L.) Root Growth and Development. Plant Cell Physiol. 2006, 47, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hronková, M.; Zahradníčková, H.; Šimková, M.; Šimek, P.; Heydová, A. The Role of Abscisic Acid in Acclimation of Plants Cultivated in vitro to ex vitro Conditions. Biol. Plant. 2003, 46, 535–541. [Google Scholar] [CrossRef]
- Pospíšilová, J.; Haisel, D.; Synková, H.; Baťková-Spoustová, P. Improvement of ex vitro transfer of tobacco plantlets by addition of abscisic acid to the last subculture. Biol. Plant. 2009, 53, 617–624. [Google Scholar] [CrossRef]
- Shi, W.-G.; Li, H.; Liu, T.-X.; Polle, A.; Peng, C.-H.; Luo, Z.-B. Exogenous abscisic acid alleviates zinc uptake and accumulation in Populus × canescens exposed to excess zinc. Plant. Cell Environ. 2015, 38, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.-G.; Liu, W.; Yu, W.; Zhang, Y.; Ding, S.; Li, H.; Mrak, T.; Kraigher, H.; Luo, Z.-B. Abscisic acid enhances lead translocation from the roots to the leaves and alleviates its toxicity in Populus × canescens. J. Hazard. Mater. 2019, 362, 275–285. [Google Scholar] [CrossRef]
- Li, C.; Yin, C.; Liu, S. Different responses of two contrasting Populus davidiana populations to exogenous abscisic acid application. Environ. Exp. Bot. 2004, 51, 237–246. [Google Scholar] [CrossRef]
- Arshad, M.; Biswas, K.; Bisgrove, S.; Schroeder, W.R.; Thomas, B.R.; Mansfield, S.D.; Mattsson, J.; Plant, A. Differences in drought resistance in nine North American hybrid poplars. Trees 2019, 33, 1111–1128. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žiauka, J.; Striganavičiūtė, G.; Szyp-Borowska, I.; Kuusienė, S.; Niemczyk, M. Differences in Environmental and Hormonal Regulation of Growth Responses in Two Highly Productive Hybrid Populus Genotypes. Forests 2022, 13, 183. https://doi.org/10.3390/f13020183
Žiauka J, Striganavičiūtė G, Szyp-Borowska I, Kuusienė S, Niemczyk M. Differences in Environmental and Hormonal Regulation of Growth Responses in Two Highly Productive Hybrid Populus Genotypes. Forests. 2022; 13(2):183. https://doi.org/10.3390/f13020183
Chicago/Turabian StyleŽiauka, Jonas, Greta Striganavičiūtė, Iwona Szyp-Borowska, Sigutė Kuusienė, and Marzena Niemczyk. 2022. "Differences in Environmental and Hormonal Regulation of Growth Responses in Two Highly Productive Hybrid Populus Genotypes" Forests 13, no. 2: 183. https://doi.org/10.3390/f13020183
APA StyleŽiauka, J., Striganavičiūtė, G., Szyp-Borowska, I., Kuusienė, S., & Niemczyk, M. (2022). Differences in Environmental and Hormonal Regulation of Growth Responses in Two Highly Productive Hybrid Populus Genotypes. Forests, 13(2), 183. https://doi.org/10.3390/f13020183






