Abstract
Tree stand dynamics, changes in the ground vegetation and soils, and species diversity of wood-decaying fungi were studied in pristine middle boreal spruce forests affected by a surface fire in the Vodlozersky National Park (Arkhangelsk Region, Russia) in 2011. In the third year after the fire, the burnt area was dominated by birch, which contributed an average of 72% to the total amount of major tree species regeneration. In sites affected by a high-severity fire, the ground vegetation cover did not exceed 40%, with Chamaenerion angustifolium (L.) Scop. and Marchantia polymorpha L. dominating in the first years after. By the tenth year, the diversity of the newly forming tree layer increased from 5 to 11 species and natural thinning of deciduous tree regeneration was already underway, although its amount was still over 100,000 plants per hectare throughout. By the end of the first post-fire decade, Picea abies (L.) H. Karst. and Pinus sylvestris L. accounted for 11% of the total regeneration. The occurrence and cover of pyrogenic species Chamaenerion angustifolium and Marchantia polymorpha declined sharply at this stage. Vegetation in sites affected by mid-severity fire was mostly regenerating through propagation of the survivor Avenella flexuosa (L.) Drejer, Vaccinium myrtillus L., V. vitis-idaea, etc. In the burnt area, the species diversity of wood-destroying fungi was reduced compared to the adjacent unburned areas, and it was the same in both heavily and moderately burnt areas. This is probably due to the fact that the downed deadwood in post-fire sites was trunks of the same age and in the same degree of decay whereas the total amount of downed deadwood in the control (unburnt forest) was lower but featuring all stages of decay and, furthermore, there were plenty of fungi-populated dead standing and weakened overmature trees.
1. Introduction
Fires define the successional dynamics of forests, their mosaic patterns, structure, quantitative and qualitative characteristics of tree stands and tree regeneration [1,2,3,4,5,6,7]. In spite of all fire prevention measures, protected areas in European Russia occasionally suffer from naturally arising local-scale fires. The damaged forest areas then become objects for the study of natural vegetation regeneration processes since economic activities are not allowed in the territory by the conservation regime. The ground vegetation in burnt forest areas can recover through spreading of undamaged underground organs and soil-preserved seeds of the species that had occupied the site prior to the fire as well as owing to colonization of the burnt site by species from the outside [8,9,10,11,12,13,14,15,16,17].
The duration of the post-fire vegetation regeneration period depends on the site conditions and fire severity [18,19,20,21]. In the boreal zone, the species diversity of vascular plants and wood-decaying fungi usually reaches a maximum 10–15 years after the fire [22,23,24,25,26,27]. Communities in a regeneration stage can comprise species of vascular plants, insects and fungi with fire-associated life cycles [28]. Nowadays, the technological development and availability of high-resolution geospatial data leads to an easy assessment of vegetation recovery and forest succession trends with remote sensing [29,30].
Fire-induced changes in the plant community structure strongly influence xylophylic insects too, primarily by generating extra sources of food in the form of deadwood and weakened trees [31] (and references therein). A medium-severity fire weakens a substantial part of trees in the stand, turning them into a readily available resource for the most active species of xylophagous insects, which attack the still-living trees. The attacked trees usually die, causing deadwood volumes to increase further during one or two years after the fire. After dead standing trees fall to the ground, the amount of the substrate suitable for a wider spectrum of xylophilic organisms is augmented.
A fire not only augments deadwood volumes but also alters its composition—slowly decaying charred wood is formed [32,33,34,35,36] and the share of wood in late stages of decay prior to the fire declines [37]—all of these factors influence the composition and abundance of wood-decaying fungi [36,38,39].
In the aftermath of wildfire, accelerated erosion occurs, and it threatens natural regeneration and ecosystem recovery [40,41]. A fire disturbs the morphological profile of soils, changes the direction of organic matter transformation and the course of the soil-formation process in general [42]. Soil surface mineralization and alteration of the thermal regime [43] after a surface fire significantly reshapes the environment for the microbiota [44,45]. The severity of soil disturbance controls the rate at which vegetation will recover after the fire [46,47]. The reverse is also true—gradual recovery of vegetation after a fire event is one of the key factors for regeneration of fire-damaged soils [48]. Organic matter accumulation on the soil surface also contributes to the restoration of the properties and depth of the active soil layer to their pre-fire condition [48,49].
The article describes a unique situation for Europe—a fire in a pristine forest initiated by a dry (without rain) thunderstorm. We present an integrated study of early stages in the natural regeneration of a bilberry-type spruce forest. We performed a comparative analysis of the response of soils, the tree-shrub, herb-subshrub, moss-lichen layers and the aphyllophoroid fungi community in sites affected by a medium- and high-severity surface fire.
The aim of the work was to identify patterns of natural restoration of forest ecosystems after the surface fire of different severity.
We put forward three hypothesis. First, we proposed that (H1) fire severity affects the pattern of shrub and ground layer recovery. Second, we proposed that (H2) fire caused an increase in species diversity during the 10 years following the disturbance (i.e., the biological diversity of communities in early post-fire regeneration stages was higher than in pristine bilberry-type spruce stands). Finally, we suggested that (H3) the post-fire diversity of aphyllophoroid fungi depended on the tree species composition and availability of wood in different stages of decay.
2. Materials and Methods
2.1. Study Area
Surveys were carried out in the Vodlozersky National Park situated in the south-eastern margin of Fennoscandia (middle taiga (mid-boreal) subzone). This park is one of the largest biosphere reserves in Europe with an area of 468,300 hectares [50].
According to climate zonation, this territory belongs to the north-western part of the temperate climate belt, which is characterized by a prevalence of Atlantic and Arctic air masses. The region’s climate is temperate-cold, transitional from maritime to continental. The number of days with mean daily temperatures above +5 °C is 145–155 and with temperatures above +10 °C is 95–100; the annual sum of air temperatures is 1700–1900 °C. Total annual precipitation is 550–600 mm of which 155–165 mm fall during the growing season. Relative air humidity changes from winter toward summer from 50 to 90%. Thunderstorm activity is the higher in the warm period—from May through August [51,52].
Topographically, the park is a rolling plain in the watersheds of the Baltic and the White Seas, adjoining the Windy Belt (Vetreny Poyas) mountain range macroslope on the south. The highest elevation of the range inside the park is 284 m above sea level. The lowest topographic positions are occupied by Vama and Sukhaya Vodla river valleys (124 m a.s.l.). The total difference in elevation is approximately 160 m [53].
Soils in the study area are Humic and Ferric Podzols (Podzols according to WRB) with the diagnostic podzolic horizon up to 5 cm thick. Mean soil temperature in July is 16.6–17.5 °C [52].
Natural forests of the national park (NP) constitute one of the largest and best preserved pristine coniferous forest expanses in the European North with variable species and age structure and high biological diversity [54,55]. The state and natural dynamics of pristine forests in the NP territory have been monitored regularly since 1992 [56]. Data from peat deposit stratigraphic analysis and other indirect indicators suggest that large forest fires happened in the territory once or twice in a millennium and small-scale fires—up to five times in a millennium [57]. During a thunderstorm in July 2011 one such fire broke out in the north-eastern part of the national park (62°45′50″ N, 37°10′20″ E) covering 198 ha in total (Figure 1). The average fire severity over the forest area was medium, with scorch height of 0.5–1.5 m on trunks [58]. In some sites, the fire severity was high, scorching trunks up to 4.0 m height.
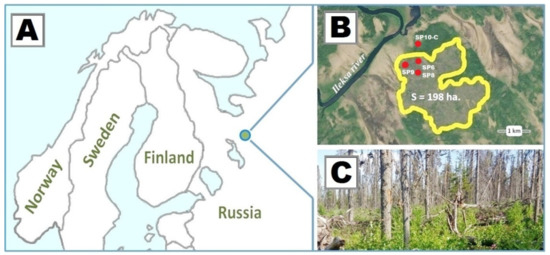
Figure 1.
Location of the forest area burnt and sample plots (SP) in the Vodlozersky National Park (A,B). View of the spruce forest after the fire in 2011 (C).
2.2. Methods
The first phase of the study of the affected forest ecosystems was implemented 3 years after the fire—in 2014. Moderately and heavily affected sites were selected. Fire severity was determined according to trunk scorch height and forest floor depth reduction [58,59]. Permanent sample plots (SPs) 100 m2 (10 × 10 m2) each were established following a standard procedure [60]. All in all, four SPs were outlined: SP 6 and SP 8—in sites burnt by medium-severity fire (up to 0.5 m scorch height, 80%–85% forest floor depth reduction), SP 9—in a site burnt by high-severity fire (over 1.5 m scorch height, 100% forest floor depth reduction), SP 10-C was the control (in a site outside of fire impact). All the surveyed sites were situated on a morainic hill: SPs 6 and 9—on a north-facing gentle slope, SP 8—on flat top.
In 2020, soil pits were made to study the properties of soils in the four SPs. Seven soil samples were taken from each genetic horizon and then combined into one. Thus, one mixed sample was produced for each horizon of the soil profile (O, E, BF) within each site. Physical and chemical properties of the soil were analyzed separately for each sample. Soil samples were dried and crushed to be sieved through 2 mm mesh. The fraction < 2 mm was analyzed. Ash content was determined gravimetrically after incinerating the sample in a muffle furnace. The acidity of salt extracts was determined potentiometrically with a pH meter (Hanna Instruments, Germany). Total nitrogen (N) and carbon (C) concentrations were determined by a CNH analyzer. Soil base saturation was calculated as suggested by Vorobyova [61]. Labile phosphorus (P) was measured by spectrophotometry. The data were obtained using equipment of the Core Facility Analytical Laboratory of the Forest Research Institute of the Karelian Research Centre of the Russian Academy of Sciences.
To facilitate the study of vegetation regeneration processes each SP was subdivided into a hundred 1 m2 subplots. The number (pcs./m2) and height (m) of woody plants was determined in each subplot. Regenerating trees and undergrowth were grouped into height classes: small—up to 0.5 m, medium—0.6–1.5 m, and large—over 1.5 m [62]. The descriptions of the herb-subshrub and moss-lichen layers recorded the composition of species, their occurrence and percentage cover. Other parameters recorded for each subplot were the downed deadwood percentage cover, stone content, etc. Altogether, 300 subplots were described in 2014 and the same subplots were revisited in 2020.
Names of vascular plant species are according to the International Plant Names Index [63]. The species nomenclature of aphyllophoroid fungi is according to Index Fungorum [64], moss species nomenclature follows N. Hodgetts et al. [65].
The survey of wood-decaying basidiomycetes covered the entire burnt area. In 2020, 40 downed trunks were randomly selected in each of heavily and moderately burnt sites separately to determine the frequency of occurrence of fungal species. The presence-absence of any species on trunks was recorded, and one or several fruit bodies of a given species on a single downed trunk were regarded as one case [66,67]. The tree species, diameter and degree of decay of the trunks were taken into account during the sampling. Decay classes were distinguished as suggested by Shorokhova and Shorokhov [68]. Fungi identification in the laboratory was done using LOMO Micmed-6 microscope (Russia) and standard reagents—5% KOH, Melzer’s reagent and 2% Cotton Blue. Specimens of aphyllophoroid fungi and vascular plants are deposited in the Karelian Research Centre RAS Herbarium (PTZ).
The stands were examined for colonization by wood borers in 2014 in three temporary sample plots. The samples were designed as circular plots with 12.62 m radius, which corresponds to 500 m2 area [69]. The stand condition was evaluated by inventorying trees by species, diameter classes, and health category (1, 2—healthy; 3, 4—weakened; 5—current-year dead standing; 6—old dead standing; 7—uprooted; 8—wind-snapped) with special focus on trees inhabited or abandoned by pests [70]. Trees of all health categories were examined for signs of wood borer infection, such as pitch tubes, entrance and emergence holes in bark, tunneling, as well as larvae and adult insects under bark, frass or sawdust on bark, under bark and around the base of the tree.
The hypothesis that fire severity influences vegetation regeneration was tested by running an ordination of geobotanic relevés using detrended correspondence analysis [71,72] in PC-ORD program. The percentage covers of moss-lichen and herb-subshrub layer species were estimated for each subplot. Axis loadings were calculated using Sørensen’s floristic similarity index (Ks).
Cole’s index of association was determined for vascular plant species with occurrence over 5% [73].
3. Results
3.1. State of the Tree Layer after Surface Fire
Prior to the fire, the woodland was an uneven-aged spruce stand with pine contributing 20% and with a minor (5%–10%) contribution of birch and aspen. The stand quality class was IV-V, relative density was 0.5–0.7. Such stands are quite common in the north of European Russia.
The survey of SP 6, which was burnt with medium severity, 3 years after the surface fire (in 2014) showed the forest floor depth reduction was 85%, all spruce was damaged or had died back: 40% of the trees (in volume) became uprooted, the rest of died-back trees remained standing (Figure 2A). Pine and birch survived after the fire in the immediate vicinity of the SP and served as the main source of seeds. By 2020, almost all previously dead standing spruce trees were uprooted with only solo died-back specimens remaining standing, their volume being some 10% of all spruce trees that died back after the fire (Figure 2B).

Figure 2.
Spruce forest site burnt by medium-severity surface fire (SP 6) 3 years (A) and 10 year (B) after the disturbance.
The fire in SP 8 was of medium severity according to fire impact indicators; the forest floor depth reduction was 80%. The fire impact (and the subsequent pest infection) killed the spruce component of the stand almost entirely, but 3 years after the fire 90% of it (in volume) still remained standing, probably due to less severe fire damage to root systems (Figure 3A). Occasional undersized living spruce trees have survived. The cover of downed deadwood was 5.0%. The survey of the plot in 2020 showed that over 70% by volume of spruce trees that had died back after the fire were uprooted. The remaining dead standing tree volume was 29% of the original (Figure 3B).

Figure 3.
Studied spruce forest burnt by medium-severity fire in SP 8 in 2014 (A) and in 2020 (B).
The stand in SP 9 was affected by high-severity fire. The root systems of spruce trees were heavily damaged because of 100% forest floor reduction and 3 years after the fire all spruce had died back, but 78% of the volume still remained standing by 2014 (Figure 4A); downed deadwood cover was 6.0%. In 2020, 32% of the spruce volume in this plot remained as dead standing trees while the rest of spruce was uprooted (Figure 4B). The cover of downed deadwood was 19.0%.

Figure 4.
Pristine spruce forest burnt by high-severity fire in SP 9 in 2014 (A) and in 2020 (B).
Overall, by 2020 the percentage cover of downed deadwood in plots after medium-severity fire increased 1.6–2-fold and in heavily affected plots the increase was 3-fold.
3.2. Biotic Factors Influencing Tree Stand Die-Back after Surface Fires
The fire severely affected spruce. The weakened trees were then attacked by various wood borers and eventually died by 2014, joining the dead standing, uprooted, or wind-snapped categories (Figure 5). Thus, spruce tree mortality in the burnt sites was 100%. At the time of survey, most dead spruce trees were almost totally abandoned by insects; longhorn and bark beetles and their larvae were present on some trunks. Outside the samples, we observed occasional trees infected with six-toothed spruce bark beetle (Pityogenes chalcographus L.), eight-toothed spruce bark beetle (Ips typographus L.), and longhorn beetles of the genus Monochamus.
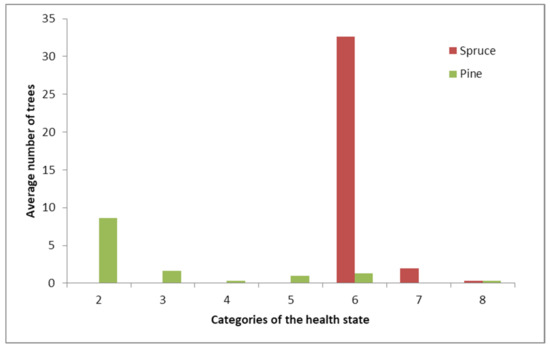
Figure 5.
Distribution of spruce and pine by health categories. 2—weakened, 3—heavily weakened, 4—dying, 5—current-year dead standing, 6—previous-years (old) dead standing, 7—uprooted, 8—wind-snapped.
Pine was much less severely affected by fire; most of the observed pine mortality dating back to pre-fire times. Our inventory found only occasional recently died trees attacked by pine shoot beetles (Tomicus minor Hart. and T. piniperda L.).
Old dead standing trees excluded, pine mortality was 7.5%. There were also pitch tubes of pine shoot beetles on some trunks, indicating failed infestation attempts (Figure 6A). The bottom trunk section of a pine tree not included in the sample carried pupae of the great spruce bark beetle—Dendroctonus micans Kug. (Figure 6B).

Figure 6.
Pine tree infestation attempts: (A)—pitch tube of a pine shoot beetle; (B)—Dendroctonus micans pupa.
Apart from wood borers, which cause tree die-back, the burnt area was populated by a wide range of other species associated directly or indirectly with deadwood. This is, first of all, the group of coleopterans that colonize weakened or recently died-back trees. Pine and spruce trees carried quite a lot of larvae of Rhagium inquisitor L. and Pytho depressus L., and spruce also harbored adult Callidium coriaceum Paykull (Figure 7A) and larvae of Diacanthous undulatus De Geer. Quite abundant were horntails Sirex juvencus L. and Xeris spectrum L. (Figure 7B). At the time of the survey, the burnt area was already colonized by other insects, superseding the first settlers. Quite common among them were predaceous beetle species (Thanasimus formicarius L. and Th. femoralis Zett.) and dipterans (members of the families Asilidae, Hybotidae, Pallopteridae), as well as various parasitic hymenopterans. Noteworthy representatives of the latter were large ichneumon wasps Megarhyssa rixator Shell. (Figure 7C) and Rhyssa persuasoria L., which infect horntail larvae. We also observed the smaller Ephialtes manifestator L., Ischnoceros rusticus Geoffr., Neoxorides montanus Oehlke, Xorides ater Grav., X. irrigator F., Odontocolon spinipes Grav. (Ichneumonidae), Atanycolus spp. and Helconidea dentator F. (Braconidae) and other species that parasitize longhorn beetles of genera Monochamus, Callidium, Tetropium, etc.

Figure 7.
Xylobiotic insects: longhorn beetle Callidium coriaceum (A), horntail Xeris spectrum (B), ichneumon wasp Megarhyssa rixator (C).
3.3. Regeneration of the Tree-Shrub and Herb-Subshrub Layers
Records of the newly forming vegetation in the fire-affected spruce forest area during the entire period of surveys include 34 species, viz., 12 trees and shrubs, 11 subshrubs and herbs, and 11 species of bryophytes (Table 1 and Table 2). In the control plot (10-C), Betula pubescens and Picea abies prevailed among woody plants while the lower layers were dominated by 6 species of vascular plants and 3 moss species. In the first post-fire decade, the burnt area exhibited a notable (3-fold) rise in the species diversity of higher plants compared to the undisturbed control site. By the end of the first decade, the burnt area retained all the vascular plant species and 80% of the moss species found there 3 years after the fire.

Table 1.
Species composition and amounts of tree advance regeneration in burnt sites.

Table 2.
Occurrence and percentage covers of species of the herb-subshrub and moss-lichen layers in permanent sample plots in 2014 and 2020.
In 2014, species of three life forms were represented among vascular plants in the burnt area (Figure 8): the woody and semi-woody plant group prevailed (72.7%). No shrubs were encountered. Among herbaceous life forms, only light-loving perennial herbaceous plants were present, such as Avenella flexuosa, Chamaenerion angustifolium, and Luzula pilosa. By the end of the first post-fire decade, the diversity of plant life forms in the burnt area gradually increased—there appeared shrubs and annual herbs.
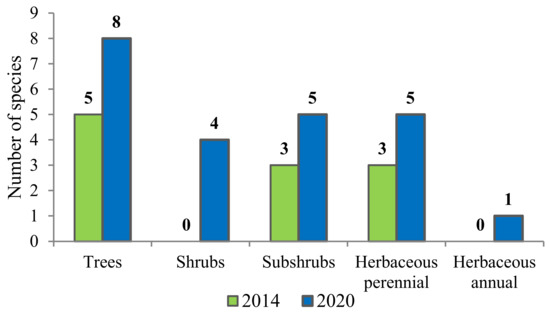
Figure 8.
Changes in the number of vascular plant species with different life forms in the first post-fire decade.
Three years after the fire (in 2014), advance regeneration in each sample plot, irrespective of fire severity and forest floor depth reduction, was represented by five woody plant species. The dominant species in burnt sites in terms of abundance were deciduous species Betula pubescens, Salix caprea, Populus tremula—89.7% of all regeneration. The amount of regenerating Picea abies and Pinus sylvestris 3 years after the fire was 8.7 times lower than that of deciduous species, contributing 10.3% to the total number of plants.
By 2020, the tree-shrub layer was joined by seven more species (Alnus incana, Betula pendula, Salix aurita, S. myrsinifolia, S. pentandra, S. phylicifolia, Sorbus aucuparia). They occurred as solo plants in burnt spots.
Heavily and moderately affected sites, where downed deadwood amounts differ, follow different scenarios of deciduous and coniferous tree regeneration. In 2020, the amount of deciduous regeneration in the plot affected by medium-severity fire and containing a relatively high percentage cover of downed deadwood (SP 6) was nearly halved versus 2014, mainly at the expense of birch, and the amount of coniferous regeneration also decreased by 16%. In a similar plot with a lower downed deadwood cover (SP 8), on the contrary, the amount of regenerating birch increased sharply (2.3-fold). Regenerating pine and spruce amounts there also increased by 29 %. Willow and aspen regeneration in this SP decreased somewhat—by 17 and 3%, respectively. In the plot affected by high-severity fire, which reduced the organic layer by nearly 100% and left behind a low percentage cover of downed deadwood (SP 9), the die-back of all deciduous regeneration 10 years after the fire was 14.5 %, whereas the amount of coniferous regeneration increased by 44% (by 67% for pine).
Ten years after the fire, approximately 2/3 of the tree regeneration was saplings 0.6 to 1.5 m tall. The share of small regeneration (up to 0.5 m) was somewhat lower (26%), whereas large specimens, taller than 1.5 m (chiefly birch and pine), were singular (Table 3).

Table 3.
Size distribution of the most common species of woody regeneration 10 years after surface fire.
The average height of regenerating trees (Hav) in the burnt area 10 years after the fire was 0.5–0.67 m for deciduous and 0.23–0.65 m for coniferous species in moderately affected plots (SPs 6, 8), while in the heavily affected plot (SP 9) it was almost uniform for all deciduous species (0.75 m) but varied for conifers from 0.3 m (spruce) to 0.62 m (pine).
Regeneration of the herb-subshrub layer 3 years after the fire mainly proceeded through spreading of the surviving subshrub and herb species that had dominated the community prior to the fire and through massive expansion of Chamaenerion angustifolium—a pioneer species of disturbed habitats. In moderately affected sites, Avenella flexuosa, Luzula pilosa, Vaccinium myrtillus, and V. vitis-idaea demonstrated comparatively high occurrence and total cover. In heavily affected sites however, these species sharply declined in abundance (occurrence/cover: 2.3–6-/2–8-fold decline in Avenella flexuosa, 10-/2-fold in Luzula pilosa, 10–16.5-/18–20-fold in Vaccinium myrtillus, 8–17-/2–6-fold in V. vitis-idaea). Chamaenerion angustifolium, on the contrary, responded in the opposite manner: the species occurrence and total cover increased 1.1–1.3- and 2-fold, respectively, in heavily affected versus moderately affected sites. The total cover of the herb-subshrub layer in this period still remained below 1/3 of that in undisturbed communities irrespective of the organic layer depth.
Ten years after the fire, the species diversity of the ground vegetation increased 1.7-fold owing to the arrival of Dactylorhiza maculata, Linnaea borealis, Lycopodium clavatum, Maianthemum bifolium, and Melampyrum pratense. The herb-subshrub layer was still dominated by Avenella flexuosa, Luzula pilosa, Vaccinium myrtillus, and V. vitis-idaea but with some changes in their contributions to the cover. Avenella flexuosa became 4.8 times more frequent and several times more abundant in heavily affected sites. The occurrence of Vaccinium myrtillus in heavily burnt sites increased 2-fold and its percentage cover 5–10 fold. As to Vaccinium vitis-idaea, its occurrence increased 1.8-fold in moderately affected sites and 6.6-fold in sites with 100% forest floor depth reduction, but the species cover remained unchanged (<1%) since 2014. The occurrence of Chamaenerion angustifolium showed no sharp change by 2020, but its cover declined significantly (5–10 times). The total cover of the herb-subshrub layer by the end of the first post-fire decade was within 10–20%. The moss-lichen layer dominants in this succession stage were the liverwort Marchantia polymorpha and Polytrichum juniperinum. Marchantia polymorpha recruitment in the ground vegetation of burnt sites in the first post-fire years has been reported previously by other researchers [15]. The occurrence of Polytrichum juniperinum was quite high irrespectively of the organic layer rate, whereas that of Marchantia polymorpha was 2.6 times higher in heavily affected sites. The percentage cover of Polytrichum juniperinum in moderately affected sites was several times that of heavily burnt sites, whereas the trend for Marchantia polymorpha was the reverse—its cover was six times higher in heavily affected sites. The usually very common moss species Dicranum scoparium, Hylocomium splendens, and Pleurozium schreberi exhibited extremely low occurrence and cover and were so far present only in moderately affected sites. Overall, the total cover of the moss-lichen layer in the first several years after the fire was 4–11-fold lower than in undisturbed in forest areas, depending on fire impact severity.
Ten years after the fire impact, Polytrichum juniperinum retained its dominant role in the moss-lichen layer. A sharp rise (6–7-fold) was seen in the occurrence of Pleurozium schreberi, which in this period started colonizing heavily affected sites as well; the percentage cover of this species however remained very low. Moderately affected sites showed also a significant rise in the occurrence of Dicranum scoparium and Hylocomium splendens, but these species have not yet arrived in heavily affected sites. In this succession stage, the community no longer contained Marchantia polymorpha, but there appeared the multi-substrate moss species—Pohlia nutans. No thalli of macroscopic lichen species have formed in the sites in the first post-fire decade.
3.4. Diversity of Aphyllophoroid Fungi
The survey in 2014 detected 13 species of wood-decaying fungi in the burnt area and the corresponding number for 2020 was 41 species (Table 4). In 2020, moderately affected SPs featured 32 species (27 in SP 6, 18 in SP 8) while SP 9 contained 25 species. Most of them were found on downed spruce trunks—28; birch trunks carried 11 species, pine trunks—2 species. Fungal abundance differences between plots were insignificant. The most common species in the burnt area were Fomes fomentarius, Fomitopsis pinicola, Gloeophyllum sepiarium, Phellinidium ferrugineofuscum, Trichaptum fuscoviolaceum, and T. abietinum. Less frequent were Daedalea xantha, Pycnoporus cinnabarinus, Peniophora septentrionalis, Phellinus nigricans, and Rhodofomes roseus. Veluticeps abietina was encountered only in moderately affected plots. A majority of the species grew on logs in decay classes 1 and 2, and only some 30% of species were found on downed deadwood in decay class 4.
Amylocystis lapponica and Daldinia concentrica were detected only in the first post-fire years (2014 survey). The rest of the species were spotted in both 2014 and 2020.
In 2020, both moderately and heavily affected SPs contained Peniophora septentrionalis, which is listed among rare and protected species in the Republic of Karelia but is not red-listed in the Arkhangelsk Region [74].
The diversity of xylotrophic fungi in old-growth forests around the burnt area was notably higher—there occurred 45 species (SP 10-C) of which only 16 (35.6%) were present in the burnt sites. The high richness of wood-decaying fungi in pristine spruce forests is due to the plenitude of downed coniferous and deciduous deadwood in different stages of decay and to the characteristic microclimate of these forests [75,76,77,78]. The intact forests adjoining the burnt area contained Junghuhnia collabens—a rare species red-listed in the Arkhangelsk Region and the Republic of Karelia [74,79], as well as 15 fungal indicator-species of old-growth forest [80].


Table 4.
Species composition and occurrence of aphyllophoroid fungi in the sample plots.
Table 4.
Species composition and occurrence of aphyllophoroid fungi in the sample plots.
| Species | Log Species Identity | 2014 | Diameter, cm | Decay Class | 2020 | SP 10-C | ||
|---|---|---|---|---|---|---|---|---|
| SP 6 | SP 8 | SP 9 | ||||||
| Amphinema byssoides (Pers.) J. Erikss. | S | – | 8 | 4 | 1 | – | – | – |
| Amylocorticium suaveolens Parmasto | S | – | – | – | – | – | – | + |
| ** Amylocystis lapponica (Romell) Bondartsev et Singer | S | + | – | – | – | – | – | + |
| Antrodia sinuosa (Fr.) P. Karst. | S | – | 8 | 4 | 1 | 1 | – | – |
| * Asterodon ferruginosus Pat. | S | – | 18–20 | 3–4 | – | – | – | + |
| ** Byssomerulius albostramineus(Torrend) Hjortstam | P | – | – | – | – | – | – | + |
| Cerrena unicolor (Bull.) Murrill | B | – | 15 | 3 | 1 | – | – | + |
| ** Chaetodermella luna (Romell ex D.P. Rogers et H.S. Jacks.) Rauschert | P | – | – | – | – | – | – | + |
| Coltricia perennis (L.) Murrill | litter | – | – | – | – | – | – | + |
| Coniophora arida (Fr.) P. Karst. | S | – | – | – | – | – | – | + |
| C. olivacea (Fr) P. Karst. | S | – | 18 | 2 | 1 | – | – | + |
| ** Crustoderma dryinum (Berk. et M.A. Curtis) Parmasto | S | – | 24 | 2 | – | – | 1 | + |
| Cyanosporus caesius (Schrad.) McGinty | S | – | 8–17 | 2–4 | – | 1 | 1 | – |
| Daedalea xantha (Fr.) A. Roy et A.B. De | S | – | 10–70 | 2–4 | 1 | 2 | 1 | + |
| Daldinia concentrica (Bolton) Ces. et De Not. | B | + | – | – | – | – | – | – |
| Dichomitus squalens (P. Karst.) D.A. Reid | S | – | 20 | 1 | – | – | 1 | – |
| Diplomitoporus flavescens (Bres.) Domański | P | – | – | – | – | – | – | + |
| Fibrodontia brevidens (Pat.) Hjortstam et Ryvarden | S | – | 20 | 2 | – | – | – | + |
| Fomes fomentarius (L.) Fr. | B | + | 12–22 | 1–3 | 3 | 1 | 7 | + |
| Fomitiporia punctata (P. Karst.) Murrill | B | – | 12 | 2 | – | – | 1 | – |
| Fomitopsis betulina (Bull.) B.K. Cui, M.L. Hanet Y.C. Dai | B | + | 14–16 | 1–2 | – | 1 | 3 | + |
| F. pinicola (Sw.) P. Karst. | B, S | + | 11–26 | 1–3 | 11 | 6 | 7 | + |
| Gloeophyllum odoratum (Wulfen) Imazeki | S | – | 28 | 4 | – | 1 | – | – |
| G. sepiarium (Wulfen) P. Karst. | S | + | 10–26 | 1–4 | 4 | 12 | 15 | + |
| * Hericium coralloides (Scop.) Pers. | A, B | – | 18 | 2 | 1 | – | – | + |
| Hydnomerulius pinastri (Fr.) Jarosch et Besl | S | – | – | – | – | – | – | + |
| Hyphoderma setigerum (Fr.) Donk | S | – | 5 | 1 | 1 | – | – | – |
| Incrustoporia chrysella (Niemelä) Zmitr. | S | – | – | – | – | – | – | + |
| ** Inonotus leporinus (Fr.) Gilb. et Ryvarden | S | – | – | – | – | – | – | + |
| I. obliquus (Fr.) Pilát | B | – | – | – | – | – | – | + |
| ** Junghuhnia collabens (Fr.) Ryvarden | S | – | – | – | – | – | – | + |
| Mycoacia livida (Pers.) Zmitr. | S | – | – | – | – | – | – | + |
| Neoantrodia serialis (Fr.) Audet | S | – | – | – | – | – | – | + |
| Peniophora cinerea (Pers.) Cooke | B | – | – | – | – | – | – | + |
| P. pithya (Pers.) J. Erikss. | S | – | 12 | 2–3 | – | – | 1 | – |
| P. septentrionalis Laurila | S | – | 8–18 | 2–4 | 1 | 1 | 4 | – |
| Phanerochaete sordida (P. Karst.) J. Erikss. et Ryvarden | S | – | – | – | – | – | – | + |
| * Phellinidium ferrugineofuscum (P. Karst.) Fiasson et Niemelä | S | + | 4–26 | 2–4 | 4 | 5 | 1 | + |
| * Phellinus chrysoloma (Fr.) Donk | S | + | 12–18 | 2–3 | – | – | 4 | + |
| P. igniarius (L.) Quél. | W | – | – | – | – | – | – | + |
| P. laevigatus (P. Karst.) Bourdot et Galzin | B | – | 10 | 2 | – | – | 1 | – |
| P. lundellii Niemelä | B | – | – | – | – | – | – | + |
| P. nigricans (Fr.) P. Karst. | B | – | 12–24 | 1–2 | 1 | 1 | 4 | – |
| P. tremulae (Bondartsev) Bondartsev et P.N. Borisov | A | – | – | – | – | – | – | + |
| * P. viticola (Schwein.) Donk | S | + | 4–70 | 2 | 2 | – | – | + |
| ** Phellopilus nigrolimitatus (Romell) Niemelä, T. Wagner et M. Fisch. | S | – | – | – | – | – | 4 | + |
| Piloderma bicolor (Peck) Jülich | S | – | 6–18 | 2–4 | 1 | 1 | – | – |
| Porodaedalea pini (Brot.) Murrill | P | – | 32 | 1 | – | 1 | – | – |
| * Pycnoporellus fulgens (Fr.) Donk | S | – | 19–20 | 2 | 2 | – | – | + |
| Pycnoporus cinnabarinus (Jacq.) P. Karst. | B | – | 7–24 | 1–2 | 1 | 1 | 3 | – |
| * Rhodofomes roseus (Alb. et Schwein.) Kotl. et Pouzar | S | + | 5–22 | 2–4 | 4 | 3 | 1 | + |
| Scytinostroma odoratum (Fr.) Donk | S | – | 38 | 1 | 1 | – | – | – |
| Stereum sanguinolentum (Alb. et Schwein.) Fr. | S | – | – | – | – | – | – | + |
| Thelephora terrestris Ehrh. ex Fr. | S, litter | + | 6 | 2 | 1 | – | – | – |
| Thelephora wakefieldiae Zmitr., Shchepin, Volobuev et Myasnikov [= Tomentella sublilacina (Elliset Holw.) Wakef.] | S | – | 6 | 1 | 1 | – | – | – |
| Tomentella coerulea Höhn. et Litsch. | S | – | 12 | 4 | 1 | – | 1 | – |
| T. lapida (Pers.) Stalpers | S | – | – | – | 1 | – | – | – |
| Trametes ochracea (Pers.) Gilb. et Ryvarden | B | – | 16 | 2 | – | – | 1 | + |
| Trechispora sp. | S | – | – | – | – | – | – | + |
| Trichaptum abietinum (Pers. ex J.F. Gmel.) Ryvarden | S | + | 5–26 | 1–4 | 5 | 2 | 2 | + |
| T. biforme (Fr.) Ryvarden | B | – | 16–30 | 2–3 | 2 | – | 2 | – |
| T. fuscoviolaceum (Ehrenb.) Ryvarden | S | + | 8–32 | 2–3 | 4 | 5 | 2 | + |
| T. laricinum (P. Karst.) Ryvarden | P, S | – | 18–20 | 1–2 | – | 1 | 2 | + |
| Tubulicrinis borealis J. Erikss. | S | – | 12 | 4 | – | – | 1 | – |
| T. calothrix (Pat.) Donk | S | – | 12 | 4 | – | – | – | + |
| ** Tyromyces odorus (Sacc.) Zmitr. | S | – | – | – | – | – | – | + |
| Veluticeps abietina (Pers.) Hjortstam et Tellería | S | – | 70 | 2 | 1 | – | – | – |
| Xylodon asper (Fr.) Hjortstam et Ryvarden | S | – | 12 | 4 | – | – | – | + |
Note. *, **—indicator species for boreal old-growth forests according to [80]. Log species identity: A—aspen, B—birch, P—pine, S—spruce, W—willow. +—species present; “–“—species absent.
3.5. Soils 10 Years after Surface Fire
In 2020, the most pronounced changes were found in the soils (Table 5) that had experienced the heaviest pyrogenic disturbance (SP 9). Their top horizon demonstrated a rise in ash content to 25.7% and a reduction in C, N, P content, pH, and base saturation. Analysis of the podzolic horizon (E) of soils in the most severely burnt plot revealed an increase in ash content and P content. Soils in this plot had a reduced content of K and C. The illuvial horizon (BF) of the soils showed a trend for a reduction in K and P content with a simultaneous rise in pH.

Table 5.
Chemical properties of soils 10 years after surface fire.
4. Discussion
Our study corroborates one of the general consequences of a forest fire: ash content in the topsoil is augmented as a result of the litter and ground vegetation combustion and the input of burnt woody debris. Stable pyrogenetic products can be preserved in the soil for decades causing direct and indirect effects on soil properties [81]. Vegetation regeneration after a heavy forest fire is constrained by a deficit of available C and N [82,83]. On the other hand, our results revealed no sharp change in the content of the studied elements, possibly due to a compensatory effect of deciduous (primarily birch) litter from young plants and of downed tree trunks, which contain large amounts of carbon [84]. Another putative factor is the relatively low fire severity and the short duration of the fire impact on soil [85]. Regeneration of soil properties is influenced also by the length of time since the fire, so there may prove to be no changes in the properties of fire-disturbed soils [86].
The factors governing the post-fire recovery of the forest plant community are the site conditions, pre-fire community structure, and forest floor depth reduction [11,27,87,88]. The amount of coniferous (Picea abies and Pinus sylvestris) regeneration by the end of the first post-fire decade was relatively low but increased gradually, reaching 1/8 of the total number of regenerating trees. This two-tiered structure, where coniferous regeneration lags significantly behind deciduous species in growth and birch is the absolute dominant, is typical of young stands forming in burnt spruce forests [89,90], and is reason to expect the community will regenerate to its original state through a birch-stand stage. In our case, coniferous regeneration can be regarded as successful—the amount of spruce regeneration was 16,900 plants per ha, that of pine—13,000 plants per ha. To compare, Gavrilova and Pak [91] reported that coniferous regeneration in mid-boreal bilberry-type spruce forests in the south of the Republic of Karelia 9 years after a fire was very poor (if at all present).
The height distribution of advance regeneration was influenced by surface fire severity. Where the difference between the amounts of small and medium regeneration in the tenth year after the medium-severity fire was within 60%, the heavily affected plot had almost ten times as much medium-size regeneration (3,390,000 plants per ha) as small-size regeneration (33,100 plants per ha).
Irrespective of fire severity there were of regenerating Picea abies in amounts 11,800–20,300 plants per ha, which would likely survive under the birch canopy [92,93,94]. The dominance of birch in fire-affected sites and its faster height growth [90,95,96,97] does not in the long term impede spruce regeneration.
One of the most widespread and active fire-site colonizer species is Chamaenerion angustifolium, which the most intensively invades heavily burnt sites in the first post-fire years [9,13,98], as confirmed also by our surveys. The ground vegetation in moderately affected sites mostly regenerated through spreading of the vegetation dominants surviving from the pre-fire bilberry-type spruce stand (Avenella flexuosa, Vaccinium myrtillus, Vaccinium vitis-idaea).
Mosses and, especially so, lichens are highly vulnerable to fire impact and are often totally destroyed by fire of any intensity [87]. The fire of 2011 killed mosses and epigeic lichens almost throughout the burnt area except for small fire-bypassed spots retaining intact forest floor, where occasional Dicranum scoparium and Pleurozium schreberi plants have survived.
The results of DCA ordination reflect the effects of forest floor depth reduction rate, abundance of advance regeneration and downed deadwood on the formation patterns of the herb-subshrub and moss-lichen layers (Figure 9). Four groups of relevés can be visually distinguished, forming a left-to-right “heavily affected—moderately affected” gradient.
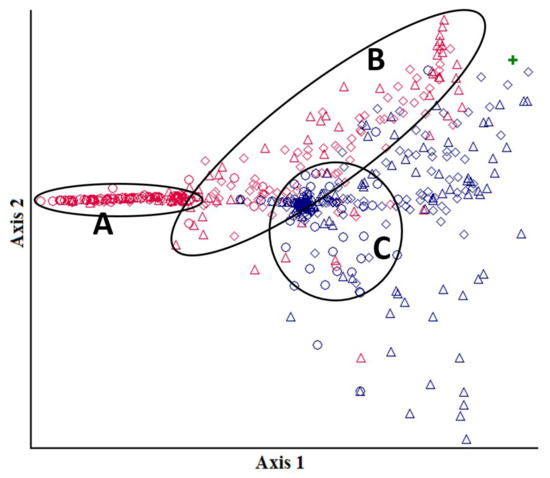
Figure 9.
Ordination plot for geobotanic relevés of ground vegetation (similarity in the space formed by the first two DCA axes) in a bilberry-type spruce stand site 3 and 10 years after surface fire. Legend:  —SP 6 in 2014,
—SP 6 in 2014,  —SP 6 in 2020,
—SP 6 in 2020,  —SP 8 in 2014,
—SP 8 in 2014,  —SP 8 in 2020,
—SP 8 in 2020,  —SP 9 in 2014,
—SP 9 in 2014,  —SP 9 in 2020. A–C—see text for explanations. Axis loadings: axis 1—0.33, axis 2—0.09.
—SP 9 in 2020. A–C—see text for explanations. Axis loadings: axis 1—0.33, axis 2—0.09.
 —SP 6 in 2014,
—SP 6 in 2014,  —SP 6 in 2020,
—SP 6 in 2020,  —SP 8 in 2014,
—SP 8 in 2014,  —SP 8 in 2020,
—SP 8 in 2020,  —SP 9 in 2014,
—SP 9 in 2014,  —SP 9 in 2020. A–C—see text for explanations. Axis loadings: axis 1—0.33, axis 2—0.09.
—SP 9 in 2020. A–C—see text for explanations. Axis loadings: axis 1—0.33, axis 2—0.09.
Polygons “A” and “B” encompass groups of ground vegetation relevés 3 years after the fire (2014). Polygon “A” comprises relevés from subplots with the forest floor most heavily disturbed by the fire (high-severity fire—SP 9). The values therein are quite closely packed, and lined up can be explained by the high occurrence and cover of dominant pioneer species: in 61% of subplots with Chamaenerion angustifolium, it had a total cover of 20%–70%; in 48% of subplots with Marchantia polymorpha, its cover was 20%–90%. Polygon “B” contains relevés from plots after medium-severity fire. The ground vegetation in moderately affected sites in any part of the burnt area followed similar development scenarios in the first post-fire years, as indicated by close packing of relevés from both SP 6 and SP 8 within contour “B”. In contrast to the heavily burnt sites (A), ground vegetation relevés from SP 6 and SP 8 in contour “B” are scattered over the space formed by the two axes as a result of highly diverse species combinations.
Ten years after high-severity fire (contour “C”), the sites still have the ground vegetation regeneration trends similar to those in early succession stages, as indicated by the relatively high floristic similarity index for SP 9 in 2014 and in 2020 (Ks = 0.75). Furthermore, geobotanic relevés from SP 9 in 2020 are also densely packed, as in 2014, aligned with contour “A” and situated very close to it. These are all indicators of a slow regeneration of the herb-subshrub and moss-lichen layers in sites affected by a high-severity surface fire.
Sites after medium-severity fire, on the contrary, exhibited clear differentiation in ground vegetation regeneration 10 years after the fire. The floristic similarity between SP 6 and SP 8 decreased from Ks= 0.84 in 2014 to Ks= 0.72 in 2020 as pre-fire species re-appeared (Linnaea borealis, Maianthemum bifolium, Melampyrum pratense, etc.). Relevés from the moderately affected SP 6 and SP 8 become more sparsely scattered over the ordination plot than in 2014. The scatter along axis 2 is due to differences in the moss-lichen layer (abundance of the shade-tolerant Dicranum scoparium and light-loving Pleurozium schreberi and Polytrichum juniperinum), which depend on the density of tree regeneration and the cover of downed deadwood. By the tenth post-fire year, almost all fire-damaged trees have fallen down, lying horizontally one over another, but most of the trunks were not yet in contact with the ground. This could have generated a mock-up forest “canopy” in SP 6, with its typical shading and, hence, a different environment for ground vegetation formation compared to the less shaded SP 8.
Analysis of interspecific association has demonstrated a strongly positive association (Cole’s index is 1.0) only with each other and a negative association with all other species in the community to exist for two pioneer species—Chamaenerion angustifolium and Marchantia polymorpha. These species employ a strategy of a rapid yet short-term (just for several post-fire years) “seizure” of disturbed habitats. Ten years after the fire, already the occurrence and/or cover of such species drop sharply (Chamaenerion angustifolium) or the species totally vanish from the vegetation layer (Marchantia polymorpha).
Predominantly strong positive associations were demonstrated also by typical ground vegetation dominants: the closest association in the herb-subshrub layer was between the subshrubs Vaccinium myrtillus, Vaccinium vitis-idaea and the perennial forest grass Avenella flexuosa. The species with the strongest positive correlation in the moss-lichen layer were Hylocomium splendens (maximum association with subshrubs) and Polytrichum juniperinum, which was closely associated with Luzula pilosa and Melampyrum pratense, whose occurrence grew sharply ten years after the fire. A typical inhabitant of boreal forests Pleurozium schreberi, which showed a sharp rise in occurrence ten years after the fire, proved to be the most closely associated (0.7) with Maianthemum bifolium—a characteristic species of bilberry-type spruce forests, which responded very negatively to the disturbance and re-appeared in the burnt area only by the end of the first post-fire decade. The association of Pleurozium schreberi with other species in the moss-lichen layer (Dicranum scoparium, Hylocomium splendens, Pohlia nutans) was positive but minor (0.2–0.4), and its association with the dominant of post-fire communities Polytrichum juniperinum was negative (−0.1). The data on interspecies associations suggest that co-habitation of species in the first post-fire decade is determined by a shared response to the fire impact and similar extent of forest floor depth reduction.
Fire intensity had a little effect on the composition of wood-decaying fungi, influencing only their abundances. The fungi diversity in fire-affected sites was less than in intact pristine forests, one of the reasons being the absence of downed aspen trunks.
In the future, as the tree layer forms, the slightly damaged uprooted trunks overhanging the ground will turn into a good substrate to be colonized by new fungal species and to promote the abundance of those already present in the burnt area [78,99,100,101]. Our results agree with the statement that many xylophylic species are sensitive to properties of downed deadwood, such as the species of the tree [100,102], log size [103] and stage of decay [104]. Other important factors are the cover of vegetation (mostly mosses and lichens) on the surface of trunks [105], their contact with the ground [106], and wood charring [104].
5. Conclusions
During the first decade after mixed severity fires in pristine uneven-aged spruce stands, spruce almost disappeared from the canopy. Mortality of spruce trees was due to fire damage to the conducting tissues in the lower part of the trunk and to the species’ shallow root system, and as a consequence of infestation of fire-weakened trees by insect pests, namely, Ips typographus. Some pine and birch survived the fire and served as seed sources for the burnt sites, influencing the composition of the newly forming young stands. During early succession stages (3–10 years after the fire) the absolute dominant in the natural tree regeneration is birch (80%–90% of the total number of plants). As fire severity grows, the share of deciduous species in the advance regeneration tends to increase.
A significant increase in the species diversity of trees and shrubs in the first post-fire decade was accompanied by thinning of the deciduous canopy. While remaining in a subordinate position, coniferous regeneration gradually augments its absolute numbers and even more so its share in the structure of advance regeneration. We can thus predict that the bilberry-type spruce forest will regenerate through a deciduous dominance stage—the shade-tolerant spruce capable of growing under canopy cover for a century will move to the first story after the currently dominant birch trees have dropped out. Pine, getting outgrown by birch, will likely experience increased mortality under the birch canopy.
Recovery of ground vegetation after fire depends on the degree of burning of the organic layer: the community appearance in the first years after a high-severity fire is predominantly shaped by pioneer species Chamaenerion angustifolium, Marchantia polymorpha. By the tenth post-fire year, the occurrence of typical forest-dwelling species increases, but their cover remains low. In moderately affected sites, active participants of vegetation renewal on the disturbed substrate in the first post-fire years are the species that had occupied the site prior to the impact (Avenella flexuosa, Vaccinium myrtillus, Vaccinium vitis-idaea).
Ten years after the fire, the transformation of the upper soil horizon is preserved, while alterations of the deeper-lying mineral horizons are minor.
The fact that soils in fire-affected sites had no major differences in properties compared to the control site ten years after the fire suggests a compensatory effect of litter of deciduous young growth.
The diversity of woody and ground vegetation species in the affected sites even 3 years after the fire was higher than in the control and continued rising by the tenth year. There were species typical of pristine bilberry-type spruce forests as well as pioneer species that occupied disturbed sites in the first post-fire 10 years. So, the diversity of wood-decaying fungi in the first post-fire 10 years did not depend on the surface fire severity but was determined by the availability of downed deadwood in different stages of decay—the number of species was the highest on fallen spruce trunks in all sites irrespective of fire impact severity. Ten years after the fire, fungal diversity in the burnt area was lower than in the control.
The article presents the results of comprehensive studies on vegetation restoration in the first 10 years after the fire, which will be continued.
Author Contributions
Conceptualization, V.A.A. and A.M.K.; methodology, V.A.A., V.V.T., A.M.K., A.V.R., M.V.M., E.E.K., A.V.P. and A.E.H.; software, E.E.K.; investigation, V.A.A., V.V.T., A.M.K., A.N.P., E.E.K., A.V.R., S.A.M., M.V.M., E.E.K., A.V.P. and A.E.H.; writing—original draft preparation, V.V.T.; writing—review and editing, V.A.A., V.V.T., A.M.K., A.N.P., E.E.K., A.V.R., M.V.M., E.E.K., A.V.P. and A.E.H. All authors have read and agreed to the published version of the manuscript.
Funding
The study was carried out under state assignment to the Forest Research Institute of the Karelian Research Centre (FRI KarRC RAS) with support from the Vodlozersky National Park and the Scientific and Educational Center “Russian Arctic: New Materials, Technologies and Research Methods” (to A.M.K.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Soil chemical analyses were performed using the equipment of the Analytical Laboratory of FRI KarRC RAS. We thank M.A. Boychuk (Cand. Sci. (Biol.), Institute of Biology KarRC RAS), whom we consulted for moss species identification, and I. Drobyshev (SLU) for valuable advice during preparation of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dyrenkov, S.A. Structure and Dynamics of Taiga Spruce Forests; Nauka: Leningrad, Russia, 1984; p. 174. (In Russian) [Google Scholar]
- Bakhtin, A.A. Formation of deciduous-spruce forest stands on the burned areas of the Arkhangelsk region. In Flora of the North and Plant Resources of the European Part of the USSR; Arkhangelsk, Russia, 1997; pp. 81–83. (In Russian) [Google Scholar]
- Ryan, K.C. Dynamic interactions between forest structure and fire behavior in boreal ecosystems. Silva Fenn. 2002, 36, 13–39. [Google Scholar] [CrossRef]
- Lundqvist, L.; Nilson, K. Regeneration dynamics in an uneven-aged virgin Norway Spruce forest in northern Sweden. Scand. J. For. Res. 2007, 22, 304–309. [Google Scholar] [CrossRef]
- Cai, W.H.; Yang, J. High-severity fire reduces early successional boreal larch forest aboveground productivity by shifting stand density in north-eastern China. Int. J. Wildland Fire 2016, 25, 861–875. [Google Scholar] [CrossRef]
- Chambers, M.E.; Fornwalt, P.J.; Malone, S.L.; Battaglia, M.A. Patterns of conifer regeneration following high severity wildfire in ponderosa pine—dominated forests of the Colorado Front Range. For. Ecol. Manag. 2016, 378, 57–67. [Google Scholar] [CrossRef]
- Stavrova, N.I.; Gorshkov, V.V.; Katyutin, P.N.; Bakkal, I.J. The Structure of Northern Siberian Spruce–Scots Pine Forests at Different Stages of Post-Fire Succession. Forests 2020, 11, 558. [Google Scholar] [CrossRef]
- Foster, D.R. Vegetation development following fire in Picea mariana (black spruce) Pleurozium forests of south-eastern Labrador, Canada. J. Ecol. 1985, 73, 517–534. [Google Scholar] [CrossRef]
- Stickney, P.F. First Decade Plant Succession Following the Sundance Fire, Northern Idaho; General Technical Report INT-197; US Department of Agriculture, Forest Service, Intermountain Research Station: Ogden, UT, USA, 1986; p. 26.
- Schimmel, J.; Granstrom, A. Fire severity and vegetation response in the boreal Swedish forest. Ecology 1996, 77, 1436–1450. [Google Scholar] [CrossRef]
- Turner, M.G.; Romme, W.H.; Gardner, R.H.; Hargrove, W.W. Effects of fire size and pattern on early succession in Yellowstone National Park. Ecol. Monogr. 1997, 67, 411–433. [Google Scholar] [CrossRef]
- Hart, B.T.; Burgman, M.; Grace, M.; Pollino, C.; Thomas, C.; Webb, J.A.; Allison, G.A.; Chapman, M.; Duivenvoorden, L.; Feehan, P.; et al. Ecological Risk Management Framework for the Irrigation Industry; Technical Report; Land and Water Australia: Canberra, Australia, 2005. [Google Scholar]
- Ruokolainen, L.; Salo, K. The effect of the fire intensity on vegetation succession on a sub-xeric heath during ten years after wildfire. Ann. Bot. Fenn. 2009, 46, 30–42. [Google Scholar] [CrossRef]
- Kovaleva, N.M.; Ivanova, G.A. Recovery of living ground vegetation at the initial stage of pyrogenic succession. Contemp. Probl. Ecol. 2013, 2, 203–213. (In Russian) [Google Scholar]
- Kovaleva, N.M. Effect of surface fires on ground layer in larch stands of the lower Angara region. Bot. Zhurn. 2014, 11, 1269–1277. (In Russian) [Google Scholar]
- Khapugin, A.A.; Vargot, E.V.; Chugunov, G.G. Vegetation recovery in fire-damaged forests: A case study at the southern boundary of the taiga zone. For. Stud. Metsanduslikud Uurim. 2016, 64, 39–50. [Google Scholar] [CrossRef]
- Ivanova, G.A.; Ivanov, V.A.; Kovaleva, N.M.; Konard, S.G.; Zhila, S.V.; Tarasov, P.A. Succession of vegetation after the high intensity fire in a pine forest with lichens. Contemp. Probl. Ecol. 2017, 17, 61–71. (In Russian) [Google Scholar] [CrossRef]
- Kuleshova, L.V.; Korotkov, V.N.; Potapova, N.A.; Evstigneev, O.I.; Kozlenko, A.B.; Rusanova, O.M. Comprehensive analysis of post-fire successions in the forests of the Kostomuksha Reserve. Bull. Mosc. Soc. Naturalists. Biol. Ser. 1996, 101, 3–15. (In Russian) [Google Scholar]
- Zyryanova, O.A.; Bugaenko, T.N. Species diversity of larch associations in the permafrost zone of Central Siberia and its post-fire transformation. Structural and functional organization and dynamics of forests. In Proceedings of the All-Russian Conference, Krasnoyarsk, Russia, 1–3 September 2004; pp. 301–303. (In Russian). [Google Scholar]
- Ivanova, G.A.; Perevoznikova, V.D.; Ivanov, V.A. Transformation of the lower tiers of forest vegetation after ground fires. Russ. J. For. Sci. 2002, 2, 30–35. (In Russian) [Google Scholar]
- Zyryanova, O.A.; Bugaenko, T.N.; Abaimov, A.P.; Bugaenko, N.N. Pyrogenic transformation of species diversity in the larch forests of the cryolithozone. In Forest Ecosystems of the Yenisey Meridian; Publishing House SO RAS: Novosibirsk, Russia, 2002; pp. 135–146. (In Russian) [Google Scholar]
- Isaeva, L.G.; Himich, Y.R.; Kostina, V.A. Diversity of spruce forests and aphyllophoroid fungi in the Murmansk region. In Coniferous Forests of Northern Latitudes—From Research to Environmentally Responsible Forestry; Kauhanen, H., Neshataev, V., Huhta, E., Vuopio, M., Eds.; Kopijyvä: Jyväskylä, Finland, 2009; pp. 49–60. (In Russian) [Google Scholar]
- Himich, Y.R. Polypore Fungi in the Process of Succession of Spruce Forests of the Murmansk Region. Ph.D. Thesis, Syktyvkar, Russia, 2011; p. 20. (In Russian). [Google Scholar]
- Suominen, M.; Junninen, K.; Heikkala, O.; Kouki, J. Combined effects of retention forestry and prescribed burning on polypore fungi. J. Appl. Ecol. 2015, 52, 1001–1008. [Google Scholar] [CrossRef]
- Komarova, T.A. Post-Fire Successions in the Forests of Southern Sikhote-Alin; Publishing House of the Far Eastern Scientific Center: Vladivostok, Russia, 1992; p. 222. (In Russian) [Google Scholar]
- Abaimov, A.P.; Prokushkin, S.G.; Zyryanova, O.A. Ecological and phytocenotic assessment of the impact of fires on the forests of the permafrost zone of Central Siberia. Contemp. Probl. Ecol. 1996, 1, 51–601. (In Russian) [Google Scholar]
- Kalinin, K.K. Vegetation cover successions on large burnt areas of the Middle Trans-Volga. Vestn. Mari State Univ. Ser. For. Ecology. Nat. Manag. 2008, 1, 19–28. (In Russian) [Google Scholar]
- Engelmark, O.; Hytteborn, H. Coniferous forests. Acta Phytogeogr. Suec. 1999, 84, 55–74. [Google Scholar]
- Oikonomakis, N.; Ganatsas, P. Land cover changes and forest succession trends in a site of Natura 2000 network (Elatia forest), in northern Greece. For. Ecol. Manag. 2012, 285, 153–163. [Google Scholar] [CrossRef]
- Christopoulou, A.; Mallinis, G.; Vassilakis, E.; Farangitakis, G.P.; Fyllas, N.M.; Kokkoris, G.D.; Arianoutsou, M. Assessing the impact of different landscape features on post-fire forest recovery with multitemporal remote sensing data: The case of Mount Taygetos (southern Greece). Int. J. Wildland Fire 2019, 28, 521–532. [Google Scholar] [CrossRef]
- Hjältén, J.; Dynesius, M.; Hekkala, A.-M.; Karlsson-Tiselius, A.; Löfroth, T.; Mugerwa-Pettersson, R. Saproxylic Insects and Fire. In Saproxylic Insects; Diversity, Ecology and Conservation, Zoological Monographs; Ulyshen, M.D., Ed.; Springer: Cham, Switzerland, 2018; pp. 669–691. [Google Scholar] [CrossRef]
- Kamdem, D.P.; Pizzi, A.; Jermannaud, A. Durability of heat-treated wood. Holz Roh Werkst 2002, 60, 1–6. [Google Scholar] [CrossRef]
- Weiland, J.J.; Guyonnet, R. Study of chemical modifications and fungi degradation of thermally modified wood using DRIFT spectroscopy. Holz Roh Werkst 2003, 61, 216–220. [Google Scholar] [CrossRef]
- Hakkou, M.; Pétrissans, M.; Gérardin, P.; Zoulalian, A. Investigation of the reasons for fungal durability of heat-treated beech wood. Polym. Degrad. Stabil. 2006, 91, 393–397. [Google Scholar] [CrossRef]
- Shorokhova, E.; Kapitsa, E.; Vanha-Majamaa, I. Decomposition of stumps in a chronosequence after clear-felling vs. clear-felling with prescribed burning in a southern boreal forest in Finland. For. Ecol. Manag. 2008, 255, 3606–3612. [Google Scholar] [CrossRef]
- Edman, M.; Eriksson, A.-M. Competitive outcomes between wood decaying fungi are altered in burnt wood. FEMS Microbiol. Ecol. 2016, 92, fiw068. [Google Scholar] [CrossRef]
- Eriksson, A.M.; Olsson, J.; Jonsson, B.G.; Toivonen, S.; Edman, M. Effects of restoration fire on dead wood heterogeneity and availability in three Pinus sylvestris forests in Sweden. Silva Fenn. 2013, 47, 954. [Google Scholar] [CrossRef]
- Van der Wal, A.; Ottosson, E.; de Boer, W. Neglected role of fungal community composition in explaining variation in wood decay rates. Ecology 2015, 96, 124–133. [Google Scholar] [CrossRef]
- Carlsson, F.; Edman, M.; Jonsson, B.G. Increased CO2 evolution caused by heat treatment in wood-decaying fungi. Mycol. Prog. 2017, 16, 513–519. [Google Scholar] [CrossRef]
- Godfree, R.C.; Knerr, N.; Encinas-Viso, F.; Albrecht, D.; Bush, D.; Christine Cargill, D.; Broadhurst, L.M. Implications of the 2019–2020 megafires for the biogeography and conservation of Australian vegetation. Nat. Commun. 2021, 12, 1023. [Google Scholar] [CrossRef]
- Stefanidis, S.; Alexandridis, V.; Spalevic, V.; Mincato, R.L. Wildfire Effects on Soil Erosion Dynamics: The Case of 2021 Megafires in Greece. Agric. For. 2022, 68, 49–63. [Google Scholar] [CrossRef]
- Bogorodskaya, A.V.; Kukavskaya, E.A. State of microbial communities in soils of deciduous and light coniferous forests of Central Siberia after logging and fires. Russ. J. For. Sci. 2016, 5, 383–396. (In Russian) [Google Scholar]
- Narita, K.; Harada, K.; Saito, K.; Sawada, Y.; Fukuda, M.; Tsuyuzaki, S. Vegetation and Permafrost Thaw Depth 10 Years after a Tundra Fire in 2002, Seward Peninsula, Alaska. Arctic Antarct. Alp. Res. 2015, 47, 547–559. [Google Scholar] [CrossRef]
- Vázquez, F.J.; Acea, M.J.; Carballas, T. Soil Microbial Populations after Wildfire. FEMS Microbiol. Ecol. 1993, 13, 93–103. [Google Scholar] [CrossRef]
- Bezkorovaynaya, I.N.; Tarasov, P.A.; Krasnoshchekova, E.N. Ecological state of soils after a fire in the pine forests of the middle taiga of the Krasnoyarsk territory. Bull. KrasGAU 2006, 13, 178–183. (In Russian) [Google Scholar]
- Bogdanov, V.V.; Prokushkin, A.S.; Prokushkin, S.G. The influence of grass-roots fires on the mobility of soil organic matter in the larch forests of the cryolithozone of Central Siberia. Bull. KrasGAU 2009, 2, 88–93. (In Russian) [Google Scholar]
- Dymov, A.A.; Dubrovsky, Y.A.; Gabov, D.N.; Zhangurov, E.V.; Nizovtsev, N.A. Influence of a fire in a northern taiga spruce forest on soil organic matter. Russ. J. For. Sci. 2015, 1, 52–62. (In Russian) [Google Scholar]
- Michaelides, R.; Schaefer, K.; Zebker, H.; Parsekian, A.; Liu, L.; Chen, J.; Natali, S.M.; Ludwig, S.; Schaefer, S. Inference of the impact of wildfire on permafrost and active layer thickness in a discontinuous permafrost region using the remotely sensed active layer thickness (ReSALT) algorithm. Environ. Res. Lett. 2018, 14, 035007. [Google Scholar] [CrossRef]
- Heim, R.J.; Bucharova, A.; Rieker, D.; Yurtaev, A.; Kamp, J.; Hölzel, N. Long-term effects of fire on Arctic tundra vegetation in Western Siberia. Sci. Total Environ. 2019, 760, 143425. [Google Scholar] [CrossRef]
- Ananyev, V.A. National Park “Vodlozerskii”. In Karelia: Encyclopedia; PetroPress Publishing House: Petrozavodsk, Russia, 2009; Volume 2, pp. 270–272. (In Russian) [Google Scholar]
- Nazarova, L.E. Climate. In Biodiversity of Karelia: Formation Conditions, Communities, Species; Gromtsev, A.N., Kitaev, S.P., Krutov, V.I., Eds.; Karelian Scientific Center of the Russian Academy of Sciences: Petrozavodsk, Russia, 2003; pp. 6–8. [Google Scholar]
- Filatov, N.N.; Derusova, O.V.; Zhukov, A.I. Atlas of the Republic of Karelia; Verso: Petrozavodsk, Russia, 2021; p. 48. (In Russian) [Google Scholar]
- Kipruhin, I.; Valdaev, V.; Volkova, A. Protected Natural Territories of the Republic of Karelia; Petrozavodsk, Russia, 2017; p. 432. (In Russian) [Google Scholar]
- Gromtsev, A.N. Primeval Forests in Karelia: Present State and Protection Prospects. Primeval Forests in the European Taiga Zone: The Present State and Conservation Problems; Review of the Proceedings of the International Scientific and Practical Conference; KarNC: Petrozavodsk, Russia, 1999; pp. 18–22. [Google Scholar]
- Pellikka, J.; Kurhinen, J.; Danilov, P.; Lindén, H.; Ovaskainen, O.; Gromtsev, A. Dimensions of the wildlife richness in Eastern Fennoscandia. Vestn. Ohotovedeniya 2014, 11, 266–269. [Google Scholar]
- Ananyev, V.A.; Raevsky, B.V.; Grabovik, S.I. Monitoring of biodiversity in the primeval spruce forests of Vodlozero national park. In Biodiversity and Conservation of Boreal Nature, Proceedings of the 10th Year Anniversary of the National Reserve Friendship, Vantaa, Finland, 2 October 2003; Heikkilä, R., Lindholm, T., Eds.; 485, Dark Oy; Kainuu Regional Environment Centre: Kajaani, Finland, 2003; pp. 113–116. [Google Scholar]
- Gromtsev, A.N. Fundamentals of Landscape Ecology of European Taiga Forests in Russia; KarRC of RAS: Petrozavodsk, Russia, 2008; p. 250. (In Russian) [Google Scholar]
- Zalesov, S.V. Forest Pyrology: Textbook; UGLTU: Ekaterinburg, Russia, 1998; p. 296. (In Russian) [Google Scholar]
- Zalesov, S.V. Forest Pyrology: Textbook; UGLTU: Ekaterinburg, Russia, 2021; p. 396. (In Russian) [Google Scholar]
- Andreeva, E.; Bakkal, I.; Gorshkov, V.; Lianguzova, I.; Maznaia, E.; Neshataev, V.; Neshataeva, V.; Stavrova, N.; Iarmishko, V.; Iarmishko, M. Methods for Studying Forest Communities; NIIKhimii: Saint Petersburg State University: Saint Petersburg, Russia, 2002; p. 240. (In Russian) [Google Scholar]
- Vorobyeva, L.A. Theory and Methods of Chemical Analysis of Soils; Publishing House of Moscow State University: Moscow, Russia, 1995; p. 136. [Google Scholar]
- Rules for Reforestation; Approved by order of the Ministry of Natural Resources of the Russian Federation, N 118, dated 25 March 2019; Ministry of Natural Resources of the Russian Federation: Moscow, Russia, 2020; p. 138.
- International Plant Names Index. The Royal Botanic Gardens, Kew, Harvard University Herbaria & Libraries and Australian National Botanic Gardens. 2021. Available online: http://www.ipni.org (accessed on 1 October 2021).
- Index Fungorum. CABI Bioscience. 2022. Available online: http://www.indexfungorum.org (accessed on 23 March 2022).
- Hodgetts, N.G.; Söderström, L.; Blockeel, T.L.; Caspari, S.; Ignatov, M.S.; Konstantinova, N.A.; Lockhart, N.; Papp, B.; Schröck, C.; Sim-Sim, M.; et al. An annotated checklist of bryophytes of Europe, Macaronesia and Cyprus. J. Bryol. 2020, 42, 1–116. [Google Scholar] [CrossRef]
- Lindblad, I. Wood-inhabiting fungi on fallen logs of Norway spruce: Relations to forest management and substrate quality. Nord. J. Bot. 1998, 18, 243–255. [Google Scholar] [CrossRef]
- Junninen, K.; Kouki, J. Are woodland key-habitats in Finland hotspots for polypores (Basidiomycota)? Scand. J. For. Res. 2006, 21, 32–40. [Google Scholar] [CrossRef]
- Shorokhova, E.V.; Shorokhov, A.A. Coarse woody debris dynamics and stores in a boreal virgin spruce forest. Ecol. Bull. 2001, 49, 129–137. [Google Scholar]
- Tuzov, V.K. (Ed.) Methods of Monitoring of Forest Pests and Diseases. Reference Book; VNIILM: Moscow, Russia, 2004; Volume III, p. 200. (In Russian) [Google Scholar]
- Vorontsov, A.I.; Mozolevskaya, E.G.; Sokolova, E.S. Technology of Forest Protection; Ecologia: Moscow, Russia, 1991; p. 304. [Google Scholar]
- Hill, M.O. DECORANA—A FORTRAN Program for Detrended Correspondence Analysis and Reciprocal Averaging. Ecology and Systematics; Cornell University: Ithaca, NY, USA, 1979; p. 52. [Google Scholar]
- Hill, M.O.; Gauch, H.G. Detrended correspondence analysis: An improved ordination technique. Vegetatio 1980, 42, 47–58. [Google Scholar] [CrossRef]
- Vasilevich, V.I. Statistical Methods in Geobotany; Nauka: Leningrad, Russia, 1969; p. 232. (In Russian) [Google Scholar]
- Anufriev, V.; Bespalaia, I.; Bolotov, I.; Ezhov, O.; Mamontov, V.; Mizin, I.; Novoselov, A.; Potapov, G.; Puchnina, L.; Pystina, T.; et al. (Eds.) Red Data Book of the Arkhangelsk Region; Northern (Arctic) Federal University: Arkhangelsk, Russia, 2020; p. 490. (In Russian) [Google Scholar]
- Kubartova, A.; Ottosson, E.; Dahlberg, A.; Stenlid, J. Patterns of fungal communities among and within decaying logs, revealed by 454 sequencing. Mol. Ecol. 2012, 21, 4514–4532. [Google Scholar] [CrossRef]
- Juutilainen, K.; Mönkkönen, M.; Kotiranta, H.; Halme, P. Resource use of wood-inhabiting fungi in different boreal forest types. Fungal Ecol. 2017, 27, 96–106. [Google Scholar] [CrossRef]
- Ruokolainen, A.; Shorokhova, E.; Penttilä, R.; Kotkova, V.; Kushnevskaya, E. A continuum of dead wood with various habitat elements maintains the diversity of wood-inhabiting fungi in an old-growth boreal forest. Eur. J. For. Res. 2018, 137, 707–718. [Google Scholar] [CrossRef]
- Yang, S.; Limpens, J.; Sterck Frank, J.; Sass-Klaassen, U.; Cornelissen, J.H.C.; Hefting, M.; Richard van Logtestijn, S.P.; Goudzwaard, L.; Dam, N.; Dam, M.; et al. Dead wood diversity promotes fungal diversity. Oikos 2021, 130, 2202–2216. [Google Scholar] [CrossRef]
- Kuznetsov, O.L. (Ed.) The Red Data Book of the Republic of Karelia; KONSTANTA: Belgorod, Russia, 2020; p. 448. (In Russian) [Google Scholar]
- Andersson, L.; Mikhailova, N.; Kuznetsova, E. (Eds.) Survey of Biologically Valuable Forests in North-Western European Russia; Identification Manual of Species to be Used During Survey at Stand Level; Pobeda: Saint-Petersburg, Russia, 2009; Volume 2, p. 258. (In Russian) [Google Scholar]
- Dymov, A.A.; Abakumov, E.V.; Bezkorovaynaya, I.N.; Prokushkin, A.S.; Kuzyakov, Y.V.; Milanovskyy, E.Y. Impact of forest fire on soil properties (review). Theor. Ecol. 2018, 4, 13–23. [Google Scholar] [CrossRef]
- Fernandes, P.M.; Vega, J.A.; Jiménez, E.; Rigolot, E. Fire resistance of European pines. For. Ecol. Manag. 2008, 256, 246–255. [Google Scholar] [CrossRef]
- Majder-Łopatka, M.; Szulc, W.; Rutkowska, B.; Ptasiński, D.; Kazberuk, W. Influence of fire on selected physico-chemical properties of forest soil. Soil Sci. Annu. 2019, 70, 39–43. [Google Scholar] [CrossRef]
- Tzvetkova, N.; Hadjiivanova, C. Chemical composition and biochemical changes in needles of Scots pine (Pinus sylvestris L.) stands at different stages of decline in Bulgaria. Trees Struct. Funct. 2006, 20, 405–409. [Google Scholar] [CrossRef]
- Barid, M.; Everett, R.; Zabowski, D. Wildfire effects on carbon and nitrogen in inland coniferous forests. Plant Soil 1999, 209, 233–243. [Google Scholar] [CrossRef]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef]
- Kovaleva, N.M.; Ivanova, G.A.; Kukavskaya, E.A. Restoration of ground cover after ground fires in middle taiga pine forests. Russ. J. For. Sci. 2011, 5, 30–35. (In Russian) [Google Scholar]
- Zhila, S.V.; Ivanova, G.A.; Ivanov, V.A.; Tsvetkov, P.A. Reforestation after fires of different intensity in pine forests of Central Siberia. Sib. Lesn. Zurnal 2019, 6, 53–62. (In Russian) [Google Scholar]
- Degteva, S.V.; Dubrovsky, Y.A. Vegetation cover dynamics during regenerative successions on burnt areas of dark coniferous forests of the Pechoro-Ilychsky Reserve. Proc. Pechoro-Ilychsky Reserve 2010, 16, 35–41. (In Russian) [Google Scholar]
- Kalinin, K.K. Natural reforestation and formation of young stands in spruce and birch plantations on large burnt areas of the Middle Trans-Volga region. Bull. Mari State Tech. Univ. Ser. Forest. Ecology. Nat. Manag. 2010, 1, 5–15. (In Russian) [Google Scholar]
- Gavrilova, O.I.; Pak, K.A. Natural forest restoration after fires in the Republic of Karelia. Adv. Curr. Nat. Sci. 2017, 12, 38–44. (In Russian) [Google Scholar]
- Man, R.; Lieffers, V.J. Are mixtures of aspen and white spruce more productive than single species stands? For. Chron. 1999, 75, 505–513. [Google Scholar] [CrossRef]
- Gryazkin, A.V. Renewal Potential of Taiga Forests (On the Example of Spruce Forests in the North-West of Russia); SPbGLTA: Saint-Petersburg, Russia, 2001; p. 188. (In Russian) [Google Scholar]
- Ranade, S.S.; García-Gil, M.R. Molecular signatures of local adaptation to light in Norway Spruce. Planta 2021, 253, 53. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, Y. Species and stand dynamics in the mixed woods of Quebec’s southern boreal forest. Ecology 2000, 81, 1500–1516. [Google Scholar] [CrossRef]
- Farber, S.K. The impact of fires on the forests of Eastern Siberia. For. Inventory For. Plan. 2012, 1, 131–141. (In Russian) [Google Scholar]
- Parro, K.; Metslaid, M.; Renel, G.; Sims, A.; Stanturf, J.A.; Jõgiste, K.; Köster, K. Impact of postfire management on forest regeneration in a managed hemiboreal forest, Estonia. Can. J. For. Res. 2015, 45, 1192–1197. [Google Scholar] [CrossRef]
- Green, D.G. Simulated effects of fire, dispersal and spatial pattern on competition within forest mosaics. Vegetatio 1989, 82, 139–153. [Google Scholar] [CrossRef]
- Rajala, T.; Tuomivirta, T.; Pennanen, T.; Mäkipää, R. Habitat models of wood-inhabiting fungi along a decay gradient of Norway spruce logs. Fungal Ecol. 2015, 18, 48–55. [Google Scholar] [CrossRef]
- Müller, J.; Ulyshen, M.; Seibold, S.; Cadotte, M.; Chao, A.; Bässler, C.; Vogel, S.; Hagge, J.; Weiß, I.; Baldrian, P.; et al. Primary determinants of communities in deadwood vary among taxa but are regionally consistent. Oikos 2020, 129, 1579–1588. [Google Scholar] [CrossRef]
- Oberle, B.; Lee, M.R.; Myers, J.A.; Osazuwa-Peters, O.L.; Spasojevic, M.J.; Walton, M.L.; Young, D.F.; Zanne, A.E. Accurate forest projections require long-term wood decay experiments because plant trait effects change through time. Glob. Chang. Biol. 2020, 26, 864–875. [Google Scholar] [CrossRef]
- Jonsell, M.; Weslien, J.; Ehnström, B. Substrate requirements of red-listed saproxylic invertebrates in Sweden. Biodivers. Conserv. 1998, 7, 749–764. [Google Scholar] [CrossRef]
- Økland, B.; Bakke, A.; Hågvar, S.; Kvamme, T. What factors influence the diversity of saproxylic beetles? A multiscaled study from a spruce forest in southern Norway. Biodivers. Conserv. 1996, 5, 75–100. [Google Scholar] [CrossRef]
- Renvall, P. Community structure and dynamics of wood-rotting Basidiomycetes on decomposing conifer trunks in northern Finland. Karstenia 1995, 35, 1–51. [Google Scholar] [CrossRef]
- Vanha-Majamaa, I.; Lilja, S.; Ryömä, R.; Kotiaho, J.S.; Laaka-Lindberg, S.; Lindberg, H.; Puttonen, P.; Tamminen, P.; Toivanen, T.; Kuuluvainen, T. Rehabilitating boreal forest structure and species composition in Finland through logging, dead wood creation and fire: The EVO experiment. For. Ecol. Manag. 2007, 250, 77–88. [Google Scholar] [CrossRef]
- Dynesius, M.; Gibb, H.; Hjältén, J. Surface Covering of Downed Logs: Drivers of a Neglected Process in Dead Wood Ecology. PLoS ONE 2010, 5, e13237. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).