Oak Competition Dominates Interspecific Interactions in Growth and Water-Use Efficiency in a Mixed Pine–Oak Mediterranean Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Meteorological Data
2.3. Individual Basal Area, Local Density, and Water Isotopes Data
2.4. Tree-Ring Analyses
2.5. Carbon Isotope Analyses
2.6. Statistical Analysis
3. Results
3.1. Growth Responses to Climate and Competition
3.2. Individual Growth and Δ13C during Wet and Dry Decades
3.3. Association between Mid-Term Responses and Seasonal Water Uptake Patterns
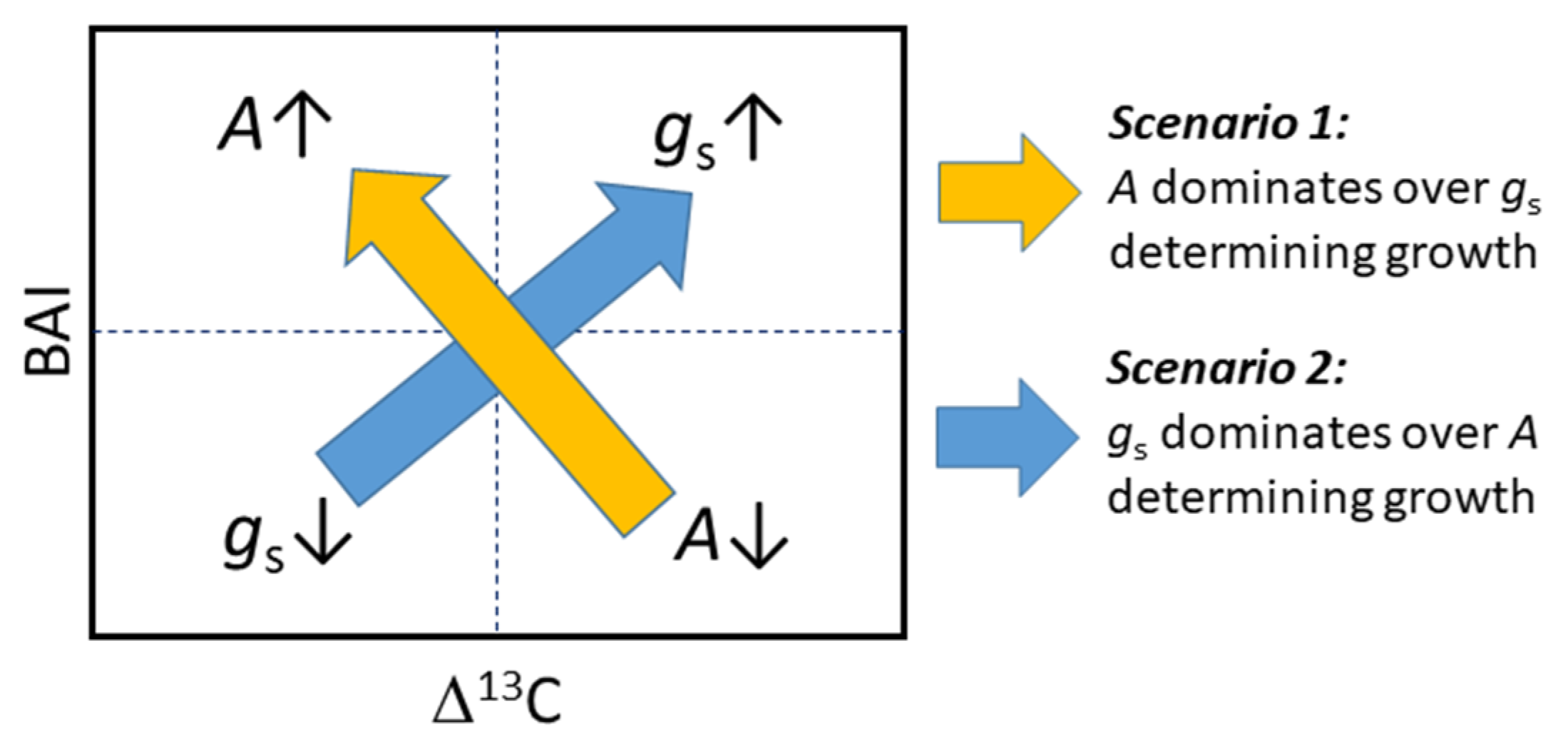
3.4. Interaction between Growth, Δ13C, and Water Uptake
4. Discussion
4.1. Climate Responses in Mixed Forests Are Modulated by Competition
4.2. Pines Modulate the Climatic Response of Oaks, but Oaks Rule in Long-Term Growth
4.3. Trade-Offs and Synergies between Growth, Water-Use Efficiency, and Water Uptake
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Station/Variable | Common Period | n | Intercept | Slope | r2 |
|---|---|---|---|---|---|
| Flix-Vinebre/Tmax | 2008–2019 | 141 | 3.622 | 0.940 | 0.941 |
| Flix-Vinebre/Tmin | 2008–2019 | 141 | −6.119 | 0.844 | 0.915 |
| Cabacés/P | 2008–2019 | 141 | 5.122 | 0.952 | 0.847 |
| Period | P25 | P50 | P75 | P < mean | Pmin | Pmax |
|---|---|---|---|---|---|---|
| Long-term (1970–2019) | 421 mm | 502 mm | 593 mm | 56% | 306 mm | 798 mm |
| Wet decade (1994–2003) | 498 mm | 598 mm | 676 mm | 30% | 409 mm | 744 mm |
| Dry decade (2004–2013) | 426 mm | 472 mm | 532 mm | 70% | 306 mm | 644 mm |
| log(BAI) (1994–2003) | log(BAI) (2004–2013) | Δ13C (1994–2003) | Δ13C (2004–2013) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| df. | F | p > F | F | p > F | F | p > F | F | p > F | |
| Species | 1 | 15.3 | <0.001 | 9.8 | 0.002 | 41.2 | <0.001 | 8.2 | 0.007 |
| X | 1 | 2.5 | 0.118 | 6.0 | 0.016 | 1.9 | 0.173 | 1.9 | 0.178 |
| Y | 1 | 1.5 | 0.219 | 1.4 | 0.235 | 2.8 | 0.106 | 2.3 | 0.139 |
| Age | 1 | 3.9 | 0.051 | 1.9 | 0.170 | 0.0 | 0.852 | 0.0 | 0.934 |
| Pine BA | 1 | 0.0 | 0.958 | 0.5 | 0.460 | 0.5 | 0.488 | 0.9 | 0.357 |
| Oak BA | 1 | 3.0 | 0.089 | 3.1 | 0.082 | 3.2 | 0.083 | 3.9 | 0.058 |
| Species × X | 1 | 0.2 | 0.648 | 0.9 | 0.342 | 4.4 | 0.043 | 1.9 | 0.178 |
| Species × Y | 1 | 2.1 | 0.147 | 4.1 | 0.046 | 1.9 | 0.177 | 1.0 | 0.321 |
| Species × Age | 1 | 0.4 | 0.521 | 0.9 | 0.353 | 0.7 | 0.411 | 0.4 | 0.508 |
| Species × Pine BA | 1 | 4.8 | 0.032 | 6.5 | 0.012 | 0.3 | 0.611 | 0.5 | 0.471 |
| Species × Oak BA | 1 | 0.2 | 0.697 | 0.0 | 0.844 | 0.1 | 0.791 | 2.4 | 0.130 |
References
- Giorgi, F. Climate change hot-spots. Geophys. Res. Lett. 2006, 33, L08707. [Google Scholar] [CrossRef]
- Pausas, J.G.; Millán, M.M. Greening and browning in a climate change hotspot: The Mediterranean sasin. Bioscience 2019, 69, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Valladares, F.; Benavides, R.; Rabasa, S.G.; Diaz, M.; Pausas, J.G.; Paula, S.; Simonson, W.D. Global change and Mediterranean forests: Current impacts and potential responses. In Forests and Global Change; Coomes, D.A., Burslem, D.F.R.P., Simonson, W.D., Eds.; Cambridge University Press: Cambridge, UK, 2014; pp. 47–75. [Google Scholar]
- Moreno-Gutiérrez, C.; Battipaglia, G.; Cherubini, P.; Saurer, M.; Nicolás, E.; Contreras, S.; Querejeta, J.I. Stand structure modulates the long-term vulnerability of Pinus halepensis to climatic drought in a semiarid Mediterranean ecosystem. Plant Cell Environ. 2012, 35, 1026–1039. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Shpringer, I.; Fikler, B.; Elbaz, G.; Cohen, S.; Yakir, D. Relationships between stomatal regulation, water-use, and water-use efficiency of two coexisting key Mediterranean tree species. For. Ecol. Manag. 2013, 302, 34–42. [Google Scholar] [CrossRef]
- Barbeta, A.; Mejía-Chang, M.; Ogaya, R.; Voltas, J.; Dawson, T.E.; Peñuelas, J. The combined effects of a long-term experimental drought and an extreme drought on the use of plant-water sources in a Mediterranean forest. Glob. Chang. Biol. 2015, 21, 1213–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gracia, C.A.; Sabate, S.; Tello, E. Modelling the responses to climate change of a Mediterranean forest managed at different thinning intensities: Effects on growth and water fluxes. Impacts Glob. Chang. Tree Physiol. For. Ecosyst. 1998, 52, 243–252. [Google Scholar]
- de Dios, R.S.; Velázquez, J.C.; Ollero, H.S. Classification and mapping of Spanish Mediterranean mixed forests. IForest 2019, 12, 480–487. [Google Scholar] [CrossRef] [Green Version]
- Rivas-Martinez, S. Map of series, geoseries and geopermaseries of Spanish vegetation. Itinera Geobot. 2011, 18, 425–800. [Google Scholar]
- Zavala, M.A.; Espelta, J.M.; Retana, J. Constraints and trade-offs in Mediterranean plant communities: The case of holm oak-Aleppo pine forests. Bot. Rev. 2000, 66, 119–149. [Google Scholar] [CrossRef]
- del Castillo, J.; Comas, C.; Voltas, J.; Ferrio, J.P. Dynamics of competition over water in a mixed oak-pine Mediterranean forest: Spatio-temporal and physiological components. For. Ecol. Manag. 2016, 382, 214–224. [Google Scholar] [CrossRef] [Green Version]
- Filella, I.; Peñuelas, J. Partitioning of water and nitrogen in co-occurring Mediterranean woody shrub species of different evolutionary history. Oecologia 2003, 137, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gutiérrez, C.; Dawson, T.E.; Nicolás, E.; Querejeta, J.I. Isotopes reveal contrasting water use strategies among coexisting plant species in a Mediterranean ecosystem. New Phytol. 2012, 196, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Jucker, T.; Bouriaud, O.; Avacaritei, D.; Dǎnilǎ, I.; Duduman, G.; Valladares, F.; Coomes, D.A. Competition for light and water play contrasting roles in driving diversity-productivity relationships in Iberian forests. J. Ecol. 2014, 102, 1202–1213. [Google Scholar] [CrossRef] [Green Version]
- Grossiord, C.; Granier, A.; Ratcliffe, S.; Bouriaud, O.; Bruelheide, H.; Checko, E.; Forrester, D.I.; Dawud, S.M.; Finer, L.; Pollastrini, M.; et al. Tree diversity does not always improve resistance of forest ecosystems to drought. Proc. Natl. Acad. Sci. USA 2014, 111, 14812–14815. [Google Scholar] [CrossRef] [Green Version]
- Granda, E.; Rossatto, D.R.; Camarero, J.J.; Voltas, J.; Valladares, F. Growth and carbon isotopes of Mediterranean trees reveal contrasting responses to increased carbon dioxide and drought. Oecologia 2014, 174, 307–317. [Google Scholar] [CrossRef]
- Maestre, F.T.; Valladares, F.; Reynolds, J.F. The stress-gradient hypothesis does not fit all relationships between plant-plant interactions and abiotic stress: Further insights from arid environments. J. Ecol. 2006, 94, 17–22. [Google Scholar] [CrossRef]
- Zavala, M.A.; De La Parra, R.B. A mechanistic model of tree competition and facilitation for Mediterranean forests: Scaling from leaf physiology to stand dynamics. Ecol. Model. 2005, 188, 76–92. [Google Scholar] [CrossRef]
- De Cáceres, M.; Mencuccini, M.; Martin-StPaul, N.; Limousin, J.M.; Coll, L.; Poyatos, R.; Cabon, A.; Granda, V.; Forner, A.; Valladares, F.; et al. Unravelling the effect of species mixing on water use and drought stress in Mediterranean forests: A modelling approach. Agric. For. Meteorol. 2021, 296, 108233. [Google Scholar] [CrossRef]
- Comas, C.; del Castillo, J.; Voltas, J.; Ferrio, J.P. Point processes statistics of stable isotopes: Analysing water uptake patterns in a mixed stand of Aleppo pine and Holm oak. For. Syst. 2015, 24, e009. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Forn, D.; Peguero-Pina, J.J.; Ferrio, J.P.; Mencuccini, M.; Mendoza-Herrer, Ó.; Sancho-Knapik, D.; Gil-Pelegrín, E. Contrasting functional strategies following severe drought in two Mediterranean oaks with different leaf habit: Quercus faginea and Quercus ilex subsp. rotundifolia. Tree Physiol. 2021, 41, 371–387. [Google Scholar] [CrossRef]
- Klein, T.; Hoch, G.; Yakir, D.; Körner, C. Drought stress, growth and nonstructural carbohydrate dynamics of pine trees in a semi-arid forest. Tree Physiol. 2014, 34, 981–992. [Google Scholar] [CrossRef] [Green Version]
- Campelo, F.; Gutiérrez, E.; Ribas, M.; Sánchez-Salguero, R.; Nabais, C.; Camarero, J.J. The facultative bimodal growth pattern in Quercus ilex–A simple model to predict sub-seasonal and inter-annual growth. Dendrochronologia 2018, 49, 77–88. [Google Scholar] [CrossRef]
- Campelo, F.; Ribas, M.; Gutiérrez, E. Plastic bimodal growth in a Mediterranean mixed-forest of Quercus ilex and Pinus halepensis. Dendrochronologia 2021, 67, 125836. [Google Scholar] [CrossRef]
- Palacio, S.; Azorín, J.; Montserrat-Martí, G.; Ferrio, J.P. The crystallization water of gypsum rocks is a relevant water source for plants. Nat. Commun. 2014, 5, 4660. [Google Scholar] [CrossRef] [Green Version]
- Martín-Gómez, P.; Barbeta, A.; Voltas, J.; Peñuelas, J.; Dennis, K.; Palacio, S.; Dawson, T.E.; Ferrio, J.P. Isotope-ratio infrared spectroscopy: A reliable tool for the investigation of plant-water sources? New Phytol. 2015, 207, 914–927. [Google Scholar] [CrossRef]
- Friedman, J.H. A Variable Span Smoother; Technical Report 5; Stanford University: Stanford, CA, USA, 1984. [Google Scholar] [CrossRef]
- Cook, E.R.; Krusic, P.J. Program ARSTAN: A Tree Ring Standardization Program Based on Detrending and Autoregressive Time Series Modeling, with Interactive Graphics; Columbia University: Palisades, NY, USA, 2005. [Google Scholar]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T.; Ehleringer, R.; Hubic, K.T. Carbon Isotope Discrimination and Photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Bioi 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Ferrio, J.P.; Araus, J.L.; Buxó, R.; Voltas, J.; Bort, J. Water management practices and climate in ancient agriculture: Inferences from the stable isotope composition of archaeobotanical remains. Veg. Hist. Archaeobot. 2005, 14, 510–517. [Google Scholar] [CrossRef]
- Verbeke, G.; Molenberghs, G. Linear Mixed Models for Longitudinal Data; Springer, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Koenker, R. Quantreg: Quantile Regression. Available online: https://CRAN.R-project.org/package=quantreg (accessed on 15 May 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 15 May 2021).
- Valentini, R.; Mugnozza, G.E.S.; Ehleringer, J.R. Hydrogen and Carbon Isotope Ratios of Selected Species of a Mediterranean Macchia Ecosystem. Funct. Ecol. 1992, 6, 627–631. [Google Scholar] [CrossRef]
- Ferrio, J.P.; Florit, A.; Vega, A.; Serrano, L.; Voltas, J. Δ13C and tree-ring width reflect different drought responses in Quercus ilex and Pinus halepensis. Oecologia 2003, 137, 512–518. [Google Scholar] [CrossRef]
- Gutiérrez, E.; Campelo, F.; Camarero, J.J.; Ribas, M.; Muntán, E.; Nabais, C.; Freitas, H. Climate controls act at different scales on the seasonal pattern of Quercus ilex L. stem radial increments in NE Spain. Trees-Struct. Funct. 2011, 25, 637–646. [Google Scholar] [CrossRef]
- Serre, F. The relation between growth and climate in Aleppo pine (Pinus halepensis). I. Methods. Cambial activity and climate. Oecologia Plant 1976, 11, 143–171. [Google Scholar]
- Del Castillo, J.; Aguilera, M.; Voltas, J.; Ferrio, J.P. Isoscapes of tree-ring carbon-13 perform like meteorological networks in predicting regional precipitation patterns. J. Geophys. Res. Biogeosci. 2013, 118, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Liphschitz, N.; Lev-Yadun, S. Cambial activity of evergreen and seasonal dimorphics around the Mediterranean. IAWA Bull. NS 1986, 7, 145–153. [Google Scholar] [CrossRef]
- De Luis, M.; Čufar, K.; Di Filippo, A.; Novak, K.; Papadopoulos, A.; Piovesan, G.; Rathgeber, C.B.K.; Raventós, J.; Saz, M.A.; Smith, K.T. Plasticity in dendroclimatic response across the distribution range of Aleppo pine (Pinus halepensis). PLoS ONE 2013, 8, e83550. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, T.J.G.; Del Campo, A.D.; Herrera, R.; Molina, A.J. Simultaneous assessment, through sap flow and stable isotopes, of water use efficiency (WUE) in thinned pines shows improvement in growth, tree-climate sensitivity and WUE, but not in WUEi. For. Ecol. Manag. 2016, 361, 298–308. [Google Scholar] [CrossRef]
- Forner, A.; Aranda, I.; Granier, A.; Valladares, F. Differential impact of the most extreme drought event over the last half century on growth and sap flow in two coexisting Mediterranean trees. Plant Ecol. 2014, 215, 703–719. [Google Scholar] [CrossRef] [Green Version]
- Klein, T.; Hemming, D.; Lin, T.; Grünzweig, J.M.; Maseyk, K.; Rotenberg, E.; Yakir, D. Association between tree-ring and needle δ13C and leaf gas exchange in Pinus halepensis under semi-arid conditions. Oecologia 2005, 144, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Nicault, A.; Rathgeber, C.; Tessier, L.; Thomas, A. Observations on the development of rings of Aleppo pine (Pinus halepensis Mill.): Confrontation between radial growth, density and climatic factors. Ann. For. Sci. 2001, 58, 769–784. [Google Scholar] [CrossRef] [Green Version]
- Voltas, J.; Camarero, J.J.; Carulla, D.; Aguilera, M.; Ortiz, A.; Ferrio, J.P. A retrospective, dual-isotope approach reveals individual predispositions to winter-drought induced tree dieback in the southernmost distribution limit of Scots pine. Plant Cell Environ. 2013, 36, 1435–1448. [Google Scholar] [CrossRef]
- de Andrés, E.G.; Camarero, J.J. Disentangling mechanisms of drought-induced dieback in Pinus nigra arn. From growth and wood isotope patterns. Forests 2020, 11, 1339. [Google Scholar] [CrossRef]
- Petrucco, L.; Nardini, A.; Von Arx, G.; Saurer, M.; Cherubini, P. Isotope signals and anatomical features in tree rings suggest a role for hydraulic strategies in diffuse drought-induced die-back of Pinus nigra. Tree Physiol. 2017, 37, 523–535. [Google Scholar] [CrossRef]
- Galiano, L.; Martínez-Vilalta, J.; Lloret, F. Carbon reserves and canopy defoliation determine the recovery of Scots pine 4yr after a drought episode. New Phytol. 2011, 190, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Peguero-Pina, J.J.; Alquézar-Alquézar, J.M.; Mayr, S.; Cochard, H.; Gil-Pelegrín, E. Embolism induced by winter drought may be critical for the survival of Pinus sylvestris L. near its southern distribution limit. Ann. For. Sci. 2011, 68, 565–574. [Google Scholar] [CrossRef]
- Klein, T.; Cohen, S.; Yakir, D. Hydraulic adjustments underlying drought resistance of Pinus halepensis. Tree Physiol. 2011, 31, 637–648. [Google Scholar] [CrossRef] [Green Version]
- Shestakova, T.A.; Camarero, J.J.; Ferrio, J.P.; Knorre, A.A.; Gutiérrez, E.; Voltas, J. Increasing drought effects on five European pines modulate Δ13C-growth coupling along a Mediterranean altitudinal gradient. Funct. Ecol. 2017, 31, 1359–1370. [Google Scholar] [CrossRef] [Green Version]
- Klein, T.; Di Matteo, G.; Rotenberg, E.; Cohen, S.; Yakir, D. Differential ecophysiological response of a major Mediterranean pine species across a climatic gradient. Tree Physiol. 2013, 33, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Warren, C.R.; McGrath, J.F.; Adams, M.A. Water availability and carbon isotope discrimination in conifers. Oecologia 2001, 127, 476–486. [Google Scholar] [CrossRef]
- Mcdowell, N.; Brooks, J.R.; Fitzgerald, S.A.; Bond, B.J. Carbon isotope discrimination and growth response of old Pinus ponderosa trees to stand density reductions. Plant Cell Environ. 2003, 26, 631–644. [Google Scholar] [CrossRef]
- Fardusi, M.J.; Ferrio, J.P.; Comas, C.; Voltas, J.; de Dios, V.R.; Serrano, L. Intra-specific association between carbon isotope composition and productivity in woody plants: A meta-analysis. Plant Sci. 2016, 251, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Voltas, J.; Chambel, M.R.; Prada, M.A.; Ferrio, J.P. Climate-related variability in carbon and oxygen stable isotopes among populations of Aleppo pine grown in common-garden tests. Trees-Struct. Funct. 2008, 22, 759–769. [Google Scholar] [CrossRef]
- Corcuera, L.; Camarero, J.J.; Gil-Pelegrín, E. Effects of a severe drought on Quercus ilex radial growth and xylem anatomy. Trees-Struct. Funct. 2004, 18, 83–92. [Google Scholar] [CrossRef]
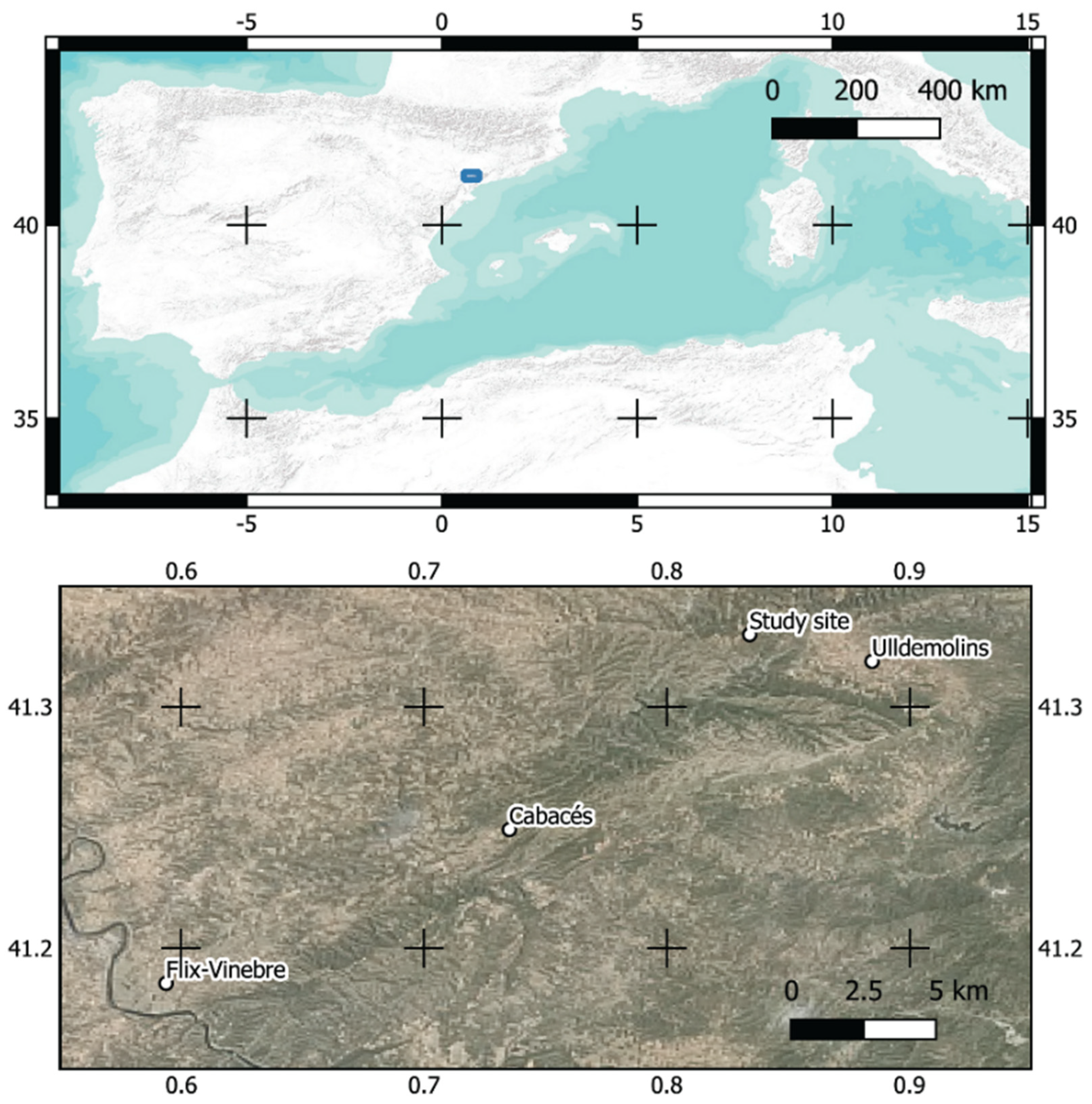
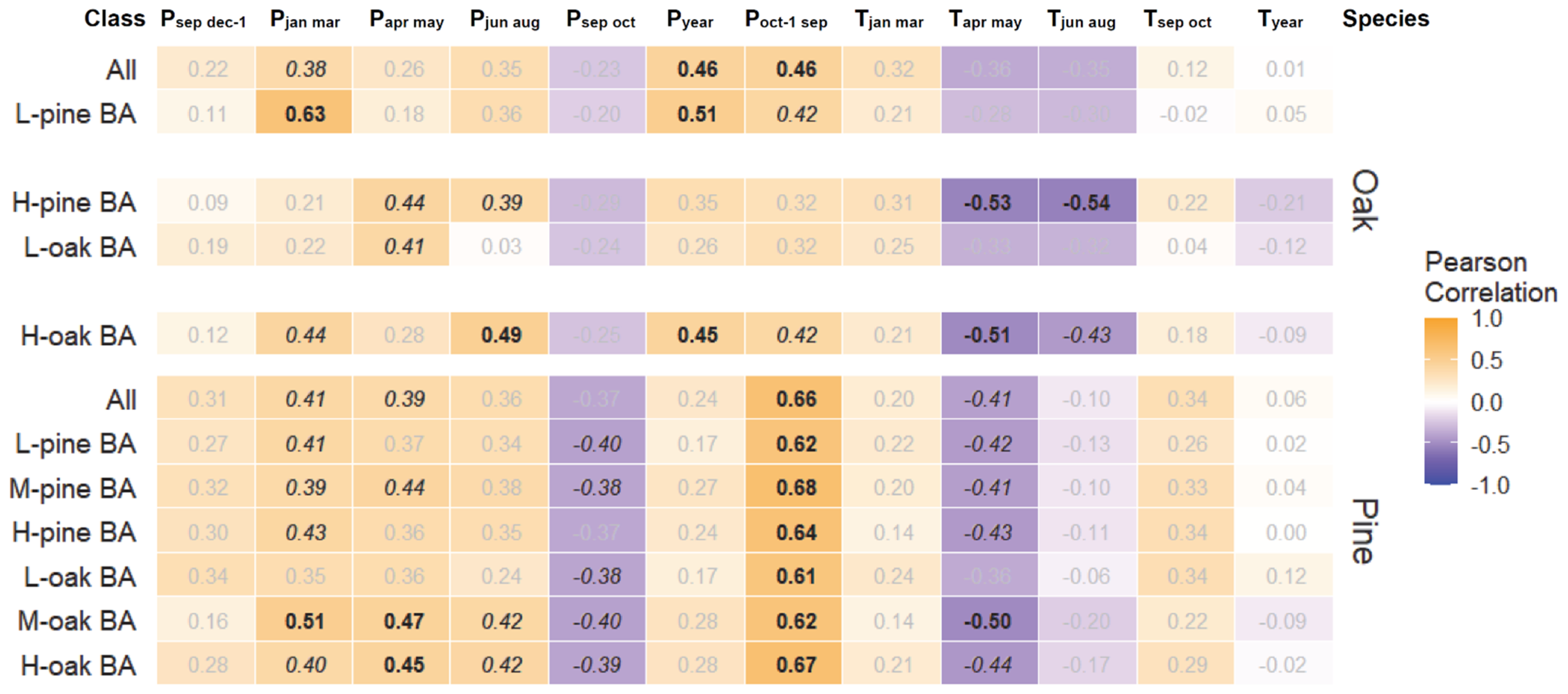
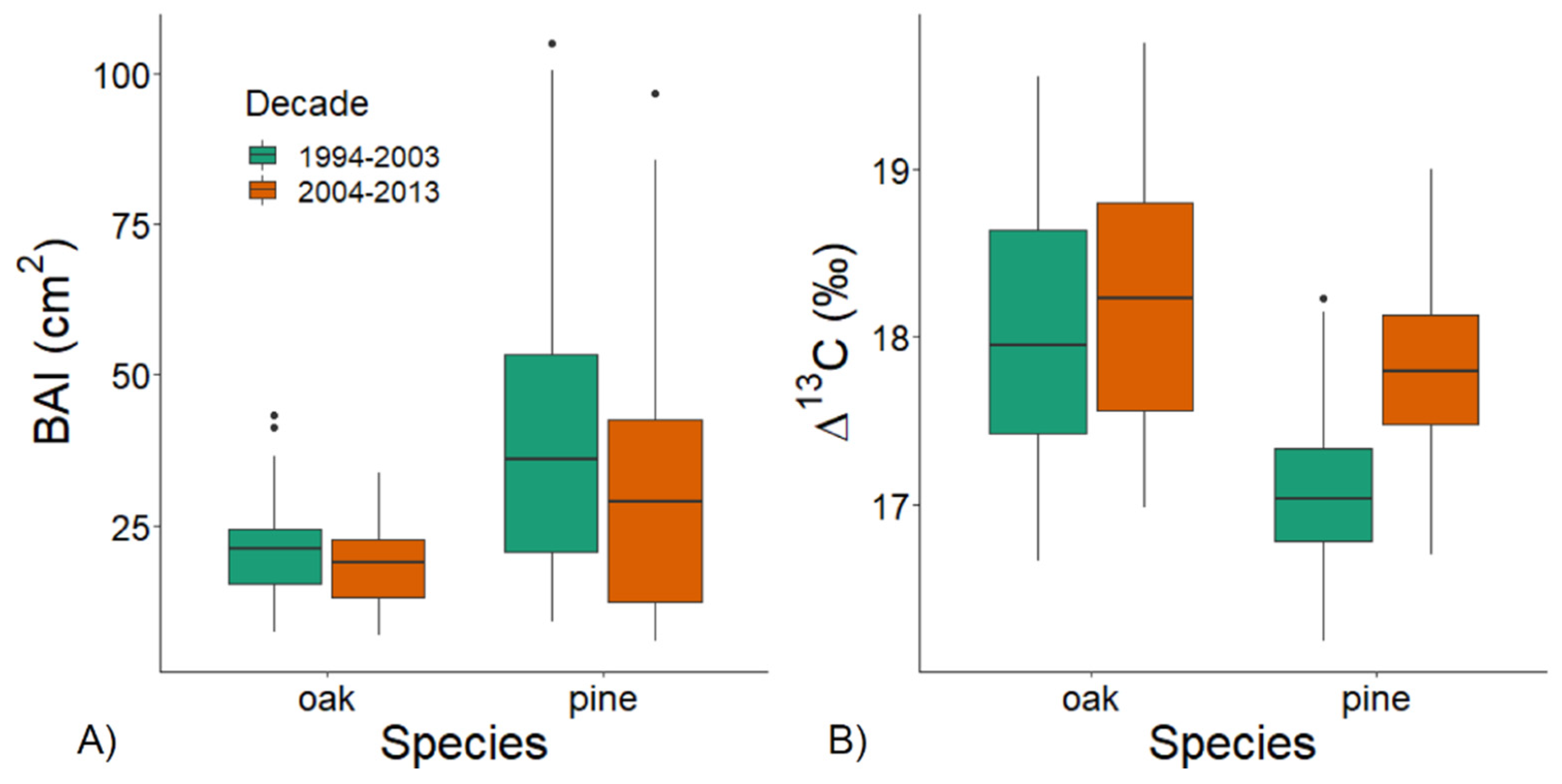
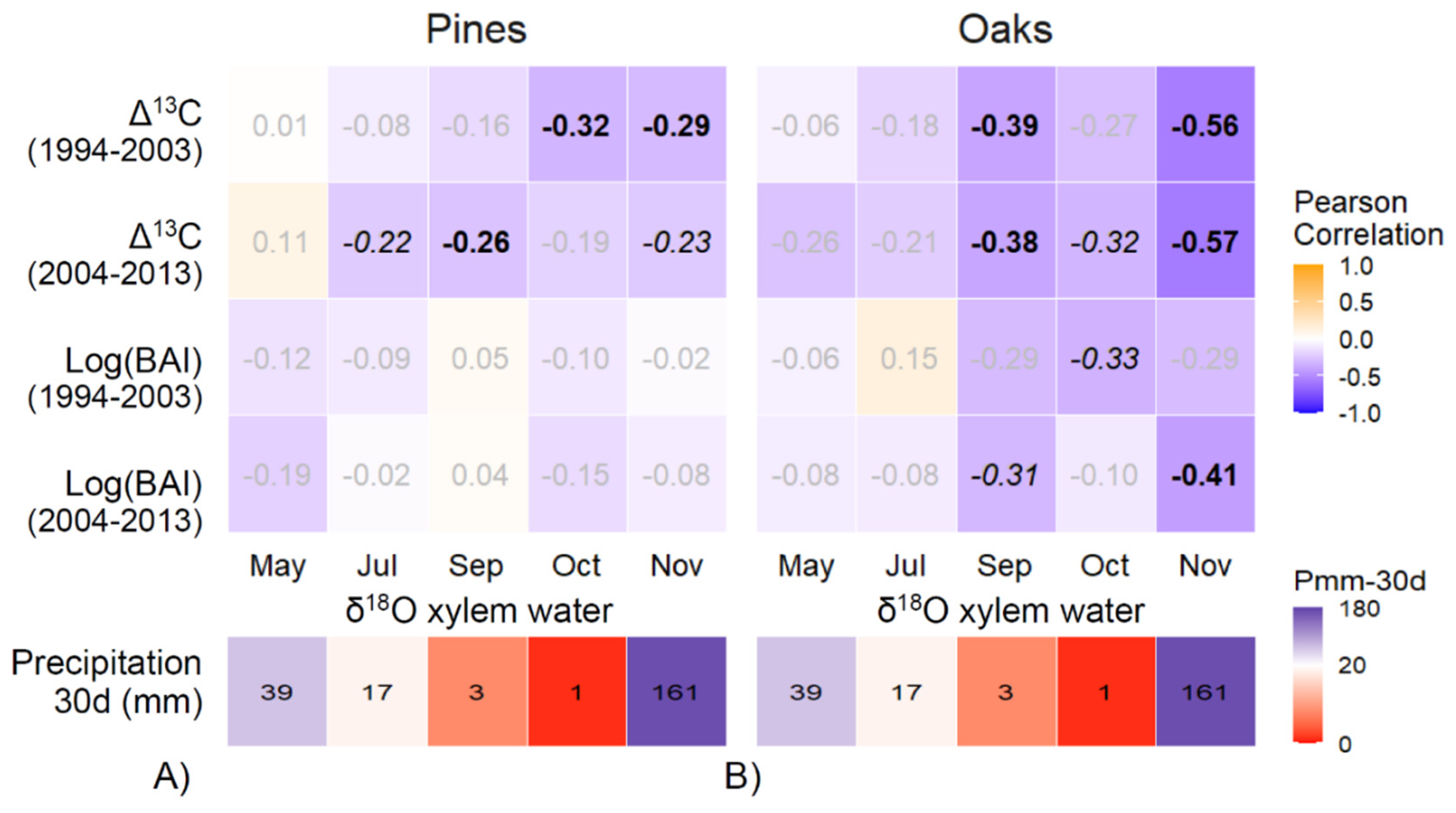
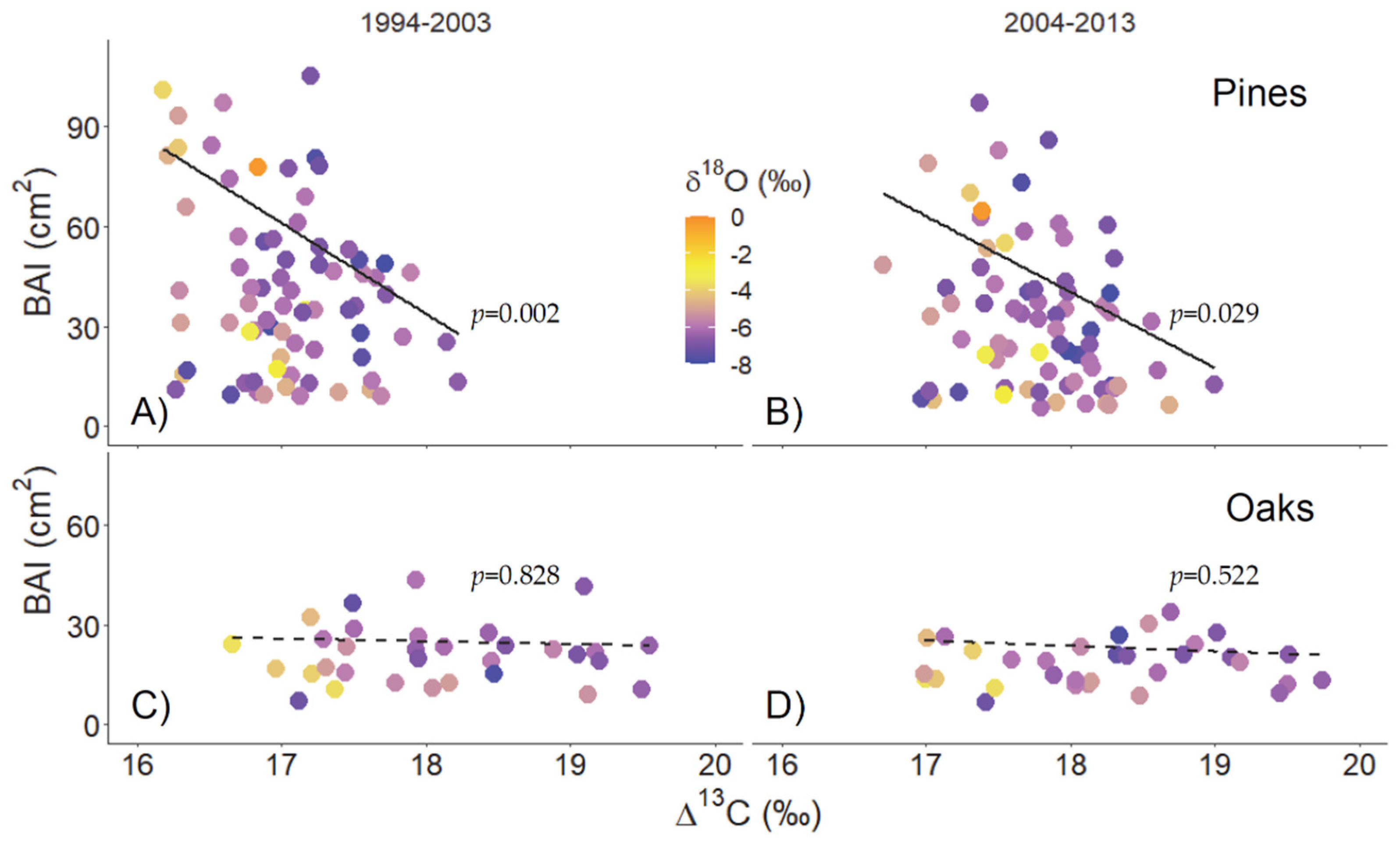
| Mean Temperature/Precipitation | |||||
|---|---|---|---|---|---|
| Period | Annual | Winter | Spring | Summer | Autumn |
| Long-term (1970–2019) | 13.5 °C/ 520 mm | 7.4 °C/ 110 mm | 13.8 °C/ 119 mm | 21.5 °C/ 78 mm | 16.7 °C/ 117 mm |
| Wet decade (1994–2003) | 14.0 °C/ 584 mm | 8.2 °C/ 101 mm | 14.3 °C/ 134 mm | 21.9 °C/ 76 mm | 16.6 °C/ 139 mm |
| Dry decade (2004–2013) | 13.8 °C/ 479 mm | 7.2 °C/ 111 mm | 14.4 °C/ 123 mm | 22.1 °C/ 62 mm | 17.2 °C/ 102 mm |
| % change 1 | −1%/−18% | −12%/+10% | +1%/−8% | +1%/−17% | +4%/−26% |
| Species | Grouped by | Density Class | n | Local Density within a 5 m Radius (cm2) Mean (Range) | |
|---|---|---|---|---|---|
| Pine BA | Oak BA | ||||
| Pine | Pine BA | low | 26 | 1048 (0–1560) | 404 (0–1127) |
| medium | 23 | 1925 (1583–2310) | 570 (0–1156) | ||
| high | 26 | 3055 (2397–4467) | 492 (0–1042) | ||
| Oak BA | low | 27 | 1970 (414–3563) | 116 (0–259) | |
| medium | 23 | 1818 (388–3640) | 449 (312–662) | ||
| high | 25 | 2238 (0–4467) | 918 (666–1156) | ||
| Oak | Pine BA | low | 17 | 1191 (0–2053) | 430 (0–1049) |
| high | 16 | 2798 (2059–4217) | 378 (0–1261) | ||
| Oak BA | low | 17 | 1953 (0–3315) | 132 (0–357) | |
| high | 16 | 1988 (357–4217) | 695 (360–1261) | ||
| log(BAI) (1994–2003) | log(BAI) (2004–2013) | Δ13C (1994–2003) | Δ13C (2004–2013) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| df. | F | p > F | F | p > F | F | p > F | F | p > F | |
| Species | 1 | 14.6 | <0.001 | 9.8 | 0.002 | 51.2 | <0.001 | 10.4 | 0.003 |
| X | 1 | - | - | 6.0 | 0.016 | 3.1 | 0.087 | 3.4 | 0.073 |
| Y | 1 | - | - | 1.4 | 0.235 | 4.2 | 0.048 | 3.4 | 0.075 |
| Age | 1 | 1.8 | 0.183 | 1.9 | 0.170 | - | - | - | - |
| Pine BA | 1 | 0.3 | 0.611 | 0.5 | 0.460 | - | - | - | - |
| Oak BA | 1 | - | - | 3.1 | 0.082 | 2.7 | 0.108 | 3.3 | 0.076 |
| Species × X | 1 | - | - | 0.9 | 0.342 | 6.1 | 0.018 | 3.0 | 0.090 |
| Species × Y | 1 | - | - | 4.1 | 0.046 | 1.7 | 0.202 | 1.0 | 0.331 |
| Species × Age | 1 | 0.1 | 0.815 | 0.9 | 0.353 | - | - | - | - |
| Species × Pine BA | 1 | 4.5 | 0.036 | 6.5 | 0.012 | - | - | - | - |
| Species × Oak BA | 1 | - | - | 0.0 | 0.844 | 0.1 | 0.787 | 2.31 | 0.137 |
| AICsim | 197.6 | - | 171.4 | 166.9 | |||||
| AICfull | 205.8 | 212.4 | 179.6 | 181.0 | |||||
| Mean ± SE | Res. ± SE | Mean ± SE | Res. ± SE | Mean ± SE | Res. ± SE | Mean ± SE | Res. ± SE | ||
| Pines | 32 ± 1.1 | 1.6 ± 1.1 | 26 ± 1.1 | 1.8 ± 1.1 | 17.07 ± 0.05 | 0.21 ± 0.03 | 17.80 ± 0.05 | 0.20 ± 0.03 | |
| Oaks | 20 ± 1.1 | 1.2 ± 1.1 | 17 ± 1.1 | 1.1 ± 1.0 | 18.17 ± 0.14 | 0.42 ± 0.11 | 18.28 ± 0.14 | 0.40 ± 0.11 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrio, J.P.; Shestakova, T.A.; del Castillo, J.; Voltas, J. Oak Competition Dominates Interspecific Interactions in Growth and Water-Use Efficiency in a Mixed Pine–Oak Mediterranean Forest. Forests 2021, 12, 1093. https://doi.org/10.3390/f12081093
Ferrio JP, Shestakova TA, del Castillo J, Voltas J. Oak Competition Dominates Interspecific Interactions in Growth and Water-Use Efficiency in a Mixed Pine–Oak Mediterranean Forest. Forests. 2021; 12(8):1093. https://doi.org/10.3390/f12081093
Chicago/Turabian StyleFerrio, Juan Pedro, Tatiana A. Shestakova, Jorge del Castillo, and Jordi Voltas. 2021. "Oak Competition Dominates Interspecific Interactions in Growth and Water-Use Efficiency in a Mixed Pine–Oak Mediterranean Forest" Forests 12, no. 8: 1093. https://doi.org/10.3390/f12081093
APA StyleFerrio, J. P., Shestakova, T. A., del Castillo, J., & Voltas, J. (2021). Oak Competition Dominates Interspecific Interactions in Growth and Water-Use Efficiency in a Mixed Pine–Oak Mediterranean Forest. Forests, 12(8), 1093. https://doi.org/10.3390/f12081093








