Comparison of Seasonally Adaptive Metabolic Response Strategies of Two Acer Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Leaf Colour and Pigment Content Determination
2.3. Antioxidant Enzyme Measurement
2.4. Element Analysis
2.5. GC–MS Analysis
2.6. LC–MS Analysis
2.7. Statistical Analysis
3. Results
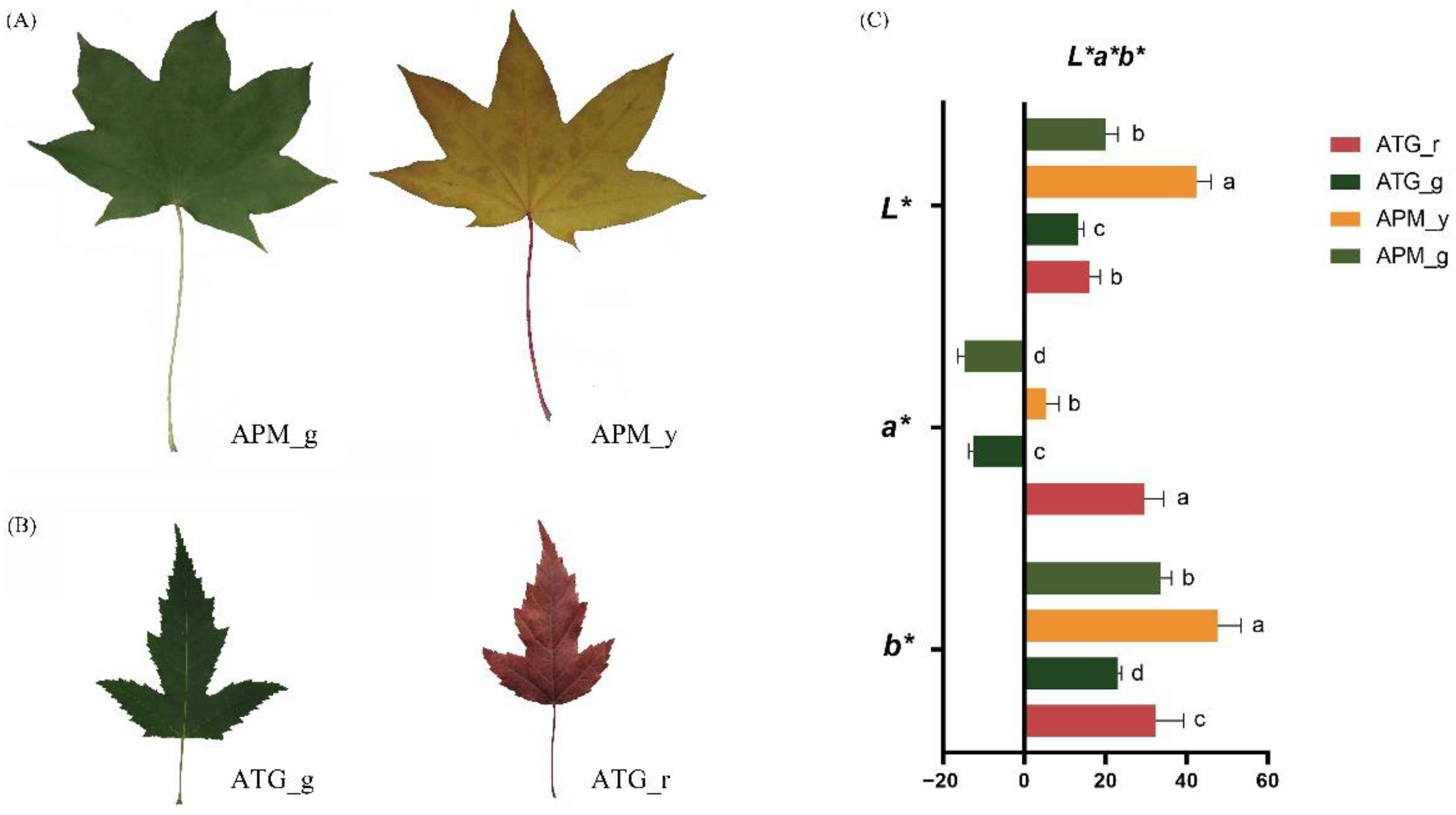
3.1. Comparison of Colour Parameters and Pigments of APM and ATP Leaves
3.2. Comparison of Antioxidant Enzymes of APM and ATP Leaves
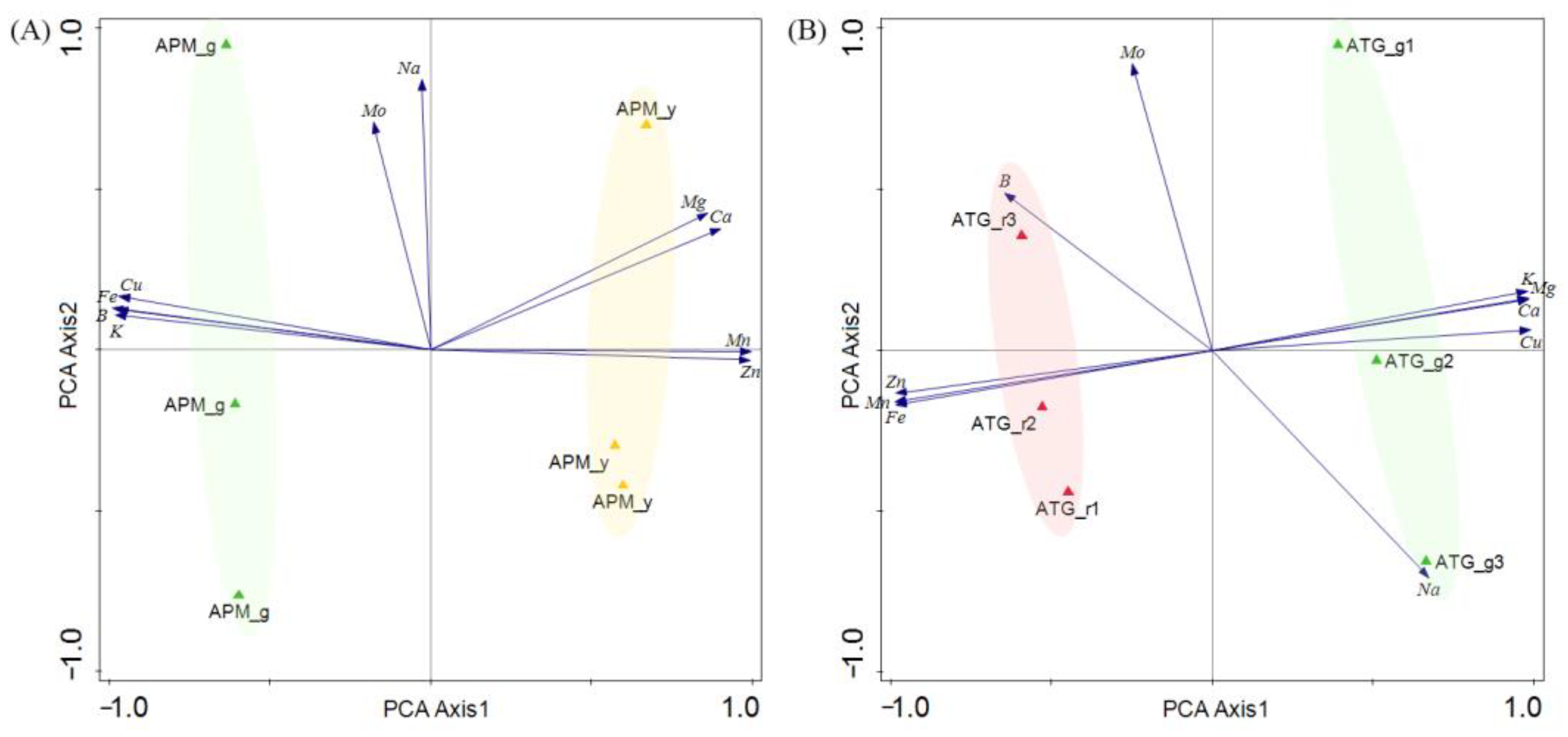
3.3. Comparison of Elements of APM and ATP Leaves
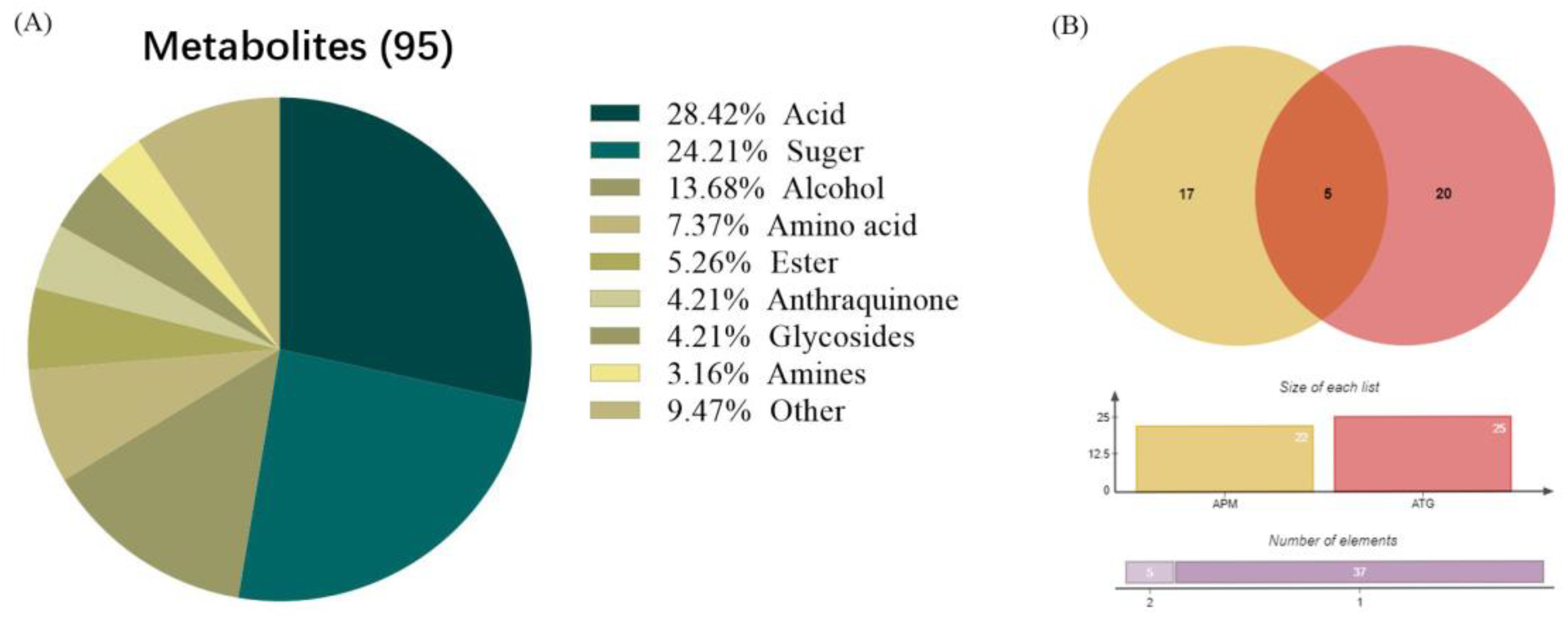
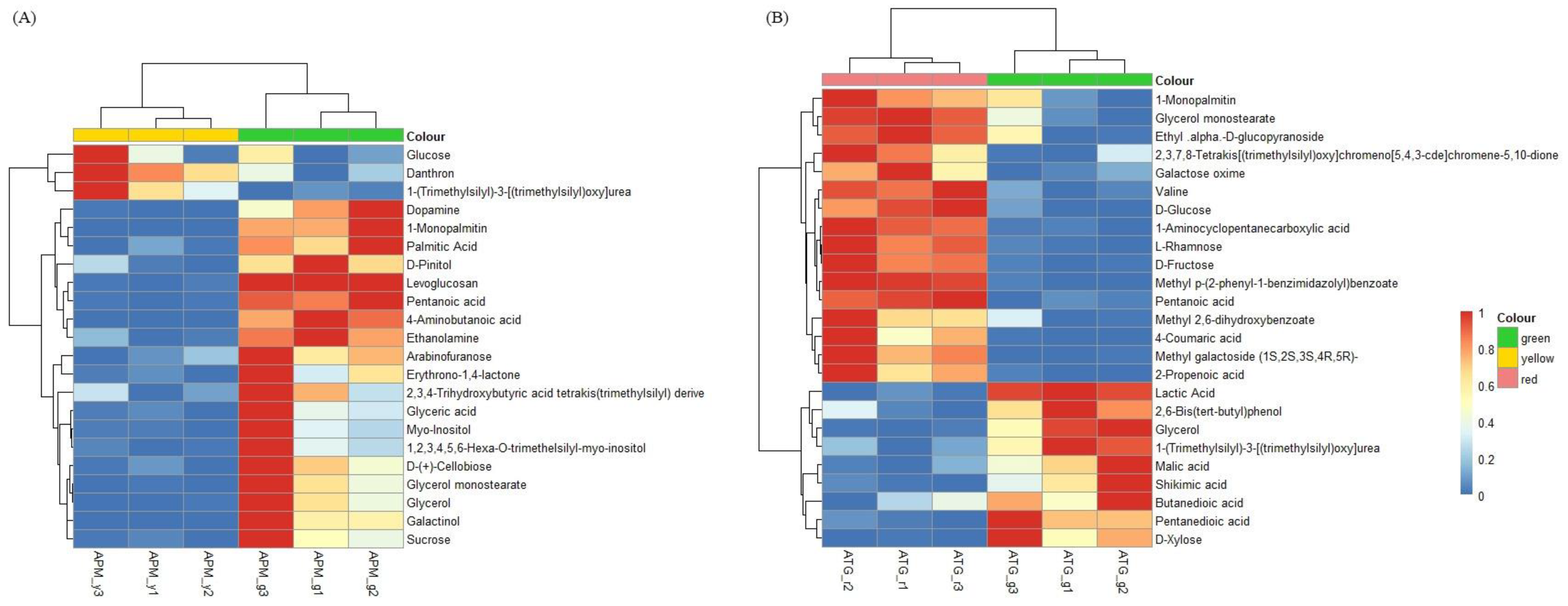
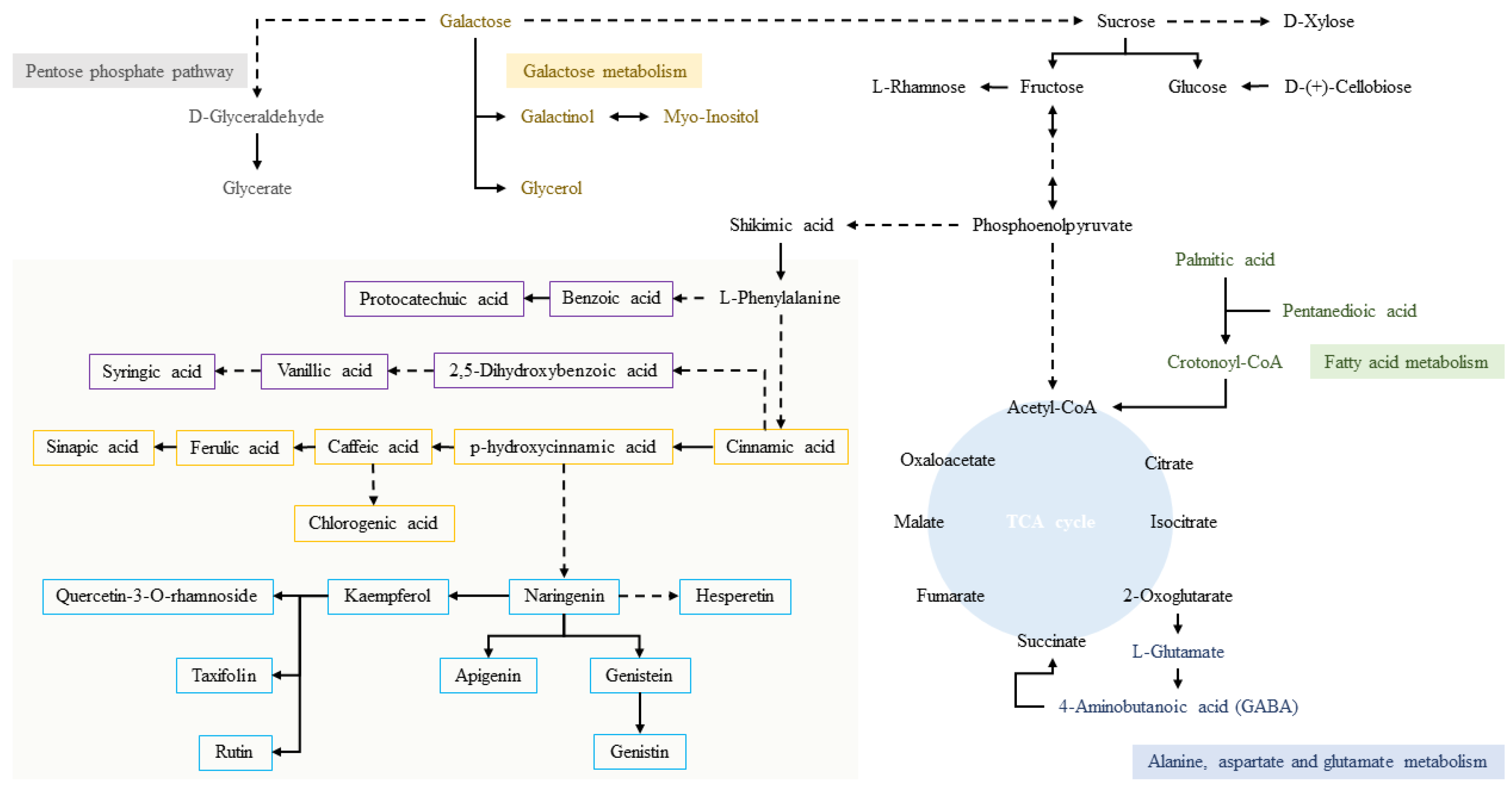
3.4. Comparison of Primary Metabolic of APM and ATP Leaves
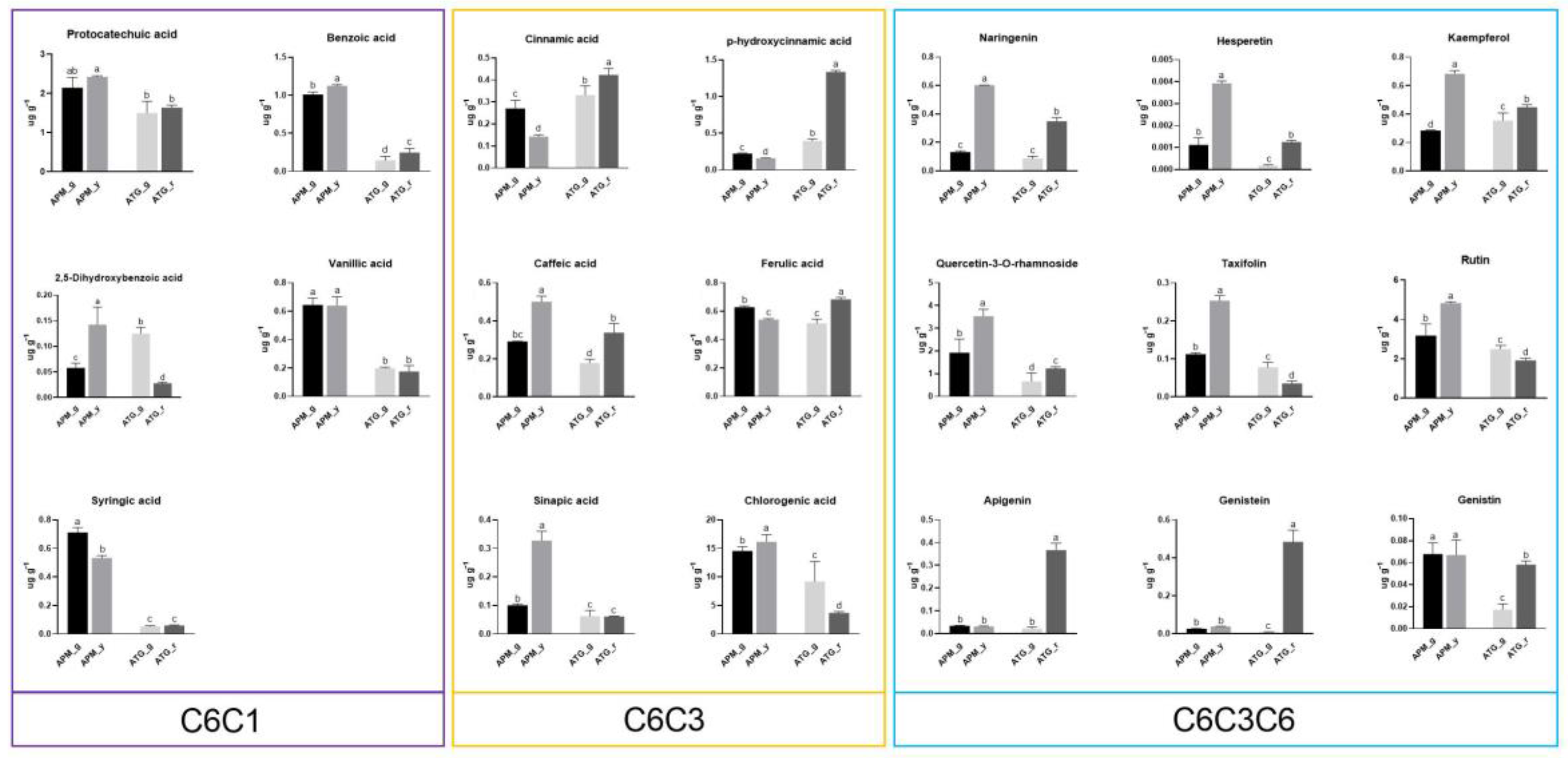
3.5. Comparison of Phenolic Compound Metabolism in APM and ATP Leaves
3.6. Metabolic Network and Correlation Analysis of APM and ATP Leaves
4. Discussion
4.1. Analysis of Pigments and Antioxidant Enzymes in APM and ATP Leaves
4.2. Analysis of Elements in APM and ATP Leaves
4.3. Analysis of Metabolites in APM and ATP Leaves
4.4. Integrative Analysis of the Difference in Leaf Discolouration between APM and ATP
4.5. Limitations and Future
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lev-Yadun, S. The phenomenon of red and yellow autumn leaves: Hypotheses, agreements and disagreements. J. Evol. Biol. 2022, 35, 1245–1282. [Google Scholar] [CrossRef] [PubMed]
- Matile, P. Biochemistry of Indian summer: Physiology of autumnal leaf coloration. Exp. Gerontol. 2000, 35, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Marin, A.; Ferreres, F.; Barbera, G.G.; Gil, M.I. Weather Variability Influences Color and Phenolic Content of Pigmented Baby Leaf Lettuces throughout the Season. J. Agric. Food Chem. 2015, 63, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Macias, P.; Ordidge, M.; Vysini, E.; Waroonphan, S.; Battey, N.H.; Gordon, M.H.; Hadley, P.; John, P.; Lovegrove, J.A.; Wagstaffe, A. Changes in the flavonoid and phenolic acid contents and antioxidant activity of red leaf lettuce (Lollo Rosso) due to cultivation under plastic films varying in ultraviolet transparency. J. Agric. Food Chem. 2007, 55, 10168–10172. [Google Scholar] [CrossRef] [PubMed]
- Koubaier, H.B.; Snoussi, A.; Essaidi, I.; Chaabouni, M.M.; Thonart, P.; Bouzouita, N. Betalain and Phenolic Compositions, Antioxidant Activity of Tunisian Red Beet (Beta vulgaris, L. Conditiva) Roots and Stems Extracts. Int. J. Food Prop. 2014, 17, 1934–1945. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Wang, Y.; Abozeid, A.; Zu, Y.G.; Tang, Z.H. The integration of GC-MS and LC-MS to assay the metabolomics profiling in Panax ginseng and Panax quinquefolius reveals a tissue- and species-specific connectivity of primary metabolites and ginsenosides accumulation. J. Pharm. Biomed. Anal. 2017, 135, 176–185. [Google Scholar] [CrossRef]
- Wu, K.-X.; Liu, J.; Liu, Y.; Guo, X.-R.; Mu, L.-Q.; Hu, X.-H.; Tang, Z.-H. A Comparative Metabolomics Analysis Reveals the Tissue-Specific Phenolic Profiling in Two Acanthopanax Species. Molecules 2018, 23, 2078. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Wang, Y.; Abozeid, A.; Tian, D.M.; Zhang, X.N.; Tang, Z.-H. The Different Resistance of Two Astragalus Plants to UV-B Stress is Tightly Associated with the Organ-specific Isoflavone Metabolism. Photochem. Photobiol. 2018, 94, 115–125. [Google Scholar] [CrossRef]
- Miller, R.; Owens, S.J.; Rorslett, B. Plants and colour: Flowers and pollination. Opt. Laser Technol. 2011, 43, 282–294. [Google Scholar] [CrossRef]
- Ferreres, F.; Pereira, D.M.; Valentao, P.; Andrade, P.B.; Seabra, R.M.; Sottomayor, M. New Phenolic Compounds and Antioxidant Potential of Catharanthus roseus. J. Agric. Food Chem. 2008, 56, 9967–9974. [Google Scholar] [CrossRef]
- Pan, Q.F.; Dai, Y.T.; Nuringtyas, T.R.; Mustafa, N.R.; Schulte, A.E.; Verpoorte, R.; Choi, Y.H. Investigation of the Chemomarkers Correlated with Flower Colour in Different Organs of Catharanthus roseus Using NMR-based Metabolomics. Phytochem. Anal. 2014, 25, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, M.J.; Liu, Y.X.; Ding, H.H.; Du, H.Y.; Sun, Z.Q.; Zhu, J. Metabolome and Transcriptome Analyses Reveal Different Flavonoid Biosynthesis and Chlorophyll Metabolism Profiles between Red Leaf and Green Leaf of Eucommia ulmoides. Forests 2021, 12, 1260. [Google Scholar] [CrossRef]
- Malien-Aubert, C.; Dangles, O.; Amiot, M.J. Color stability of commercial anthocyanin-based extracts in relation to the phenolic composition. Protective effects by intra- and intermolecular copigmentation. J. Agric. Food Chem. 2001, 49, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.L.; Melzer, M.; Zheng, S.J.; Benke, A.; Stich, B.; von Wiren, N. Iron Retention in Root Hemicelluloses Causes Genotypic Variability in the Tolerance to Iron Deficiency-Induced Chlorosis in Maize. Front. Plant Sci. 2018, 9, 557. [Google Scholar] [CrossRef] [PubMed]
- Lev-Yadun, S.; Yamazaki, K.; Holopainen, J.K.; Sinkkonen, A. Spring versus autumn leaf colours: Evidence for different selective agents and evolution in various species and floras. Flora 2012, 207, 80–85. [Google Scholar] [CrossRef]
- Avila-Ospina, L.; Clement, G.; Masclaux-Daubresse, C. Metabolite Profiling for Leaf Senescence in Barley Reveals Decreases in Amino Acids and Glycolysis Intermediates. Agronomy 2017, 7, 15. [Google Scholar] [CrossRef]
- Podzimska-Sroka, D.; O’Shea, C.; Gregersen, P.L.; Skriver, K. NAC Transcription Factors in Senescence: From Molecular Structure to Function in Crops. Plants 2015, 4, 412–448. [Google Scholar] [CrossRef]
- Avila-Ospina, L.; Moison, M.; Yoshimoto, K.; Masclaux-Daubresse, C. Autophagy, plant senescence, and nutrient recycling. J. Exp. Bot. 2014, 65, 3799–3811. [Google Scholar] [CrossRef]
- Hollmann, J.; Gregersen, P.L.; Krupinska, K. Identification of predominant genes involved in regulation and execution of senescence-associated nitrogen remobilization in flag leaves of field grown barley. J. Exp. Bot. 2014, 65, 3963–3973. [Google Scholar] [CrossRef]
- Watanabe, M.; Balazadeh, S.; Tohge, T.; Erban, A.; Giavalisco, P.; Kopka, J.; Mueller-Roeber, B.; Fernie, A.R.; Hoefgen, R. Comprehensive Dissection of Spatiotemporal Metabolic Shifts in Primary, Secondary, and Lipid Metabolism during Developmental Senescence in Arabidopsis. Plant Physiol. 2013, 162, 1290–1310. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.L.; Li, X.X.; Zhang, F.X.; Liu, C.; Du, Y.M.; Gao, X.; Zhang, Z.; Zhang, X.; Hou, Z.; et al. Intergrative metabolomic and transcriptomic analyses unveil nutrient remobilization events in leaf senescence of tobacco. Sci. Rep. 2017, 7, 12126. [Google Scholar] [CrossRef] [PubMed]
- Horbowicz, M.; Szablinska-Piernik, J.; Goraj-Koniarska, J.; Miyamoto, K.; Ueda, J.; Saniewski, M. Changes in Polar Metabolites Content during Natural and Methyl-Jasmonate-Promoted Senescence of Ginkgo biloba Leaves. Int. J. Mol. Sci. 2021, 23, 266. [Google Scholar] [CrossRef] [PubMed]
- Paaso, U.; Keski-Saari, S.; Keinanen, M.; Karvinen, H.; Silfver, T.; Rousi, M.; Mikola, J. Intrapopulation Genotypic Variation of Foliar Secondary Chemistry during Leaf Senescence and Litter Decomposition in Silver Birch (Betula pendula). Front. Plant Sci. 2017, 8, 1074. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Areces-Berazain, F.; Hinsinger, D.D.; Strijk, J.S. Genome-wide supermatrix analyses of maples (Acer, Sapindaceae) reveal recurring inter-continental migration, mass extinction, and rapid lineage divergence. Genomics 2021, 113, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wariss, H.M.; Tao, L.; Zhang, R.; Yun, Q.; Hollingsworth, P.; Dao, Z.; Luo, G.; Guo, H.; Ma, Y.; et al. De novo genome assembly of the endangered Acer yangbiense, a plant species with extremely small populations endemic to Yunnan Province, China. Gigascience 2019, 8, giz085. [Google Scholar] [CrossRef]
- Bi, W.; Gao, Y.; Shen, J.; He, C.N.; Liu, H.B.; Peng, Y.; Zhang, C.; Xiao, P. Traditional uses, phytochemistry, and pharmacology of the genus Acer (maple): A review. J. Ethnopharmacol. 2016, 189, 31–60. [Google Scholar] [CrossRef]
- Gonzalez-Sarrias, A.; Li, L.; Seeram, N.P. Effects of maple (Acer) plant part extracts on proliferation, apoptosis and cell cycle arrest of human tumorigenic and non-tumorigenic colon cells. Phytother. Res. 2012, 26, 995–1002. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Rose, K.N.; DaSilva, N.A.; Johnson, S.L.; Seeram, N.P. Isolation, Identification, and Biological Evaluation of Phenolic Compounds from a Traditional North American Confectionery, Maple Sugar. J. Agric. Food Chem. 2017, 65, 4289–4295. [Google Scholar] [CrossRef]
- Lu, X.Y.; Chen, Z.; Deng, X.Y.; Gu, M.Y.; Zhu, Z.Y.; Ren, J.; Fu, S. Transcriptomic and metabolomic analyses of non-structural carbohydrates in red maple leaves. Funct. Integr. Genomic 2021, 21, 265–281. [Google Scholar] [CrossRef]
- Harris, A.J.; Chen, Y.; Olsen, R.T.; Lutz, S.; Wen, J. On merging Acer sections Rubra and Hyptiocarpa: Molecular and morphological evidence. PhytoKeys 2017, 86, 9–42. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Rodriguez, Y.L.; Urbatsch, L.E.; Karaman-Castro, V. Taxonomy and phylogenetic insights for Mexican and Central American species of Acer (Sapindaceae). J. Torrey Bot. Soc. 2020, 147, 49–86. [Google Scholar] [CrossRef]
- Liu, W.; Ouyang, Y.; Wan, C.P. Flavonoids of the Genus of Acer. Asian J. Chem. 2013, 25, 7075–7078. [Google Scholar] [CrossRef]
- Ren, H.J.; Feng, Z. Comparison and correlation analysis of flavonoids and chlorogenic acid contents in different strains of Acer truncatum. Food Sci. Technol. 2018, 38, 319–326. [Google Scholar] [CrossRef]
- Liu, C.; Tsuda, Y.; Shen, H.; Hu, L.; Saito, Y.; Ide, Y. Genetic structure and hierarchical population divergence history of Acer mono var. mono in South and Northeast China. PLoS ONE 2014, 9, e87187. [Google Scholar] [CrossRef][Green Version]
- Yang, H.; Sung, S.H.; Kim, Y.C. Two new hepatoprotective stilbene glycosides from Acer mono leaves. J. Nat. Prod. 2005, 68, 101–103. [Google Scholar] [CrossRef]
- Jin, Y.J.; Ji, Y.; Jang, Y.P.; Choung, S.Y. Acer tataricum subsp. ginnala Inhibits Skin Photoaging via Regulating MAPK/AP-1, NF-kappaB, and TGFbeta/Smad Signaling in UVB-Irradiated Human Dermal Fibroblasts. Molecules 2021, 26, 662. [Google Scholar]
- Bi, W.; He, C.N.; Li, X.X.; Zhou, L.Y.; Liu, R.J.; Zhang, S.; Li, G.Q.; Chen, Z.C.; Zhang, P.F. Ginnalin A from Kujin tea (Acer tataricum subsp. ginnala) exhibits a colorectal cancer chemoprevention effect via activation of the Nrf2/HO-1 signaling pathway. Food Funct. 2018, 9, 2809–2819. [Google Scholar]
- Bi, W.; Shen, J.; Gao, Y.; He, C.N.; Peng, Y.; Xiao, P.G. Ku-jin tea (Acer tataricum subsp ginnala or A. tataricum subsp theiferum), an underestimated functional beverage rich in antioxidant phenolics. J. Funct. Foods 2016, 24, 75–84. [Google Scholar] [CrossRef]
- Bi, W.; Liu, H.; Shen, J.; Zhang, L.H.; Li, P.; Peng, B.; Cao, L.; Zhang, P.; He, C.; Xiao, P. Chemopreventive effects of Ku-jin tea against AOM-induced precancerous colorectal lesions in rats and metabolomic analysis. Sci. Rep. 2017, 7, 15893. [Google Scholar] [CrossRef]
- Liu, R.Y.; Dong, X.C.; Zhang, M.M.; Qu, Y.; Jin, W.J.; Li, X.; Zhou, L.; Yu, L.; Li, W. Comparative transcriptome analysis reveals candidate genes for leaf color formation in a thermo-sensitive leaf-color mutant generated by carbon-ion beam in green wandering Jew (Tradescantia fluminensis). Sci. Hortic. 2021, 288, 110300. [Google Scholar] [CrossRef]
- Kohler, H.; Contreras, R.A.; Pizarro, M.; Cortes-Antiquera, R.; Zuniga, G.E. Antioxidant Responses Induced by UVB Radiation in Deschampsia antarctica Desv. Front. Plant Sci. 2017, 8, 921. [Google Scholar] [CrossRef]
- Yang, N.; Song, X.Q.; Lu, X.Y.; Chen, Q.; Liu, J.; Liu, Y.; Wang, H.; Zhang, Z.; Tang, Z. Comparative study on metabolites and elements of two dominant plant communities in saline-alkali grassland. Environ. Exp. Bot. 2021, 190, 104587. [Google Scholar] [CrossRef]
- Liu, J.; Kang, R.; Liu, Y.; Wu, K.X.; Yan, X.; Song, Y.; Pan, L.B.; Tang, Z.H. Differential Metabolite Accumulation in Different Tissues of Gleditsia sinensis under Water Stress and Rehydration Conditions. Forests 2020, 11, 542. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Wang, H.Z.; Wu, K.X.; Tang, Z.H. Comparison of the global metabolic responses to UV-B radiation between two medicinal Astragalus species: An integrated metabolomics strategy. Environ. Exp. Bot. 2020, 176, 104094. [Google Scholar] [CrossRef]
- Luo, J.R.; Shi, Q.Q.; Niu, L.X.; Zhang, Y.L. Transcriptomic Analysis of Leaf in Tree Peony Reveals Differentially Expressed Pigments Genes. Molecules 2017, 22, 324. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.Z.; Sun, X.Q.; Li, C.M.; Huan, R.; Sun, C.H.; Wang, Y.; Xiao, F.; Wang, Q.; Chen, P.; Ma, F.; et al. Map-based cloning and characterization of the novel yellow-green leaf gene ys83 in rice (Oryza sativa). Plant Physiol. Biochem. 2017, 111, 1–9. [Google Scholar] [CrossRef]
- Shi, X.Y.; Xu, S.S.; Mu, D.; Sadeghnezhad, E.; Li, Q.; Ma, Z.H.; Zhao, L.; Zhang, Q.; Wang, L. Exogenous Melatonin Delays Dark-Induced Grape Leaf Senescence by Regulation of Antioxidant System and Senescence Associated Genes (SAGs). Plants 2019, 8, 366. [Google Scholar] [CrossRef]
- Kraj, W. Antioxidative enzyme activity as the factor causing differential autumn senescence in phenological forms of beech (Fagus sylvatica L.). Acta Physiol. Plant 2017, 39, 1–16. [Google Scholar] [CrossRef]
- Watanabe, T.; Broadley, M.R.; Jansen, S.; White, P.J.; Takada, J.; Satake, K.; Takamatsu, T.; Tuah, S.J.; Osaki, M. Evolutionary control of leaf element composition in plants. New Phytol. 2007, 174, 516–523. [Google Scholar] [CrossRef]
- Wang, L.L.; Xiong, F.; Yang, L.C.; Xiao, Y.M.; Zhou, G.Y. A Seasonal Change of Active Ingredients and Mineral Elements in Root of Astragalus membranaceus in the Qinghai-Tibet Plateau. Biol. Trace Element Res. 2021, 199, 3950–3959. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Y.; Tang, J.; Yang, Q.L. Effects of geographical origin, variety, harvest season, and their interactions on multi-elements in cereal, tuber, and legume crops for authenticity. J. Food Compos. Anal. 2021, 100, 103900. [Google Scholar] [CrossRef]
- Zhang, W.W.Q.; Ouyang, Z.; Zhao, M.; Wei, Y.; Peng, H.S.; Wang, Q.; Guo, L.; Zhang, W. The influences of inorganic elements in soil on the development of famous—region Atractylodes lancea (Thunb.) DC. Pharmacogn. Mag. 2015, 11, 337–344. [Google Scholar] [PubMed]
- Ning, L.H.; Kan, G.Z.; Shao, H.B.; Yu, D.Y. Physiological and transcriptional responses to salt stress in salt-tolerant and salt-sensitive soybean (Glycine max [L.] Merr.) seedlings. Land Degrad. Dev. 2018, 29, 2707–2719. [Google Scholar] [CrossRef]
- Medveckiene, B.; Kulaitiene, J.; Vaitkeviciene, N.; Levickiene, D.; Buneviciene, K. Effect of Harvesting in Different Ripening Stages on the Content of the Mineral Elements of Rosehip (Rosa spp.) Fruit Flesh. Horticulturae 2022, 8, 467. [Google Scholar] [CrossRef]
- Moschen, S.; Bengoa Luoni, S.; Di Rienzo, J.A.; Caro, M.D.; Tohge, T.; Watanabe, M.; Hollmann, J.; González, S.; Rivarola, M.; García-García, F.; et al. Integrating transcriptomic and metabolomic analysis to understand natural leaf senescence in sunflower. Plant Biotechnol. J. 2016, 14, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Obata, T.; Fernie, A.R. The use of metabolomics to dissect plant responses to abiotic stresses. Cell. Mol. Life Sci. 2012, 69, 3225–3243. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.C.; Fan, Y.H.; Rao, Z.M.; Li, Y.; Qian, H.F.; Zhang, H.; Wu, G.; Qi, X.; Wang, L. Comparative investigation on metabolite changes in ‘wu mi’ production by Vaccinium bracteatum Thunb. leaves based on multivariate data analysis using UPLC-QToF-MS. Food Chem. 2019, 286, 146–153. [Google Scholar] [CrossRef]
- Fan, M.C.; Lian, W.J.; Li, T.T.; Fan, Y.H.; Rao, Z.M.; Li, Y.; Qian, H.; Zhang, H.; Wu, G.; Qi, X.; et al. Metabolomics approach reveals discriminatory metabolites associating with the blue pigments from Vaccinium bracteatum thunb. leaves at different growth stages. Ind. Crops Prod. 2020, 147, 112252. [Google Scholar] [CrossRef]
- Adams, E.; Miyazaki, T.; Moon, J.Y.; Sawada, Y.; Sato, M.; Toyooka, K.; Hirai, M.; Shin, R. Syringic Acid Alleviates Cesium-Induced Growth Defect in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 9116. [Google Scholar] [CrossRef]
- Luo, J.; Butelli, E.; Hill, L.; Parr, A.; Niggeweg, R.; Bailey, P.; Weisshaar, B.; Martin, C. AtMYB12 regulates caffeoyl quinic acid and flavonol synthesis in tomato: Expression in fruit results in very high levels of both types of polyphenol. Plant J. 2008, 56, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.H.; Lin, K.H.; Lin, H.H.; Chu, W.X.; Lai, Y.C.; Chao, P.Y. Analysis of Chlorogenic Acid in Sweet Potato Leaf Extracts. Plants 2022, 11, 2063. [Google Scholar] [CrossRef] [PubMed]
- Li, P.H.; Dong, Q.; Ge, S.J.; He, X.Z.; Verdier, J.; Li, D.Q.; Zhao, J. Metabolic engineering of proanthocyanidin production by repressing the isoflavone pathways and redirecting anthocyanidin precursor flux in legume. Plant Biotechnol. J. 2016, 14, 1604–1618. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Shayganfar, A.; Azizi, M.; Rasouli, M. Various strategies elicited and modulated by elevated UV-B radiation and protectant compounds in Thymus species: Differences in response over treatments, acclimation and interaction. Ind. Crops Prod. 2018, 113, 298–307. [Google Scholar] [CrossRef]
- Sgherri, C.; Cosi, E.; Navari-Izzo, F. Phenols and antioxidative status of Raphanus sativus grown in copper excess. Physiol. Plant. 2003, 118, 21–28. [Google Scholar] [CrossRef]
- Goto, T.; Kondo, T. Structure and Molecular Stacking of Anthocyanins—Flower Color Variation. Angew. Chem. Int. Ed. 1991, 30, 17–33. [Google Scholar] [CrossRef]
- Lv, X.; Li, L.; Lu, X.; Wang, W.; Sun, J.; Liu, Y.; Mu, J.; Ma, Q.; Wang, J. Effects of organic acids on color intensification, thermodynamics, and copigmentation interactions with anthocyanins. Food Chem. 2022, 396, 133691. [Google Scholar] [CrossRef]
- Shoji, K.; Miki, N.; Nakajima, N.; Momonoi, K.; Kato, C.; Yoshida, K. Perianth bottom-specific blue color development in Tulip cv. Murasakizuisho requires ferric ions. Plant Cell Physiol. 2007, 48, 243–251. [Google Scholar] [CrossRef]
- Ben Abdallah, H.; Mai, H.J.; Slatni, T.; Fink-Straube, C.; Abdelly, C.; Bauer, P. Natural Variation in Physiological Responses of Tunisian Hedysarum carnosum under Iron Deficiency. Front. Plant Sci. 2018, 9, 1383. [Google Scholar] [CrossRef]
- Ren, L.J.; Eller, F.; Lambertini, C.; Guo, W.Y.; Sorrell, B.K.; Brix, H. Minimum Fe requirement and toxic tissue concentration of Fe in Phragmites australis: A tool for alleviating Fe-deficiency in constructed wetlands. Ecol. Eng. 2018, 118, 152–160. [Google Scholar] [CrossRef]
- Singh, D.P.; Beloy, J.; McInerney, J.K.; Day, L. Impact of boron, calcium and genetic factors on vitamin C, carotenoids, phenolic acids, anthocyanins and antioxidant capacity of carrots (Daucus carota). Food Chem. 2012, 132, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Fracheboud, Y.; Luquez, V.; Bjorken, L.; Sjodin, A.; Tuominen, H.; Jansson, S. The Control of Autumn Senescence in European Aspen. Plant Physiol. 2009, 149, 1982–1991. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Abrol, E.; Koul, S.; Vyas, D. Seasonal low temperature plays an important role in increasing metabolic content of secondary metabolites in Withania somnifera (L.) Dunal and affects the time of harvesting. Acta Physiol. Plant 2012, 34, 2027–2031. [Google Scholar] [CrossRef]
- Vitasse, Y.; Porte, A.J.; Kremer, A.; Michalet, R.; Delzon, S. Responses of canopy duration to temperature changes in four temperate tree species: Relative contributions of spring and autumn leaf phenology. Oecologia 2009, 161, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Lev-Yadun, S. Avoiding rather than resisting herbivore attacks is often the first line of plant defence. Biol. J. Linn. Soc. 2021, 134, 775–802. [Google Scholar] [CrossRef]
- Doring, T.F.; Archetti, M.; Hardie, J. Autumn leaves seen through herbivore eyes. Proc. R. Soc. B Biol. Sci. 2009, 276, 121–127. [Google Scholar] [CrossRef]
- Hughes, N.M.; Lev-Yadun, S. Red/purple leaf margin coloration: Potential ecological and physiological functions. Environ. Exp. Bot. 2015, 119, 27–39. [Google Scholar] [CrossRef]
- Baisden, E.C.; Tallamy, D.W.; Narango, D.L.; Boyle, E. Do Cultivars of Native Plants Support Insect Herbivores? HortTechnology 2018, 28, 596. [Google Scholar] [CrossRef]
- Sinkkonen, A.; Somerkoski, E.; Paaso, U.; Holopainen, J.K.; Rousi, M.; Mikola, J. Genotypic variation in yellow autumn leaf colours explains aphid load in silver birch. New Phytol. 2012, 195, 461–469. [Google Scholar] [CrossRef]



| K | Ca | Mg | Na | Fe | Cu | Zn | Mn | Mo | B | |
|---|---|---|---|---|---|---|---|---|---|---|
| APM_g | 13.456 ± 0.328 a | 37.117 ± 0.695 b | 4.733 ± 0.130 b | 0.281 ± 0.024 a | 0.190 ± 0.006 b | 0.010 ± 0.000 a | 0.009 ± 0.000 c | 0.141 ± 0.003 c | 0.001 ± 0.000 a | 0.061 ± 0.002 a |
| APM_y | 9.425 ± 0.147 b | 41.228 ± 0.758 a | 5.233 ± 0.074 a | 0.275 ± 0.040 a | 0.128 ± 0.002 c | 0.008 ± 0.000 b | 0.016 ± 0.000 b | 2.304 ± 0.025 a | 0.001 ± 0.000 a | 0.025 ± 0.001 c |
| ATG_g | 13.361 ± 0.035 a | 23.367 ± 0.121 c | 4.767 ± 0.029 b | 0.283 ± 0.026 a | 0.176 ± 0.004 b | 0.008 ± 0.000 b | 0.014 ± 0.000 b | 0.081 ± 0.001 d | 0.001 ± 0.000 a | 0.024 ± 0.011 c |
| ATG_r | 7.847 ± 0.108 c | 11.483 ± 0.107 d | 1.833 ± 0.012 c | 0.239 ± 0.008 a | 0.471 ± 0.003 a | 0.006 ± 0.000 c | 0.027 ± 0.000 a | 0.322 ± 0.002 b | 0.001 ± 0.000 a | 0.039 ± 0.000 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.; Liu, Y.; Liu, J.; Ren, Y.; Liu, Y.; Sun, F.; Abozeid, A.; Tang, Z.; Mu, L. Comparison of Seasonally Adaptive Metabolic Response Strategies of Two Acer Species. Forests 2022, 13, 2141. https://doi.org/10.3390/f13122141
Wu K, Liu Y, Liu J, Ren Y, Liu Y, Sun F, Abozeid A, Tang Z, Mu L. Comparison of Seasonally Adaptive Metabolic Response Strategies of Two Acer Species. Forests. 2022; 13(12):2141. https://doi.org/10.3390/f13122141
Chicago/Turabian StyleWu, Kexin, Yang Liu, Jia Liu, Yanjun Ren, Yuli Liu, Feiyang Sun, Ann Abozeid, Zhonghua Tang, and Liqiang Mu. 2022. "Comparison of Seasonally Adaptive Metabolic Response Strategies of Two Acer Species" Forests 13, no. 12: 2141. https://doi.org/10.3390/f13122141
APA StyleWu, K., Liu, Y., Liu, J., Ren, Y., Liu, Y., Sun, F., Abozeid, A., Tang, Z., & Mu, L. (2022). Comparison of Seasonally Adaptive Metabolic Response Strategies of Two Acer Species. Forests, 13(12), 2141. https://doi.org/10.3390/f13122141







