The Responses of Four Typical Annual Desert Species to Drought and Mixed Growth
Abstract
1. Introduction
2. Research Methods
2.1. Study Site and Experimental Materials
2.2. Experimental Design
2.3. Indicator Measurement and Methods
2.4. Data Analysis
3. Results
3.1. Emergence Characteristics of Annual Herbs
3.1.1. Germination Rate
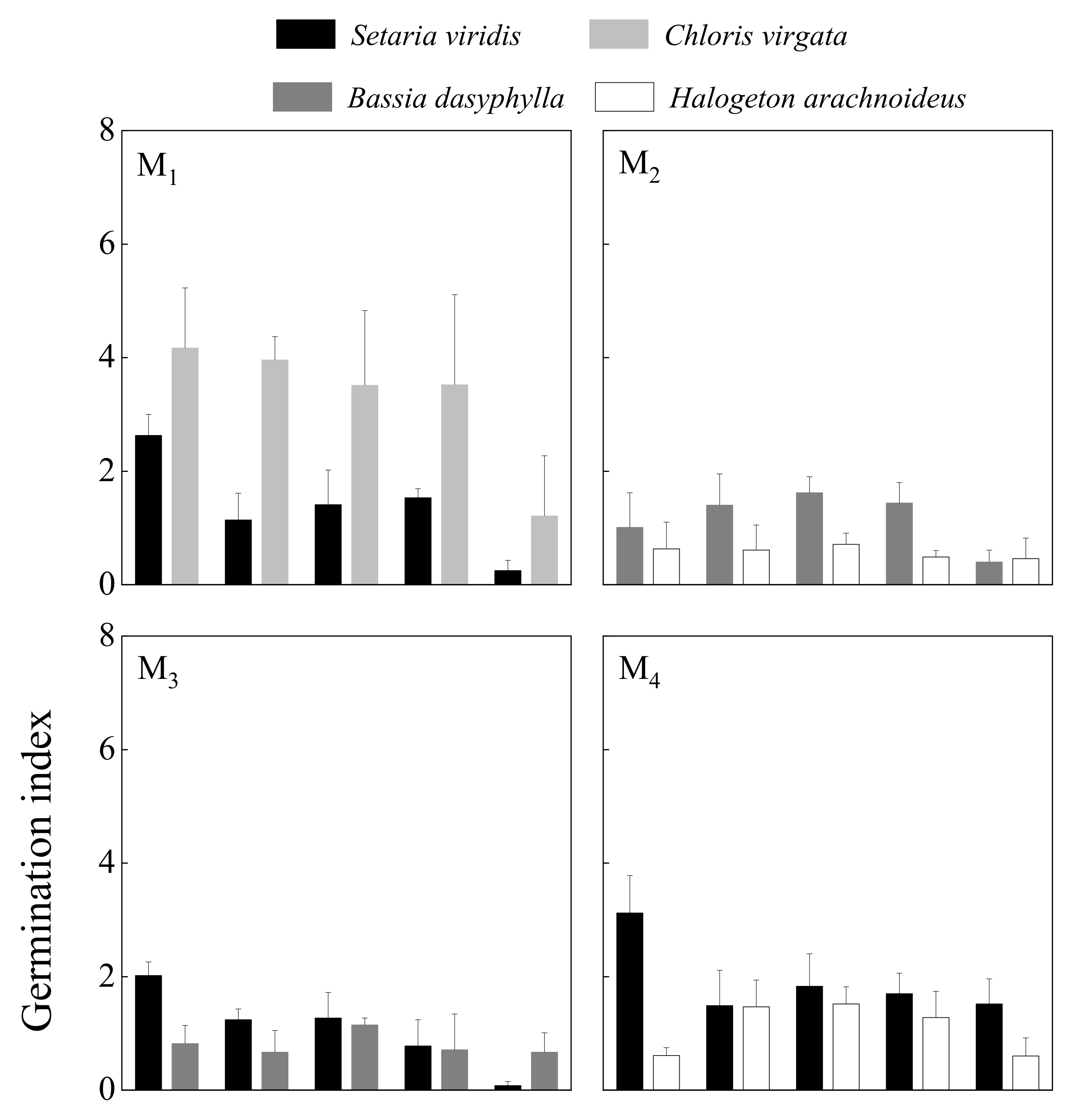
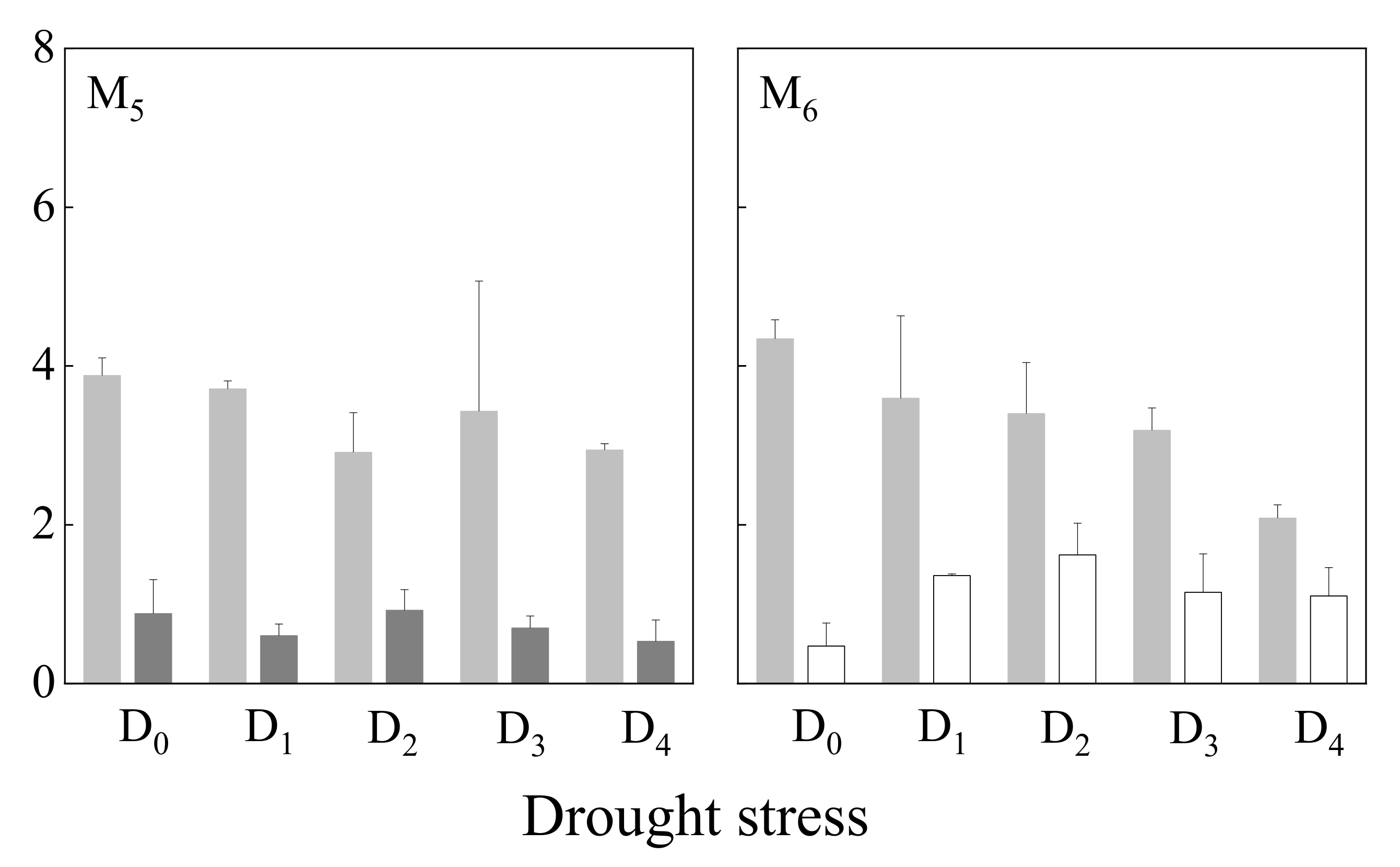
3.1.2. Germination Index
3.2. Physiological Response of Annual Herbaceous Plants
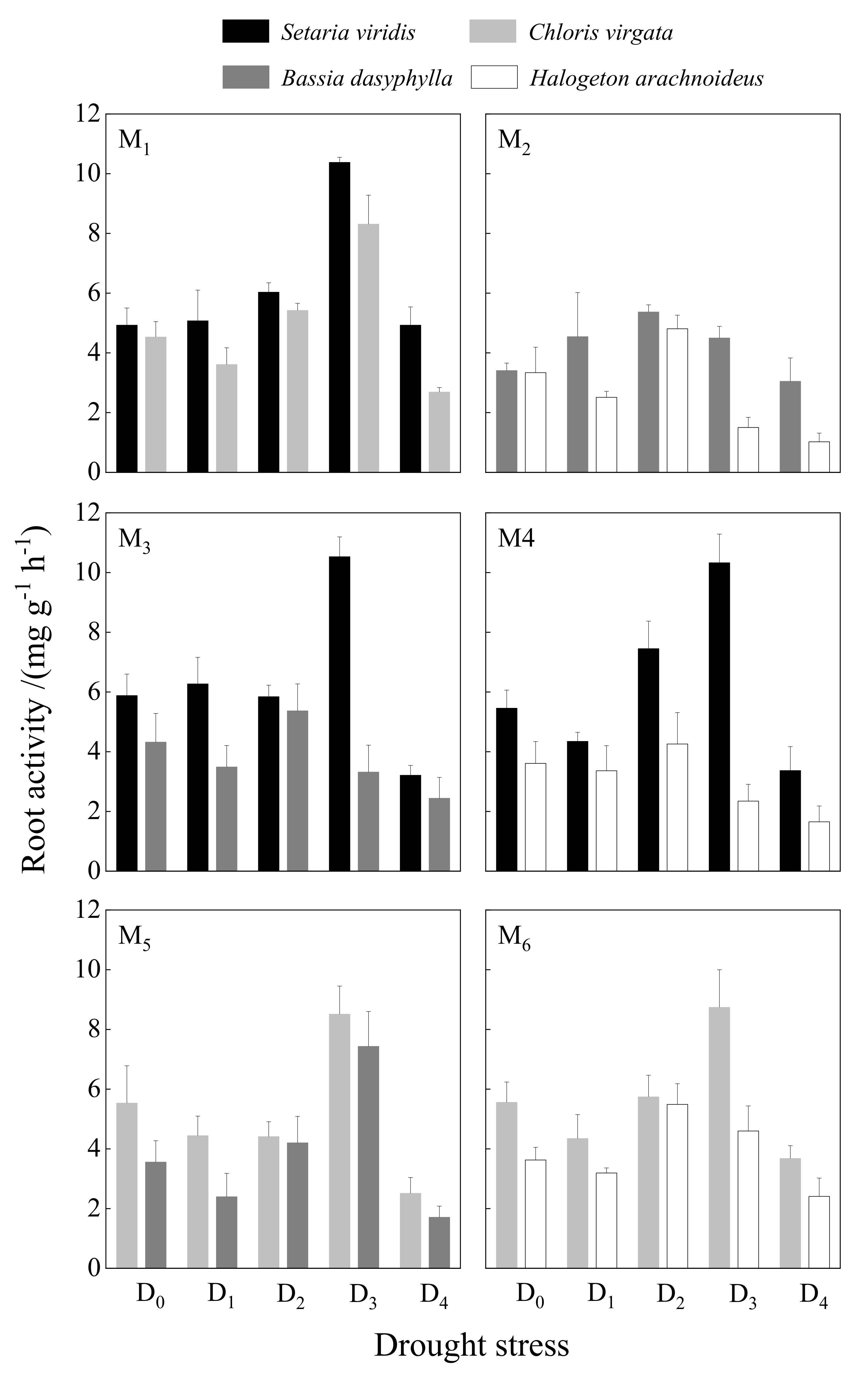
3.2.1. Root System Vigor
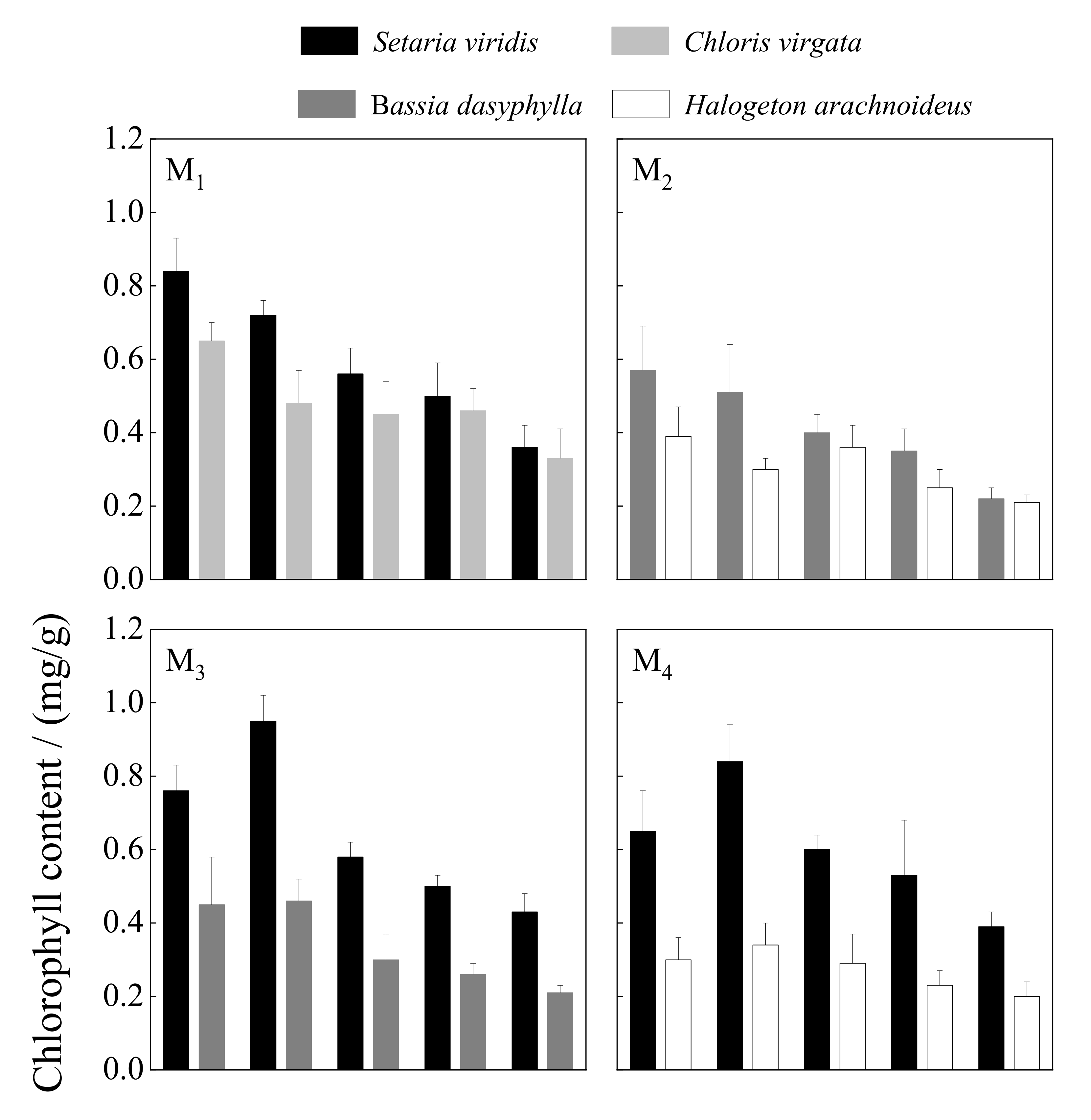
3.2.2. Chlorophyll
3.3. Growth Response of Annual Herbaceous Plants
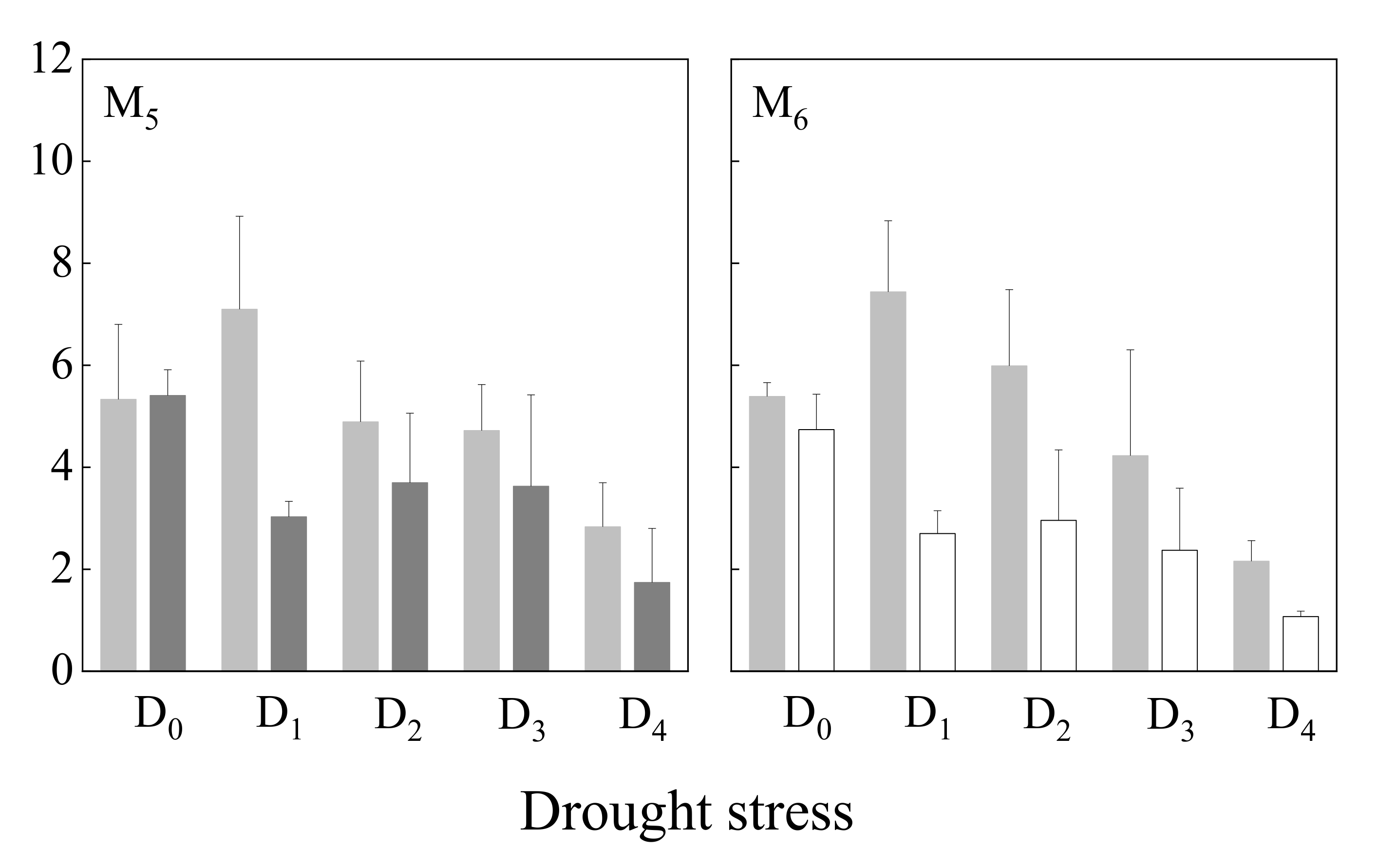
3.3.1. Root Length
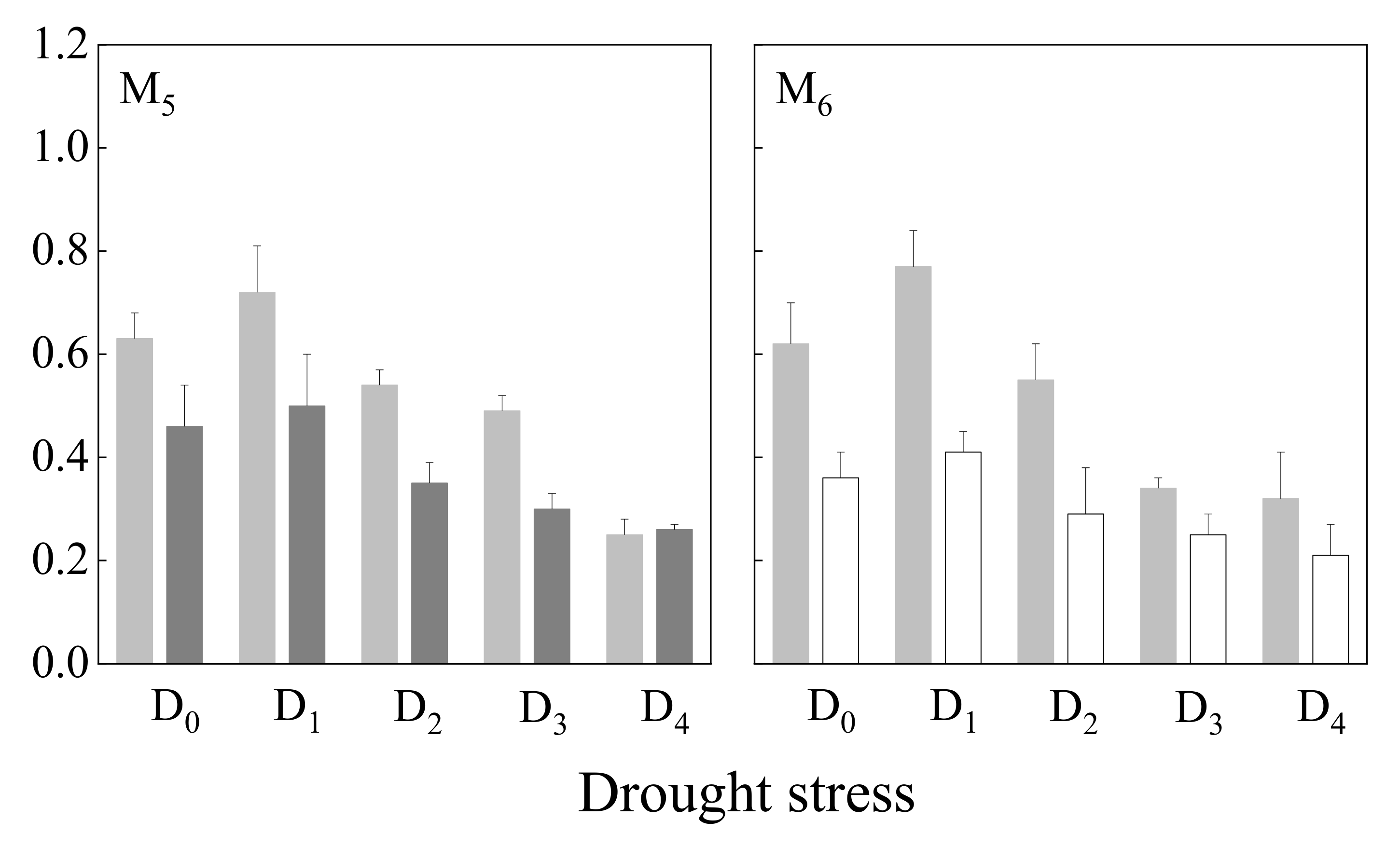
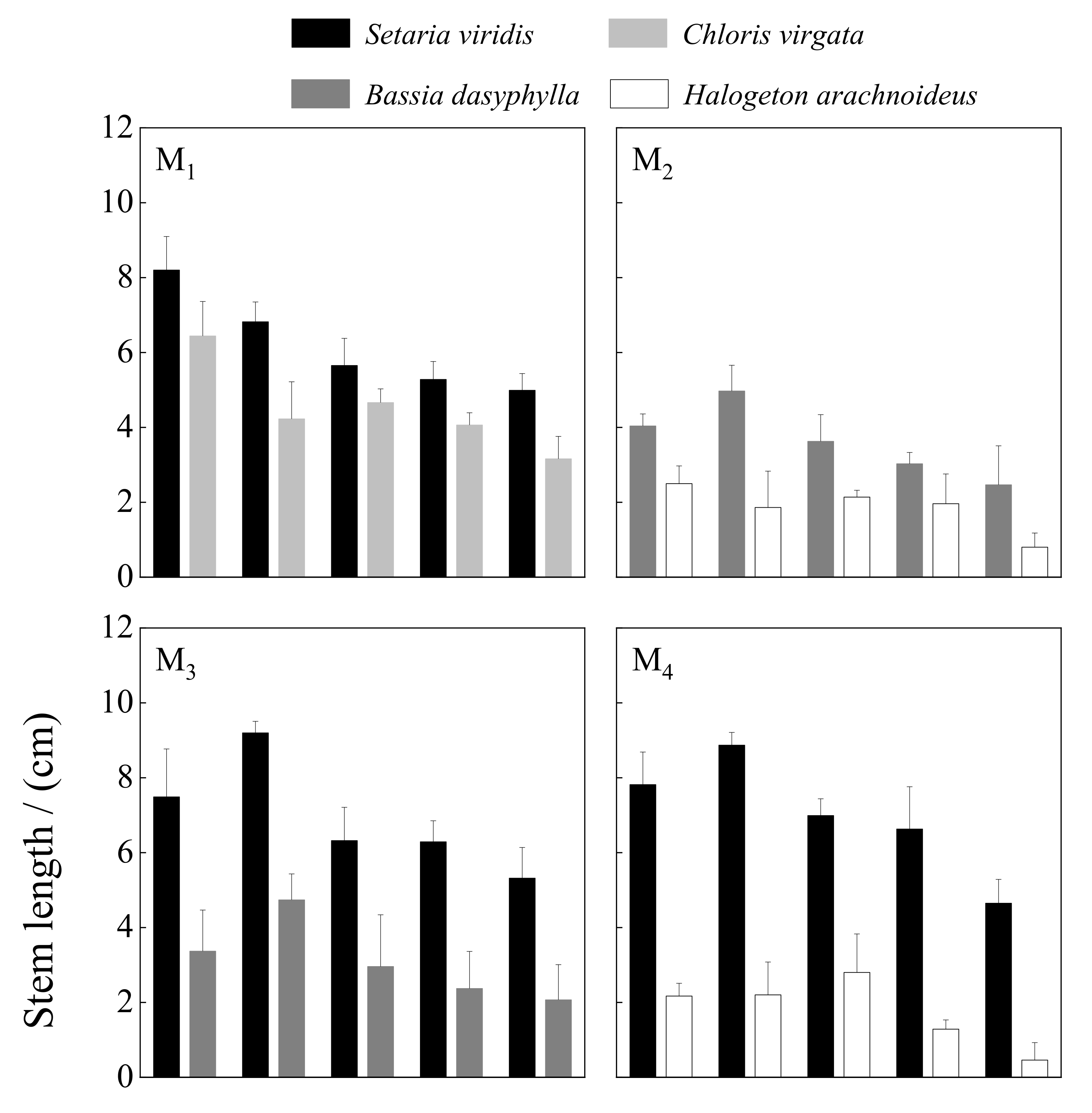
3.3.2. Stem Length
4. Discussion
4.1. Germination Characteristics of Annual Herbaceous Plants
4.2. Physiological Response of Annual Herbaceous Plants
4.3. Growth Response of Annual Herbaceous Plants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, B.; Zhao, W.Z.; Chang, X.X.; Li, S.B. Response of Soil Moisture to Rainfall Pulse in Desert Region of the Heihe River Basin. J. Desert Res. 2011, 31, 716–722. [Google Scholar]
- Liang, C.Z.; Wang, W.; Zhu, Z.Y.; Liu, Z.L. The Organization Patterns and Ecological Adaptive Modes of Annual Plant Synusia in Desert Regions. J. Arid Land Resour. Environ. 2002, 16, 77–83. [Google Scholar]
- Jia, M.-Y.; Li, X.-H.; Wu, Z.-X.; Piao, H.-Z.; Miao, C.-P.; Han, X. Spatial pattern and coexistence of plants with different life forms in inter-dune low land of Horqin Sandy Land. Chin. J. Ecol. 2016, 35, 111–117. [Google Scholar]
- Chen, L.; Xin, J.N.; Su, Y.; Li, Y.F.; Song, N.P.; Wang, L.; Bian, Y.Y. Effects of heterogeneous habitats on community composition and niche characteristics of different plant populations in the desert steppe of China. Acta Ecol. Sin. 2019, 39, 6187–6205. [Google Scholar] [CrossRef]
- Fang, C. Analysis of Plant Growth Dynamics and Ecological Benefits in Flower Meadow; Beijing Forestry University: Beijing, China, 2013. [Google Scholar]
- Dale, M.P.; Causton, D.R. The Ecophysiology of Veronica Chamaedrys, V. Montana and V. Officinalis. I. Light Quality and Light Quantity. J. Ecol. 1992, 80, 483–492. [Google Scholar] [CrossRef]
- Tremmel, D.C.; Bazzaz, F.A. How Neighbor Canopy Architecture Affects Target Plant Performance. Ecology 1993, 74, 2114–2124. [Google Scholar] [CrossRef]
- Seabloom, E.W.; Harpole, W.S.; Reichman, O.J.; Tilman, D. Invasion, Competitive Dominance, and Resource Use by Exotic and Native California Grassland Species. Proc. Natl. Acad. Sci. USA 2003, 100, 13384–13389. [Google Scholar] [CrossRef]
- Hitchmough, J.; Wagner, M. The dynamics of designed plant communities of rosette forming forbs for use in supra-urban drainage swales. Landsc. Urban Plan. 2013, 117, 122–134. [Google Scholar] [CrossRef]
- Albayrak, S.; Türk, M. Changes in the forage yield and quality of legume–grass mixtures throughout a vegetation period. Turk. J. Agric. For. 2013, 37, 139–147. [Google Scholar] [CrossRef]
- Ren, W.; Zhang, Z.; Lin, C. Effects of mixed seeding ratio on biomass allocation and competition of Onobrychis viciifolia and Elymus nutans under cold conditions in the Tianzhu alpine region. Pratacultural Sci. 2020, 37, 2035–2048. [Google Scholar]
- Ren, K.; Guo, K.; Zheng, J.; Zhou, J. Growth of four herbaceous species in different mixing modes for restoration of desertificated grassland around Cuona Lake, Tibet. Acta Ecol. Sin. 2020, 40, 910–920. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, L.; Gao, J.; Ding, Y. Effects of interspecific competition on the growth of invasive and native species. Chin. J. Ecol. 2016, 35, 1504–1510. [Google Scholar]
- Wang, S.; Jia, H.; Zhang, Z.; Hu, L.; Chen, G. Competitive Effects between Invasive Plant Phytolacca americana L. and Three Pasture Species. Acta Agrestia Sin. 2021, 29, 95–102. [Google Scholar]
- Guo, Y.; Li, S.; Xu, W.; Wu, A.; Chen, J.; Xu, B. Photosynthetic response of Bothriochloa ischaemum (L.) Keng to drought andshort-term rewatering when intercropped with Lespedeza davurica (Laxm.) Schindl. in a pot experiment. Chin. J. Eco-Agric. 2015, 23, 579–588. [Google Scholar]
- Li, X.; He, Y.; Yang, W.; Wang, C.; Yan, J.; Cui, Z.; Li, C. Effects of different water conditions and plant density on the growth and interspecific competition of Hemarthria compressa and Cynodon dactylon. Acta Ecol. Sin. 2018, 38, 3046–3058. [Google Scholar] [CrossRef]
- Shan, L.; Su, M.; Zhang, Z.; Wang, Y.; Wang, S.; Li, Y. Vertical distribution pattern of mixed root systems of desert plants Reaumuria soongarica and Salsola passerina under different environmental gradients. Chin. J. Plant Ecol. 2018, 42, 475–486. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Zhao, W.; Shi, X. Tracking analysis on changes of ecological patterns in Hexi Corridor Region. Water Resour. Prot. 2015, 31, 5–10. [Google Scholar]
- Wang, G.; Guo, W.; Gou, Q. Effects of sodium salt stress on seed germination of typical annuals in a desert-oasis ecotoneof Hexi Corridor, China. Chin. J. Appl. Ecol. 2020, 31, 1941–1947. [Google Scholar]
- Austin, A.T.; Yahdjian, L.; Stark, J.M.; Belnap, J.; Porporato, A.; Norton, U.; Ravetta, D.A.; Schaeffer, S.M. Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia 2004, 141, 221–235. [Google Scholar] [CrossRef]
- Hulme, M.; Osborn, T.J.; Johns, T.C. Precipitation sensitivity to global warming: Comparison of observations with HadCM2 simulations. Geophys. Res. Lett. 1998, 25, 3379–3382. [Google Scholar] [CrossRef]
- Reynolds, J.F.; Kemp, P.R.; Ogle, K.; Fernández, R.J. Modifying the ‘pulses-reserve’ paradigm for deserts of North America: Precipitation pulses, soil water, and plant responses. Oecologia 2004, 141, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Houghton, J.T.; Ding YD, J.G.; Griggs, D.J.; Noguer, M.; van der Linden, P.J.; Dai, X.; van der Linden, J.; Maskell, K.; Johnson, C.A. Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Meehl, G.A.; Arblaster, J.M.; Tebaldi, C. Understanding future patterns of increased precipitation intensity in climate model simulations. Geophys. Res. Lett. 2005, 32, L18719. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2007: The Physical Science Basis; Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Vile, D.; Pervent, M.; Belluau, M.; Vasseur, F.; Bresson, J.; Muller, B.; Granier, C.; Simonneau, T. Arabidopsis growth under prolonged high temperature and water deficit: Independent or interactive effects? Plant Cell Environ. 2011, 35, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Walck, J.L.; Hidayati, S.N.; Dixon, K.W.; Thompson, K.E.N.; Poschlod, P. Climate change and plant regeneration from seed. Glob. Change Biol. 2011, 17, 2145–2161. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Fang, G.; Li, Z.; Wang, F.; Qin, J.; Sun, F. Potential risks and challenges of climate change in the arid region of northwestern China. Reg. Sustain. 2020, 1, 20–30. [Google Scholar] [CrossRef]
- Eziz, A.; Yan, Z.; Tian, D.; Han, W.; Tang, Z.; Fang, J. Drought effect on plant biomass allocation: A meta-analysis. Ecol. Evol. 2017, 7, 11002–11010. [Google Scholar] [CrossRef]
- Guo, C.; Ma, L.; Yuan, S.; Wang, R. Morphological, physiological and anatomical traits of plant functional types in temperate grasslands along a large-scale aridity gradient in northeastern China. Sci. Rep. 2017, 7, 40900. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Wali, N.; Wang, S.-L.; Jing, P.-C.; Lu, W.-H. Effect of Different Treatment Methods on Seed Dormancy and Seedling Characteristics of Seven Kinds of Leguminous Plants. Acta Agrestia Sin. 2017, 25, 823–831. [Google Scholar]
- Qin, W.; Liang, Z. Response and drought resistance of four leguminous pastures to drought during seed germination. Acta Prataculturae Sin. 2010, 19, 61–70. [Google Scholar]
- Zhao, W.; Chang, X. The effect of hydrologic process changes on NDVI in the desert-oasis ecotone of the Hexi Corridor. Earth Sci. 2014, 44, 1561–1571. [Google Scholar] [CrossRef]
- Yan, Q. Seed Science; China Agriculture Press: Beijing, China, 2001. [Google Scholar]
- Ishibashi, Y.; Koda, Y.; Zheng, S.-H.; Yuasa, T.; Iwaya-Inoue, M. Regulation of soybean seed germination through ethylene production in response to reactive oxygen species. Ann. Bot. 2012, 111, 95–102. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Zhao, L. Physiological and biochemical responses of Lespedeza cuneata seedlings to different calcium and drought stresses in karst habitats. Jiangsu Agric. Sci. 2014, 42, 335. [Google Scholar]
- Cai, X.Y.; Chen, X.D.; Li, C.Z.; Liu, C. Effects of exogenous Ca2+ on the seed germination of Koelreuteria paniculata in limestone area of Southwest China under drought stress. Chin. J. Appl. Ecol. 2013, 24, 1341–1346. [Google Scholar]
- Yao, C.J.; Guo, S.M.; Ma, Y.C.; Lai, X.L.; Yang, X.H. Effect of drought stress on characteristics of photosynthesis and chlorophyll fluorescence of four species of Cassia. Pratacultural Sci. 2017, 34, 1880–1888. [Google Scholar]
- Guan, Y.X.; Dai, J.Y.; Lin, Y. The Photosynthetic Stomatal and Nonstomatal Limitation of Plant Leaves Under Water Stress. Plant Physiol. J. 1995, 4, 293–297. [Google Scholar]
- Jin, R.; Zhang, A.; Shi, X.; Tang, Z.; Chen, X. Photosynthetic characteristics and root activity of drought-stressed sweetpotato exposed to potassiumv. Jiangsu J. Agric. Sci. 2014, 30, 992–996. [Google Scholar]
- Yunus, Q.; Zari, M.; Ahmat, T. Root activity and photosynthetic characteristics of Elaeagnus oxycarpa seedlings underdrought stress. Chin. J. Appl. Ecol. 2011, 22, 1789–1795. [Google Scholar]
- Sharp, R.E.; Poroyko, V.; Hejlek, L.G.; Spollen, W.G.; Springer, G.K.; Bohnert, H.J.; Nguyen, H.T. Root growth maintenance during water deficits: Physiology to functional genomics. J. Exp. Bot. 2004, 55, 2343–2351. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.E. Evolutionary Implications of Phenotypic Plasticity in Plants. In Evolutionary Biology; Springer: Boston, MA, USA, 1987; pp. 127–178. [Google Scholar]
- Franklin, K.A. Shade avoidance. New Phytol. 2008, 179, 930–944. [Google Scholar] [CrossRef]
- Ge, J.; Bu, Z.; Zheng, X.; Ma, J. Morphological responses of three Sphagnum species to drought and interspecific interaction. Chin. J. Ecol. 2014, 33, 2363–2368. [Google Scholar]
- Zhai, S.H.; Wang, P.; Sheng, L.X. Phenotypic Plasticity of Plant Functional Traits in Competition Environments. J. Beihua Univ. Nat. Sci. 2017, 18, 538–546. [Google Scholar]
- Arndal, M.F.; Tolver, A.; Larsen, K.S.; Beier, C.; Schmidt, I.K. Fine Root Growth and Vertical Distribution in Response to Elevated CO2, Warming and Drought in a Mixed Heathland–Grassland. Ecosystems 2018, 21, 15–30. [Google Scholar] [CrossRef]
- Zamora, D.S.; Jose, S.; Nair, P.K.R. Morphological plasticity of cotton roots in response to interspecific competition with pecan in an alleycropping system in the southern United States. Agrofor. Syst. 2006, 69, 107–116. [Google Scholar] [CrossRef]
- Lopez-Zamora, I.; Comerford, N.; Muchovej, R. Root development and competitive ability of the invasive species Melaleuca quinquenervia (Cav.) S.T. Blake in the South Florida flatwoods. Plant Soil 2004, 263, 239–247. [Google Scholar] [CrossRef]
- Jia, J.; Huang, C.; Bai, J.; Zhang, G.; Zhao, Q.; Wen, X. Effects of drought and salt stresses on growth characteristics of euhalophyte Suaeda salsa in coastal wetlands. Phys. Chem. Earth Parts A B C 2017, 103, 68–74. [Google Scholar] [CrossRef]
- Lu, Y.W.; Miao, X.L.; Song, Q.Y.; Peng, S.M.; Duan, B.L. Morphological and ecophysiological plasticity in dioecious plant Populus tomentosa under drought and alkaline stresses. Photosynthetica 2018, 56, 1353–1364. [Google Scholar] [CrossRef]
- Coleman, M. Spatial and temporal patterns of root distribution in developing stands of four woody crop species grown with drip irrigation and fertilization. Plant Soil 2007, 299, 195–213. [Google Scholar] [CrossRef]
- Ogbonnaya, C.I.; Nwalozie, M.C.; Roy-Macauley, H.; Annerose, D.J.M. Growth and water relations of Kenaf (Hibiscus cannabinus L.) under water deficit on a sandy soil. Ind. Crops Prod. 1998, 8, 65–76. [Google Scholar] [CrossRef]


| Mixed Growing Treatments | Drought Treatments (PEG-6000) | ||||
|---|---|---|---|---|---|
| 0% | 2% | 5% | 10% | 15% | |
| Setaria viridis and Chloris virgata | M1D0 | M1D1 | M1D2 | M1D3 | M1D4 |
| Bassia dasyphylla and Halogeton arachnoideus | M2D0 | M2D1 | M2D2 | M2D3 | M2D4 |
| Setaria viridis and Bassia dasyphylla | M3D0 | M3D1 | M3D2 | M3D3 | M3D4 |
| Setaria viridis and Halogeton arachnoideus | M4D0 | M4D1 | M4D2 | M4D3 | M4D4 |
| Chloris virgata and Bassia dasyphylla | M5D0 | M5D1 | M5D2 | M5D3 | M5D4 |
| Chloris virgata and Halogeton arachnoideus | M6D0 | M6D1 | M6D2 | M6D3 | M6D4 |
| Plant Species | Indicators | Drought | Mixed Sowing | Drought and Mixed-Sowing Interactions | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | F | p | ||
| Setaria viridis | Germination rate | 4 | 11.151 | <0.001 | 1 | 2.597 | 0.116 | 4 | 0.720 | 0.584 |
| Germination index | 12.650 | <0.001 | 0.352 | 0.557 | 0.576 | 0.682 | ||||
| Root Vitality | 71.170 | <0.001 | 0.000 | 0.995 | 2.780 | <0.05 | ||||
| Chlorophyll | 40.506 | <0.001 | 1.114 | 0.298 | 3.879 | <0.05 | ||||
| Length of root | 49.716 | <0.001 | 4.857 | <0.05 | 1.573 | 0.203 | ||||
| Stem length | 26.005 | <0.001 | 11.187 | <0.01 | 4.381 | <0.01 | ||||
| Chloris virgata | Germination rate | 8.454 | <0.001 | 1.293 | 0.263 | 0.532 | 0.713 | |||
| Germination index | 8.693 | <0.001 | 0.075 | 0.786 | 1.379 | 0.261 | ||||
| Root Vitality | 56.609 | <0.001 | 3.080 | 0.088 | 0.881 | 0.486 | ||||
| Chlorophyll | 28.974 | <0.001 | 5.112 | <0.05 | 7.121 | <0.001 | ||||
| Length of root | 37.350 | <0.001 | 0.806 | 0.375 | 0.122 | 0.974 | ||||
| Stem length | 10.538 | <0.001 | 2.078 | 0.158 | 4.326 | <0.01 | ||||
| Bassia dasyphylla | Germination rate | 2.4300 | 0.063 | 36.737 | <0.001 | 3.047 | <0.05 | |||
| Germination index | 2.193 | 0.075 | 13.271 | <0.001 | 3.456 | <0.05 | ||||
| Root Vitality | 5.905 | <0.01 | 0.840 | 0.366 | 1.463 | 0.234 | ||||
| Chlorophyll | 20.578 | <0.001 | 5.339 | <0.05 | 1.287 | 0.294 | ||||
| Length of root | 7.746 | <0.001 | 11.262 | <0.01 | 3.811 | <0.05 | ||||
| Stem length | 5.470 | <0.01 | 0.874 | 0.356 | 0.483 | 0.748 | ||||
| Halogetonarachnoideus | Germination rate | 1.795 | 0.152 | 19.267 | <0.001 | 2.829 | <0.05 | |||
| Germination index | 1.814 | 0.148 | 21.133 | <0.001 | 2.748 | <0.05 | ||||
| Root Vitality | 18.906 | <0.001 | 10.454 | <0.01 | 1.738 | 0.164 | ||||
| Chlorophyll | 12.007 | <0.001 | 0.231 | 0.634 | 0.894 | 0.478 | ||||
| Length of root | 6.710 | <0.001 | 7.309 | <0.05 | 0.172 | 0.951 | ||||
| Stem length | 6.525 | <0.001 | 2.171 | 0.150 | 0.551 | 0.699 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gou, Q.; Xi, L.; Li, Y.; Wang, G. The Responses of Four Typical Annual Desert Species to Drought and Mixed Growth. Forests 2022, 13, 2140. https://doi.org/10.3390/f13122140
Gou Q, Xi L, Li Y, Wang G. The Responses of Four Typical Annual Desert Species to Drought and Mixed Growth. Forests. 2022; 13(12):2140. https://doi.org/10.3390/f13122140
Chicago/Turabian StyleGou, Qianqian, Lulu Xi, Yuda Li, and Guohua Wang. 2022. "The Responses of Four Typical Annual Desert Species to Drought and Mixed Growth" Forests 13, no. 12: 2140. https://doi.org/10.3390/f13122140
APA StyleGou, Q., Xi, L., Li, Y., & Wang, G. (2022). The Responses of Four Typical Annual Desert Species to Drought and Mixed Growth. Forests, 13(12), 2140. https://doi.org/10.3390/f13122140




