Regional, Site, and Tree Variations of Wood Density and Growth in Thuja occidentalis L. in the Quebec Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Material
2.2. Collection and Preparation of Wood Samples
2.3. Ring Width and Wood Density Measurement
2.4. Statistical Analysis
3. Results and Discussions
3.1. T. occidentalis Wood Density Characteristics in the Québec Forest
3.2. Regional Variation of Ring Widths and Ring Density Components
3.3. Inter-Site, Among-Tree, and with Cambial Age Variations of Ring Width and Wood Density Components
3.4. Relationships of Wood Density and Ring Width with Ecological Factors and Their Variation along a South–North Latitudinal Gradient in Abitibi Témiscamingue
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koubaa, A.; Zhang, S.Y. White Cedar, Thuja occidentalis L. In Softwoods of Eastern Canada. Their Sylvics, Characteristics, Manufacturing and End-Use; Koubaa, A., Zhang, S.Y., Eds.; Special Publication, SP-526E, Chapter 11; FPInnovations: Québec, QC, Canada, 2008; 18p. [Google Scholar]
- Taylor, A.M.; Gartner, B.L.; Morrell, J.J. Heartwood formation and natural durability—A review. Wood Fiber. Sci. 2002, 34, 587–611. [Google Scholar]
- Johnston, W.F. Thuja occidentalis L. Northern white-cedar. In Silvics of North America; Forest Service, Agricultural Handbook; Volume 1: Conifers; Burns, R.M., Honkala, B.H., Eds.; United States Department of Agriculture: Washington, DC, USA, 1990; pp. 580–589. [Google Scholar]
- Behr, E.A. Special physical and chemical properties of northern white cedar. In Proceedings of the National Northern White Cedar Conference; No. 3-76; Michigan State University Publication: East Lansing, MI, USA, 1976; pp. 11–15. [Google Scholar]
- Wan, H.; Wang, X.M.; Yang, D.Q. Utilizing eastern white cedar to improve the resistance of strand boards to mold and decay fungi. For. Prod. J. 2007, 57, 54–59. [Google Scholar]
- Behr, E.A. Decay and termite resistance of medium-density fiberboards made from wood residue. For. Prod. J. 1972, 22, 48–51. [Google Scholar]
- Haataja, B.A.; Laks, P.E. Properties of flakeboard made from northern white cedar. For. Prod. J. 1995, 45, 68–70. [Google Scholar]
- Panshin, A.J.; De Zeeuw, C. Textbook of Wood Technology; McGraw-Hill Book Co.: New York, NY, USA, 1980; p. 772. [Google Scholar]
- Zobel, B.J.; Van Buijtenen, J.P. Wood Variation: Its Causes and Control; Springer: Berlin, Germany, 1989; p. 363. [Google Scholar]
- Zhang, S.Y.; Nepveu, G.; Owoundi, R.E. Intratree and intertree variation in selected wood quality characteristics of European oak (Quercus petraea and Quercus robur). Can. J. For. Res. 1994, 24, 1818–1823. [Google Scholar] [CrossRef]
- Koga, S.; Zhang, S.Y. Inter-tree and intra-tree variations in ring width and wood density components in balsam fir [Abies balsamea]. Wood Sci. Technol. 2004, 38, 149–162. [Google Scholar] [CrossRef]
- Guller, B.; Isik, K.; Cetinay, S. Variations in the radial growth and wood density components in relation to cambial age in 30-year-old Pinus brutia Ten. at two test sites. Trees 2012, 26, 975–986. [Google Scholar] [CrossRef]
- Barbour, J. Wood formation and properties. In Wood Quality, in Encyclopedia of Forest Sciences; Jeffery, B., Ed.; Elsevier: Oxford, UK, 2004; pp. 1840–1846. [Google Scholar]
- Bao, F.C.; Jiang, Z.H.; Jiang, X.M.; Lu, X.X.; Luo, X.Q.; Zhang, S.Y. Differences in wood properties between juvenile wood and mature wood in 10 species grown in China. Wood Sci. Technol. 2001, 35, 363–375. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Morgenstern, E.K. Genetic variation and inheritance of wood density in black spruce (Picea mariana) and its relationship with growth: Implications for tree breeding. Wood Sci. Technol. 1996, 30, 63–75. [Google Scholar] [CrossRef]
- Chave, J.; Muller-Landau, H.C.; Baker, T.R.; Easdale, T.A.; Steege, H.; Webb, C.O. Regional and phylogenetic variation of wood density across 2456 neotropical tree species. Ecol. Appl. 2006, 16, 2356–2367. [Google Scholar] [CrossRef] [PubMed]
- Savva, Y.; Koubaa, A.; Tremblay, F.; Bergeron, Y. Effects of radial growth, tree age, climate, and seed origin on wood density of diverse jack pine populations. Trees-Struct. Funct. 2010, 24, 53–65. [Google Scholar] [CrossRef]
- Bergès, L.; Nepveu, G.; Franc, A. Effects of ecological factors on radial growth and wood density components of sessile oak (Quercus petraea Liebl.) in Northern France. For. Ecol. Manag. 2008, 255, 567–579. [Google Scholar] [CrossRef]
- Oliva, A.G.; Merino, V.B.; Seco, J.I.F.G.; Garcia, M.C.; Prieto, E.H. Effect of growth conditions on wood density of Spanish Pinus nigra. Wood Sci. Technol. 2006, 40, 190–204. [Google Scholar] [CrossRef]
- DeRose, R.J.; Seymour, R.S. The effect of site quality on growth efficiency of upper crown class Picea rubens and Abies balsamea in Maine, USA. Can. J. For. Res. 2009, 39, 777–784. [Google Scholar] [CrossRef]
- Muller-Landau, H.C. Interspecific and inter-site variation in wood specific gravity of tropical trees. Biotropica 2004, 36, 20–32. [Google Scholar] [CrossRef]
- Ackermann, F. Relationship between forest site and intra-ring wood density components for the pedunculate oak (Quercus robur L.) of southwestern France. Ann. For. Sci. 1995, 52, 635–652. [Google Scholar] [CrossRef]
- Guilley, E.; Hervé, J.C.; Nepveu, G. The influence of site quality, silviculture and region on wood density mixed model in Quercus petraea Liebl. For. Ecol. Manag. 2004, 189, 111–121. [Google Scholar] [CrossRef]
- Knapic, S.; Louzada, J.L.; Pereira, H. Variation in wood density components within and between Quercus faginea trees. Can. J. For. Res. 2011, 41, 1212–1219. [Google Scholar] [CrossRef]
- Tardif, J.; Stevenson, D. Radial growth-climate association of Thuja occidentalis L. at the northwestern limit of its distribution, Manitoba, Canada. Dendrochronologia 2001, 19, 179–187. [Google Scholar]
- Tardif, J.; Bergeron, Y. Comparative dendroclimatological analysis of two black ash and two white cedar populations from contrasting sites in the Lake Duparquet region, northwestern Quebec. Can. J. For. Res. 1997, 27, 108–116. [Google Scholar] [CrossRef]
- Housset, J.M.; Girardin, M.P.; Baconnet, M.; Carcaillet, C.; Bergeron, Y. Unexpected warming-induced growth decline in Thuja occidentalis at its northern limits in North America. J. Biogeogr. 2015, 42, 1233–1245. [Google Scholar] [CrossRef]
- Archambault, S.; Bergeron, Y. An 802-year tree-ring chronology from the Quebec boreal forest. Can. J. For. Res. 1992, 22, 674–682. [Google Scholar] [CrossRef]
- Maeglin, R.R. Wisconsin Wood Density Suarvey; Forest Products Lab: Madison, WI, USA, 1973. [Google Scholar]
- Bouslimi, B.; Koubaa, A.; Bergeron, Y. Anatomical properties in Thuja occidentalis: Variation and relationship to biological processes. IAWA 2014, 35, 363–384. [Google Scholar] [CrossRef]
- Xu, H.; Tremblay, F.; Bergeron, Y.; Paul, V.; Chen, C. Genetic consequences of fragmentation in arbor vitae, eastern white cedar (Thuja occidentalis L.), toward the northern limit of its distribution range. Ecol. Evol. 2012, 2, 2506–2520. [Google Scholar] [CrossRef]
- Paul, V. Les Facteurs Ecologiques Limitant la Répartition Nordique du Thuja de L’est (Thuja occidentalis L.); Université du Québec en Abitibi-Témiscamingue: Rouyn-Noranda, QC, Canada, 2011; p. 84. [Google Scholar]
- Régnière, J.; St-Amant, R. Stochastic simulation of daily air temperature and precipitation from monthly normals in North America north of Mexico. Int. J. Biometeorol. 2007. 51, 415–430. [CrossRef]
- Bergeron, Y.; Gauthier, S.; Flannigan, M.; Kafka, V. Fire regimes at the transition between mixedwood and coniferous boreal forest in northwestern Quebec. Ecology 2004, 85, 1916–1932. [Google Scholar] [CrossRef]
- Bouslimi, B.; Koubaa, A.; Bergeron, Y. Variation of brown rot decay in eastern white cedar (Thuja occidentalis L.). BioResources 2013, 8, 4735–4755. [Google Scholar] [CrossRef]
- Koubaa, A.; Zhang, S.Y.; Makni, S. Defining the transition from earlywood to latewood in black spruce based on intra-ring wood density profiles from X-ray densitometry. Ann. For. Sci. 2002, 59, 511–518. [Google Scholar] [CrossRef]
- Singleton, R.; DeBell, D.S.; Gartner, B.L. Effect of extraction on wood density of western hemlock (Tsuga heterophylla (Raf.) Sarg.). Wood Fiber Sci. 2003, 35, 363–369. [Google Scholar]
- Grabner, M.; Wimmer, R.; Gierlinger, N.; Evans, R.; Downes, G. Heartwood extractives in larch and effects on X-ray densitometry. Can. J. For. Res. 2005, 35, 2781–2786. [Google Scholar] [CrossRef]
- Bouslimi, B.; Koubaa, A.; Bergeron, Y. Intra-Ring Variations and Interrelationships for Selected Wood Anatomical and Physical Properties of Thuja occidentalis L. Forests 2019, 10, 339. [Google Scholar] [CrossRef]
- Smith, D. Maximum Moisture Content Method for Determining Specific Gravity of Small Wood Samples; Forest Products Laboratory Report 2014; Forest Products Laboratory: Madison, WI, USA, 1954. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–75. [Google Scholar]
- Littell, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D.; Schabenberber, O. SAS for Mixed Models, 2nd ed.; SAS Institute, Inc.: Cary, NC, USA, 2006; p. 814. [Google Scholar]
- SAS Institute Inc. Statistical Analysis Software. Users’ Guide Statistics Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- Park, Y.I.; Koubaa, A.; Brais, S.; Mazerolle, M.J. Effects of cambial age and stem height on wood density and growth of jack pine grown in boreal stands. Wood Fiber Sci. 2009, 41, 346–358. [Google Scholar]
- Koubaa, A.; Zhang, S.Y.; Isabel, N.; Beaulieu, J.; Bousquet, J. Phenotypic correlations between juvenile-mature wood density and growth in black spruce. Wood Fiber Sci. 2000, 32, 61–71. [Google Scholar]
- Jozsa, L.A.; Middleton, G.R. A Discussion of Wood Quality Attributes and Their Practical Implications; No. SP-34; Forintek Canada Corp.: Ottawa, ON, Canada, 1994; p. 42. [Google Scholar]
- Larson, P.R.; Kretschmann, D.E.; Clark, A., III; Isebrands, J.G. Formation and Properties of Juvenile Wood in Southern Pines: A Synopsis; USDA Forest Service, Forest Products Laboratory: Madison, WI, USA, 2001; p. 42. [Google Scholar]
- Alteyrac, J.; Cloutier, A.; Zhang, S.Y. Characterization of juvenile wood to mature wood transition age in black spruce (Picea mariana (Mill.) B.S.P.) at different stand densities and sampling heights. Wood Sci. Technol. 2006, 40, 124–138. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Simpson, D.; Morgenstern, E.K. Variation in the relationship of wood density with growth in 40 black spruce (Picea mariana) families grown in New Brunswick. Wood Fiber Sci. 1996, 28, 91–99. [Google Scholar]
- Koga, S.; Zhang, S.Y. Relationships between wood density and annual growth rate components in balsam fir (Abies balsamea). Wood Fiber Sci. 2002, 34, 146–157. [Google Scholar]
- Bergeron, Y. Species and stand dynamics in the mixed woods of Quebec’s southern boreal forest. Ecology 2000, 81, 1500–1516. [Google Scholar] [CrossRef]
- Larson, D.W.; Doubt, J.; Matthes-Sears, U. Radially Sectored Hydraulic Pathways in the Xylem of Thuja occidentalis as Revealed by the Use of Dyes. Int. J. Plant Sci. 1994, 155, 569–582. [Google Scholar] [CrossRef]
- Matthes-Sears, U.; Nash, C.H.; Larson, D.W. Constrained growth of trees in a hostile environment: The role of water and nutrient availability for Thuja occidentalis on cliff faces. Int. J. Plant Sci. 1995, 156, 311–319. [Google Scholar] [CrossRef]
- Matthes-Sears, U.; Larson, D.W. Growth and physiology of Thuja occidentalis L. from cliffs and swamps: Is variation habitat or site-specific? Bot. Gaz. 1991, 152, 500–508. [Google Scholar] [CrossRef]
- Koubaa, A.; Isabel, N.; Zhang, S.Y.; Beaulieu, J.; Bousquet, J. Transition from juvenile to mature wood in black spruce (Picea mariana (Mill.) B.S.P.). Wood Fiber Sci. 2005, 37, 445–455. [Google Scholar]
- Paul, V.; Bergeron, Y.; Tremblay, F. Does climate control the northern range limit of eastern white cedar (Thuja occidentalis L.)? Plant Ecol. 2014, 215, 181–194. [Google Scholar] [CrossRef]
- Bergeron, Y. Les conséquences des changements climatiques sur la fréquence des feux et la composition forestière au sud-ouest de la forêt boréale québécoise. Géogr. Phys. Quat. 1998, 52, 167–174. [Google Scholar] [CrossRef][Green Version]
- Schulz, K. Northern White-Cedar Stand Structure, Composition, and Ecophysiology: Implications for Management and Climate Change Resilience. Electronic Theses and Dissertations. 3694. p. 90. Available online: https://digitalcommons.library.umaine.edu/etd/3694 (accessed on 4 October 2022).
- Fromm, J. Cellular Aspects of Wood Formation; Springer: Berlin/Heidelberg, Germany, 2013; p. 264. [Google Scholar]
- Larocque, G.R. Importance of different climatic parameters on growth and wood formation of red pine (Pinus resinosa Ait) in Ontario (Canada). Ann. Des. Sci. For. 1997, 54, 51–63. [Google Scholar] [CrossRef]
- Fei, B.H.; Ruan, X.G. Effects of temperature and precipitation on tree-ring and wood density of Ginkgo in Beijing. For. Res. 2001, 14, 176–180. [Google Scholar]
- Gindl, W.; Grabner, M.; Wimmer, R. The influence of temperature on latewood lignin content in treeline Norway spruce compared with maximum density and ring width. Trees 2000, 14, 409–414. [Google Scholar] [CrossRef]
- Zhang, S.Y. Variations and correlations of various ring width and ring density features in European oak: Implications in dendroclimatology. Wood Sci. Technol. 1997, 31, 63–72. [Google Scholar] [CrossRef]
- Plomion, C.; Leprovost, G.; Stokes, A. Wood formation in trees. Plant Physiol. 2001, 127, 1513–1523. [Google Scholar] [CrossRef]
- Hofmeyer, P.V.; Kenefic, L.S.; Seymour, R.S. Northern white-cedar ecology and silviculture in the northeastern United States and southeastern Canada: A synthesis of knowledge. North. J. Appl. For. 2009, 26, 21–27. [Google Scholar] [CrossRef]
- Rooney, T.P.; Solheim, S.L.; Waller, D.M. Factors affecting the regeneration of northern white cedar in lowland forests of the Upper Great Lakes region, USA. For. Ecol. Manag. 2002, 163, 119–130. [Google Scholar] [CrossRef]
- Rosner, S. Hydraulic and biomechanical optimization in Norway spruce trunkwood—A review. IAWA J. 2013, 34, 365–390. [Google Scholar] [CrossRef]
- Hacke, U.G.; Sperry, J.S.; Pockman, W.T.; Davis, S.D.; McCulloh, K.A. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 2001, 126, 457–461. [Google Scholar] [CrossRef]
- Tyree, M.T.; Ewers, F.W. The hydraulic architecture of trees and other woody plants. New Phytol. 1991, 119, 345–360. [Google Scholar] [CrossRef]
- Larson, D.W.; Matthes-Sears, U.; Kelly, P.E. Cambial Dieback and Partial Shoot Mortality in Cliff-Face Thuja occidentalis: Evidence for Sectored Radial Architecture. Int. J. Plant Sci. 1993, 154, 496–505. [Google Scholar] [CrossRef]
- Fujiwara, S.; Yang, K. The relationship between cell length and ring width and circumferential growth rate in five Canadian species. IAWA J. 2000, 21, 335–346. [Google Scholar] [CrossRef]
- Hofmeyer, P.V.; Kenefic, L.S.; Seymour, R.S. Historical stem development of northern White-cedar (Thuja occidentalis L.) in Maine. North. J. Appl. For. 2010, 27, 92–96. [Google Scholar] [CrossRef]
- Mansfield, S.D.; Parish, R.; Goudie, J.W.; Kang, K.Y.; Ott, P. The effects of crown ratio on the transition from juvenile to mature wood production in lodgepole pine in western Canada. Can. J. For. Res. 2007, 37, 1450–1459. [Google Scholar] [CrossRef]
- Kuprevicius, A.P. Quantifying the Influence of Crown Size on Mechanical Wood Properties in White Spruce (Picea glauca); University of Toronto: Toronto, ON, Canada, 2011; p. 72. [Google Scholar]
- Briand, C.H.; Posluszny, U.; Larson, D.W. Influence of age and growth rate on radial anatomy of annual rings of Thuja occidentalis L. (eastern white cedar). Int. J. Plant Sci. 1993, 154, 406–411. [Google Scholar] [CrossRef]
- Koubaa, A.; Zhang, S.Y. Jack pine (Pinus banksiana Lamb). In Softwoods of Eastern Canada. Their Sylvics, Characteristics, Manufacturing and End-Uses; Koubaa, A., Zhang, S.Y., Eds.; Special Publication, SP-526F, Chapter 4; FPInnovations: Quebec, QC, Canada, 2008; 35p. [Google Scholar]
- Koubaa, A.; Zhang, S.Y. White spruce (Picea glauca (Moench) Voss). In Softwoods of Eastern Canada. Their Sylvics, Characteristics, Manufacturing and End-Uses; Zhang, S.Y., Koubaa, A., Eds.; Special Publication, SP-526F, Chapter 2; FPInnovations: Quebec, QC, Canada, 2008; 33p. [Google Scholar]
- Gryc, V.; Vavrčík, H.; Horn, K. Density of juvenile and mature wood of selected coniferous species. J. For. Sci. 2011, 57, 123–130. [Google Scholar] [CrossRef]
- Knapic, S.; Louzada, J.L.; Leal, S.; Pereira, H. Within-tree and between-tree variation of wood density components in cork oak trees in two sites in Portugal. Forestry 2008, 81, 465–473. [Google Scholar] [CrossRef]
- Bouriaud, O.; Leban, J.-M.; Bert, D.; Deleuze, C. Intra-annual variations in climate influence growth and wood density of Norway spruce. Tree Physiol. 2005, 25, 651–660. [Google Scholar] [CrossRef]
- Spicer, R.; Gartner, B.L. The effects of cambial age and position within the stem on specific conductivity in Douglas-fir (Pseudotsuga menziesii) sapwood. Trees-Struct. Funct. 2001, 15, 222–229. [Google Scholar] [CrossRef]
- Hofmeyer, P.V.; Kenefic, L.S.; Seymour, R.S.; Brissette, J.C. Growth Comparison of Northern White-Cedar to Balsam Fir and Red Spruce by Site Class; Forest Science; University of Maine: Orono, ME, USA, 2006; p. 04469. [Google Scholar]
- Gartner, B.L.; Robbins, J.M.; Newton, M. Effects of pruning on wood density and tracheid length in young Douglas-fir. Wood Fiber Sci. 2005, 37, 304–313. [Google Scholar]
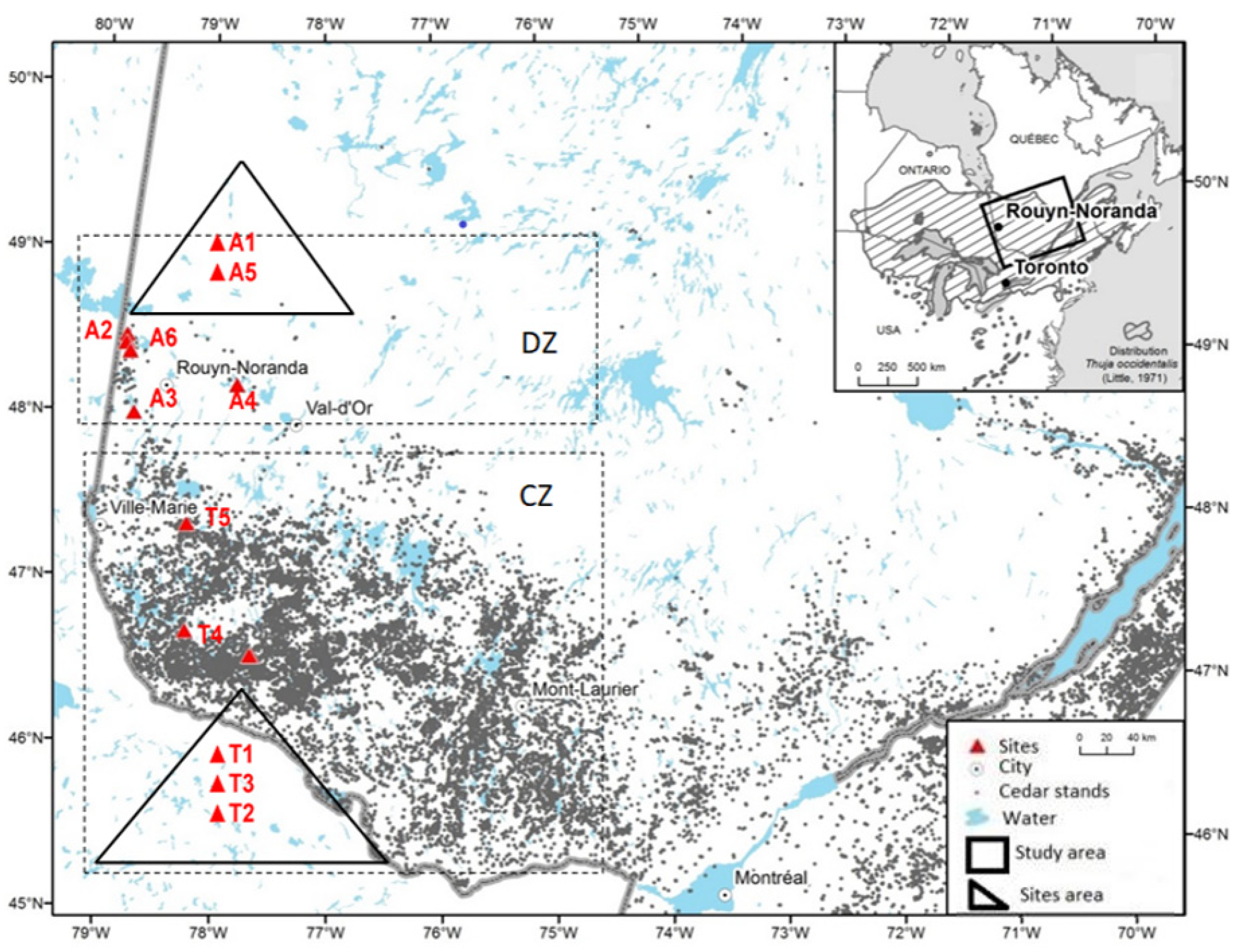
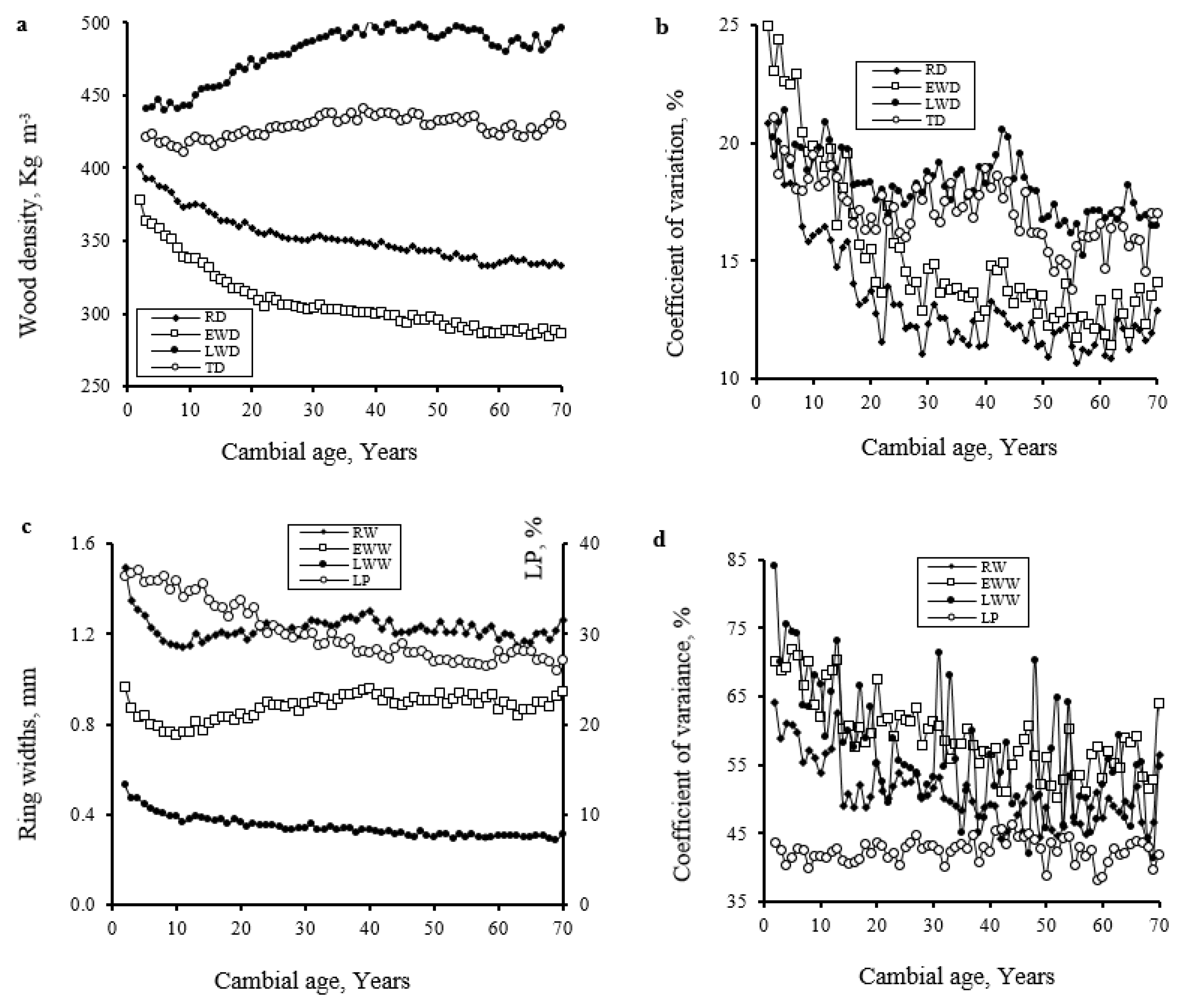


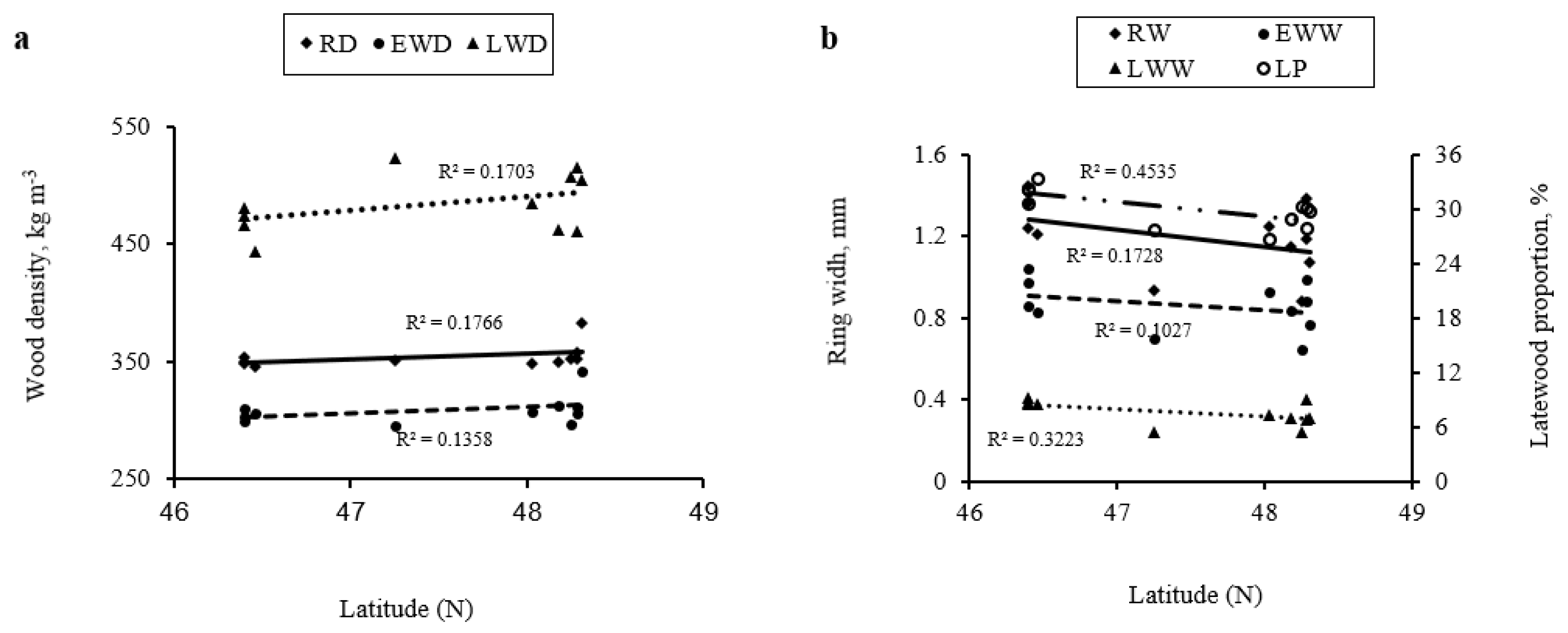
| Regions | Abitibi (Discontinuous Zone) | Témiscamingue (Continuous Zone) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sites | A1 | A2 | A3 | A4 | A5 | A6 | T1 | T2 | T3 | T4 | T5 |
| Localization | FERLD | FERLD | Lac Preissac | Lac Long | FERLD | FERLD | Maniwaki | Maniwaki | Maniwaki | Maniwaki | Laforce |
| Latitude | 48°52′ N | 48°47′ N | 48°05′ N | 48°30′ N | 48°47′ N | 48°42′ N | 46°67′ N | 46°67′ N | 46°67′ N | 46°77′ N | 47°42′ N |
| Longitude | 79°45′ W | 79°43′W | 79°28′ W | 78°38′ W | 79°45′ W | 79°40′ W | 77°95′ W | 77°93′ W | 77°95′ W | 78°55′ W | 78°67′ W |
| Elevation(m) | 317 | 308 | 289 | 349 | 314 | 272 | 388 | 351 | 352 | 280 | 333 |
| Altitude (m) | 1550 | 1620 | 1530 | 1690 | 1630 | 1540 | 1790 | 1700 | 1710 | 1560 | 1970 |
| Mean annual Temperature (°C) a | 1.62 | 1.65 | 1.95 | 1.39 | 1.65 | 1.64 | 2.35 | 2.76 | 2.75 | 3.63 | 2.33 |
| Precipitation (mm) a | 857.82 | 852.48 | 855.25 | 892.63 | 854.1 | 840.66 | 882.95 | 874.15 | 882.95 | 902.87 | 932.28 |
| Number of sampled trees | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| Tree height (m) | 17 (8–22) b | 17 (10–3) | 15 (6–20) | 15 (8–20) | 12 (9–15) | 11 (7–14) | 28 (17–22) | 25 (8–40) | 35 (12–52) | 19 (8–32) | 11 (7–14) |
| DHP (cm) | 17 (10–41) | 16 (10–41) | 17 (10–41) | 17 (10–40) | 16 (10–42) | 16 (10–41) | 17 (6–41) | 18 (6–41) | 19 (8–41) | 20 (14–41) | 17 (16–29) |
| Tree age (years) | 68 (40–140) | 66 (34–95) | 67 (34–93) | 69 (38–103) | 96 (58–134) | 121 (73–198) | 72 (32–156) | 75 (37–138) | 79 (37–160) | 89 (58–170) | 93 (75–127) |
| Number of analyzed trees | 26 | 16 | 29 | 33 | 15 | 15 | 27 | 36 | 36 | 39 | 15 |
| Soil Type | Sand deposit | Clay deposit | Clay deposit | Clay deposit | Clay deposit | Clay deposit | Glacial tills | Glacial tills | Glacial tills | Glacial tills | Sand deposit |
| EWC percentage in the stand (%) | 61–81 | 41–60 | 41–60 | 41–60 | 73 | 84 | 61–80 | 41–60 | 41- 60 | 41- 60 | 70 |
| Ecological Type | Spruce stand | Cedar-fir stand | Cedar-fir stand. | Cedar-fir stand | Cedar-fir stand | Cedar-fir stand | Mixed stand dominated by birch | Conifer stand dominated by cedar associated only with birch | Mixed stand dominated by yellow birch and cedar | Cedar-fir stand | |
| Ring Density Components (kg m−3) | Ring Width Components (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N 1 | RD | EWD | LWD | TD | RW | EWW | LWW | LP (%) | |
| Abitibi-Témiscamingue | |||||||||
| Whole tree | 15651 | 354 (15) | 311 (18) | 478 (19) | 428 (17) | 1.22 (52) | 0.87 (60) | 0.35 (62) | 30.7 (44) |
| Juvenile wood | 6662 | 362 (12) | 319 (15) | 468 (17) | 423 (14) | 1.27 (49) | 0.87 (59) | 0.40 (59) | 32.80 (41) |
| Mature wood | 8989 | 346 (10) | 295 (12) | 497 (15) | 437 (14) | 1.29 (45) | 0.95 (53) | 0.34 (47) | 27.30 (40) |
| Abitibi stands | |||||||||
| A1 | 1435 | 379 (17) a | 338 (19) a | 500 (24) a | 448 (21) a | 1.10 (46) a | 0.79 (51) a | 0.32 (59) a | 29.7 (42) a |
| A2 | 722 | 355 (16) bc | 314 (14) bc | 461 (15) bc | 417 (14) bc | 1.36 (52) bc | 0.96 (59) bc | 0.40 (58) bc | 30.7 (40) bc |
| A3 | 1620 | 351 (15) b | 310 (19) b | 484 (17) bd | 431 (16) bd | 1.25 (43) bd | 0.92 (48) bc | 0.33 (59) ac | 27.0 (42) bd |
| A4 | 1924 | 352 (18) b | 315 (22) bc | 464 (18) bc | 419 (18) bc | 1.15 (52) a | 0.83 (59) a | 0.32 (58) ac | 29.0 (42) ab |
| 5A | 983 | 361 (18) a | 310 (22) bc | 513 (16) be | 456 (16) a | 1.21 (42) bd | 0.89 (50) b | 0.31 (60) a | 29.2 (47) a |
| A6 | 778 | 360 (17) a | 306 (21) bd | 508 (18) ae | 450 (18) a | 0.93 (52) be | 0.67 (70) bd | 0.25 (76) bd | 31.2 (44) bd |
| Témiscamingue stands | |||||||||
| T1 | 1568 | 350 (12) b | 301 (14) be | 470 (17) bc | 418 (16) bc | 1.23 (47) bd | 0.84 (55) a | 0.39 (58) ac | 33.4 (41) be |
| T2 | 1695 | 354 (12) b | 311 (14) be | 466 (18) bc | 421 (17) bc | 1.37 (56) bc | 0.98 (68) bc | 0.38 (57) ac | 32.1 (45) be |
| T3 | 2012 | 353 (10) b | 305 (11) bd | 484 (17) bd | 431 (15) bd | 1.43 (52) bc | 1.02 (63) be | 0.25 (62) bd | 31.0 (44) bc |
| T4 | 2027 | 348 (14) bd | 308 (17) b | 442 (18) be | 401 (17) be | 1.21 (48) bd | 0.82 (59) a | 0.38 (60) ac | 34.4 (43) be |
| T5 | 887 | 354 (13) b | 301 (17) be | 521 (18) be | 460 (17) bf | 0.94 (54) be | 0.69 (62) bd | 0.40 (61) be | 28.3 (43) be |
| Means (CV) 1 | Ring Density Components (kg m−3) | Ring Widths (mm) | ||||||
|---|---|---|---|---|---|---|---|---|
| RD | EWD | LWD | TD | RW | EWW | LWW | LP (%) | |
| Whole tree (Rings 2–70) | ||||||||
| Abitibi | 356 (17) a | 313 (20) a | 490 (19) a | 437 (18) a | 1.14 (50) a | 0.84 (57) a | 0.31 (61) a | 29 (43) a |
| Témiscamingue | 352 (12) a | 304 (15) b | 474 (18) b | 423 (17) b | 1.28 (53) b | 0.90 (63) b | 0.38 (63) b | 32 (44) b |
| F-value | 0.04 ns | 17 *** | 60.84 *** | 58.78 *** | 60.84 *** | 1.53 ns | 351.77 *** | 187.61 *** |
| Juvenile wood (Rings 2–30) | ||||||||
| Abitibi | 378 (19) a | 340 (23) a | 470 (20) a | 432 (19) a | 1.13 (53) a | 0.79 (60) a | 0.35 (66) a | 32 (41) a |
| Témiscamingue | 359 (13) b | 316 (16) b | 458 (19) a | 415 (17) b | 1.30 (55) b | 0.88 (67) b | 0.42 (64) b | 35 (45) b |
| F-value | 60.80 *** | 121.34 *** | 3.14 ns | 24.10 *** | 89.28 *** | 24.96 *** | 154.12 *** | 22.98 *** |
| Mature wood (Rings 30–70) | ||||||||
| Abitibi | 345 (13) a | 296 (15) a | 503 (18) a | 440 (17) a | 1.14 (48) a | 0.86 (55) a | 0.28 (54) a | 27 (43) a |
| Témiscamingue | 343 (11) b | 294 (12) b | 486 (17) b | 429 (16) b | 1.26 (51) b | 0.92 (59) b | 0.34 (57) b | 29 (43) b |
| F-value | 4.10 ns | 6.25 ns | 55.72 *** | 23.49 *** | 24.10 *** | 3.99 * | 172.04 *** | 165.80 *** |
| Fixed Effects | Random Effects | |||||||
|---|---|---|---|---|---|---|---|---|
| Source of variation | Cambial age | Site | Tree nested within the site | residuals | ||||
| Characteristics | F-value | VAR | F-value | VAR | Z-value | VAR | Z-value | VAR |
| All data: Rings 2–70 | ||||||||
| Ring density | 8.1 *** | 12.7 | 6.1 *** | 3.1 | 5.6 *** | 15 | 43.9 *** | 69.0 |
| Earlywood density | 13.0 *** | 18.4 | 5.7 *** | 3.0 | 6.3 *** | 14.3 | 46.4 *** | 64.3 |
| Latewood density | 4.3 *** | 6.2 | 6.1 *** | 5.8 | 9.2 *** | 31.6 | 46.0 *** | 56.9 |
| Transition density | 1.5 ** | 1.5 | 6.0 *** | 5.8 | 8.8 *** | 27.8 | 47.2 *** | 64.9 |
| Ring width | 1.8 *** | 0.6 | 5.6 *** | 7.2 | 7.6 *** | 27.3 | 39.1 *** | 65.0 |
| Earlywood width | 2.3 *** | 2.7 | 3.7 *** | 3.8 | 7.8 *** | 26.9 | 42.2 *** | 66.5 |
| Latewood width | 2.9 *** | 3.1 | 8.3 *** | 9.9 | 8.2 *** | 22.9 | 43.0 *** | 64.0 |
| Latewood proportion | 7.4 *** | 8.7 | 2.5 *** | 1.2 | 9.1 *** | 22.8 | 57.9 *** | 67.3 |
| Juvenile wood: Rings 2–30 | ||||||||
| Ring density | 8.5 *** | 7.24 | 4.6 *** | 3.64 | 5.5 *** | 26.12 | 31.8 *** | 62.91 |
| Earlywood density | 17.2 *** | 14.28 | 6.1 *** | 4.39 | 6.3 *** | 23.03 | 31.9 *** | 58,30 |
| Latewood density | 7.5 *** | 5.47 | 4.6 *** | 6.56 | 9.4 *** | 43.84 | 32.4 *** | 44.12 |
| Transition density | 2.2 ** | 1.23 | 4.9 *** | 7.10 | 9.2 *** | 39.84 | 35.9 *** | 51.83 |
| Ring width | 2.7 *** | 0.40 | 3.9 *** | 7.92 | 8.2 *** | 40.23 | 30.3 *** | 51.44 |
| Earlywood width | 2.0 *** | 1.04 | 3.6 ** | 4.44 | 8.6 *** | 40.17 | 33.4 *** | 54.36 |
| Latewood width | 7.0 *** | 1.78 | 4.9 *** | 8.56 | 8.5 *** | 28.35 | 42.9 *** | 61.30 |
| Latewood proportion | 7.0 *** | 3.39 | 3.0 ** | 2.23 | 9.1 *** | 27.06 | 46.5 *** | 67.32 |
| Mature wood: Rings 30–70 | ||||||||
| Ring density | 2.5 *** | 3.2 | 5.0 *** | 5.0 | 6.8 *** | 32.5 | 31,9 *** | 59.27 |
| Earlywood density | 2.5 *** | 2.6 | 5.5 *** | 6.1 | 7.3 *** | 33.8 | 33.4 *** | 57.48 |
| Latewood density | 0.9 ns | 0 | 4.3 *** | 5.4 | 8.5 *** | 42.5 | 32.9 *** | 52.1 |
| Transition density | 1.0 ns | 0 | 4.0 *** | 3.6 | 7.6 *** | 37.7 | 32.7 *** | 58.7 |
| Ring width | 1.0 ns | 0 | 3.9 *** | 6.9 | 6.6 *** | 36.8 | 27.4 *** | 56.3 |
| Earlywood width | 1.1 ns | 0 | 2.60 * | 2.9 | 6.2 *** | 37.0 | 27.1 *** | 60.1 |
| Latewood width | 1.3 ns | 0.7 | 6.8 *** | 13.2 | 9.0 *** | 36.7 | 45.5 *** | 49.4 |
| Latewood proportion | 1.6 * | 1.1 | 2.7 ** | 2.8 | 8.5 *** | 38.4 | 38.7 *** | 57.7 |
| RD | RW | LA | LO | E | T | P | SD | DHP | TH | |
|---|---|---|---|---|---|---|---|---|---|---|
| Latitude (LA) | 0.08 * | −0.12 * | 1 | |||||||
| Longitude (LO) | 0.076 * | −0.12 * | 0.85 * | 1 | ||||||
| Elevation (E) | −0.01 ns | 0.07 * | −0.37 * | −0.73 * | 1 | |||||
| Temperature (T) | −0.07 * | 0.04 * | −0.82 * | −0.49 * | −0.09 * | 1 | ||||
| Precipitation (P) | −0.07 * | 0.03 * | −0.50 * | −0.54 * | 0.25 * | 0.65 * | 1 | |||
| Stand Density (SD) | 0.09 * | −0.15 * | 0.29 * | 0.35 * | 0.014 ns | −0.28 * | −0.26 * | 1 | ||
| DHP | −0.073 * | 0.08 * | −0.79 * | −0.53 * | −0.11 * | 0.87 * | 0.57 * | −0.49 * | 1 | |
| Tree height (TH) | −0.04 * | 0.17 * | −0.78 * | −0.80 * | 0.57 * | 0.40 * | 0.14 * | −0.33 * | 0.52 * | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouslimi, B.; Koubaa, A.; Bergeron, Y. Regional, Site, and Tree Variations of Wood Density and Growth in Thuja occidentalis L. in the Quebec Forest. Forests 2022, 13, 1984. https://doi.org/10.3390/f13121984
Bouslimi B, Koubaa A, Bergeron Y. Regional, Site, and Tree Variations of Wood Density and Growth in Thuja occidentalis L. in the Quebec Forest. Forests. 2022; 13(12):1984. https://doi.org/10.3390/f13121984
Chicago/Turabian StyleBouslimi, Besma, Ahmed Koubaa, and Yves Bergeron. 2022. "Regional, Site, and Tree Variations of Wood Density and Growth in Thuja occidentalis L. in the Quebec Forest" Forests 13, no. 12: 1984. https://doi.org/10.3390/f13121984
APA StyleBouslimi, B., Koubaa, A., & Bergeron, Y. (2022). Regional, Site, and Tree Variations of Wood Density and Growth in Thuja occidentalis L. in the Quebec Forest. Forests, 13(12), 1984. https://doi.org/10.3390/f13121984







