High Seedling Mortality of Scots Pine Caused by Heterobasidion annosum s.s.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Monitoring Heterobasidion Root Rot in the Previous Tree Generation
2.3. Determination of Heterobasidion Infections in the Subsequent Pine Regeneration
2.4. Somatic Incompatibility Tests
2.5. Measurement of the Soil pH
2.6. Calculations and Statistics
3. Results
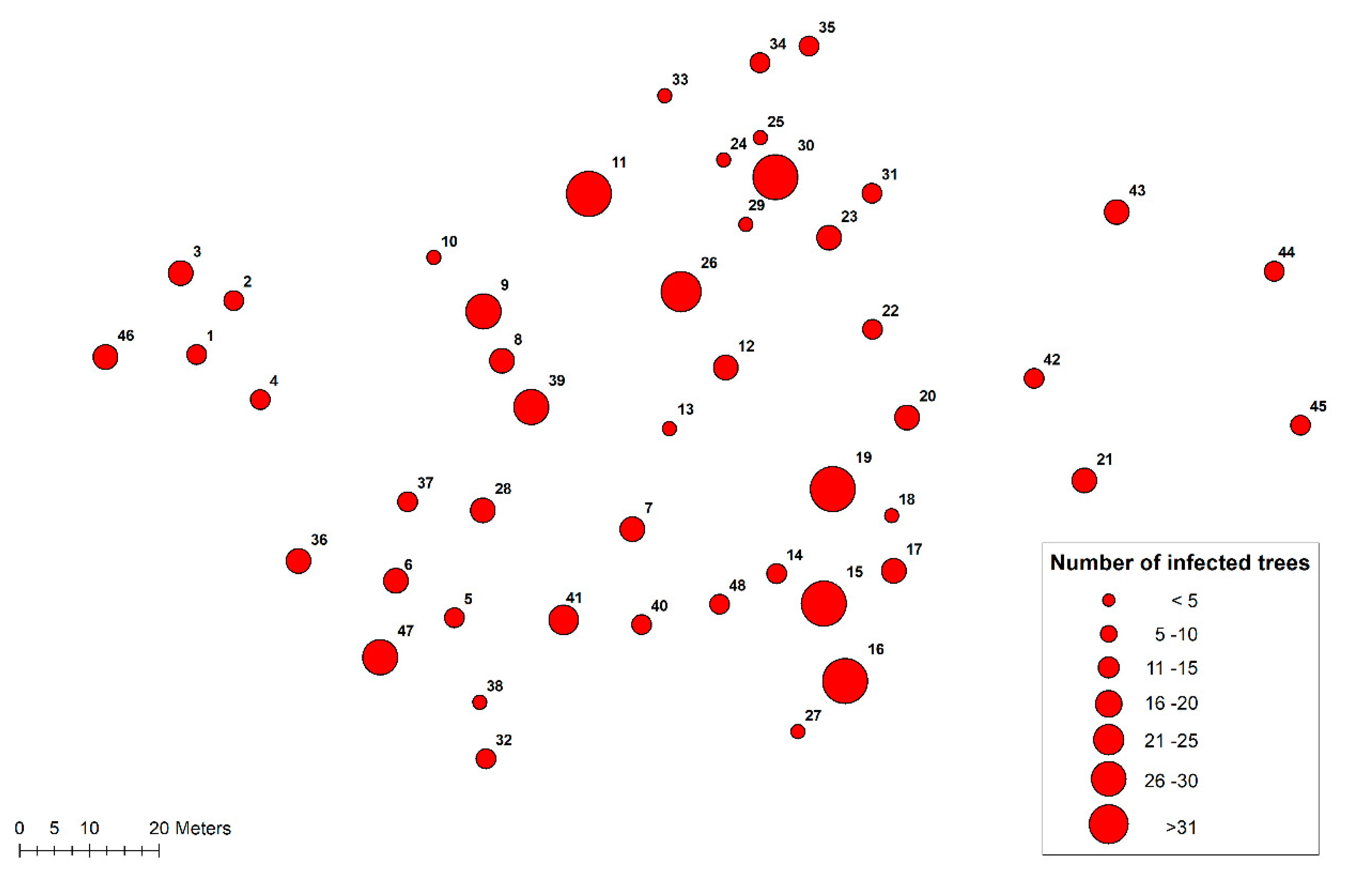
3.1. Heterobasidion Root Rot in the Previous Tree Generation
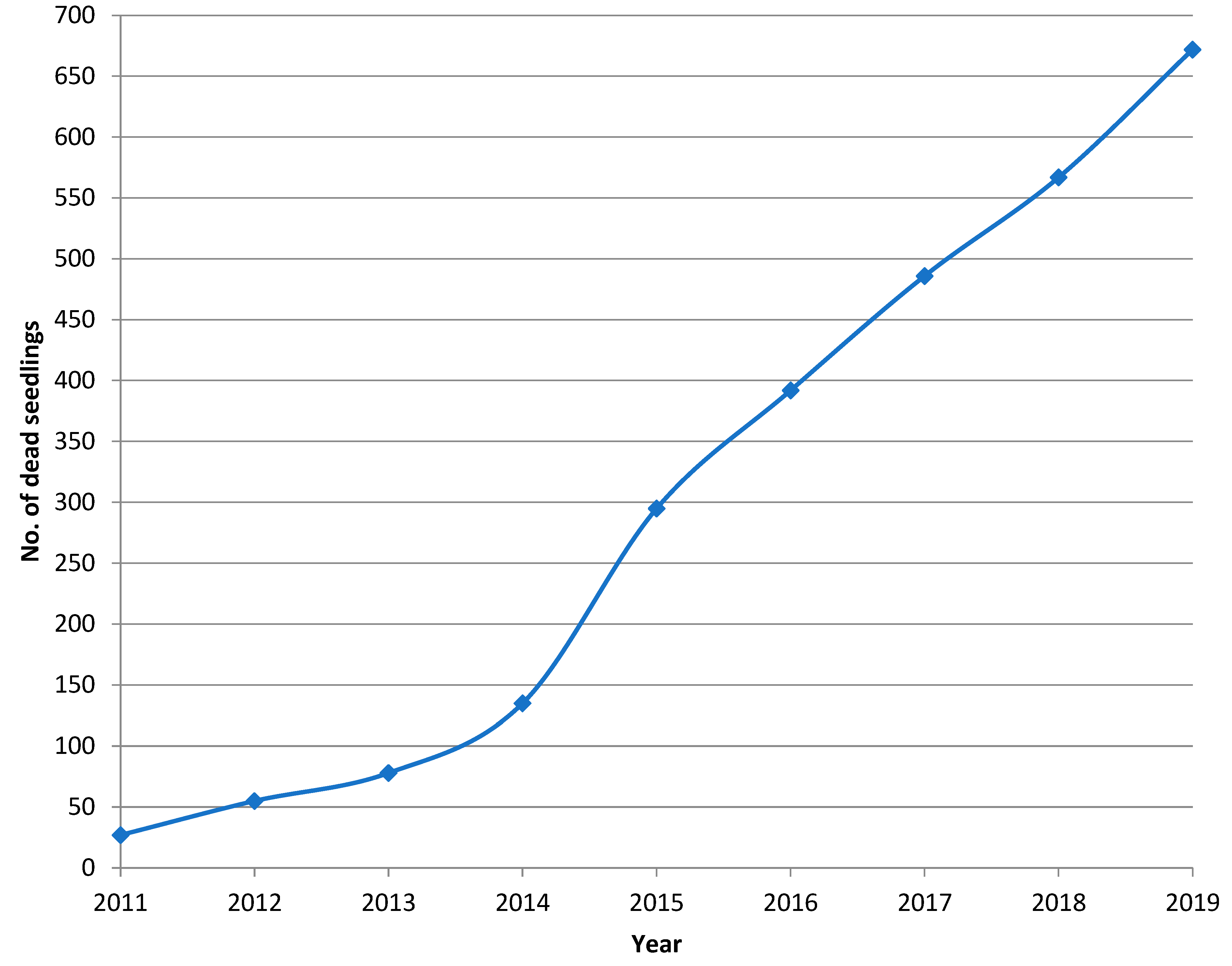
3.2. Progression of Heterobasidion Root Rot in the Subsequent Pine Regeneration
3.3. Occurrence of Heterobasidion Fruiting Bodies
3.4. Soil pH in Disease Centers and Healthy Parts of the Stand
4. Discussion
4.1. Incidence of Heterobasidion Root Rot in the Previous Scots Pine Rotation
4.2. Progress of Heterobasidion Root Rot in the Subsequent Pine Regeneration
4.3. Heterobasidion Populations in Disease Centers
4.4. Occurrence of Heterobasidion Fruiting Bodies
4.5. Variation of Soil pH in the Regeneration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garbelotto, M.; Gonthier, P. Biology, epidemiology, and control of Heterobasidion species worldwide. Annu. Rev. Phytopathol. 2013, 51, 39–59. [Google Scholar] [CrossRef] [Green Version]
- Korhonen, K. Intersterility groups of Heterobasidion annosum. Commun. Inst. For. Fenn. 1978, 94, 1–25. [Google Scholar]
- Bendz-Hellgren, M.; Lipponen, K.; Solheim, H.; Thomsen, I.M. The Nordic Countries. In Heterobasidion annosum: Biology, Ecology, Impact and Control; Woodward, S., Stenlid, J., Karjalainen, R., Hüttermann, A., Eds.; CAB International: Wallingford, UK, 1998; pp. 333–345124. [Google Scholar]
- Mäkinen, H.; Hallaksela, A.-M.; Isomäki, A. Increment and decay in Norway spruce and Scots pine after artificial logging damage. Can. J. For. Res. 2007, 37, 2130–2141. [Google Scholar] [CrossRef]
- Redfern, D.B.; Stenlid, J. Spore dispersal and infection. In Heterobasidion annosum: Biology, Ecology, Impact and Control; Woodward, S., Stenlid, J., Karjalainen, R., Hüttermann, A., Eds.; CAB International: Wallingford, UK, 1998; pp. 105–124. [Google Scholar]
- Kallio, T. Aerial distribution of the root-rot fungus Fomes annosus (Fr.) Cooke in Finland. Acta For. Fenn. 1970, 107, 1–20. [Google Scholar] [CrossRef] [Green Version]
- La Porta, N.; Capretti, P.; Thomsen, I.M.; Kasanen, R.; Hietala, A.M.; Von Weissenberg, K. Forest pathogens with higher damage potential due to climate change in Europe. Can. J. Plant Pathol. 2008, 30, 177–195. [Google Scholar] [CrossRef]
- Queloz, V.; Holdenrieder, O. Wie gross wird Heterobasidion annosum s.l.?—Eine Literaturübersicht. Schweiz. Z. Forstwes. 2005, 156, 395–398. [Google Scholar] [CrossRef]
- Hodges, C.S. Modes of infection and spread of Fomes annosus. Annu. Rev. Phytopathol. 1969, 7, 247–266. [Google Scholar] [CrossRef]
- Fiodorov, N. Eastern Europe and Baltic Countries. In Heterobasidion annosum: Biology, Ecology, Impact and Control; Woodward, S., Stenlid, J., Karjalainen, R., Hüttermann, A., Eds.; CAB International: Wallingford, UK, 1998; pp. 387–403. [Google Scholar]
- Kurkela, T. Crown condition as an indicator of the incidence of root rot caused by Heterobasidion annosum in Scots pine stands. Silva Fenn. 2002, 36, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Rönnberg, J.; Petrlaité, E.; Nilsson, G.; Pratt, J. Two studies to assess the risk to Pinus sylvestris from Heterobasidion spp. in southern Sweden. Scand. J. For. Res. 2006, 21, 405–413. [Google Scholar] [CrossRef]
- Wang, L.Y.; Zhang, J.; Drobyshev, I.; Cleary, M.; Rönnberg, J. Incidence and impact of root infection by Heterobasidion spp., and the justification for preventative silvicultural measures on Scots pine trees: A case study in southern Sweden. For. Ecol. Manag. 2014, 315, 153–159. [Google Scholar] [CrossRef]
- Allikmäe, E.; Laarmann, D.; Korjus, H. Vitality assessment of visually healthy trees in Estonia. Forests 2017, 8, 223. [Google Scholar] [CrossRef] [Green Version]
- Laine, L. The occurrence of Heterobasidion annosum (Fr.) Bref. in woody plants in Finland. Commun. Inst. For. Fenn. 1976, 90, 1–53. [Google Scholar]
- Korhonen, K.; Piri, T. The main hosts and distribution of the S and P groups of Heterobasidion annosum in Finland. In Proceedings of the 8th IUFRO Conference on Root and Butt Rots, Wik, Sweden; Haikko, Finland, 9–16 August 1993; Johansson, M., Stenlid, J., Eds.; Swedish University of Agricultural Sciences: Uppsala, Sweden, 1994; pp. 260–267. [Google Scholar]
- Delatour, C.; von Weissenberg, K.; Dimitri, L. Host resistance. In Heterobasidion annosum: Biology, Ecology, Impact and Control; Woodward, S., Stenlid, J., Karjalainen, R., Hüttermann, A., Eds.; CAB International: Wallingford, UK, 1998; pp. 143–166. [Google Scholar]
- Cajander, A.K. Forest types and their significance. Acta For. Fenn. 1949, 56, 1–71. [Google Scholar] [CrossRef] [Green Version]
- Hyder, R.; Piri, T.; Hantula, J.; Nuorteva, H.; Vainio, E.J. Distribution of viruses inhabiting Heterobasidion annosum in a pine-dominated forest plot in southern Finland. Microb. Ecol. 2018, 75, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Piri, T.; Korhonen, K.; Sairanen, A. Occurrence of Heterobasidion annosum in pure and mixed spruce stands in southern Finland. Scand. J. For. Res. 1990, 5, 113–125. [Google Scholar] [CrossRef]
- Stenlid, J. Population structure of Heterobasidion annosum as determined by somatic incompatibility, sexual incompatibility, and isoenzyme patterns. Can. J. Bot. 1985, 63, 2268–2273. [Google Scholar] [CrossRef]
- Hansen, E.M.; Stenlid, J.; Johansson, M. Genetic control of somatic incompatibility in the root-rotting basidiomycete Heterobasidion annosum. Mycol. Res. 1993, 97, 1229–1233. [Google Scholar] [CrossRef]
- Rishbeth, J. Observations on the biology of Fomes annosus, with particular reference to East Anglian pine plantations. III. Natural and experimental infection of pines, and some factors affecting severity of the disease. Ann. Bot. 1951, 15, 221–246. [Google Scholar] [CrossRef]
- Gibbs, J.N.; Greig, B.J.W.; Pratt, J.E. Fomes root rot in Thetford Forest, East Anglia: Past, present and future. Forestry 2002, 75, 191–202. [Google Scholar] [CrossRef]
- Müller, M.M.; Sievänen, R.; Beuker, E.; Meesenburg, H.; Kuuskeri, J.; Hamberg, L.; Korhonen, K. Predicting the activity of Heterobasidion parviporum on Norway spruce in warming climate from its respiration rate at different temperatures. For. Pathol. 2014, 44, 325–336. [Google Scholar] [CrossRef]
- Piri, T. Response of compensatory-fertilized Pinus sylvestris to infection by Heterobasidion annosum. Scand. J. For. Res. 2000, 15, 218–224. [Google Scholar] [CrossRef]
- Gibbs, J.N. A study of the epiphytic growth habit of Fomes annosus. Ann. Bot. 1967, 31, 755–774. [Google Scholar] [CrossRef]
- Korhonen, K.; Stenlid, J. Biology of Heterobasidion annosum. In Heterobasidion annosum: Biology, Ecology, Impact and Control; Woodward, S., Stenlid, J., Karjalainen, R., Hüttermann, A., Eds.; CAB International: Wallingford, UK, 1998; pp. 43–70. [Google Scholar]
- Bendz-Hellgren, M.; Brandtberg, P.O.; Johansson, M.; Swedjemark, G.; Stenlid, J. Growth rate of Heterobasidion annosum in Picea abies established on forest land and arable land. Scand. J. For. Res. 1999, 14, 402–407. [Google Scholar] [CrossRef]
- Morrison, D.J.; Johnson, A.L.S. Stump colonization and spread of Fomes annosus five years after thinning. Can. J. For. Res. 1978, 8, 177–180. [Google Scholar] [CrossRef]
- Froelich, R.C.; Dell, T.R.; Walkinshaw, C.H. Soil factors associated with Fomes annosus in the Gulf States. For. Sci. 1966, 12, 356–361. [Google Scholar] [CrossRef]
- Alexander, S.A.; Skelly, J.M.; Morris, C.L. Edaphic factors associated with the incidence and severity of disease caused by Fomes annosus in loblolly pine plantations in Virginia. Phytopathology 1975, 65, 585–591. [Google Scholar] [CrossRef]
- Kovalchuk, A.; Zhu, L.; Keriö, S.; Asiegbu, F.O. Differential responses of Scots pine stilbene synthase and chalcone synthase genes to Heterobasidion annosum infection. For. Pathol. 2017, 47, e12348. [Google Scholar] [CrossRef]
- Mukrimin, M.; Kovalchuk, A.; Ghimire, R.P.; Kivimäenpää, M.; Sun, H.; Holopainen, J.K.; Asiegbu, F.O. Evaluation of potential genetic and chemical markers for Scots pine tolerance against Heterobasidion annosum. Planta 2019, 250, 1881–1895. [Google Scholar] [CrossRef] [Green Version]
- Jurvansuu, J.; Kashif, M.; Vaario, L.; Vainio, E.J.; Hantula, J. Partitiviruses of a fungal forest pathogen have species-specific quantities of genome segments and transcripts. Virology 2014, 462–463, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vainio, E.J.; Jurvansuu, J.; Hyder, R.; Kashif, M.; Piri, T.; Tuomivirta, T.; Poimala, A.; Xu, P.; Mäkelä, S.; Nitisa, D.; et al. The partitivirus HetPV13-an1 mediates growth debilitation and major alterations in the gene expression of a fungal forest pathogen. J. Virol. 2018, 92, e01744-17. [Google Scholar] [CrossRef] [Green Version]
- Kashif, M.; Jurvansuu, J.; Vainio, E.J.; Hantula, J. Alphapartitiviruses of Heterobasidion wood decay fungi affect each other’s transmission and host growth. Front. Cell. Infect. Microbiol. 2019, 9, 64. [Google Scholar] [CrossRef]
- Vainio, E.J.; Hakanpää, J.; Dai, Y.-C.; Hansen, E.; Korhonen, K.; Hantula, J. Species of Heterobasidion host a diverse pool of partitiviruses with global distribution and interspecies transmission. Fungal Biol. 2011, 115, 1234–1243. [Google Scholar] [CrossRef]
- Vainio, E.J.; Müller, M.M.; Korhonen, K.; Piri, T.; Hantula, J. Viruses accumulate in aging infection centers of a fungal forest pathogen. ISME J. 2015, 9, 497–507. [Google Scholar] [CrossRef]
- Zaļuma, A.; Muižnieks, I.; Gaitnieks, T.; Burņeviča, N.; Jansons, Ā.; Stenlid, J.; Vasaitis, R. Infection and spread of root rot caused by Heterobasidion spp. in Pinus contorta plantations in Northern Europe: Tree case studies. Can. J. For. Res. 2019, 48, 969–977. [Google Scholar] [CrossRef] [Green Version]
- Łakomy, P.; Broda, Z.; Werner, A. Genetic diversity of Heterobasidion spp. in Scots pine, Norway spruce and European silver fir stands. Acta Mycol. 2007, 42, 203–210. [Google Scholar] [CrossRef]
- Lygis, V.; Vasiliauskas, R.; Stenlid, J. Planting Betula pendula on pine sites infested by Heterobasidion annosum: Disease transfer, silvicultural evaluation, and community of wood-inhabiting fungi. Can. J. For. Res. 2004, 34, 120–130. [Google Scholar] [CrossRef]
- Chase, T.E.; Ullrich, R.C. Sexuality, distribution, and dispersal of Heterobasidion annosum in pine plantations of Vermont. Mycologia 1983, 75, 825–831. [Google Scholar] [CrossRef]
- Swedjemark, G.; Stenlid, J. Population dynamics of the root rot fungus Heterobasidion annosum following thinning of Picea abies. Oikos 1993, 66, 247–254. [Google Scholar] [CrossRef]
- Johannesson, H.; Stenlid, J. Nuclear reassortment between vegetative mycelia in natural populations of the basidiomycete Heterobasidion annosum. Fungal Genet. Biol. 2004, 41, 563–570. [Google Scholar] [CrossRef]
- James, T.Y.; Johansson, B.K.; Johannesson, H. Trikaryon formation and nuclear selection in pairings between heterokaryons and homokaryons of the root rot pathogen Heterobasidion parviporum. Mycol. Res. 2009, 113, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Stenlid, J. Regional differentiation in Heterobasidion annosum. In Proceedings of the 8th International Conference on Root and Butt Rots, Wik, Sweden; Haikko, Finland, 9–16 August 1993; Johansson, M., Stenlid, J., Eds.; Sveriges Lantbruksuniv.: Uppsala, Sweden, 1994; pp. 243–248. [Google Scholar]
- Jokinen, K. The spread of Heterobasidion annosum and its control using Phlebiopsis gigantea during thinning in the young stands of Scots pine. Folia For. 1984, 607, 1–12, (In Finnish with English Summary). [Google Scholar]
- Sierota, Z.; Damszel, M.; Borys, M.; Nowakowska, J.A. The couch grass rhizome with Heterobasidion annosum fruiting bodies in afforested post-agricultural land. For. Pathol. 2016, 46, 376–379. [Google Scholar] [CrossRef]
- Stenlid, J.; Redfern, D.B. Spread within the tree and stand. In Heterobasidion annosum: Biology, Ecology, Impact and Control; Woodward, S., Stenlid, J., Karjalainen, R., Hüttermann, A., Eds.; CAB International: Wallingford, UK, 1998; pp. 125–141. [Google Scholar]
- Wallis, G.W. Relation of Fomes annosus Incidence to Soil and Forest Management in East Anglian Pine Plantations; Report on Forest Research: London, UK, 1960; p. 113. [Google Scholar]
- Lahti, T.; Väisänen, R.A. Ecological gradients of boreal forests in South Finland: An ordination test of Cajander’s forest site type theory. Vegetatio 1987, 68, 145–156. [Google Scholar] [CrossRef]
- Tamminen, P. Soil factors. In Forest Condition in a Changing Environment—The Finnish Case; Mälkönen, E., Ed.; Springer: Dordrecht, The Netherlands, 2000; Volume 65, pp. 72–86. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piri, T.; Vainio, E.J.; Nuorteva, H.; Hantula, J. High Seedling Mortality of Scots Pine Caused by Heterobasidion annosum s.s. Forests 2021, 12, 1289. https://doi.org/10.3390/f12091289
Piri T, Vainio EJ, Nuorteva H, Hantula J. High Seedling Mortality of Scots Pine Caused by Heterobasidion annosum s.s. Forests. 2021; 12(9):1289. https://doi.org/10.3390/f12091289
Chicago/Turabian StylePiri, Tuula, Eeva J. Vainio, Heikki Nuorteva, and Jarkko Hantula. 2021. "High Seedling Mortality of Scots Pine Caused by Heterobasidion annosum s.s." Forests 12, no. 9: 1289. https://doi.org/10.3390/f12091289
APA StylePiri, T., Vainio, E. J., Nuorteva, H., & Hantula, J. (2021). High Seedling Mortality of Scots Pine Caused by Heterobasidion annosum s.s. Forests, 12(9), 1289. https://doi.org/10.3390/f12091289







