Predicting the Potential Distribution of Endangered Parrotia subaequalis in China
Abstract
:1. Introduction
2. Methods
2.1. Species Occurrence Data
2.2. Bioclimatic Variables
2.3. MaxEnt Modeling
2.4. Geospatial Analysis
3. Results
3.1. Model Performance
3.2. Key Bioclimatic Variables
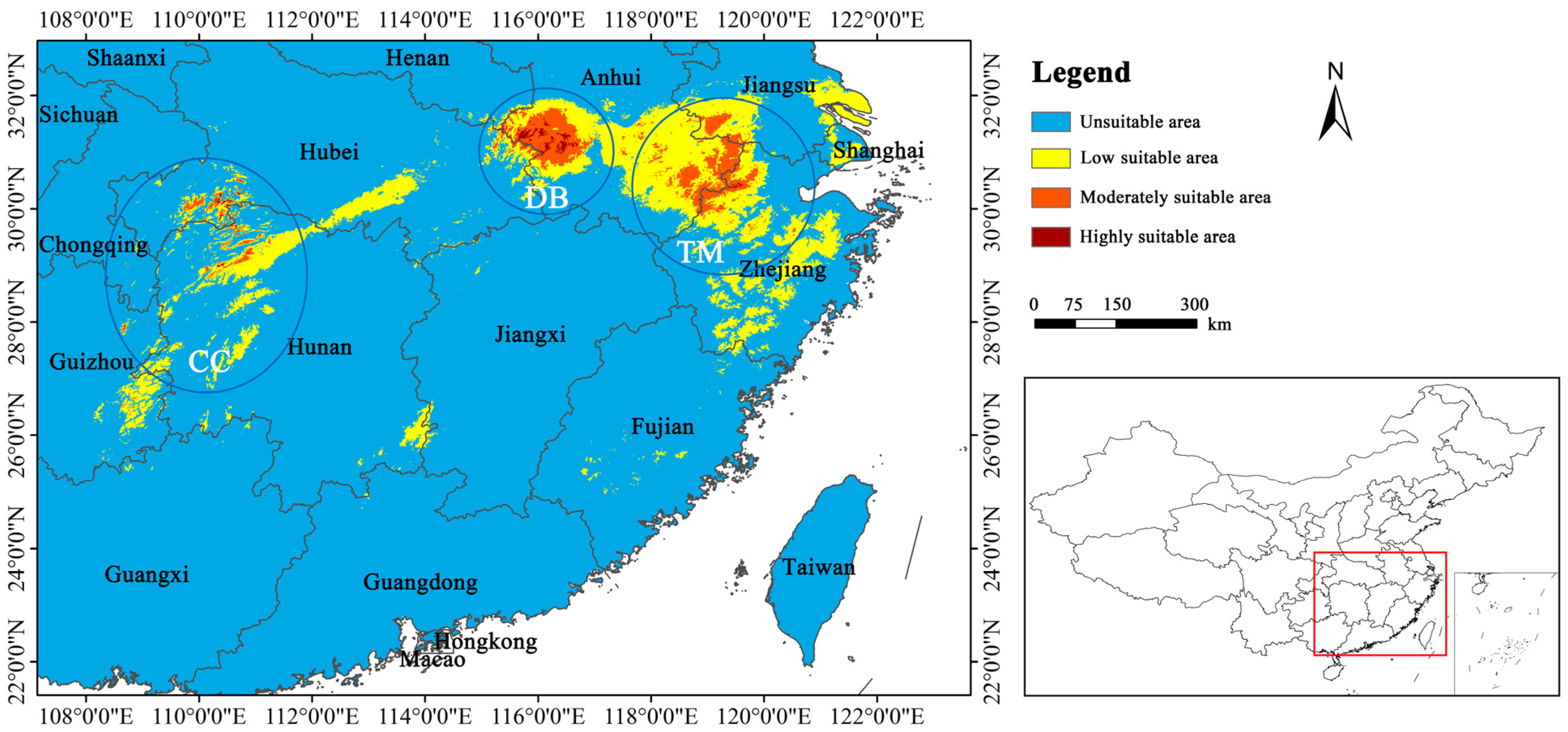
3.3. Current Distribution of Habitat Suitability
3.4. Future Changes in Habitat Suitability
4. Discussion
4.1. Modeling Evaluation and Variable Influence
4.2. Predicted Habitat Suitability for P. subaequalis under Current Scenario
4.3. Geographical Shift in Habitat Suitability under Future Climate
4.4. Conservation Implications for P. subaequalis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.H.; Tredici, P.D. The Chinese Parrotia: A sibling species of the Persian Parrotia. Arnoldia 2008, 66, 2–9. [Google Scholar]
- Li, W.; Zhang, G.F. Population structure and spatial pattern of the endemic and endangered subtropical tree Parrotia subaequalis (Hamamelidaceae). Flora 2015, 212, 10–18. [Google Scholar] [CrossRef]
- Wang, J.Q.; Zhang, G.F. Woody Plants in Liyang; Nanjing Normal University Press: Nanjing, China, 2019; ISBN 9787565142468. [Google Scholar]
- Zhang, G.F. Illustrations of the Common Plants in Tianmu Mountain; Higher Education Press: Beijing, China, 2020; ISBN 9787040543940. [Google Scholar]
- Zhang, G.F.; Xiong, T.S.; Sun, T.; Li, K.D.; Shao, L.Y. Diversity, distribution, and conservation of rare and endangered plant species in Jiangsu Province. Biodivers. Sci. 2022, 30, 21335. [Google Scholar] [CrossRef]
- Qin, H.N.; Yang, Y.; Dong, S.Y.; He, Q.; Jia, Y.; Zhao, L.N.; Yu, S.X.; Liu, H.Y.; Liu, B.; Yan, Y.H.; et al. Threatened species list of China’s higher plants. Biodivers. Sci. 2017, 25, 696–744. [Google Scholar] [CrossRef]
- Gong, B.; Xia, Y.J.; Zhang, G.F.; Lu, Y.; Sun, G. Population structure and spatial pattern of Parrotia subaequalis, a rare and endangered species endemic to China. J. Ecol. Rural Environ. 2012, 28, 628–646. [Google Scholar]
- Yan, C.; Wang, Z.S.; An, S.Q.; Chen, S.N.; Wei, N.; Lu, X.M. Differences in photosynthetic capacity among different diameter-classes of Parrotia subaequalis populations and their implications to regeneration limitation. Acta Ecol. Sin. 2008, 28, 4153–4161. [Google Scholar]
- Liu, J.; Zhang, G.F.; Li, X. Structural diversity and conservation implications of Parrotia subaequalis (Hamamelidaceae), a rare and endangered tree species in China. Nat. Conserv. 2021, 44, 99–115. [Google Scholar] [CrossRef]
- Li, L.; Zhang, G.F.; Wang, M.D.; Wang, J.Q.; Zhu, J.H. Structure, spatial pattern and regeneration of Parrotia subaequalis population in Liyang mountainous area of Jiangsu Province. J. Jiangsu For. Sci. Technol. 2018, 45, 17–28. [Google Scholar] [CrossRef]
- Wang, Q.R.; Fan, W. A new record genus and species of Hamamelidaceae in Henan. J. Zhejiang Agric. Sci. 2017, 58, 2205–2209. [Google Scholar] [CrossRef]
- Dawson, T.P.; Jackson, S.T.; House, J.I.; Prentice, I.C.; Mace, G.M. Beyond predictions: Biodiversity conservation in a changing climate. Science 2011, 332, 53–58. [Google Scholar] [CrossRef]
- Qin, A.; Liu, B.; Guo, Q.; Bussmann, R.W.; Ma, F.; Jian, Z.; Xu, G.; Pei, S. Maxent modeling for predicting impacts of climate change on the potential distribution of Thuja sutchuenensis Franch, an extremely endangered conifer from southwestern China. Glob. Ecol. Conserv. 2017, 10, 139–146. [Google Scholar] [CrossRef]
- Pounds, J.A.; Bustamante, M.R.; Coloma, L.A.; Consuegra, J.A.; Fogden, M.P.L.; Foster, P.N.; La Marca, E.; Masters, K.L.; Merino-Viteri, A.; Puschendorf, R.; et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 2006, 439, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.C.; Zagortchev, L.; Ma, J.X.; Yan, M.; Li, J.M. Predicting the potential distribution of the parasitic Cuscuta chinensis under global warming. BMC Ecol. 2020, 20, 28. [Google Scholar] [CrossRef] [PubMed]
- Abolmaali, S.M.-R.; Tarkesh, M.; Bashari, H. Maxent modeling for predicting suitable habitats and identifying the effects of climate change on a threatened species, Daphne mucronata, in central Iran. Ecol. Inform. 2018, 43, 116–123. [Google Scholar] [CrossRef]
- Vincent, H.; Bornand, C.N.; Kempel, A.; Fischer, M. Rare species perform worse than widespread species under changed climate. Biol. Conserv. 2020, 246, 108586. [Google Scholar] [CrossRef]
- Sun, J.J.; Qiu, H.; Guo, J.H.; Xu, X.; Wu, D.T.; Zhong, L.; Jiang, B.; Jiao, J.J.; Yuan, W.G.; Huang, Y.J.; et al. Modeling the potential distribution of Zelkova schneideriana under different human activity intensities and climate change patterns in China. Glob. Ecol. Conserv. 2020, 21, e00840. [Google Scholar] [CrossRef]
- Wiens, J.A.; Stralberg, D.; Jongsomjit, D.; Howell, C.A.; Snyder, M.A. Niches, models, and climate change: Assessing the assumptions and uncertainties. Proc. Natl. Acad. Sci. USA 2009, 106, 19729–19736. [Google Scholar] [CrossRef]
- Yackulic, C.B.; Chandler, R.; Zipkin, E.F.; Royle, J.A.; Nichols, J.D.; Campbell Grant, E.H.; Veran, S. Presence-only modelling using MAXENT: When can we trust the inferences? Methods Ecol. Evol. 2013, 4, 236–243. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Warren, R.; VanDerWal, J.; Price, J.; Welbergen, J.A.; Atkinson, I.; Ramirez-Villegas, J.; Osborn, T.J.; Jarvis, A.; Shoo, L.P.; Williams, S.E.; et al. Quantifying the benefit of early climate change mitigation in avoiding biodiversity loss. Nat. Clim. Change 2013, 3, 678–682. [Google Scholar] [CrossRef]
- Abdelaal, M.; Fois, M.; Fenu, G.; Bacchetta, G. Using MaxEnt modeling to predict the potential distribution of the endemic plant Rosa arabica Crép. in Egypt. Ecol. Inform. 2019, 50, 68–75. [Google Scholar] [CrossRef]
- Hills, R.; Bachman, S.; Forest, F.; Moat, J.; Wilkin, P. Incorporating evolutionary history into conservation assessments of a highly threatened group of species, South African Dioscorea (Dioscoreaceae). S. Afr. J. Bot. 2019, 123, 296–307. [Google Scholar] [CrossRef]
- Dhyani, A.; Kadaverugu, R.; Nautiyal, B.P.; Nautiyal, M.C. Predicting the potential distribution of a critically endangered medicinal plant Lilium polyphyllum in Indian Western Himalayan Region. Reg. Environ. Change 2021, 21, 30. [Google Scholar] [CrossRef]
- Zhang, G.F.; Yao, R.; Jiang, Y.Q.; Chen, F.C.; Zhang, W.Y. Intraspecific and interspecific competition intensity of Parrotia subaequalis in different habitats from Wanfoshan Nature Reserve, Anhui Province. Chin. J. Ecol. 2016, 35, 1744–1750. [Google Scholar] [CrossRef]
- Yin, H. Rare and Endangered Plants in China; China Forestry Publishing House: Beijing, China, 2013; p. 107. ISBN 9787503870262. [Google Scholar]
- Liu, Q.X. Flora of Jiangsu; Jiangsu Phoenix Science and Technology Press: Nanjing, China, 2015; Volume 2, p. 128. ISBN 9787553701073. [Google Scholar]
- Hu, Z.J.; Wang, W.G.; Song, N.B. Field survey on Parrotia subaequalis in Tianxia Mountain of Yuexi County and in situ conservation countermeasues. J. Jiangsu For. Sci. Technol. 2012, 39, 19–21. [Google Scholar]
- Zhu, B. Living conditions of and conservation strategies for Parrotia subaequalis in Huangwei Township, Yuexi County. Anhui For. Sci. Technol. 2016, 42, 51–53. [Google Scholar]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef] [PubMed]
- Radosavljevic, A.; Anderson, R.P. Making better MAXENT models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Narouei-Khandan, H.A.; Worner, S.P.; Viljanen, S.L.H.; van Bruggen, A.H.C.; Jones, E.E. Projecting the suitability of global and local habitats for myrtle rust (Austropuccinia psidii) using model consensus. Plant Pathol. 2020, 69, 17–27. [Google Scholar] [CrossRef]
- Poirazidis, K.; Bontzorlos, V.; Xofis, P.; Zakkak, S.; Xirouchakis, S.; Grigoriadou, E.; Kechagioglou, S.; Gasteratos, I.; Alivizatos, H.; Panagiotopoulou, M. Bioclimatic and environmental suitability models for capercaillie (Tetrao urogallus) conservation: Identification of optimal and marginal areas in Rodopi Mountain-Range National Park (Northern Greece). Glob. Ecol. Conserv. 2019, 17, e00526. [Google Scholar] [CrossRef]
- Chen, Q.; Yin, Y.; Zhao, R.; Yang, Y.; Teixeira da Silva, J.A.; Yu, X. Incorporating local adaptation into species distribution modeling of Paeonia mairei, an endemic plant to China. Front. Plant Sci. 2020, 10, 1717. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Jiang, R.Y.; Zhang, G.F. Predicting the potential distribution of four endangered holoparasites and their primary hosts in China under climate change. Front. Plant Sci. 2022, 13, 942448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Li, K.J.; Liu, X.F.; Yang, L.; Shen, S.K. Interspecific variance of suitable habitat changes for four alpine Rhododendron species under climate change: Implications for their reintroductions. Forests 2021, 12, 1520. [Google Scholar] [CrossRef]
- Sillero, N.; Barbosa, A.M. Common mistakes in ecological niche models. Int. J. Geogr. Inf. Sci. 2021, 35, 213–226. [Google Scholar] [CrossRef]
- Liu, L.; Guan, L.; Zhao, H.; Huang, Y.; Mou, Q.; Liu, K.; Chen, T.; Wang, X.; Zhang, Y.; Wei, B.; et al. Modeling habitat suitability of Houttuynia cordata Thunb (Ceercao) using MaxEnt under climate change in China. Ecol. Inform. 2021, 63, 101324. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Kiser, A.H.; Cummings, K.S.; Tiemann, J.S.; Smith, C.H.; Johnson, N.A.; Lopez, R.R.; Randklev, C.R. Using a multi-model ensemble approach to determine biodiversity hotspots with limited occurrence data in understudied areas: An example using freshwater mussels in Mexico. Ecol. Evol. 2022, 12, e8909. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P.; McPherson, J. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Assefa, A.; Tibebu, A.; Bihon, A.; Yimana, M. Global ecological niche modelling of current and future distribution of peste des petits ruminants virus (PPRv) with an ensemble modelling algorithm. Transbound. Emerg. Dis. 2021, 68, 3601–3610. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Arunachalam, R.; Kumar, L. Modeling potential hotspots of invasive Prosopis juliflora (Swartz) DC in India. Ecol. Inform. 2021, 64, 101386. [Google Scholar] [CrossRef]
- Kaky, E.; Nolan, V.; Alatawi, A.; Gilbert, F. A comparison between Ensemble and MaxEnt species distribution modelling approaches for conservation: A case study with Egyptian medicinal plants. Ecol. Inform. 2020, 60, 101150. [Google Scholar] [CrossRef]
- Baldwin, R.A. Use of maximum entropy modeling in wildlife research. Entropy 2009, 11, 854–866. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Srivastava, V.; Griess, V.C.; Keena, M.A. Assessing the potential distribution of Asian gypsy moth in Canada: A comparison of two methodological approaches. Sci. Rep. 2020, 10, 22. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Y.; Zhao, H.; Yang, M.; Zhuang, Y.; Ye, X. Modelling the Effects of Climate Change on the Distribution of Endangered Cypripedium japonicum in China. Forests 2021, 12, 429. [Google Scholar] [CrossRef]
- Jiang, R.; Zou, M.; Qin, Y.; Tan, G.; Huang, S.; Quan, H.; Zhou, J.; Liao, H. Modeling of the potential geographical distribution of three Fritillaria species under climate change. Front. Plant Sci. 2022, 12, 749838. [Google Scholar] [CrossRef]
- Narouei-Khandan, H.A. Ensemble Models to Assess the Risk of Exotic Plant Pathogens in a Changing Climate, in Lincoln; Lincoln University: Lincoln, New Zealand, 2014. [Google Scholar]
- Adroit, B.; Zhuang, X.; Wappler, T.; Terral, J.-F.; Wang, B. A case of long-term herbivory: Specialized feeding trace on Parrotia (Hamamelidaceae) plant species. R. Soc. Open Sci. 2020, 7, 201449. [Google Scholar] [CrossRef]
- Yue, C.L.; Jin, S.H.; Chang, J.; Jiang, H. Response of photosynthesis in Shaniodendron subaequale to soil water status. Ann. Bot. Fenn. 2006, 43, 389–393. [Google Scholar]
- Li, H.P.; Yue, C.L.; Yu, Q.J.; Yang, Z.J.; Shao, S.L.; Yu, L.P. Advance of research on Parrotia subaequalis. J. Zhejiang For. Sci. Tech. 2012, 32, 79–84. [Google Scholar]
- Cai, C.; Zhang, X.; Zha, J.; Li, J.; Li, J. Predicting climate change impacts on the rare and endangered Horsfieldia tetratepala in China. Forests 2022, 13, 1051. [Google Scholar] [CrossRef]
- Hao, R.M.; Wei, H.T. A new combination of Hamamelidaceae. Acta Phytotaxon. Sin. 1998, 36, 80. [Google Scholar]
- Wu, Z.Y.; Raven, P.H.; Hong, D.Y. Flora of China; Science Press: Beijing, China, 2003; Volume 9, ISBN 1930723148. [Google Scholar]
- Lu, Z.J.; Bao, D.C.; Liu, H.B.; Xu, Y.Z. Hunan Badagongshan Forest Dynamics Plot: Tree Species and Their Distribution Patterns; China Forestry Publishing House: Beijing, China, 2017; ISBN 9787503887659. [Google Scholar]
- Deng, T.; Zhang, D.G.; Sun, H. Flora of Shennongjia; China Forestry Publishing House: Beijing, China, 2018; Volume 2, ISBN 9787503894596. [Google Scholar]
- Dong, H.J.; Fang, Y.P.; Xiang, J.; Zhen, A.G. Illustrated Flora of Dabie Mountain in Hubei; China Forestry Publishing House: Beijing, China, 2021; ISBN 9787521910032. [Google Scholar]
- Liu, J.F.; Chen, L.; Yang, Z.H.; Zhao, Y.F.; Zhang, X.W. Unraveling the spatio-temporal relationship between ecosystem services and socioeconomic development in Dabie Mountain Area over the last 10 years. Remote Sens. 2022, 14, 1059. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Shi, E.; Yang, Z.P.; Geng, Q.F.; Qiu, Y.X.; Wang, Z.S. Development and application of genomic resources in an endangered palaeoendemic tree, Parrotia subaequalis (Hamamelidaceae) from Eastern China. Front. Plant Sci. 2018, 9, 246. [Google Scholar] [CrossRef]
- Shen, X.S. Study on the pteridophytic flora of Jiangsu, Zhejiang and Anhui provinces. J. Wuhan Bot. Res. 2001, 19, 215–219. [Google Scholar]
- Ren, J.; Zhang, G.F.; Hu, R.K.; Sun, G.; Yu, L.P. Population structure and distribution pattern of Parrotia subaequalis from Longwangshan Nature Reserve, Zhejiang Province. Bulletin Bot. Res. 2012, 32, 554–560. [Google Scholar]
- Li, K.D.; Zhang, G.F. Species diversity and distribution pattern of heritage trees in the rapidly-urbanizing province of Jiangsu, China. Forests 2021, 12, 1543. [Google Scholar] [CrossRef]
- Cai, Q.F.; Liu, Y.; Qian, H.J.; Liu, R.S. Inverse effects of recent warming on trees growing at the low and high altitudes of the Dabie Mountains, subtropical China. Dendrochronologia 2020, 59, 125649. [Google Scholar] [CrossRef]
- Yang, J.; Cai, L.; Liu, D.T.; Chen, G.; Gratzfeld, J.; Sun, W.B. China’s conservation program on plant species with extremely small populations (PSESP): Progress and perspectives. Biol. Conserv. 2020, 244, 108535. [Google Scholar] [CrossRef]




| Variable | Description | Unit | Percent Contribution (%) |
|---|---|---|---|
| Bio1 | Annual mean temperature | °C | 13.1 |
| Bio2 | Mean diurnal range (mean of monthly (max temp–min temp)) | °C | 0.9 |
| Bio3 | Isothermality (Bio2/Bio7) (×100) | % | |
| Bio4 | Temperature seasonality (standard deviation × 100) | - | |
| Bio5 | Max temperature of warmest month | °C | |
| Bio6 | Min temperature of coldest month | °C | |
| Bio7 | Temperature annual range (Bio5–Bio6) | °C | |
| Bio8 | Mean temperature of wettest quarter | °C | |
| Bio9 | Mean temperature of driest quarter | °C | 19.1 |
| Bio10 | Mean temperature of warmest quarter | °C | |
| Bio11 | Mean temperature of coldest quarter | °C | |
| Bio12 | Annual precipitation | mm | |
| Bio13 | Precipitation of wettest month | mm | 0.5 |
| Bio14 | Precipitation of driest month | mm | |
| Bio15 | Precipitation seasonality (coefficient of variation) | - | 2.1 |
| Bio16 | Precipitation of wettest quarter | mm | |
| Bio17 | Precipitation of driest quarter | mm | 64.3 |
| Bio18 | Precipitation of warmest quarter | mm | |
| Bio19 | Precipitation of coldest quarter | mm |
| Scenarios | AUCtraining | AUCtest | TSS | |
|---|---|---|---|---|
| Current | 0.994 ± 0.0002 | 0.994 ± 0.0011 | 0.971 ± 0.0115 | |
| 2050s | RCP 2.6 | 0.994 ± 0.0003 | 0.993 ± 0.0013 | 0.972 ± 0.0097 |
| RCP 4.5 | 0.994 ± 0.0003 | 0.993 ± 0.0013 | 0.971 ± 0.0178 | |
| RCP 8.5 | 0.994 ± 0.0002 | 0.994 ± 0.0008 | 0.976 ± 0.0094 | |
| 2070s | RCP 2.6 | 0.994 ± 0.0002 | 0.994 ± 0.0008 | 0.974 ± 0.0133 |
| RCP 4.5 | 0.995 ± 0.0001 | 0.994 ± 0.0008 | 0.976 ± 0.0114 | |
| RCP 8.5 | 0.995 ± 0.0002 | 0.994 ± 0.0012 | 0.976 ± 0.0078 | |
| Scenarios | Low Suitable Area | Moderately Suitable Area | Highly Suitable Area | Suitable Area (Moderately and Highly) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Area (×104 km2) | Trend (%) | Area (×104 km2) | Trend (%) | Area (×104 km2) | Trend (%) | Area (×104 km2) | Trend (%) | ||
| Current | 10.980 | - | 2.140 | - | 0.185 | - | 2.325 | - | |
| 2050s | RCP 2.6 | 11.819 | ↑7.64 | 2.467 | ↑15.28 | 0.246 | ↑32.97 | 2.713 | ↑16.69 |
| RCP 4.5 | 10.973 | ↓0.07 | 3.057 | ↑42.87 | 0.121 | ↓34.70 | 3.178 | ↑36.71 | |
| RCP 8.5 | 9.322 | ↓15.10 | 2.184 | ↑2.07 | 0.187 | ↑1.05 | 2.371 | ↑1.99 | |
| 2070s | RCP 2.6 | 9.457 | ↓13.87 | 2.411 | ↑12.67 | 0.116 | ↓37.33 | 2.527 | ↑8.70 |
| RCP 4.5 | 8.615 | ↓21.54 | 1.797 | ↓16.01 | 0.218 | ↑17.86 | 2.015 | ↓13.32 | |
| RCP 8.5 | 9.652 | ↓12.10 | 2.142 | ↑0.08 | 0.149 | ↓19.36 | 2.291 | ↓1.46 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, G.; Zhang, G. Predicting the Potential Distribution of Endangered Parrotia subaequalis in China. Forests 2022, 13, 1595. https://doi.org/10.3390/f13101595
Yan G, Zhang G. Predicting the Potential Distribution of Endangered Parrotia subaequalis in China. Forests. 2022; 13(10):1595. https://doi.org/10.3390/f13101595
Chicago/Turabian StyleYan, Ge, and Guangfu Zhang. 2022. "Predicting the Potential Distribution of Endangered Parrotia subaequalis in China" Forests 13, no. 10: 1595. https://doi.org/10.3390/f13101595
APA StyleYan, G., & Zhang, G. (2022). Predicting the Potential Distribution of Endangered Parrotia subaequalis in China. Forests, 13(10), 1595. https://doi.org/10.3390/f13101595




