Abstract
This study recommends a non-grinding measurement method of leaf pH which can reduce the destructive interference to the measured fresh-leaf pH values. To verify the accuracy of this method, we measured leaf pH with the non-grinding and grinding method and further assessed the dilution effect on leaf pH in the grinding process. Compared with the non-grinding method, the grinding method significantly increased the measured pH value; leaf pH increased with decreasing leaf–water ratio in the procedure of the grinding method, but gradually stabilized. The grinding effects of severe physical damage and thereafter oxidization of leaf samples, and the dilution effects according to the Debye–Hückel limiting law and acid-base ionization theory, may both contribute to the increased leaf pH measured with the grinding method. Thus, leaf pH measured with the non-grinding method was expected to be much closer to those of leaf sap in vivo and be more suitable to indicating the dynamic variation or instant response of leaf pH to the environmental changes. Finally, considering that non-significant difference had been proved in the measured leaf pH between dried, frozen, refrigerated, and fresh ground samples, a conversion equation was provided to facilitate mutual conversion of the results with non-grinding fresh samples (y) against those with grinding dried samples (as representative) (x): y = 1.097x − 0.722.
1. Introduction
Hydrogen ion plays a vital role in regulating the physiological function of plant organs, hydrogen ion concentration is commonly expressed as pH. The research on the pH of organisms began in the late 20th century, physiologists studied the intracellular pH (pHi) and extracellular pH (pHe) of animals and plants, and emphasized the importance of intracellular pH variation in energy conversion, metabolic regulation and signal transduction [1,2,3]. Plant pH, especially leaf pH, is a valuable plant functional trait, which is believed to be a species-specific trait with large-scale spatio-temporal patterns [4,5,6,7]. Leaf pH can not only regulate the nutrient cycling and biological relationships [5,8,9], but also indicate the air pollution [10], reveal plant responses to the environment and jointly affects plant survival strategies with other plant functional traits [6,11,12,13]. Thus, focusing our attention on leaf pH is of great significance to both plant physiology and ecology.
The accurate measurement of living organisms’ pH is a key challenge to pH value research for both physiologists and ecologists. For physiological research, pH measurement has been mainly targeted at cells or organelles, measuring methods mainly includes fluorescent dye, microelectrode, and nuclear magnetic resonance (NMR) [1]. However, ecologists, who are more interested in plant pH at the level of organs, individuals, and species locally or across large spatial scales, often need to conduct the measurement in the field; hence, convenient leaf pH measurement methods are required. The handbook of plant functional traits written by Pérez-Harguindeguy et al. (2013) proposed a method for measuring leaf pH with ground samples (oven-dried or fresh). Another recent study on leaf pH measurement reviewed some popular measurement methods based on grinding samples and presented conversion equations between them [14]. However, all these methods mentioned by Pérez-Harguindeguy et al. (2013) and Liu et al. (2022) cannot reflect the instant leaf pH in vivo due to the after-sampling destructive treatments, such as grinding, drying, or freezing, which may induce unexpected measurement error of in vivo pH values. Intracellular pH is generally in homeostasis [15], only the apoplastic pH changes dynamically in response to both environmental factors and physiological conditions [16,17]. Apoplastic pH can act as a regulator for plants to respond to external environmental stress and a messenger to meet internal physiological growth needs [17,18,19,20]. To observe the timely response of leaf pH to environmental factors requires focusing on the apoplastic pH of fresh leaves, which cannot be met by previous measurement methods. Therefore, physiological ecologists need a measurement which can both, as accurately as possible, measure the “real” leaf pH value and explore the almost real-time response of leaf pH to environmental changes.
In addition, the complex biochemical and physicochemical reactions during the grinding process, as well as other pretreatment processes (such as drying), can affect the measured leaf pH. In terms of basic chemical principles, hydrogen ion (Haq+, in the form of hydrate H(H2O)n+) in aqueous solution can be affected by the ionic strength and ionization equilibrium [21]. For strong electrolytes, according to Debye–Hückel limiting law, when the concentration of a solute is greater than 1 × 10−3 mol/L, the interactions among ions are significant, and the effective concentrations (ionic activities) are no longer equal to the real concentrations [22,23]. Ion concentrations in leaf cells (include vacuoles, cytoplasm, and apoplast) are higher than 1 × 10−3 mol/L; thus, ionic strength must affect the activities of ions [22,24]. The measured pH value reflects the activity of Haq+ in solution, procedures in grinding measurements (such as grinding and dilution) may have an impact on Haq+ activity and result in a disparity to the real fresh leaf pH. The disparity caused by grinding has not been well-estimated yet. A puncturing method (without grinding) used by agricultural study (Masoero and Cugnetto, 2018) can be employed to measure the approximate instantaneous leaf pH in vivo directly with less leaf tissue damage and monitor the change in leaf pH in real time. Based on current studies, we performed a preliminary comparison of the grinding (according to data from peer-reviewed literature) and non-grinding (Masoero and Cugnetto, 2018) leaf pH for the same species, and found that ground leaf pH was higher than unground leaf pH for the 11 collected common species (paired t-test; p < 0.05). However, due to the lack of controlled experiments, current evidence for the likely systematic differences between grinding and non-grinding measurements remains inadequate. If systemic differences exist, what causes them? What is the conversion relationship between the leaf pH measured with grinding and non-grinding methods? These questions remain to be resolved.
To address above questions, control experiments were conducted. We collected wild plants’ leaves and measured leaf pH with both the non-grinding puncturing method and grinding method, then compared the measurement results of the two methods. If there is a definite systematic difference between the results of the two methods, the conversion equation between them can be established. Moreover, we designed a dilution experiment to test the possible effects of additional deionized water on the measured leaf pH in the grinding method.
2. Materials and Methods
2.1. Leaf Sample Collection and Non-Grinding Measurement Method
Totally, 129 leaf samples were collected from the Maoer Mountain, Guangxi Province, China (25°53′05″ N, 110°29′12″ E) in August. These leaf samples included various plant functional types: herb, shrub, and tree. Fully expanded green leaves were collected from randomly selected mature healthy individuals and these leaves (without petiole) were assembled in zip-lock bags and divided into two parts.
One part of the fresh samples was measured with the non-grinding puncturing method (Figure 1): take 3–8 leaves and overlap them top to bottom, curl the leaves into a cylinder and fold the cylinder in half along the middle of it, avoiding the main vein. Insert the folded leaf cylinder into a 2 mL centrifuge tube and make sure the thickest protruding part of the folded leaf cylinder is upward. Cut a small opening at the thickest protruding part of the leaf cylinder, insert the needle-punched pH electrode into it, ensure the electrode is completely and tightly wrapped by leaves, and record the stable pH value (Experimental photographs, see Figure 1). Leaf pH was measured by a pH meter (Mettler-Toledo portable pH S8-Meter) with a precise pH electrode (Mettler-Toledo pH electrode InLab Solids Go-ISM), which was calibrated against buffer solutions (pH 4.00, 7.00 and 9.18) before measurement.
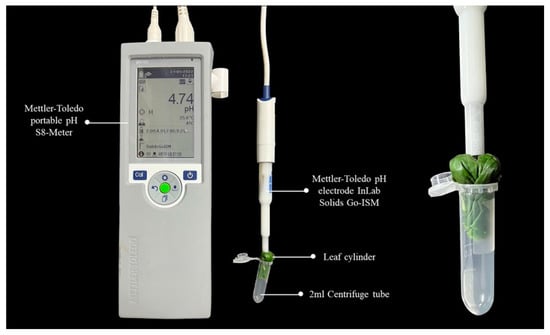
Figure 1.
Illustration of leaf pH measurement with non-grinding method.
2.2. Drying Leaf Samples and Measuring Leaf pH with the Grinding Method
Our previous study [14] showed that the three types of pretreatments (freezing, drying, and refrigeration) has no significant effects on measured leaf pH and the measurement of oven-dried leaves was also recommended in another study [7]; thus, we chose oven-dried leaves for grinding measurement in this study. Therefore, the other fresh samples were placed in the 105 °C oven for 30 m in and then oven-dried at 70 °C to constant mass for 48–72 h after recording the fresh weight, and then ground with a grinder. A total of 0.5 g oven-dried samples (dry mass) were weighed and mixed with 5 g deionized water in a 10 mL centrifuge tube. The oven-dried leaf–water mass ratio was 1:10. The mixtures were shaken at 250 rpm for 1 h and separated into solid and liquid by standing. The supernatant pH was measured by a precise pH electrode (Mettler-Toledo pH electrode LE422), which was calibrated against buffer solutions (pH 4.00, 7.00 and 9.18) before measurement. It should be noted that the dry mass here only included the oven-dried leaves, whereas in Liu et al. (2022), the dry mass included oven-dried leaves and deionized water in an appropriate proportion (see Materials and Methods in Liu et al. (2022) for details).
2.3. Effects of Dilution on Leaf pH Measurement with the Grinding Method
Additional deionized water during the grinding process might affect leaf pH, thus we designed a dilution experiment with oven-dried leaf samples from six campus plant species (Buddleja lindleyana, Lonicera maackii, Rosa cathayensis, Cedrus deodara, Amygdalus trilobal, and Viburnum opulus). Oven-dried leaf samples (3 g) were mixed with deionized water in 50 mL centrifuge tubes according to 7 kinds of leaf–water mass ratio (1:3, 1:5, 1:8, 1:10, 1:13, 1:15, and 1:17). The mixtures were shaken at 250 rpm for 1 h and separated into solid and liquid by standing. The supernatant pH of this part was measured using a calibrated precise pH meter (Mettler-Toledo pH electrode LE422).
2.4. Data Analyses
Standard major axis (SMA) regression (i.e., Model II regression) was applied to analyze the effects of different measurement methods on leaf pH. The slope and intercept of the SMA regression against the 1:1 line were used to judge the magnitude and direction of the impact on measured leaf pH. Paired t-test was used to compare the pH measurement with grinding vs. non-grinding (puncturing) methods. Nonlinear regression (quadratic regression) was used to explore the dynamics of leaf pH under different dilution ratios. All statistical analyses were performed in R 4.1.2.
3. Results
3.1. Comparison of Leaf pH Measured with Grinding and Non-Grinding Methods
Ground leaf pH was significantly higher than unground leaf pH (4.99 vs. 4.75; p < 0.01) (Table 1). We found significant positive correlation between ground leaf pH and unground leaf pH (R2 = 0.545, p < 0.001) (Figure 2). The SMA regression fitting results showed no significant difference between the fitting slope and 1 (p = 0.125). However, the regression intercept was significantly less than 0 (p = 0.031), which means grinding does have a fixed effect (increased) on measured leaf pH. The conversion equation between the two methods was: y = 1.097x − 0.722.

Table 1.
Comparison of different measurement methods on leaf pH.
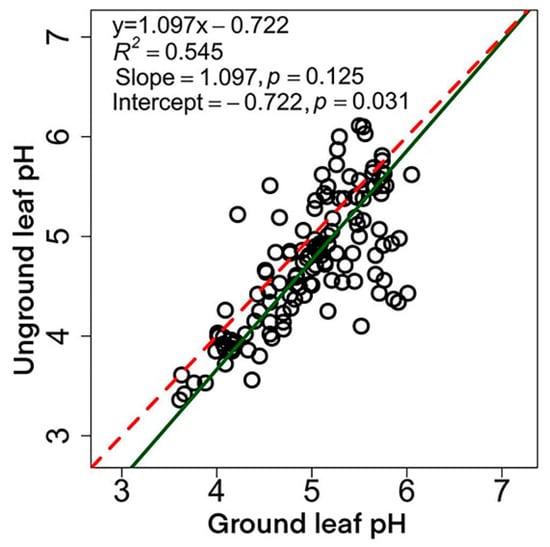
Figure 2.
Relationship between two leaf pH measuring methods. The red dash line denotes the 1:1 line, the p value in brackets indicates the difference between the SMA regression slope (intercept) and 1 (0). Fresh leaf samples were used to obtain the unground leaf pH, while the ground leaf pH using oven-dried ones. See Materials and Methods for details.
3.2. Effects of Dilution on Leaf pH in the Grinding Method
The dilution experiment showed that the measured pH increased with the addition of deionized water (the increase in dilution degree) and gradually stabilized (Figure 3). The maximum pH change in the experiment was less than 0.5 unit at the whole dilution scale (e.g., only changed 0.35 for Rosa cathayensis, from leaf:water ratio 1:3 to 1:15). When the oven-dried leaf:water ratio increased from 1:3 to 1:10, the pH of the six plant species significantly increased (p < 0.05) by 0.11–0.26.
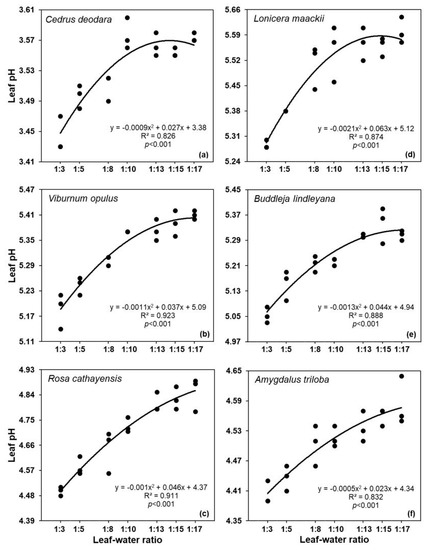
Figure 3.
Relationship between leaf–water ratio and leaf pH. Dried leaf samples of (a) Cedrus deodara, (b) Viburnum opulus, (c) Rosa cathayensis, (d) Lonicera maackii, (e) Buddleja lindleyana, and (f) Amygdalus triloba were used here. See Materials and Methods for details.
4. Discussion
4.1. Comparison of Grinding and Non-Grinding Measurement Results
In this study, leaf pH measured by the non-grinding method ranged from 3.36 to 6.16, which was consistent with the variation range of plant leaf pH (from acidic to neutral): apoplast (pH 5.5) and vacuole (pH generally 5.0–6.5) of mature leaves [25]. Our result was consistent with that measured with a needle-punched electrode in previous studies (e.g., Masoero and Cugnetto (2018): leaf pH of 49 plant species ranging from 3.06 to 6.38). The needle-punched electrode, used to measure unground-leaf pH in our study, breaks the limitation of traditional electrodes and reduces the requirements for the subjects: both liquid and semi-solid samples can be measured. The needle-punched electrode was tightly wrapped in the cross sections of leaves during the measurement process; hence, leaf pH measured with this method should be almost exactly the pH of the primordial leaf sap. Compared with grinding measurement method, the non-grinding method without additional deionized water (Haq+ was undiluted) and severe physical damage to leaves might obtain lower pH that relatively closer to the living-leaf pH in situ.
Our results indicated that the ground leaf pH was higher than that measured by the non-grinding method. We also compared the leaf pH of ground samples reported by peer-reviewed literature (11 species) with those measured for non-unground leaf samples of the same species reported by Masoero and Cugnetto (2018). The leaf pH of ground samples were significantly higher than those of the same species without grinding (5.77 vs. 5.10 in average; p < 0.01; paired t-test) (Table 2).

Table 2.
Comparison of leaf pH measured for the same plant species with ground and unground samples based on published literature and our data.
Although the leaf pH measured by grinding and non-grinding methods had significant difference, they correlated well in SMA regression: no significant difference between the regression slope and 1 while the regression intercept was significantly different from 0. For the previous grinding method in leaf pH measurement, the grinding scale usually only achieved tissue level (500–100 μm) rather than cell level (50–10 μm); the cell level requires more sophisticated equipment such as superfine ball mill [36]. Therefore, in our study, the substances dissolved in the supernatant in the grinding method were mainly from leaf apoplast. The final measured solutions of the two methods mainly came from the apoplast, thus the results correlated well in SMA regression, but due to the influence of measuring procedure (such as drying, grinding and dilution), there existed difference between the two methods as well as the fitting intercept and 0.
The two methods were suitable for different experimental situations. The grinding measurement method was recommended when conducting field experiments with a large sample size because of its modelized and standardized experimental processes [7]. For the non-grinding measurement method, the leaf pH derived from the primordial leaf sap, but mainly reflected the fresh apoplastic sap pH since less intracellular sap was released without severe physical damage. The pH of cytoplasm and vacuole were relatively stable, while the pH of apoplast changed with environment factors [25,37]; so that non-grinding leaf pH can reflect the real-time response of leaves to environmental changes more accurately. Therefore, the non-grinding measurement method was more suitable for measuring the immediate response of leaf pH to stress factors or leaf pH dynamics, such as diel, hourly, or shorter temporal variations. The non-grinding measurement method also has its limitation, e.g., the fine leaves (including pine needle) and leaves with very little water content may be not suitable for this method.
4.2. Effects of Grinding Measurement on Leaf pH
The procedure of the grinding method mainly involves drying, grinding, and dilution. These three steps may dominate the increase in leaf pH.
4.2.1. Drying
Our previous study reported that drying can cause the over-valuation of the measured leaf pH and this may be attributed to the decomposition of various acidic chemicals contained in leaves (e.g., phenolics and flavonoids) and their diverse thermal sensitivities [14,38]. Moreover, studies have shown that drying can cause the decreasing in phenolics (acid substance), so it is no wonder that drying can increase the measured leaf pH [39,40].
4.2.2. Grinding
The grinding process aerated the crushed leaves and led to the biochemical reactions between O2 and the leaf powder. On the contrary, the non-grinding measurement had less oxidation due to the weaker physical damage to leaves and shorter exposure times. Most biochemical reactions in living organisms are redox processes (e.g., photosynthesis), and molecules always have various redox potentials in biological biochemical reactions [41,42]. O2 has a high redox potential (strong oxidizer) and can readily react with Haq+ and other electron donors of these molecules, which results in Haq+ consuming (pH rising) [42]. Moreover, plant cells contain many antioxidants (such as phenols), which react easily with O2 or reactive oxide species (ROS) and protect other molecules by transferring Haq+ to oxidants [43,44] and result in an elevated pH. Research in food science have showed that with the extension of aerobic exposure time the phenolics of organisms (e.g., fruit peel and lettuce leaves) are oxidized into quinones, the color turns dark brown, and the total phenolic content decreases while the pH increases [45,46]. Therefore, in the process of grinding, post-grinding shaking extraction, and standing, Haq+ in leaf sap is consumed by the oxidation reactions with O2, leading to an increase in the final measured pH.
4.2.3. Dilution
Compared with non-grinding measurements, the addition of deionized water during the grinding process has the most direct effect on the measured electrolyte solution, which may increase the ionic strength of the solution and thus affected the measured pH value [22]. In the grinding measurement, more ions dissolve into the measured solution because leaves are ground to powder and additional deionized water is added, which causes the increase in ionic strength. In the non-grinding measurement, with little damages to leaves, less ions dissolve into the measured leaf sap and lead to lower ionic strength. A previous study revealed how changes in ionic strength affect the dissociation of acids and provided a general equation (Equation (1)) for the nth dissociation of a polyprotic acid (z is charge number) under the effect of ionic strength [22]:
where pKA(MIX),n is the mixed dissociation constant, and pKA(THERM),n denotes the thermodynamic dissociation constant; I stands for the ionic strength; z is charge number, and n is the degree of dissociation.
According to the equation, when the ionic strength (I) increases, the degree of acid dissociation decreases and leads to less Haq+ (higher measured pH).
Furthermore, for the dilution experiment with increasing additional deionized water (leaf–water ratio from 1:3 to 1:17), the acid-base ionization theory may dominate the measured pH in the electrolyte solution; that is, the dilution of acidic compounds can reduce the ionization of Haq+ [41,47]. This may cause the measured pH to increase with addition of deionized water (the increase in dilution degree). Our results confirmed that the addition of deionized water in the grinding method is one of the reasons for the disparity between the grinding and non-grinding measurement results. Moreover, with the decrease in the leaf–water ratio, leaf pH gradually tended to be stable when it was higher than 1:13 (Figure 3), which suggests that it is rational to use 1:15 mass ratio (e.g., Liu et al., 2022) in common measurement methods.
4.3. Conversion Equation between Grinding and Non-Grinding Methods
In our previous study [14], the measured leaf pH of three types of pretreatments (freezing, drying, and refrigeration) had no significant difference (see Table 2 in Liu et al. (2022) for more details). Therefore, the conversion equation of fresh samples (non-grinding (y)) and dried samples (grinding (x)) provided in this study is: y = 1.097x − 0.722, which is also applicable to the other two pretreatments (freezing and refrigeration).
5. Conclusions
This research compared the difference of leaf pH measured by the non-grinding method and grinding method, and discussed the possible reasons for the difference. Compared with grinding measurement methods, the non-grinding method, without additional deionized water (Haq+ was undiluted) and severe physical damage to leaves, might obtain lower pH that relatively closer to the living-leaf pH in situ. Given that the unground leaf pH derived from the primordial leaf sap and mainly reflected the fresh apoplastic sap pH, which is more responsive to environmental changes, the non-grinding measurement method might be closer to the living-leaf pH in situ, and be more suitable for monitoring the living-leaf pH under certain conditions, especially to observe the real-time response of leaf pH to environmental changes or leaf pH dynamics. For field experiments with a large sample size and focus only on the trend of the variation in leaf pH, grinding measurement is recommended due to its flexibility and convenience. In addition, we present a conversion equation between grinding and non-grinding measurements to facilitate mutual conversion of the results reported by previous research. This study recommends a non-grinding leaf pH measurement method, which can minimize the destructive interference and reflect the temporal leaf pH dynamics or its nearly instant response to the environment. We hope this paper further promotes the study of leaf pH in plant physiology and ecology.
Author Contributions
Conceptualization, W.H. and J.C.; methodology, J.C. and W.H.; formal analysis, J.C.; investigation, J.C. and W.H.; resources, J.C. and Y.H.; data curation, J.C.; writing—original draft preparation, J.C., S.L., and W.H.; writing—review and editing, J.C., S.L., Y.H., Y.L., and W.H.; visualization, J.C.; supervision, W.H.; project administration, W.H.; funding acquisition, J.C., W.H. and S.L.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA26040202), the Chinese Universities Scientific Fund (2021TC117), and the National Natural Science Foundation of China (32001165).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Z.J. Shi and Q.Q. Meng in our lab for participating in the field sampling for this experiment, and J.X. Yin and W. Pan for their enlightening discussions on the original draft. The authors also appreciate the anonymous reviewers whose relevant comments and suggestions have improved the readability of the manuscript, and especially one of whom for giving his professional guidance on water chemistry. We owe to G. Masoero and A. Cugnetto, whose previous research had inspired us to do this study, and all researchers and the TRY Plant Trait Database whose data had facilitated our research. We thank Guangxi Lijiangyuan Forest Ecosystem Research Station for the help during the sampling period.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Egginton, S.; Taylor, E.W.; Raven, J.A. Regulation of Tissue pH in Plants and Animals: A Reappraisal of Current Techniques; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Felle, H.H. pH: Signal and Messenger in Plant Cells. Plant Biol. 2001, 3, 577–591. [Google Scholar] [CrossRef]
- Rengel, Z. (Ed.) Handbook of Plant Growth: pH as the Master Variable; Marcel Dekker: New York, NY, USA, 2002. [Google Scholar]
- Cornelissen, J.H.C.; Sibma, F.; Van Logtestijn, R.S.P.; Broekman, R.A.; Thompson, K. Leaf pH as a Plant Trait: Species-Driven Rather than Soil-Driven Variation: Species versus Soil Chemistry Effects on Leaf pH. Funct. Ecol. 2011, 25, 449–455. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Quested, H.M.; van Logtestijn, R.S.P.; Pérez-Harguindeguy, N.; Gwynn-Jones, D.; Díaz, S.; Callaghan, T.V.; Press, M.C.; Aerts, R. Foliar pH as a New Plant Trait: Can It Explain Variation in Foliar Chemistry and Carbon Cycling Processes among Subarctic Plant Species and Types? Oecologia 2006, 147, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yan, Z.; Chen, Y.; Zhang, M.; Chen, J.; Han, W. Foliar pH, an Emerging Plant Functional Trait: Biogeography and Variability across Northern China. Glob. Ecol. Biogeogr. 2019, 28, 386–397. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New Handbook for Standardised Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2013, 61, 167. [Google Scholar] [CrossRef]
- Masoero, G.; Cugnetto, A. The Raw pH in Plants: A Multifaceted Parameter. JAR 2018, 1, 18–34. [Google Scholar] [CrossRef]
- Tao, J.; Zuo, J.; He, Z.; Wang, Y.; Liu, J.; Liu, W.; Cornelissen, J.H.C. Traits Including Leaf Dry Matter Content and Leaf pH Dominate over Forest Soil pH as Drivers of Litter Decomposition among 60 Species. Funct. Ecol. 2019, 33, 1798–1810. [Google Scholar] [CrossRef]
- Bui, H.T.; Odsuren, U.; Kwon, K.J.; Kim, S.Y.; Yang, J.C.; Jeong, N.R.; Park, B.J. Assessment of Air Pollution Tolerance and Particulate Matter Accumulation of 11 Woody Plant Species. Atmosphere 2021, 12, 1067. [Google Scholar] [CrossRef]
- Husson, O.; Audebert, A.; Benada, J.; Soglonou, B.; Tano, F.; Dieng, I.; Bousset, L.; Sarthou, J.P.; Joseph, S.; Menozzi, P.; et al. Leaf Eh and pH: A Novel Indicator of Plant Stress. Spatial, Temporal and Genotypic Variability in Rice (Oryza Sativa L.). Agronomy 2018, 8, 209. [Google Scholar] [CrossRef]
- Liu, S.; An, S.; Yan, Z.; Ren, J.; Lu, X.; Ge, F.; Han, W. Variation and Potential Influence Factors of Foliar pH in Land-Water Ecozones of Three Small Plateau Lakes. J. Plant Ecol. 2021, 14, 504–514. [Google Scholar] [CrossRef]
- Luo, Y.; Yan, Z.; Liu, S.; Chen, J.; Li, K.; Mohammat, A.; Han, W. Variation in Desert Shrub Foliar pH in Relation to Drought and Salinity in Xinjiang, China. J. Veg. Sci. 2021, 32, e13031. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.; Han, W.; Zhang, W. Comparison of Pretreatment, Preservation and Determination Methods for Foliar PH of Plant Samples. J. Plant Ecol. 2022, 15, 673–682. [Google Scholar] [CrossRef]
- Kurkdjian, A.; Guern, J. Intracellular pH: Measurement and Importance in Cell Activity. Annu. Rev. Plant Biol. 1989, 40, 271–303. [Google Scholar] [CrossRef]
- Grignon, C.; Sentenac, H. pH and Ionic Conditions in the Apoplast. Annu. Rev. Plant Biol. 1991, 42, 103–128. [Google Scholar] [CrossRef]
- Tsai, H.H.; Schmidt, W. The Enigma of Environmental pH Sensing in Plants. Nat. Plants 2021, 7, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Song, W.; Huang, S.; Jiang, K.; Moriwaki, Y.; Wang, Y.; Men, Y.; Zhang, D.; Wen, X.; Han, Z.; et al. Extracellular pH Sensing by Plant Cell-Surface Peptide-Receptor Complexes. Cell 2022, 185, 3341–3355. [Google Scholar] [CrossRef]
- Rayle, D.L.; Cleland, R.E. The Acid Growth Theory of Auxin-Induced Cell Elongation Is Alive and Well. Plant Physiol. 1992, 99, 1271–1274. [Google Scholar] [CrossRef]
- Geilfus, C.M. The pH of the Apoplast: Dynamic Factor with Functional Impact Under Stress. Mol. Plant 2017, 10, 1371–1386. [Google Scholar] [CrossRef]
- Stoyanov, E.S.; Stoyanova, I.V.; Reed, C.A. The Structure of the Hydrogen Ion (Haq+) in Water. J. Am. Chem. Soc. 2010, 132, 1484–1485. [Google Scholar] [CrossRef]
- Kennedy, C. Ionic Strength and the Dissociation of Acids. Biochem. Educ. 1990, 18, 35–40. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Sharpe, A.G. Inorganic Chemistry, 4th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2012. [Google Scholar]
- Speer, M.; Kaiser, W.M. Ion Relations of Symplastic and Apoplastic Space in Leaves from Spinacia Oleracea L. and Pisum Sativum L. under Salinity. Plant Physiol. 1991, 97, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E.; Bloom, A.J. Mineral Nutrition of Plants: Principles and Perspectives, 2nd ed.; Sinauer Associates: Sunderland, UK, 2004. [Google Scholar]
- Yang, Y. Fluoride Resistance Mechanism of Different Trees. J. Henan Agric. Univ. 1999, 33, 84–88. [Google Scholar]
- Liu, S. Studies on Resistance of Garden Tress to Damage from Sulfur Dioxide Pollutant. J. Shenyang Agric. Univ. 1988, 19, 50–57. [Google Scholar]
- Mann, K.K.; SchuMann, A.W.; Spann, T.M. Response of Citrus to Exogenously Applied Salicylate Compounds during Abiotic and Biotic Stress. Proc. Fla. State Hortic. Soc. 2011, 124, 10. [Google Scholar]
- Lohe, R.N.; Tyagi, B.; Singh, V.; Kumar Tyagi, P.; Khanna, D.R.; Bhutiani, R. A Comparative Study for Air Pollution Tolerance Index of Some Terrestrial. Glob. J. Environ. Sci. Manag. 2015, 1, 10. [Google Scholar]
- TRY Plant Trait Database. Available online: https://www.try-db.org/TryWeb/Home.php (accessed on 16 September 2019).
- Han, Z.; Sun, H.; Gu, S.; Bao, W.; Yang, L. Responses of 9 Species of Arbor Trees to the Urban Traffic Environment. J. Fujian For. Sci. Technol. 2012, 39, 95–100. [Google Scholar]
- Riga, P.; Benedicto, L. Effects of Light-Diffusing Plastic Film on Lettuce Production and Quality Attributes. Span J. Agric. Res. 2017, 15, e0801. [Google Scholar] [CrossRef]
- Alhesnawi, A.S.M.; Alsalman, I.M.; Najem, N.A. Evaluation of Air Pollution Tolerance Index of Some Plants Species in Kerbala City, Iraq. J. Pharm. Sci. 2018, 10, 6. [Google Scholar]
- Chen, D.; Zheng, M. Study on Heightening Ornamental Plants Resistance to Air Pollution. J. Zhejiang For. Sci. Technol. 1992, 12, 6–10. [Google Scholar]
- Zhang, Y.; Yan, X. Changes of Pigments and Relative Substances in Leaves of Three Red-Leafed Tree Species from Prune L. in Growing Seasons. J. Yangtze Univ. (Nat. Sci. Ed.) 2008, 5, 16–19 + 127. [Google Scholar]
- Zhang, Y.; Xiao, W.; Ji, G.; Gao, C.; Chen, X.; Cao, Y.; Han, L. Effects of Multiscale-Mechanical Grinding Process on Physicochemical Properties of Black Tea Particles and Their Water Extracts. Food Bioprod. Process. 2017, 105, 171–178. [Google Scholar] [CrossRef]
- Frohnmeyer, H.; Grabov, A.; Blatt, M.R. A Role for the Vacuole in Auxin-Mediated Control of Cytosolic pH by Vicia Mesophyll and Guard Cells: Control of Cytosolic pH. Plant J. 2002, 13, 109–116. [Google Scholar] [CrossRef]
- Jeong, S.M.; Kim, S.Y.; Kim, D.R.; Jo, S.C.; Nam, K.C.; Ahn, D.U.; Lee, S.C. Effect of Heat Treatment on the Antioxidant Activity of Extracts from Citrus Peels. J. Agric. Food Chem. 2004, 52, 3389–3393. [Google Scholar] [CrossRef] [PubMed]
- Gümüşay, Ö.A.; Borazan, A.A.; Ercal, N.; Demirkol, O. Drying Effects on the Antioxidant Properties of Tomatoes and Ginger. Food Chem. 2015, 173, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Guiné, R.P.F.; Barroca, M.J.; Gonçalves, F.J.; Alves, M.; Oliveira, S.; Mendes, M. Artificial Neural Network Modelling of the Antioxidant Activity and Phenolic Compounds of Bananas Submitted to Different Drying Treatments. Food Chem. 2015, 168, 454–459. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry and Molecular Biology of Plants; John Wiley & Sons, Ltd.: West Sussex, UK, 2015. [Google Scholar]
- Zhao, W.L. Basic Biochemistry; China Agricultural University Press: Beijing, China, 2008. [Google Scholar]
- Cheynier, V. Phenolic Compounds: From Plants to Foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Chisari, M.; Todaro, A.; Barbagallo, R.N.; Spagna, G. Salinity Effects on Enzymatic Browning and Antioxidant Capacity of Fresh-Cut Baby Romaine Lettuce (Lactuca Sativa L. Cv. Duende). Food Chem. 2010, 119, 1502–1506. [Google Scholar] [CrossRef]
- Lin, H.; Xi, Y.; Chen, S. The Relationship Between the Desiccation-Induced Browning and the Metabolism of Active Oxygen and Phenolics in Pericarp of Postharvest Longan Fruit. J. Plant Physiol. Mol. Biol. 2005, 3, 287–297. [Google Scholar]
- Yue, H. Advanced Inorganic Chemistry; China Machine Press: Beijing, China, 2002. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).