The Triggering Effect of Gaps on Seedling Germination of the Soil Seed Bank in Tropical Rain Forests, Hainan Island, South China
Abstract
1. Introduction
2. Material and Methods
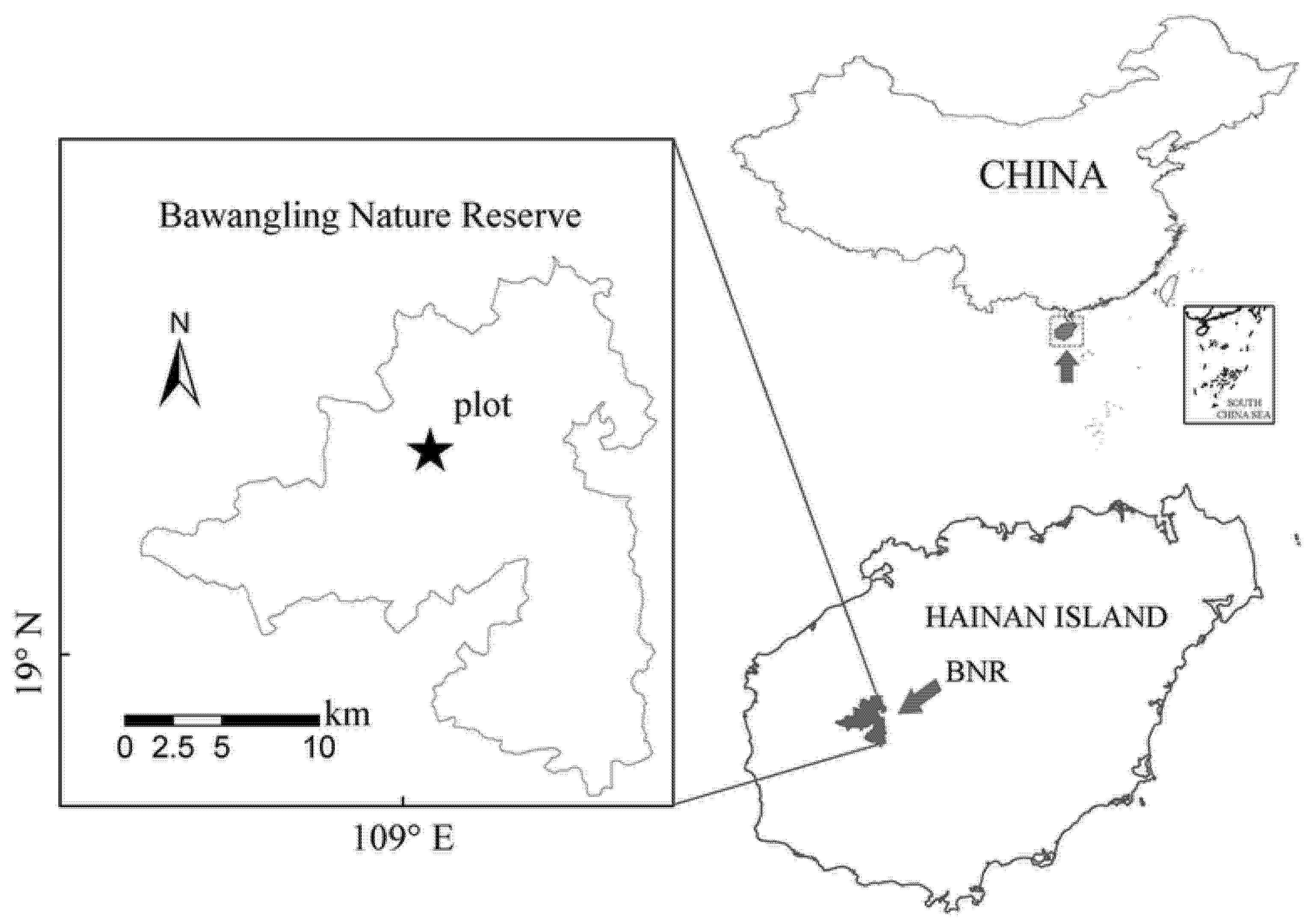
2.1. Overview of the Study Site
2.2. The Photo-Thermal Separation Method Used in the Forest Understory
2.3. Indoor Verification Experiment
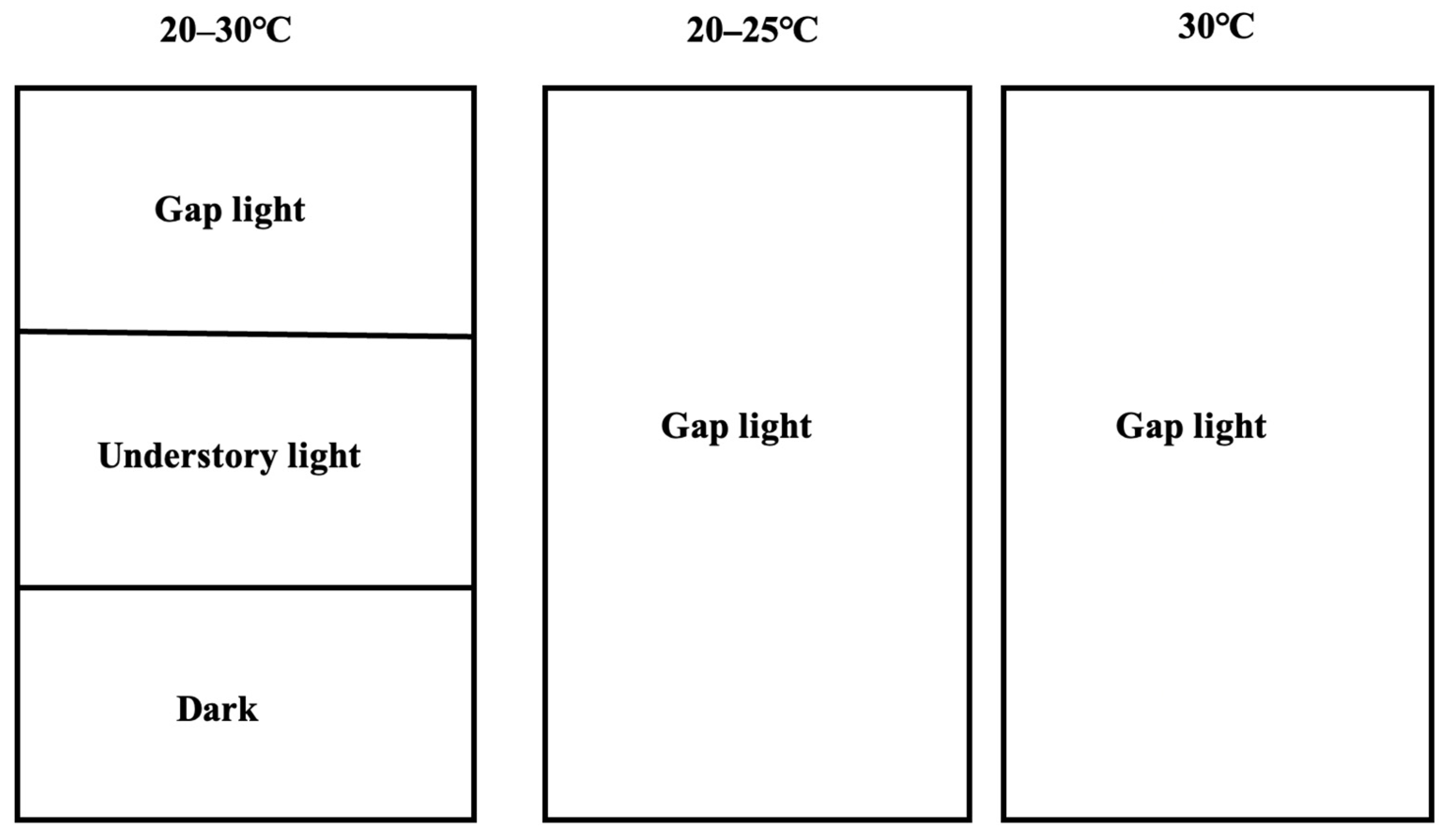
2.4. Data Analyses
- (1)
- FG = number of germinated seeds/total number of seeds × 100%;
- (2)
- GS = number of days from the start of the experiment to 1/6 of the FG;
- (3)
- MTG = Σ Ni. Di/Σ Ni.
3. Results
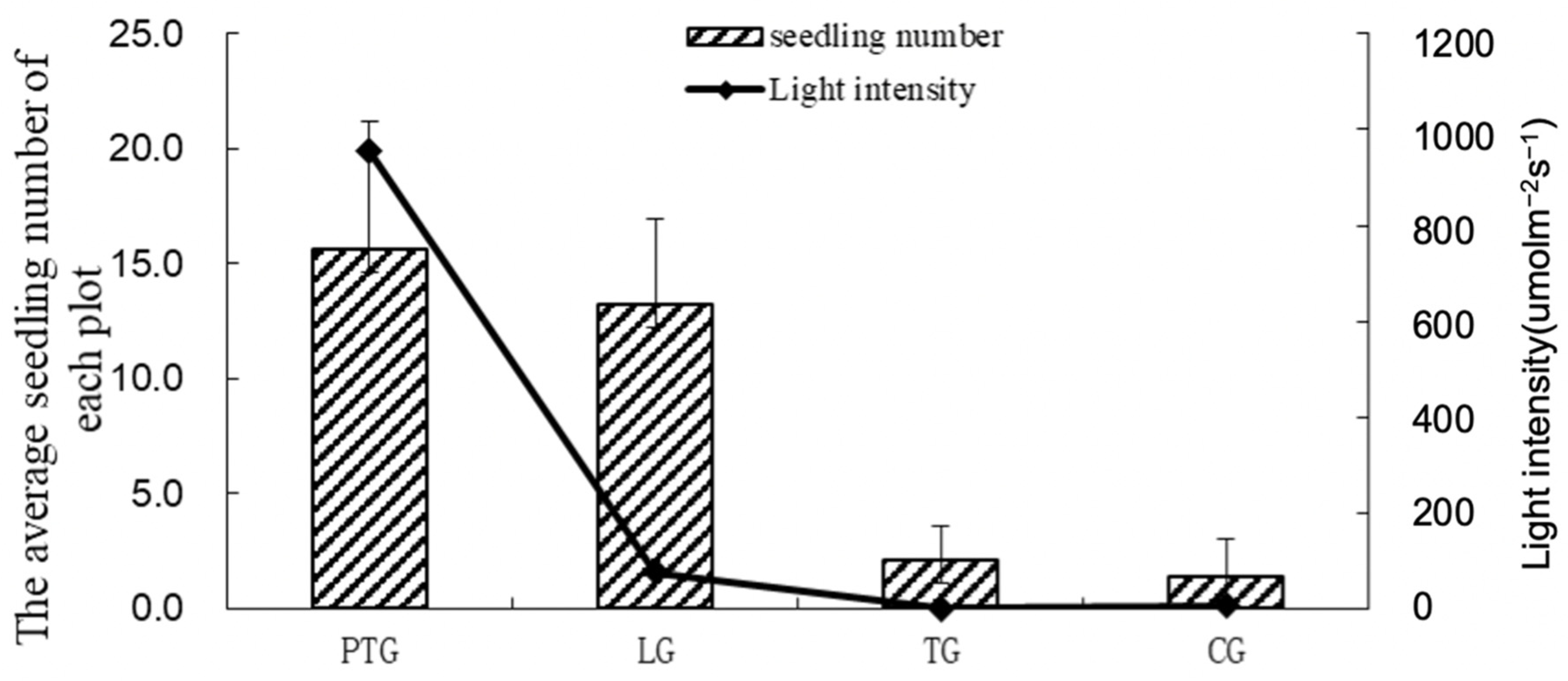
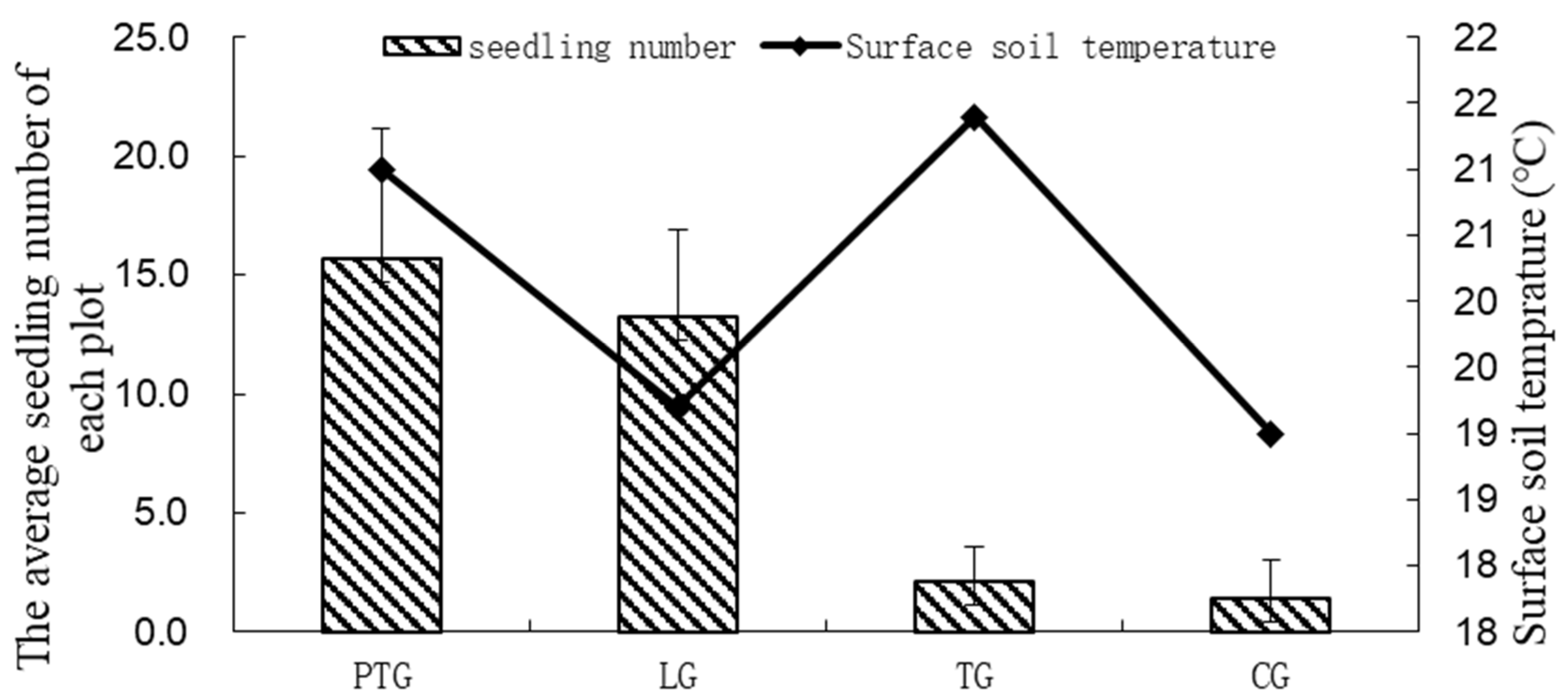
3.1. Effect of Simulated Light and Soil Temperature on Seed Germination of the Soil Seed Bank
3.1.1. Light and Temperature Characteristics under Different Treatments in the Germination Experiment of the Understory Soil Seed Bank

3.1.2. Seed Germination in the Understory Soil Seed Bank under Photo-Thermal Separation Conditions and Remaining Conditions
| Group | A (Day) | B (Day) | C (Day) | D (Seedling Number) | E (Seedling Number) | F (Seedling Number) | G (Seedling Number) |
|---|---|---|---|---|---|---|---|
| PTG | 20 a | 38 a | 142 a | 141 a | 15.7 a | 22 a | 7 a |
| LG | 31 a | 50 b | 192 a | 119 a | 13.2 a | 21 a | 10 a |
| TG | 49 a | 197 c | / b | 19 b | 2.1 b | 4 b | 0 b |
| CG | 170 b | 200 c | / b | 13 c | 1.4 b | 5 b | 0 b |
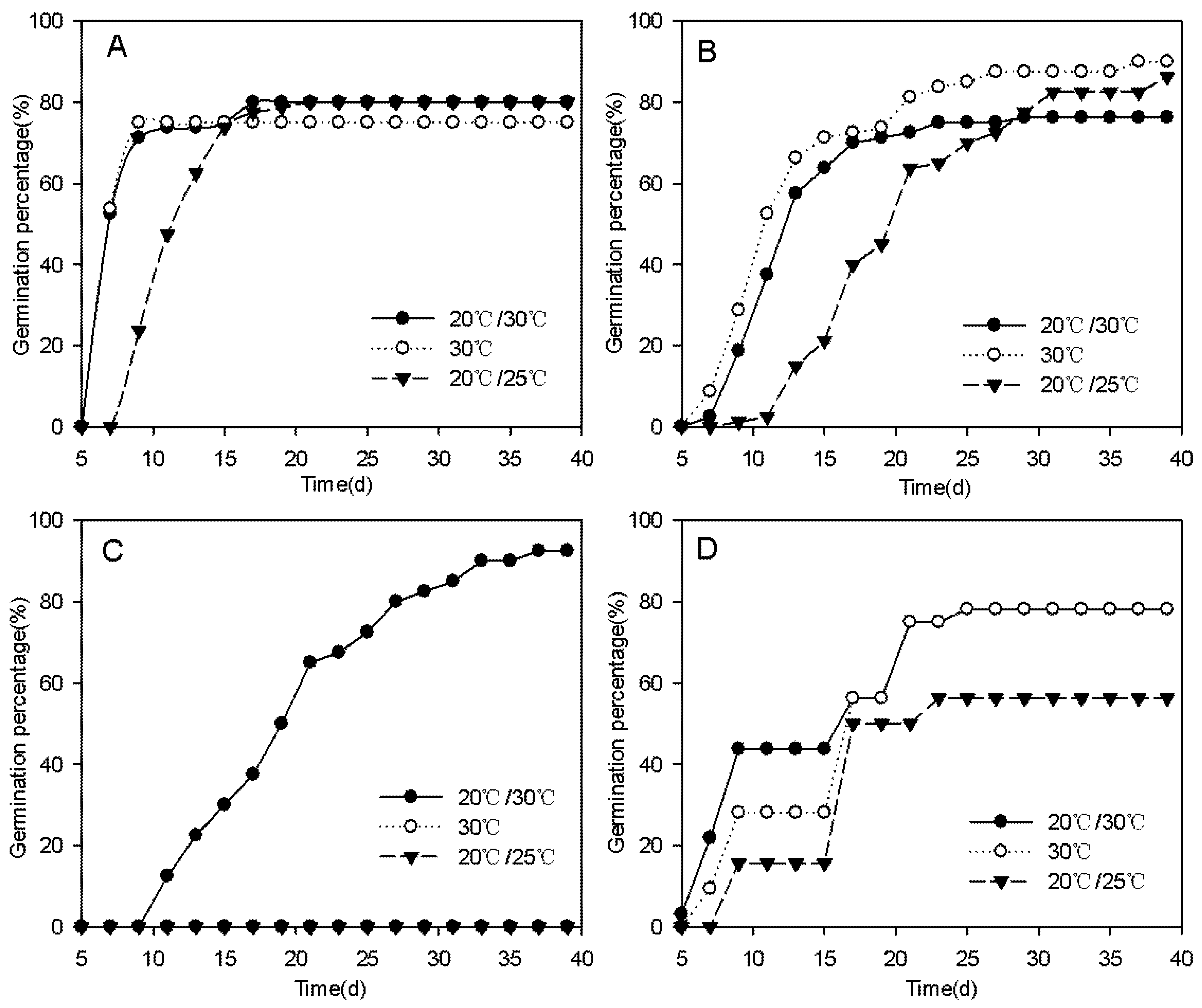
3.2. Indoor Verification Experiments
| MTG (d) | GS (d) | |||||
|---|---|---|---|---|---|---|
| Species | Dark | Understory Light | Gap Light | Dark | Understory Light | Gap Light |
| Thysanolaena latifolia | / a | 17.3 b | 9.2 b | / a | 9 b | 7.5 b |
| Melastoma sanguineum | / a | 22 b | 14.5 c | / a | 14 b | 11 c |
| Trema tomentosa | / a | 29 a | 21.8 b | / a | 25 a | 15 b |
| 20/30 ℃ | 30 ℃ | 20/25 ℃ | 20/30 ℃ | 30 ℃ | 20/25 ℃ | |
| Thysanolaena latifolia | 8.3 a | 7.6 a | 12 b | 7.5 a | 7 a | 9.5 b |
| Melastoma sanguineum | 12.8 a | 13.3 a | 20.3 b | 9.5 a | 8.5 a | 14 b |
| Trema tomentosa | 20 a | / b | / b | 15 a | / b | / b |

4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Watt, A.S. Pattern and process in the plant community. J. Ecol. 1947, 35, 1–22. [Google Scholar] [CrossRef]
- Pickett, S.T.A.; White, P.S. The Ecology of Natural Disturbance and Patch Dynamics; Academic Press: New York, NY, USA, 1985. [Google Scholar]
- Whitmore, T.C. Canopy gaps and the two major groups of forest trees. Ecology 1989, 70, 536–538. [Google Scholar] [CrossRef]
- Whitmore, T.C. Changes over twenty-one years in the Kolombangara rain forests. J. Ecol. 1989, 77, 469–483. [Google Scholar] [CrossRef]
- Everham, E.M.; Brokaw, N.V. Forest damage and recovery from catastrophic wind. Bot. Rev. 1996, 62, 113–185. [Google Scholar] [CrossRef]
- Du, Y.J.; Mi, X.C.; Ma, K.P. Comparison of seed rain and seed limitation between community understory and gaps in a subtropical evergreen forest. Acta Oecologica 2012, 44, 11–19. [Google Scholar] [CrossRef]
- Feng, D.L.; Huang, X.H.; Geng, Y.H.; Zhu, H.X. Influence of forest gaps and litter on growth of Castanopsis fargesii and Castanopsis carlesii in the Pinus massoniana pure forest of the three gorges reservoir area. Appl. Mech. Mater. 2014, 692, 33–39. [Google Scholar] [CrossRef]
- Ren, J.Y.; Kadir, A.; Yue, M. The role of tree-fall gaps in the natural regeneration of birch forests in the taibai mountains. Appl. Veg. Sci. 2015, 18, 64–74. [Google Scholar] [CrossRef]
- Meiado, M.V.; Rojas-Aréchiga, M.; de Siqueira-Filho, J.A.; Leal, I.R. Effects of light and temperature on seed germination of cacti of brazilian ecosystems. Plant Species Biol. 2016, 31, 87–97. [Google Scholar] [CrossRef]
- Vàzquez-Yanes, C.; Smith, H. Phytochrome control of seed germination in the tropical rain forest pioneer trees Cecropia obtusifolia and Piper auritum and its ecological significance. New Phytol. 1982, 92, 477–485. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M. Seeds: Physiology of Development and Germination; Plenum Press: New York, NY, USA, 1994. [Google Scholar]
- Gul, B.; Weber, D.J. Effect of dormancy relieving compounds on the seed germination of non-dormant Allenrolfea occidentalis under salinity stress. Ann. Bot. 1998, 82, 555–560. [Google Scholar] [CrossRef]
- Rosbakh, S.; Poschlod, P. Initial temperature of seed germination as related to species occurrence along a temperature gradient. Funct. Ecol. 2015, 29, 5–14. [Google Scholar] [CrossRef]
- Aghamir, F.; Bahrami, H.; Malakouti, M.J.; Eshghi, S.; Sharifi, F. Seed germination and seedling growth of bean (Phaseolus vulgaris) as influenced by magnetized saline water. Eurasian J. Soil Sci. 2016, 5, 39–46. [Google Scholar] [CrossRef]
- Carón, M.M.; Frenne, P.D.; Verheyen, K.; Quinteros, A.; Ortega-Baes, P. Germination responses to light of four Neotropical forest tree species along an elevational gradient in the southern Central Andes. Ecol. Res. 2020, 35, 550–558. [Google Scholar] [CrossRef]
- Orozco-Segovia, A.; Vàzquez-Yanes, C. Light effect on seed germination in Piper L. Acta Oecologica Oecologia Plant. 1989, 10, 123–146. [Google Scholar]
- Vàzquez-Yanes, C.; Orozco-Segovia, A. Ecological significance of light controlled seed germination in two contrasting tropical habitats. Oecologia 1990, 83, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Valio, I.F.M.; Joly, C.A. Light Sensitivity of the Seeds on the Distribution of Cecropia glaziovi Snethlage (Moraceae). Z. Pflanzenphysiol. 1979, 91, 371–376. [Google Scholar] [CrossRef]
- Vázquez-Yanes, C. Germination of a pioneer tree (Trema guineensis Ficahlo) from Equatorial Africa. Turrialba 1977, 27, 301–302. [Google Scholar]
- Vàzquez-Yanes, C.; Orozco-Segovia, A. Seed germination of a tropical rain forest pioneer tree (Heliocarpusdonnell-smithii) in response to diurnal fluctuation of temperature. Physiol. Plant 1982, 56, 295–298. [Google Scholar] [CrossRef]
- Yu, Y.; Baskin, J.M.; Baskin, C.C.; Tang, Y.; Cao, M. Ecology of seed germination of eight non-pioneer tree species from a tropical seasonal rain forest in southwest China. Plant Ecol. 2008, 197, 1–16. [Google Scholar] [CrossRef]
- Zang, R.G. Measurement and analysis on light and temperature regimes in different patch types of forest cycle in a tropical mountain rain forest, Hainan Island. J. Beijing For. Univ. 2002, 24, 125–130. [Google Scholar]
- Vàzquez-Yanes, C.; Orozco-Segovia, A. Light gap detection by the photoblastic seeds of Cecropia obtusifolia and Piper auritum, two tropical rain forest trees. Biol. Plant. 1987, 29, 234–236. [Google Scholar] [CrossRef]
- Vàzquez-Yanes, C.; Orozco-Segovia, A.; Rincón, E.; Sanchez-Coronado, M.E.; Huante, P.; Toledo, J.R.; Barradas, V.L. Light beneath the litter in a tropical forest: Effect on seed germination. Ecology 1990, 7, 1952–1958. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.; Cao, M. Effects of light and temperature on seed germination of Ficus hispida in Xishuangbanna, Southwest China. J. Plant Ecol. 2008, 32, 1084–1090. (In Chinese) [Google Scholar]
- Zang, R.G.; Tao, J.; Li, C. Within community patch dynamics in a tropical montane rain forest of Hainan Island, South China. Acta Oecologica 2005, 28, 39–48. [Google Scholar] [CrossRef]
- Duan, W.B.; Guo, Q.W.; Chen, L.X.; Feng, J.; Wang, L.X.; Du, S. Heterogeneity of soil surface temperature and shallow soil temperature in different size gaps of broadleaved Pinus koraiensis forest. J. Beijing For. Univ. 2019, 41, 108–121. [Google Scholar]
- Probert, R.J. The role of temperature in the regulation of seed dormancy and germination. In Seed: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CABI: London, UK, 2000; pp. 261–292. [Google Scholar]
- Vranckx, G.; Vandelook, F. A season and gap detection mechanism regulates seed germination of two temperate forest pioneers. Plant Biol. 2012, 14, 481–490. [Google Scholar] [CrossRef]
- Chen, H.; Cao, M.; Baskin, J.M.; Baskin, C.C. Temperature regulates positively photoblastic seed germination in four Ficus (moraceae) tree species from contrasting habitats in a seasonal tropical rainforest. Am. J. Bot. 2013, 100, 1683–1687. [Google Scholar] [CrossRef]
- Kyereh, B.; Swaine, M.; Thompson, J. Effect of light on the germination of forest trees in Ghana. J. Ecol. 1999, 87, 772–783. [Google Scholar] [CrossRef]
- Lv, X.B.; Yang, L.R.; Yang, M.; Yang, X.B.; Li, D.H.; Tao, C.; Wan, C.H. Study on the distribution patterns and dynamics of main tree population of Fagaceae dominance in tropical montane rain forest in Hainan Island. Chin. Agric. Sci. Bull. 2012, 28, 84–90. [Google Scholar]
- Liu, W.D.; Zang, R.G.; Ding, Y. Community features of two types of typical tropical monsoon forests in Bawangling Nature Reserve, Hainan Island. Acta Ecol. Sin. 2009, 29, 3465–3476. [Google Scholar]
- Ojanen, M.; Kärhä, P.; Ikonen, E. Spectral irradiance model for tungsten halogen lamps in 340–850 nm wavelength range. Appl. Opt. 2010, 49, 880–886. [Google Scholar] [CrossRef]
- Steigerwald, D.A.; Bhat, J.C.; Collins, D.; Fletcher, R.M.; Holcomb, M.O.; Ludowise, M.J.; Martin, P.S.; Rudaz, S.L. Illumination with solid state lighting technology. IEEE J. Sel. Top. Quantum Electron. 2002, 8, 310–320. [Google Scholar] [CrossRef]
- Gong, Q.; Zhou, J.S.; Liu, D.M.; Yi, W.; Xing, F.W.; Chen, H.F. Application of native plants in the ecological virescence of Guang-Wu Expressway. Ecol. Environ. 2007, 16, 486–491. [Google Scholar]
- Zhang, Z.D.; Zang, R.G. Predicting potential distribution of dominant woody plant keystone species in a natural tropical forest landscape of Bawangling, Hainan Island, South China. J. Plant Ecol. 2007, 31, 1079–1091. (In Chinese) [Google Scholar]
- Wang, Z.F.; Gao, S.H.; Tian, S.N.; Fu, S.L.; Ren, H.; Peng, S.L. Genetic structure of Cryptocarya chinensis in fragmented lower subtropical forests in China based on ISSR markers. Biodivers. Sci. 2005, 13, 324–331. [Google Scholar] [CrossRef][Green Version]
- Editorial Committee of Flora Reipublicae Popularis Sinicae. Flora Reipublicae Popularis Sinicae; Sciences Press: Beijing, China, 1959–2004.
- Chazdon, R.L.; Pearcy, R.W. The importance of sunflecks for forest understory plants. BioScience 1991, 41, 760–766. [Google Scholar] [CrossRef]
- Ritter, E.; Dalsgaard, L.; Einhorn, K.S. Light, temperature and soil moisture regimes following gap formation in a semi-natural beech-dominated forest in Denmark. For. Ecol. Manag. 2005, 206, 15–33. [Google Scholar] [CrossRef]
- Daïnou, K.; Bauduin, A.; Bourland, N.; Gillet, J.F.; Fétéké, F.; Doucet, J.L. Soil seed bank characteristics in Cameroonian rainforests and implications for post-logging forest recovery. Ecol. Eng. 2011, 37, 1499–1506. [Google Scholar] [CrossRef]
- Han, A.R.; Sohng, J.E.; Barile, J.R.; Lee, Y.K.; Woo, S.Y.; Lee, D.K.; Park, P.S. Comparison of Soil Seed Banks in Canopy Gap and Closed Canopy Areas between a Secondary Natural Forest and a Big Leaf Mahogany (Swietenia macrophylla King) Plantation in the Mt. Makiling Forest Reserve, Philippines. J. Environ. Sci. Manag. 2012, 15, 47–59. [Google Scholar]
- Miller, G.; Cummins, R. Soil seed banks of woodland, heathland, grassland, mire and montane communities, Cairngorm Mountains, Scotland. Plant Ecol. 2003, 168, 255–266. [Google Scholar] [CrossRef]
- Liu, K.; Baskin, J.M.; Baskin, C.C.; Bu, H.; Du, G.; Ma, M. Effect of diurnal fluctuating versus constant temperatures on germination of 445 species from the eastern Tibet Plateau. PLoS ONE 2013, 8, e69364. [Google Scholar] [CrossRef] [PubMed]
- Swaine, M.D.; Whitmore, T.C. On the definition of ecological species groups in tropical rain forests. Vegetatio 1988, 75, 81–86. [Google Scholar] [CrossRef]
- Vàzquez-Yanes, C.; Orozco-Segovia, A. Signals for Seeds to Sense and Respond to Gaps. Ecophysiological Processes above and below Ground; Academic Press: New York, NY, USA, 1994. [Google Scholar]
- Nishii, K.; Nagata, T.; Wang, C.N.; Mölle, M. Light as environmental regulator for germination and macrocotyledon development in Streptocarpus rexii. S. Afr. J. Bot. 2012, 81, 50–60. [Google Scholar] [CrossRef][Green Version]
- Pearson, T.R.H.; Burslem, D.F.R.P.; Mullins, C.E.; Dalling, J.W. Germination ecology of neotropical pioneers: Interacting effects of environmental conditions and seed size. Ecology 2002, 83, 2798–2807. [Google Scholar] [CrossRef]
- Pearson, T.R.H.; Burslem, D.F.R.P.; Mullins, C.E.; Dalling, J.W. Functional significance of photoblastic germination in neotropical pioneer trees: A seed’s eye view. Funct. Ecol. 2003, 17, 394–402. [Google Scholar] [CrossRef]
- Daws, M.I.; Burslem, D.F.R.P.; Crabtree, L.M.; Kirkman, P.; Mullins, C.E.; Dalling, J.W. Differences in seed germination responses may promote coexistence of four sympatric Piper species. Funct. Ecol. 2002, 16, 258–267. [Google Scholar] [CrossRef]
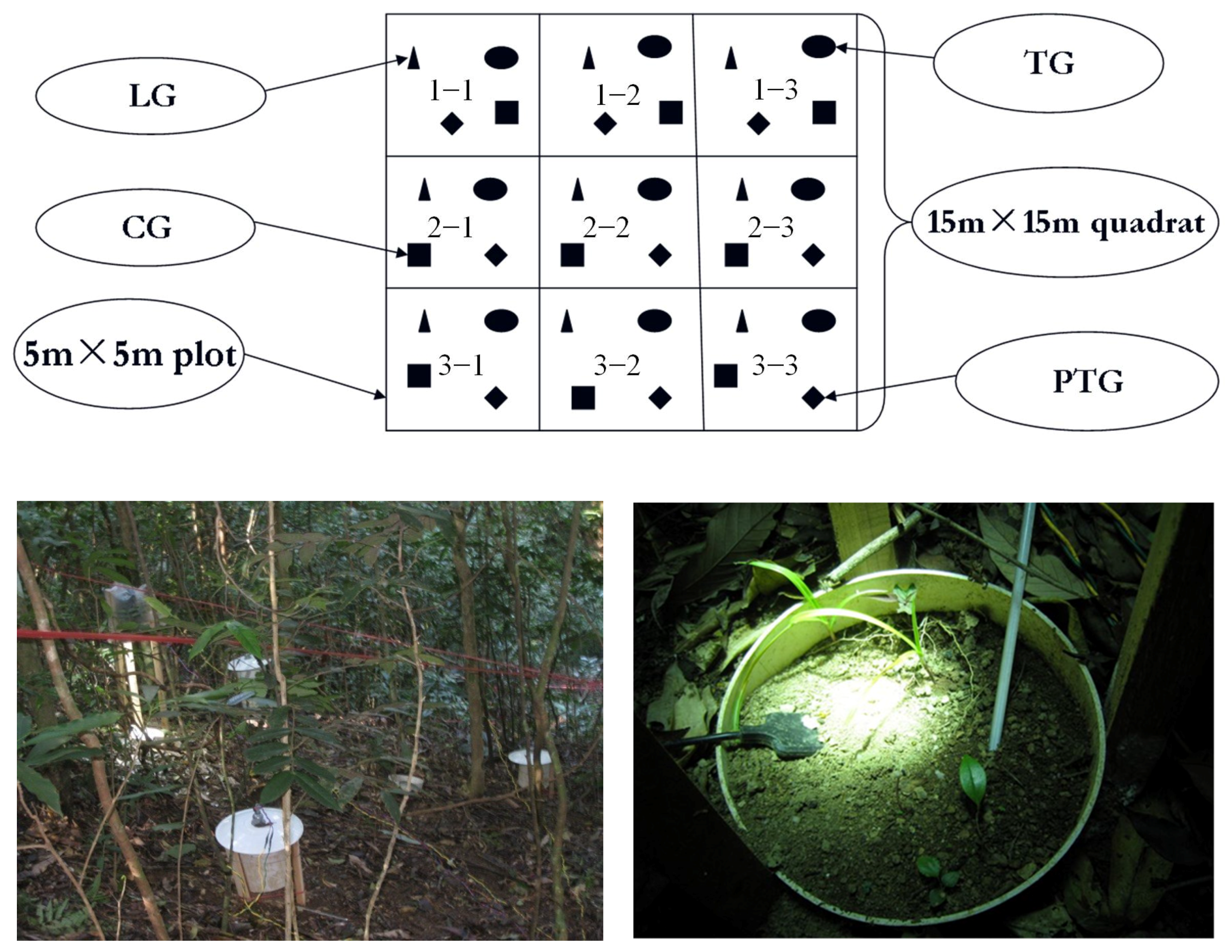
| Species | Family | Life Form | Ecological Form | Habitat | Seed Shape and Size |
|---|---|---|---|---|---|
| Thysanolaena maxima | Poaceae | H | P | Roadside and sparse forest | Circular with a diameter of 0.1 mm |
| Melastoma sanguineum | Melastomataceae | S | P | Roadside | Flat semicircle with a length of 2 mm |
| Trema tomentosa | Ulmaceae | T | P | Roadside | Circular with a diameter of 2 mm |
| Cryptocarya chinensis | Lauraceae | T | NP | Hidden forest | Circular with a diameter of 10 mm |
| Group | Tmin (°C) | Tmax (°C) | T (°C) | L (μmol m−2 s−1) |
|---|---|---|---|---|
| TG | 17.9 | 32.2 | 21.4 | 1.3 |
| PTG | 17.8 | 31.6 | 21.0 | 954 |
| LG | 17.9 | 20.8 | 19.2 | 75 |
| CG | 17.7 | 20.5 | 19.0 | 6.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Lv, X.; Yang, X.; Zhang, G.; Li, D. The Triggering Effect of Gaps on Seedling Germination of the Soil Seed Bank in Tropical Rain Forests, Hainan Island, South China. Forests 2022, 13, 1653. https://doi.org/10.3390/f13101653
Yang L, Lv X, Yang X, Zhang G, Li D. The Triggering Effect of Gaps on Seedling Germination of the Soil Seed Bank in Tropical Rain Forests, Hainan Island, South China. Forests. 2022; 13(10):1653. https://doi.org/10.3390/f13101653
Chicago/Turabian StyleYang, Lirong, Xiaobo Lv, Xiaobo Yang, Guofeng Zhang, and Donghai Li. 2022. "The Triggering Effect of Gaps on Seedling Germination of the Soil Seed Bank in Tropical Rain Forests, Hainan Island, South China" Forests 13, no. 10: 1653. https://doi.org/10.3390/f13101653
APA StyleYang, L., Lv, X., Yang, X., Zhang, G., & Li, D. (2022). The Triggering Effect of Gaps on Seedling Germination of the Soil Seed Bank in Tropical Rain Forests, Hainan Island, South China. Forests, 13(10), 1653. https://doi.org/10.3390/f13101653






