Abstract
The spotted lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae) is a polyphagous, phloem-feeding invasive forest, agricultural, and nuisance pest that is rapidly spreading through the U.S. Little is known about how fulgorids locate each other to mate. To determine if conspecific semiochemicals such as pheromones occur, whole body extracts (WBE) of adult spotted lanternflies from different physiological states were tested for attraction in a dual-choice olfactometer. In olfactometer assays, prior to mating, males were oriented to WBE from males and females. During their mating period, males were attracted to WBE of females, but not to that of males. After mating and oviposition had taken place, males were not attracted to either male or female extracts. Conversely, females did not orient to any WBE from either sex during any physiological state. These behavioral responses by males but not females to WBE from conspecifics in different physiological states suggest the possible presence of an aggregation-sex pheromone in the spotted lanternfly.
1. Introduction
The northeastern United States now confronts an invasion of spotted lanternfly Lycorma delicatula (White) (Hemiptera: Fulgoridae) (hereafter SLF), a destructive univoltine plant pest [1]. Therefore, what cues or signals mediate SLF behavior is an important topic for investigation. Signals used by insects to locate conspecifics for mating may be visual, chemical, or vibroacoustic [2]. Chemical signals used in communication between conspecifics are called pheromones. Chemicals that convey information between species in which the receiver benefits to the detriment of the sender, such as plant volatiles that attract herbivores, are called kairomones. In several families of Fulgoromorpha, such as Caliscelidae, Cixiidae, Derbidae, Delphacidae, Issidae, Tettigarctidae, and Tropiduchidae, vibroacoustic communications have been found [3,4,5,6,7,8,9,10], with few examples of pheromone use in this superfamily [11]. SLF attraction has been demonstrated in response to numerous plant-derived kairomones, reflecting their polyphagous nature [12]. These kairomones are attractive to nymphs and female adult life stages [12,13]. Methyl salicylate is one of many volatile kairomones released by their preferred host, tree-of-heaven Ailanthus altissima (Mill.) Swingle (Sapindales: Simaroubaceae), as well as secondary hosts including grape Vitis vinifera L. (Vitales: Vitaceae). Although methyl salicylate has been developed into lures to help attract SLF for detection trapping [13], when there are many other competing sources of attraction, its performance may be inadequate [14,15]. The discovery of a sex pheromone could potentially improve lure performance. Recently, chemical signals contributing to intraspecific communication were found in SLF honeydew, and these may play a role in their aggregation behavior [16]. Other sources of intraspecific chemical signals deem investigation, as the presence of a sex pheromone in SLF has not been reported [17].
Many hemipterans utilize multiple modes of communication involving both mechanical (substrate-borne vibration) and chemical (pheromones) signals to locate conspecifics [18]. Substrate-borne vibrational signals are typically utilized for sexual communication over shorter ranges [19], whereas chemical signals can be detected over greater distances and can be used for long-range orientation, or in conjunction with shorter-range vibrational signals [20,21,22]. The co-occurrence of both strategies has been demonstrated for several hemipteran species, mostly in Psyllidae [23,24,25,26,27,28] and Pentatomidae [20,29,30,31,32,33]. We recently found that SLF are capable of detecting vibroacoustic cues and respond with positive vibrotaxis [34]. Therefore, it is conceivable that SLF use multiple communication strategies to locate one another.
The primary objective of this study was to investigate attraction of SLF to volatile compounds derived from conspecifics. Therefore, we tested adult male and female SLF for attraction in a dual-choice olfactometer to conspecific volatiles from body extracts of wild-caught adult male or female SLF in different physiological phases.
2. Materials and Methods
2.1. Insects
Live adult male and female SLF for use in behavioral bioassays were field-collected weekly from A. altissima trees in Berks, Montgomery, Lehigh, and Northampton Counties in Pennsylvania. They were shipped overnight to the Forest Pest Methods Laboratory (FPML) Insect Containment Facility (ICF) in Buzzards Bay, Massachusetts every Monday, adhering to the conditions set by permits from the Pennsylvania Department of Agriculture (PDA) (PP3-0123-2015) and U.S. Department of Agriculture (USDA) (P526P-15-00152 and P526P-17-04376). Adult males and females were housed in separate cages, with up to 20 SLF per cage as previously described [12], at 25 °C under a photoperiod of 16:8 (L:D), and provided A. altissima to feed upon. Newly field-caught insects were used every week within four days of their collection.
The adult SLF stage is relatively long-lived in the wild. In northeastern Pennsylvania, adults typically emerge at the end of July or the beginning of August and die in late October or early November, a period lasting approximately 12-16 weeks, with mating taking place in mid-September [35]. No evidence exists to suggest that SLF mate more than once. Therefore, in order to separate the adult stage into physiological states relevant to their behavior, adults have been classified into three phases, “Early”, “Mid”, and “Late”, as previously described [13,16,35]. Adult phases were defined by first observations of each corresponding physiological state of SLF in the field, such as adult emergence (Early), mating (Mid), and oviposition (Late). Start dates respectively for 2018 and 2021 adult phases were: 1 August and 22 July for “Early”, 14 and 13 September for “Mid”, and 1 October and 20 September for “Late”.
2.2. Whole Body Extract
In the field, SLF adults were removed from trees, quickly sexed and placed into an oven bag (Turkey size, Reynolds Consumer Products, Field Court, Lake Forest, IL, USA) which was resting on top of dry ice in a 900-mL plastic cup. This technique separated males and females, instantly killed the insects, and preserved their body volatiles. Prior to use, oven bags were baked at 150 °C for 1–2 h to remove volatile contaminants such as caprolactam [36]. Thus, SLF were flash-frozen in the field and shipped on dry ice to the FPML to make whole body extracts (WBE).
Upon the arrival of frozen SLF at the FPML, they were removed from the dry ice and oven bags and cohorts of each sex were placed into a 500 mL glass beaker and covered with hexane (99.9% purity, Fisher Scientific, Fair Lawn, NJ, USA). The beaker was covered with foil and held at room temperature for 15 min. The resulting WBE was then decanted through glass wool, to exclude solids, into 20 mL scintillation vials. The WBE was subsequently concentrated by passing a gentle stream of nitrogen over the sample until the volume had decreased to the desired number of male or female equivalents (ME or FE) per microliter of sample. This was calculated by dividing the number of insects used to make the extract by the volume of the total extract, and resulted in a concentration of 7.1 ± 0.2 male or female SLF equivalent in 100 μL hexane. Extracts were kept in a freezer (−25 °C) for up to 3 days before use, and lures were prepared immediately before use in behavioral bioassays. Lures consisted of an open 0.5 mL microcentrifuge tube (Globe Scientific Inc., Mahwah, NJ, USA) containing 100 μL of WBE or hexane (control).
2.3. Behavioral Bioassays
A custom Teflon Y-plate olfactometer (28.6 cm long × 21.6 cm wide × 3.8 cm high, with a 5.1 cm wide Y-shaped corridor) was used to measure the walking orientation of male and female SLF in response to volatiles from the WBE or the control, identical to previously described procedures and conditions [12,13,16]. The upwind arms of the Y-plate were connected to two ground glass joint adapters to arms of two 50-mL glass Erlenmeyer flasks: one contained a WBE lure and the other contained a control lure. Air was pumped (Air Cadet, Thermo Scientific, Barrington, IL, USA) into a 2-Channel Air Delivery System (Analytical Research Systems, Gainesville, FL, USA), drawn through activated charcoal, and humidified by bubbling it through water before it entered the glass flasks containing the lures at the two upwind arms of the Y-plate. Air flowed through the y-plate at 24 cm/s.
SLF were transferred to individual release cages in the bioassay chamber approximately 30–60 min before starting the behavioral bioassays to allow acclimatization. The first 5 SLF tested were released into the Y-plate without any lures present in order to verify that the Y-plate had no directional bias. If a plate showed a directional bias without any lures, it was replaced with a clean plate and tested again. Once it was confirmed that there was no directional bias in the system, the control and WBE lures were placed into the upwind flasks and individual SLF adults of alternating sex were released into the Y-plate and allowed 3 min to respond, ensuring that both sexes received the exact same olfactory stimuli. A choice was recorded when an SLF walked more than halfway up one of the upwind arms in the allotted time. Up to 20 SLF (10 males and 10 females, alternating) were tested in each round, then a clean Y-plate was used to start a new round. Each insect was used only once. Two rounds were conducted per day with at least 2 h in between rounds. Each round used a newly cleaned Y-plate setup and a freshly made lure and took approximately 60 min to complete. The side of the assay housing the WBE and control were alternated between rounds to control for any potential unknown visual bias. The Y-plate behavioral bioassay data were analyzed by a chi-square goodness-of-fit test, with significance at α = 0.05 when the test statistic G was greater than 3.841 [37].
3. Results
Sex-Specific Responses to Whole Body Extracts
Early and Mid males were the only SLF adults that made statistically significant choices in the dual-choice bioassays. No females were attracted to any WBE from either sex at any stage (Figure 1). WBE from both sexes attracted Early males, whereas only Mid female WBE attracted Mid males (Figure 1).
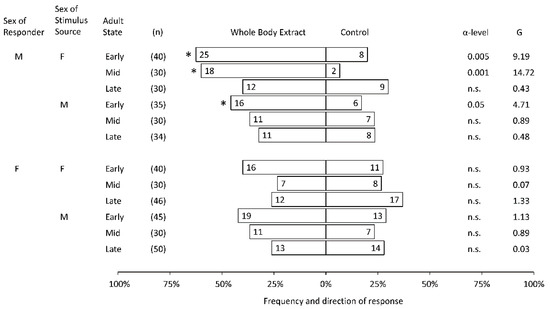
Figure 1.
Frequency and direction of choices of individual adult male (M) and female (F) spotted lanternflies (SLF), Lycorma delicatula, from different adult physiological states, in response to volatiles from whole body extracts collected from male or female SLF (7.1 insect equivalents), tested against the solvent control in a dual-choice walking olfactometer. Asterisks indicate a significant difference between sides (Chi Square test). A test statistic (G) below 3.841 was not significant (n.s.), and the level of significance (α) is shown.
Response rates, the percentage of SLF that were activated to walk upwind and make a choice, varied depending on the stimulus and responder. For instance, 83%, 67%, and 70% of males responded to female WBE, and 63%, 60%, and 56% of males responded to male WBE during Early, Mid, and Late phases, respectively. Female response rates were 68%, 50%, and 63% to female WBE, and 71%, 60%, and 54% to male WBE, during Early, Mid, and Late phases, respectively.
4. Discussion and Conclusions
We report behavioral evidence of insect-derived volatiles attracting males to the same- and opposite-sex conspecifics in olfactometer assays in the family Fulgoridae. These findings suggest the presence of pheromones that play a role in male SLF finding conspecifics to aggregate and females to mate.
Evidence of a possible aggregation of pheromones was recently reported from the honeydew secretions of SLF [16]. In that study, which combined responses over physiological states, males were found to be significantly attracted to volatiles from male-produced honeydew, and both males and females showed a trend towards attraction to honeydew volatiles from females. Same-sex attraction of free-living SLF was observed in the field using caged aggregations of all males or females as lures [35]. During mating time, marked-released male SLF oriented significantly to the combination of caged females on higher SLF population density trees, but not to caged males on higher density trees [35]. Olfactory signals mediating mating occur in several hemipteran species. Female-produced sex pheromones have been described in several species of Psyllidae such as Cacopsylla bidens [23], C. pyricola [26,38,39], Diaphorina citri [24], Bactericera cockerelli [40], scale insects [41,42], several mealybugs species [43], and aphids [44,45]. In addition, male stink bugs produce sex pheromones, and females emit vibrational signals to attract males [46,47,48,49].
To our knowledge, no other members of Fulgoridae have been investigated to determine how they locate mates. Additionally, mating behavior in SLF has not been adequately described, especially concerning what cues are involved in bringing the sexes together, or even where or what time most mating takes place. In the field, mating pairs appear approximately six weeks after the first emergence of adults [12,13,35]. Baker, et al. [50] reported that a male and female were sedentary, positioned side-by-side on an Ailanthus trunk for two hours, and then the male suddenly vibrated his wings and immediately copulated with the female. The use of vibrational communication between the sexes has been established in other families of Fulgoromorpha, such as Cixiidae, Delphacidae, Flatidae, and Issidae [3,4,6,9,10], and likely plays a role in SLF communication [34], but we should not ignore the potential of chemical signals being involved in the mating process.
Based on previous studies, we hypothesize that both SLF males and females locate their host plants using a combination of visual cues [51] and host plant volatiles [12,13]. However, more precise conspecific interactions between SLF adults must occur for them to locate one another for mating. This could occur before or after landing on the host plant. A sex pheromone can be a single compound or a blend of compounds released by one sex which is attractive to the other sex [52]. Similarly, an aggregation pheromone can be released by one or both sexes and typically attracts the same sex as the emitter [53]. Once on the same substrate, substrate vibrations may also be involved [34]. In this study, prior to mating, only males were attracted to the volatiles from both conspecific sexes, suggesting the presence of an aggregation pheromone. Then during the mating phase, males were able to distinguish female body volatiles from those of males, suggesting the presence of a sex pheromone. In the oviposition phase, Late males were no longer attracted to the body volatiles from either sex. Females during the entire process were not attracted to the body volatiles from either sex. The fact that Mid males were only attracted to female volatiles during mating time and this attraction was lost after mating could be explained by changes in the physiological state of one or both sexes. Mate-seeking males that are responsive to volatiles produced by sexually receptive females may no longer respond after mating. Likewise, females may no longer emit the sexual attractant once mated. Although these behaviors could potentially be explained by the presence of two different pheromones for aggregation and sex, a single aggregation-sex pheromone [53] could potentially be responsible if both sexes emit it prior to mating, and then sexually receptive males stop emitting it while sexually receptive females continue to emit it until after mating. We do not know if the compounds responsible for the behaviors we observed were derived from glands, cuticular hydrocarbons, honeydew, or some combination thereof, but this is a topic for future investigation.
The identification of pheromones and their development into effective lures would greatly facilitate the early detection and management of SLF. A qualitative analysis of the chemical components of the WBE was beyond the scope of this study due to large amounts of heavy, waxy, cuticular components that resulted in cloudy samples and precluded their injection into a mass spectrometer [54,55,56]. The development of different volatile collection techniques to facilitate the use of gas chromatography coupled with electroantennography (GC-EAD) and gas chromatography-mass spectrometry (GC-MS), may help to identify specific compounds composing an SLF aggregation and/or sex pheromone. Such work is currently underway.
Author Contributions
Conceptualization, M.F.C.; methodology, M.F.C. and H.F.; validation, M.F.C. and H.F.; formal analysis, M.F.C. and H.F.; investigation, I.C. and H.F.; resources, M.F.C. and D.C.; data curation, M.F.C. and H.F.; writing—original draft preparation, H.F. and M.F.C.; writing—review and editing, M.F.C. and H.F.; visualization, M.F.C. and H.F.; supervision, M.F.C. and D.C.; project administration, M.F.C.; funding acquisition, M.F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in 2017 and 2018 by USDA Farm Bill Section 10007 (6R.3.0320 and 6R.0679, respectively), and in 2019, 2020, and 2021 from Plant Protection Act 7721 (3.0386, 3.0106, and 3.0792, respectively), which supported the project at the FPML, and through cooperative agreements with University of Florida (AP17PPQS&T00C022, AP18PPQS&T00C036, AP19PPQS&T00C036, AP21PPQS&T00C065), East Stroudsburg University (AP18PPQS&T00C017, AP19PPQS&T00C016, AP21PPQS&T00C117), and Xavier University (AP18PPQS&T00C042, AP19PPQS&T00C028, AP20PPQS&T00C023).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Raw data for this study are reported in Figure 1.
Acknowledgments
Our sincere thanks are extended to numerous people for their contributions or support in the laboratory or field: Nathan Derstine, Linnea Meier, Sam Stella, Kelly Murman, Stefani Cannon, Leslie Abreu, Kyle Kaye, Sebastian Harris, Levi Morris, Aubrianna Stetina, Reannon Zangakis, Valerie Muñoz, Cole Davis, Matthew Wallace, Annie Ray, and property owners. This material was made possible, in part, by a Cooperative Agreement from the United States Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS). It may not necessarily express APHIS’ views.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Lee, D.-H.; Park, Y.-L.; Leskey, T.C. A review of biology and management of Lycorma delicatula (Hemiptera: Fulgoridae), an emerging global invasive species. J. Asia-Pac. Entomol. 2019, 22, 589–596. [Google Scholar] [CrossRef]
- Shorey, H.H. Behavioral responses to insect pheromones. Annu. Rev. Entomol. 1973, 18, 349–380. [Google Scholar] [CrossRef]
- Ichikawa, T. Mutual communication by substrate vibrations in the mating behavior of planthoppers (Homoptera: Delphacidae). Appl. Entomol. Zool. 1976, 11, 8–21. [Google Scholar] [CrossRef][Green Version]
- Ichikawa, T.; Ishii, S. Mating signal of the brown planthopper, Nilaparvata lugens Stål (Homoptera: Delphacidae): Vibration of the substrate. Appl. Entomol. Zool. 1974, 9, 196–198. [Google Scholar] [CrossRef]
- Claridge, M.F.; Morgan, J.C.; Moulds, M.S. Substrate-transmitted acoustic signals of the primitive cicada, Tettigarcta crinita Distant (Hemiptera Cicadoidea, Tettigarctidae). J. Nat. Hist. 1999, 33, 1831–1834. [Google Scholar] [CrossRef]
- Tishechkin, D.Y. New data on vibrational calling signals of Fulgoroidea (Homoptera: Auchenorrhyncha) from the Asian part of Palaearctic with new records of three species of Cixiidae. Russ. Entomol. J. 2016, 25, 307–322. [Google Scholar] [CrossRef]
- Tishechkin, D.Y. On the similarity of temporal pattern of vibrational calling signals in different species of Fulgoroidea (Homoptera: Auchenorrhyncha). Russ. Entomol. J. 2008, 17, 349–357. [Google Scholar]
- Tishechkin, D.Y. Vibrational communication in Cercopoidea and Fulgoroidea (Homoptera: Cicadina) with notes on classification of higher taxa. Russ. Entomol. J. 2003, 12, 127–181. [Google Scholar]
- Virant-Doberlet, M.; Žežlina, I. Vibrational communication of Metcalfa pruinosa (Hemiptera: Fulgoroidea: Flatidae). Ann. Entomol. Soc. Am. 2007, 100, 73–82. [Google Scholar] [CrossRef]
- Davranoglou, L.-R.; Cicirello, A.; Taylor, G.K.; Mortimer, B. Planthopper bugs use a fast, cyclic elastic recoil mechanism for effective vibrational communication at small body size. PLoS Biol. 2019, 17, e3000155. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liang, A.-P. Identification of a self-regulatory pheromone system that controls nymph aggregation behavior of rice spittlebug Callitettix versicolor. Front. Zool. 2015, 12, 10. [Google Scholar] [CrossRef]
- Derstine, N.T.; Meier, L.; Canlas, I.; Murman, K.; Cannon, S.; Carrillo, D.; Wallace, M.; Cooperband, M.F. Plant volatiles help mediate host plant selection and attraction of the spotted lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae): A generalist with a preferred host. Environ. Entomol. 2020, 49, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Cooperband, M.F.; Wickham, J.; Cleary, K.; Spichiger, S.-E.; Zhang, L.; Baker, J.; Canlas, I.; Derstine, N.; Carrillo, D. Discovery of three kairomones in relation to trap and lure development for spotted lanternfly (Hemiptera: Fulgoridae). J. Econ. Entomol. 2019, 112, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Nixon, L.J.; Leach, H.; Barnes, C.; Urban, J.; Kirkpatrick, D.M.; Ludwick, D.C.; Short, B.; Pfeiffer, D.G.; Leskey, T.C. Development of Behaviorally Based Monitoring and Biosurveillance Tools for the Invasive Spotted Lanternfly (Hemiptera: Fulgoridae). Environ. Entomol. 2020, 49, 1117–1126. [Google Scholar] [CrossRef]
- Cooperband, M.; Meier, L.; Wickham, J.; Murman, K.; Cannon, S.; Abreu, L. Tree effects: Understanding what makes “hot” trees hot to spotted lanternfly. In Otis Laboratory 2018 Annual Report; Trepanowski, N., Vieira, K., Heller, S., Booth, E., Eds.; United States Department of Agriculture: Buzzards Bay, MA, USA, 2019; pp. 62–63. [Google Scholar]
- Faal, H.; Meier, L.R.; Canlas, I.J.; Murman, K.; Wallace, M.S.; Carrillo, D.; Cooperband, M.F. Volatiles from male honeydew excretions attract conspecific male spotted lanternflies, Lycorma delicatula (Hemiptera: Fulgoridae). Front. Insect Sci. 2022, 2, 982965. [Google Scholar] [CrossRef]
- Urban, J.M. Perspective: Shedding light on spotted lanternfly impacts in the USA. Pest Manag. Sci. 2020, 76, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.; Jepson, P.C.; Howse, P.E. Long-range mate location and close-range courtship behaviour of the green stink bug, Nezara viridula and its mediation by sex pheromones. Entomol. Exp. Appl. 1987, 44, 205–212. [Google Scholar] [CrossRef]
- Čokl, A.; McBrien, H.L.; Millar, J.G. Comparison of substrate-borne vibrational signals of two stink bug species, Acrosternum hilare and Nezara viridula (Heteroptera: Pentatomidae). Ann. Entomol. Soc. Am. 2001, 94, 471–479. [Google Scholar] [CrossRef]
- Miklas, N.; Lasnier, T.; Renou, M. Male bugs modulate pheromone emission in response to vibratory signals from conspecifics. J. Chem. Ecol. 2003, 29, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Zgonik, V.; Čokl, A. The role of signals of different modalities in initiating vibratory communication in Nezara viridula. Open Life Sci. 2014, 9, 200–211. [Google Scholar] [CrossRef]
- Čokl, A.; Laumam, R.A.; Stritih, N. Substrate-borne vibratory communication. In Stink Bugs; Čokl, A., Borges, M., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 125–164. [Google Scholar]
- Soroker, V.; Talebaev, S.; Harari, A.R.; Wesley, S.D. The role of chemical cues in host and mate location in the pear psylla Cacopsylla bidens (Homoptera: Psyllidae). J. Insect Behav. 2004, 17, 613–626. [Google Scholar] [CrossRef]
- Wenninger, E.J.; Stelinski, L.L.; Hall, D.G. Behavioral evidence for a female-produced sex attractant in Diaphorina citri. Entomol. Exp. Appl. 2008, 128, 450–459. [Google Scholar] [CrossRef]
- Guédot, C.; Horton, D.R.; Landolt, P.J. Sex attraction in Bactericera cockerelli (Hemiptera: Triozidae). Environ. Entomol. 2010, 39, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Guédot, C.; Millar, J.G.; Horton, D.R.; Landolt, P.J. Identification of a sex attractant pheromone for male winterform pear psylla, Cacopsylla pyricola. J. Chem. Ecol. 2009, 35, 1437–1447. [Google Scholar] [CrossRef]
- Moghbeli Gharaei, A.; Ziaaddini, M.; Jalali, M.; Michaud, J. Sex-specific responses of Asian citrus psyllid to volatiles of conspecific and host-plant origin. J. Appl. Entomol. 2014, 138, 500–509. [Google Scholar] [CrossRef]
- Stockton, D.G.; Martini, X.; Stelinski, L.L. Male psyllids differentially learn in the context of copulation. Insects 2017, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Čokl, A.; Žunič-Kosi, A.; Laumann, R.A. Stink bug communication with multimodal signals transmitted through air and substrate. Emerg. Sci. J. 2019, 3, 407–424. [Google Scholar] [CrossRef]
- Blassioli-Moraes, M.C.; Magalhaes, D.M.; ČOkl, A.; Laumann, R.A.; Da Silva, J.P.; Silva, C.C.A.; Borges, M. Vibrational communication and mating behaviour of Dichelops melacanthus (H emiptera: P entatomidae) recorded from loudspeaker membranes and plants. Physiol. Entomol. 2014, 39, 1–11. [Google Scholar] [CrossRef]
- Borges, M.; Moraes, M.C.B.; Peixoto, M.F.; Pires, C.S.S.; Sujii, E.R.; Laumann, R.A. Monitoring the Neotropical brown stink bug Euschistus heros (F.)(Hemiptera: Pentatomidae) with pheromone-baited traps in soybean fields. J. Appl. Entomol. 2011, 135, 68–80. [Google Scholar] [CrossRef]
- Polajnar, J.; Čokl, A. The effect of vibratory disturbance on sexual behaviour of the southern green stink bug Nezara viridula (Heteroptera, Pentatomidae). Open Life Sci. 2008, 3, 189–197. [Google Scholar] [CrossRef]
- Claridge, M.F. Vibratory Signals Produced by Heteroptera—Pentatomorpha and Cimicomorpha: Matija Gogala. In Insect Sounds and Communication; CRC Press: Boca Raton, FL, USA, 2005; pp. 293–314. [Google Scholar]
- Rohde, B.B.; Cooperband, M.F.; Canlas, I.; Mankin, R.W. Evidence of receptivity to vibroacoustic stimuli in the spotted lanternfly. J. Econ. Entomol. 2022. [Google Scholar]
- Cooperband, M.F.; Murman, K. Responses of adult spotted lanternflies to artificial aggregations composed of all males or females. Front. Insect Sci. 2022, 2, 981832. [Google Scholar] [CrossRef]
- Stewart-Jones, A.; Poppy, G.M. Comparison of glass vessels and plastic bags for enclosing living plant parts for headspace analysis. J. Chem. Ecol. 2006, 32, 845–864. [Google Scholar] [CrossRef]
- Rohlf, F.J.; Sokal, R.R. Statistical Tables; Macmillan: New York, NY, USA, 1995. [Google Scholar]
- Horton, D.R.; Guédot, C.; Landolt, P.J. Attraction of male summerform pear psylla to volatiles from female pear psylla: Effects of female age, mating status, and presence of host plant. Can. Entomol. 2008, 140, 184–191. [Google Scholar] [CrossRef]
- Horton, D.R.; Landolt, P.J. Attraction of male pear psylla, Cacopsylla pyricola, to female-infested pear shoots. Entomol. Exp. Appl. 2007, 123, 177–183. [Google Scholar] [CrossRef]
- Guédot, C.; Horton, D.R.; Landolt, P.J.; Munyaneza, J.E. Effect of mating on sex attraction in B actericera cockerelli with evidence of refractoriness. Entomol. Exp. Appl. 2013, 149, 27–35. [Google Scholar] [CrossRef]
- Lanier, G.N.; Qi, Y.-T.; West, J.R.; Park, S.C.; Webster, F.X.; Silverstein, R.M. Identification of the sex pheromone of three Matsucoccus pine bast scales. J. Chem. Ecol. 1989, 15, 1645–1659. [Google Scholar] [CrossRef]
- Tashiro, H.; Chambers, D.L. Reproduction in the California red scale, Aonidiella aurantii (Homoptera: Diaspididae). I. Discovery and extraction of a female sex pheromone. Ann. Entomol. Soc. Am. 1967, 60, 1166–1170. [Google Scholar] [CrossRef]
- Bakthavatsalam, N. Semiochemicals in Mealybugs. In Mealybugs and their Management in Agricultural and Horticultural Crops; Springer: Berlin/Heidelberg, Germany, 2016; pp. 173–198. [Google Scholar]
- Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M.; Hardie, J. The chemical ecology of aphids. Annu. Rev. Entomol. 1992, 37, 67–90. [Google Scholar] [CrossRef]
- Boullis, A.; Verheggen, F.J. Chemical ecology of aphids (Hemiptera: Aphididae). Biol. Ecol. Aphids 2016, 171, 171–198. [Google Scholar] [CrossRef]
- Blassioli-Moraes, M.C.; Khrimian, A.; Michereff, M.F.F.; Magalhães, D.M.; Hickel, E.; de Freitas, T.F.S.; Barrigossi, J.A.F.; Laumann, R.A.; Silva, A.T.; Guggilapu, S.D. Male-produced sex pheromone of Tibraca limbativentris revisited: Absolute configurations of zingiberenol stereoisomers and their influence on chemotaxis behavior of conspecific females. J. Chem. Ecol. 2020, 46, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.; Birkett, M.; Aldrich, J.R.; Oliver, J.E.; Chiba, M.; Murata, Y.; Laumann, R.A.; Barrigossi, J.A.; Pickett, J.A.; Moraes, M.C.B. Sex attractant pheromone from the rice stalk stink bug, Tibraca limbativentris Stal. J. Chem. Ecol. 2006, 32, 2749–2761. [Google Scholar] [CrossRef] [PubMed]
- Moraes, M.C.B.; Laumann, R.A.; Cokl, A.; Borges, M. Vibratory signals of four Neotropical stink bug species. Physiol. Entomol. 2005, 30, 175–188. [Google Scholar] [CrossRef]
- Borges, M.; Millar, J.G.; Laumann, R.A.; Moraes, M.C.B. A male-produced sex pheromone from the neotropical redbanded stink bug, Piezodorus guildinii (W.). J. Chem. Ecol. 2007, 33, 1235–1248. [Google Scholar] [CrossRef]
- Baker, T.C.; Smyers, E.; Urban, J.; Meng, Z.; Damadaram, K.P.; Myrick, A.J.; Cooperband, M.; Domingue, M. Progression of seasonal activities of adults of the spotted lanternfly, Lycorma delicatula, during the 2017 season of mass flight dispersal behavior in eastern Pennsylvania. J. Asia-Pac. Entomol. 2019, 22, 705–713. [Google Scholar] [CrossRef]
- Baker, T.; Myrick, A.; Wolfin, M.; Wang, Y. Visual Responses of Flight-Dispersing Spotted Lanternflies, Lycorma delicatula toward a Tall Vertical Silhouette in a Vineyard. J. Insect Behav. 2021, 34, 49–60. [Google Scholar] [CrossRef]
- Cardé, R.T.; Baker, T.C. Sexual communication with pheromones. In Chemical Ecology of Insects; Bell, W.J., Cardé, R.T., Eds.; Springer: London, UK, 1984; pp. 355–383. [Google Scholar]
- Cardé, R.T. Defining attraction and aggregation pheromones: Teleological versus functional perspectives. J. Chem. Ecol. 2014, 40, 519–520. [Google Scholar] [CrossRef]
- Mason, R.T.; Fales, H.M.; Jones, T.H.; O’Brien, L.B.; Taylor, T.W.; Hogue, C.L.; Blum, M.S. Characterization of fulgorid waxes (Homoptera: Fulgoridae: Insecta). Insect Biochem. 1989, 19, 737–740. [Google Scholar] [CrossRef]
- O’Brien, L.B.; Wilson, S.W. Planthopper systematics and external morphology. In The Leafhoppers and Planthoppers; Nault, L.R., Rodriguez, J.G., Eds.; John Wiley & Sons: New York, NY, USA, 1985; pp. 61–102. [Google Scholar]
- Cho, S.-R.; Lee, J.-E.; Jeong, J.-W.; Yang, J.-O.; Yoon, C.; Kim, G.-H. Comparison of cuticular hydrocarbons of different developmental stages of the spot clothing wax cicada, Lycorma delicatula (Hemiptera: Fulgoridae). Korean J. Appl. Entomol. 2011, 50, 185–194. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).