Effects of Antimony Stress on Growth and Physiology of 10 Genotypes of Catalpa bungei
Abstract
:1. Introduction
- How do different genotypes of C. bungei grow under Sb stress?
- How do different genotypes of C. bungei differ in their Sb absorption and transport abilities?
- What are the physiological responses of different genotypes of C. bungei to different Sb concentrations?
2. Materials and Methods
2.1. Materials
2.2. Plant Material Culture and Experimental Design
2.3. Sample Collection and Characterization
2.3.1. Sb Content Measurement
2.3.2. Measurement of Physiological and Biochemical Indices
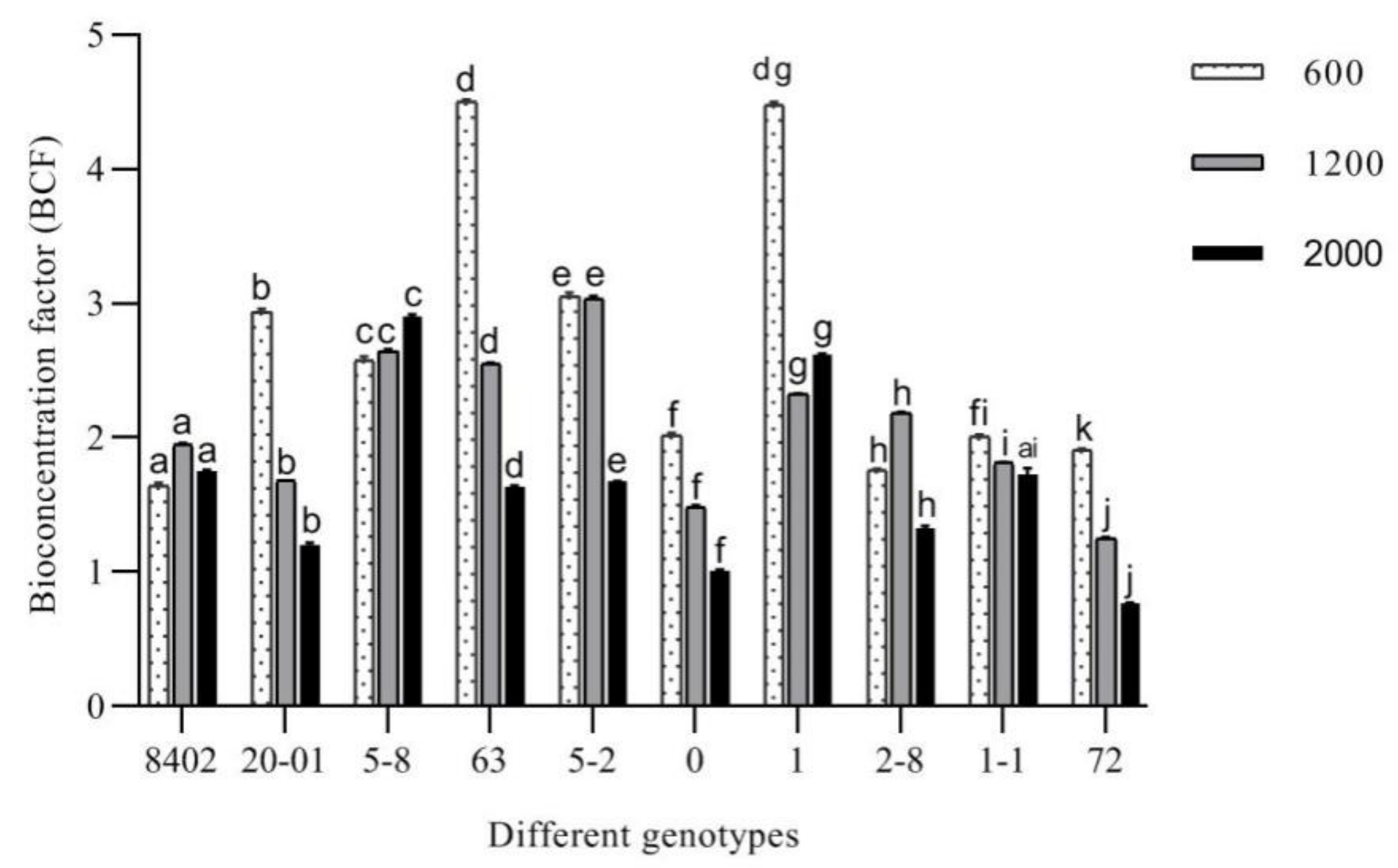
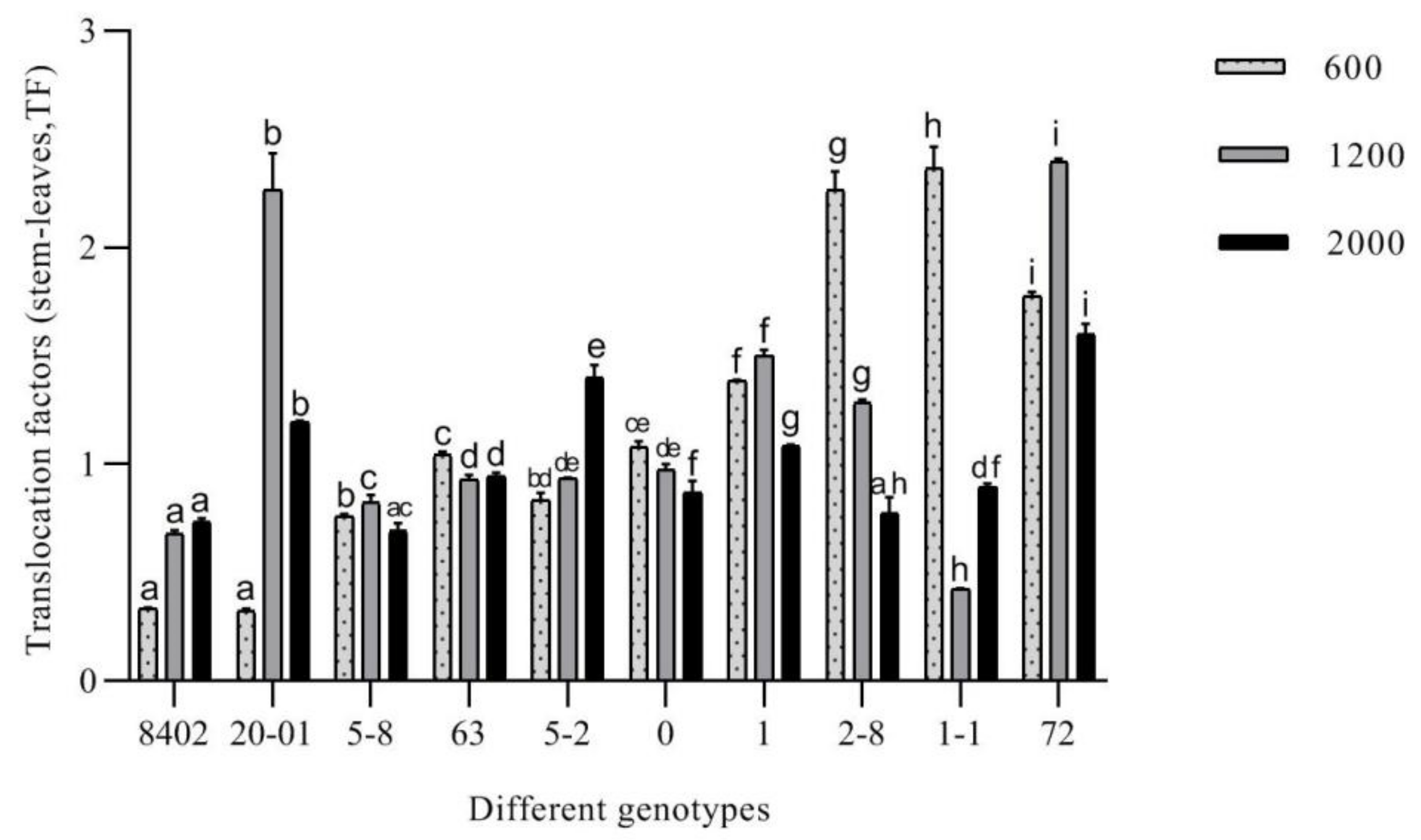
2.4. Biological Concentration Factor and Transfer Factor of Heavy Metal(loid)s
TFStem-Leaf = CLeaf-Sb/CStem-Sb
2.5. Statistical Analysis
3. Results
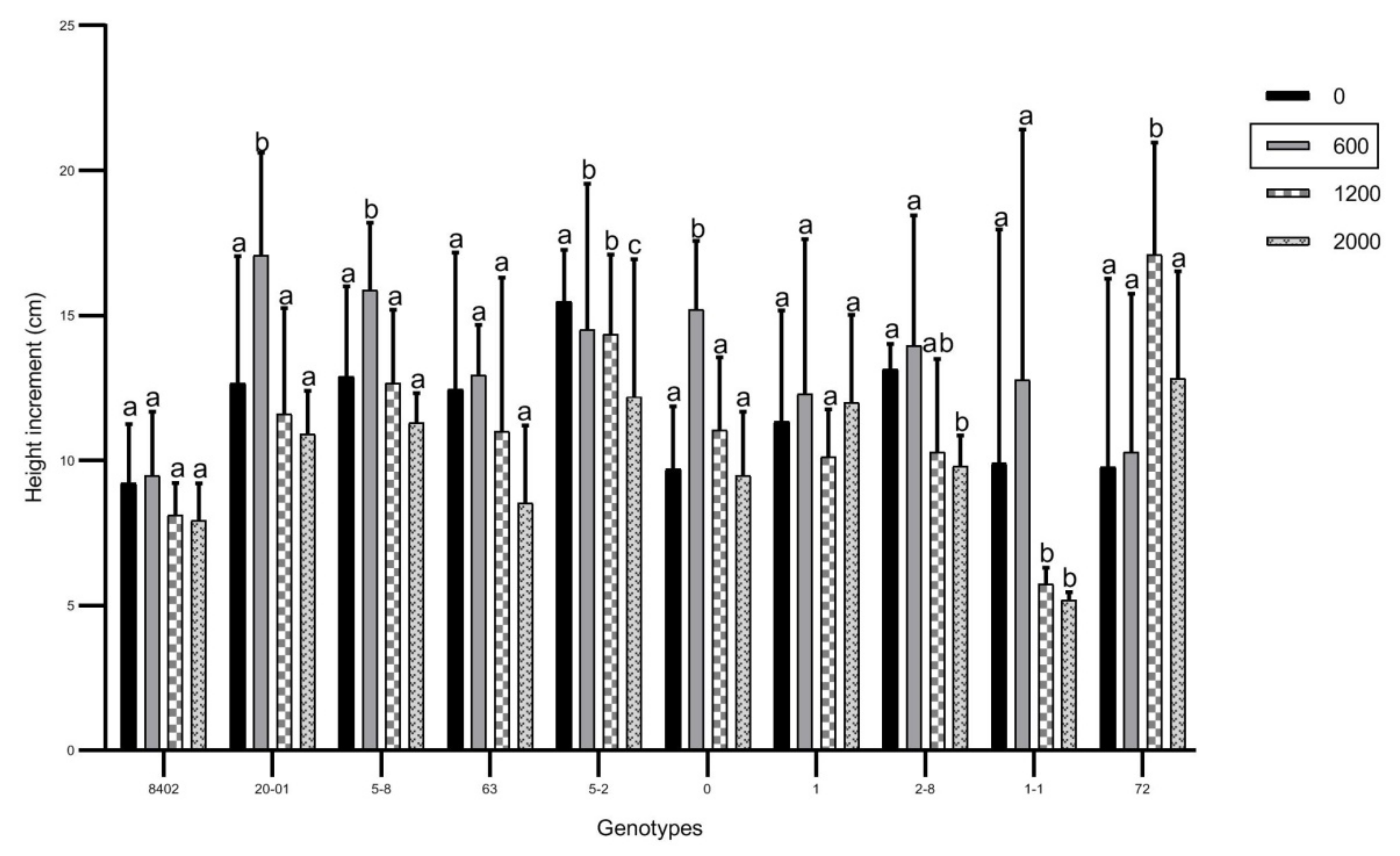
3.1. Effects of Soil Sb Concentration on Growth of C. bungei
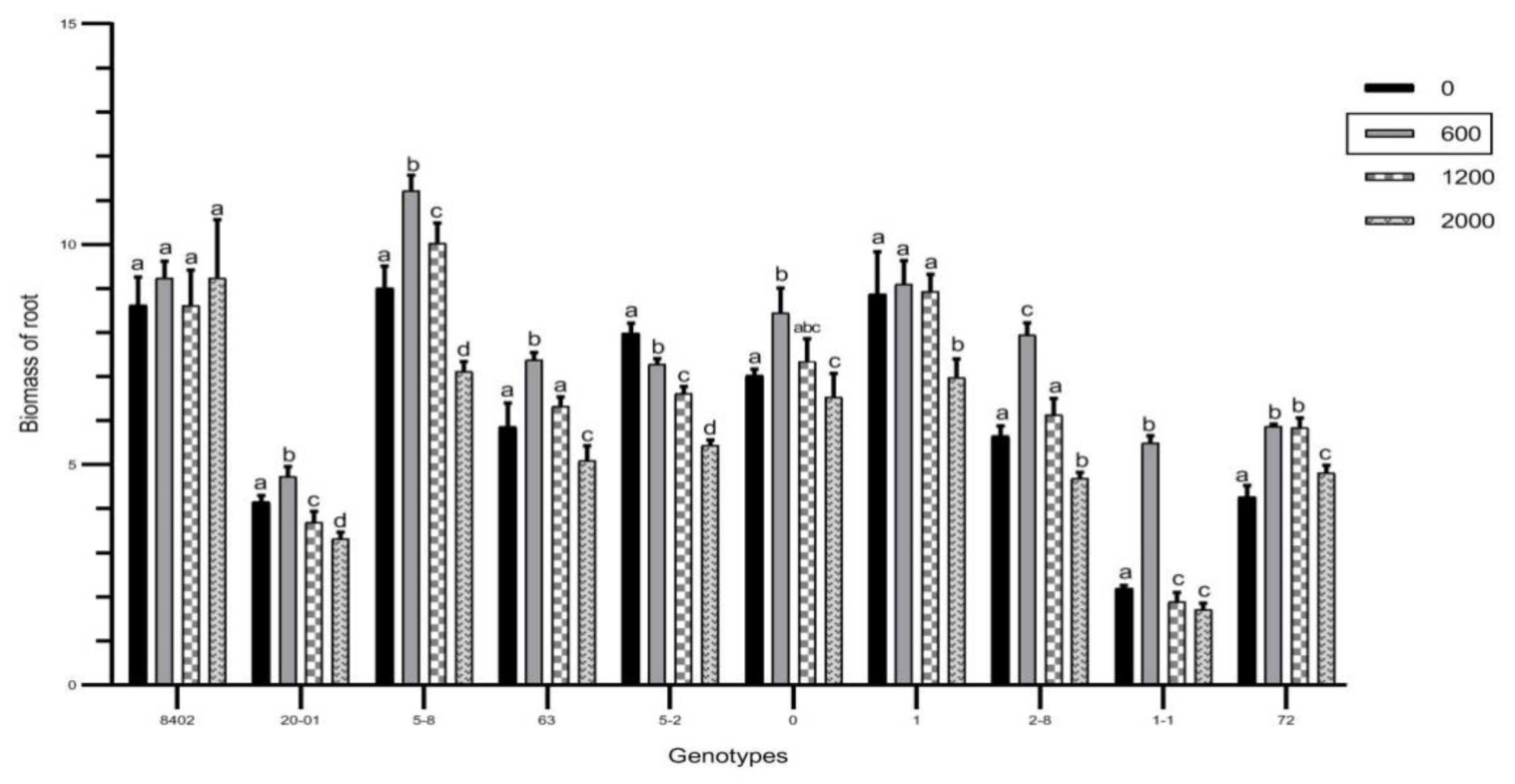
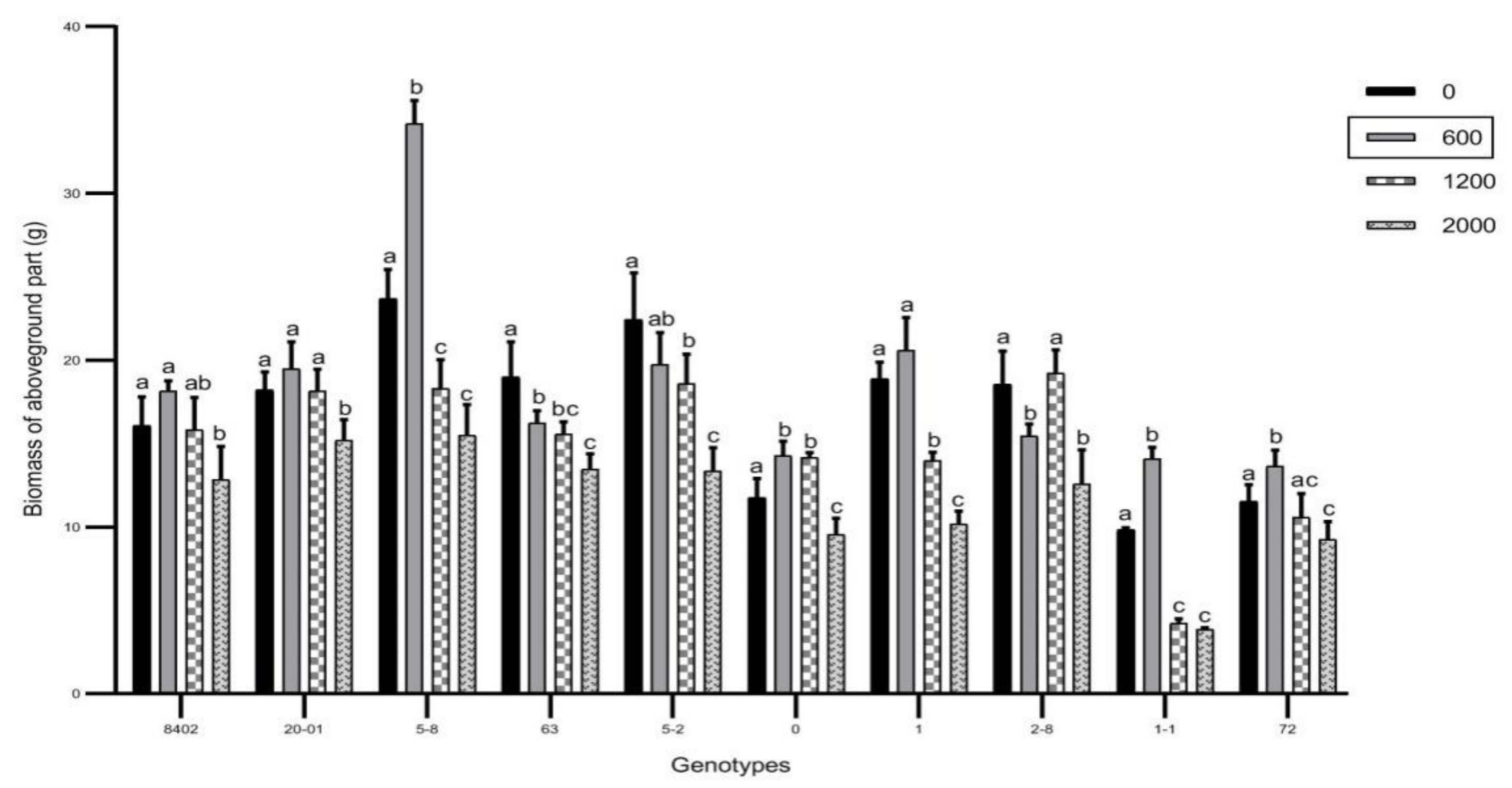
3.2. Effects of Sb on Biomass of C. bungei
3.3. Sb Concentration in C. bungei
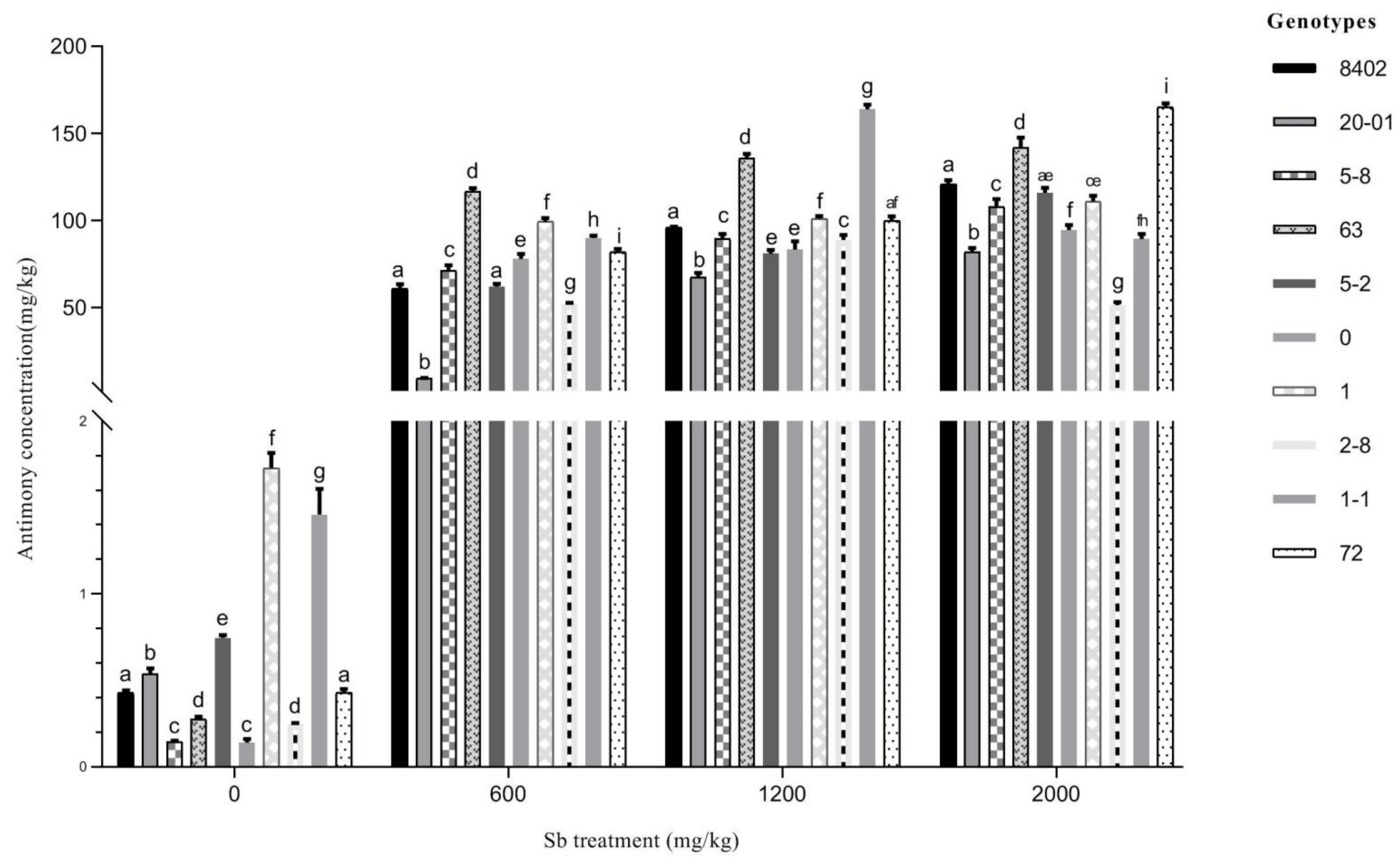
3.3.1. Sb Concentration in Roots of C. bungei
3.3.2. Sb Concentration in Stems of C. bungei
3.3.3. Sb Concentration in Leaves of C. bungei
3.3.4. Evaluation of the Remediation Potential of C. bungei
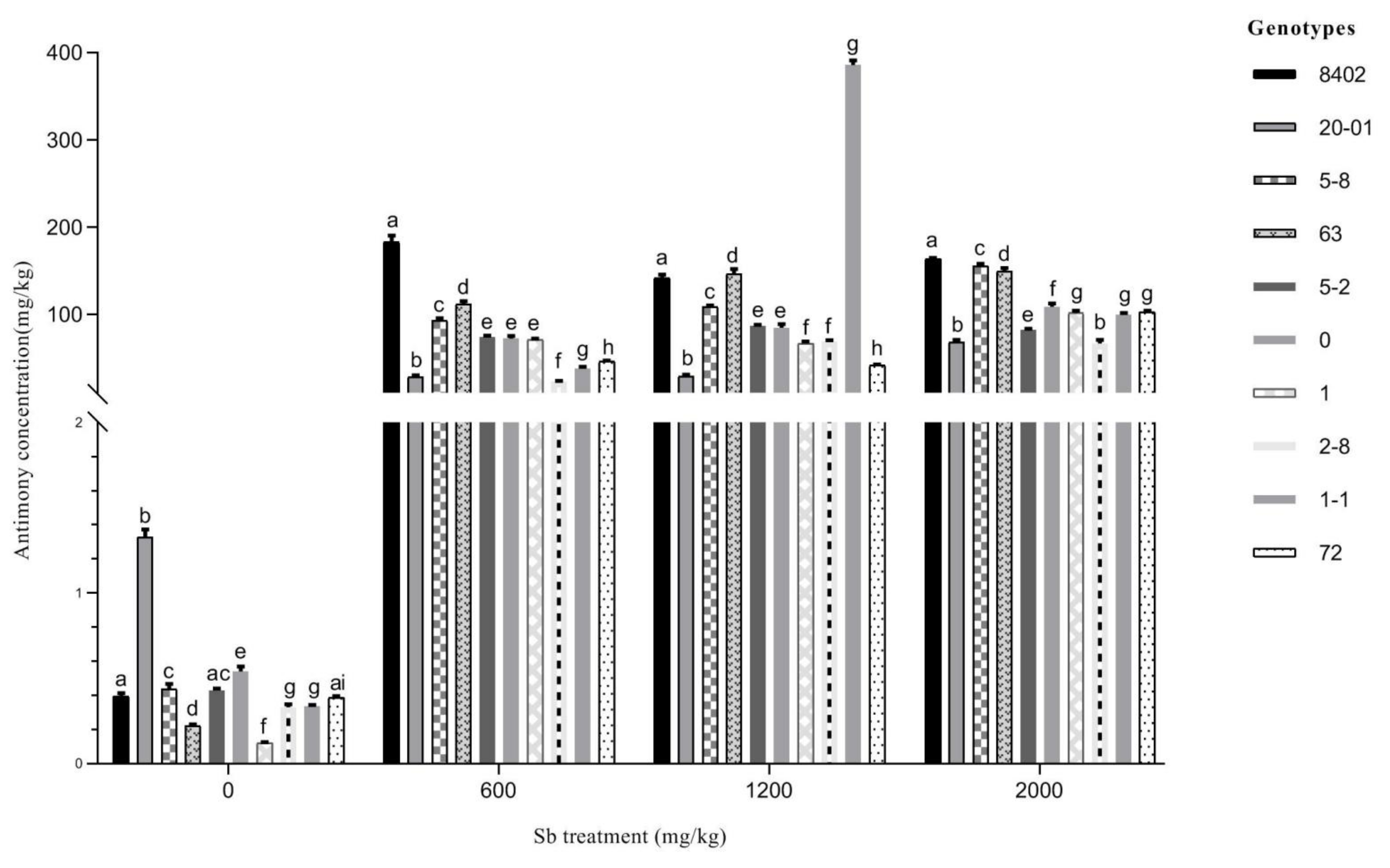
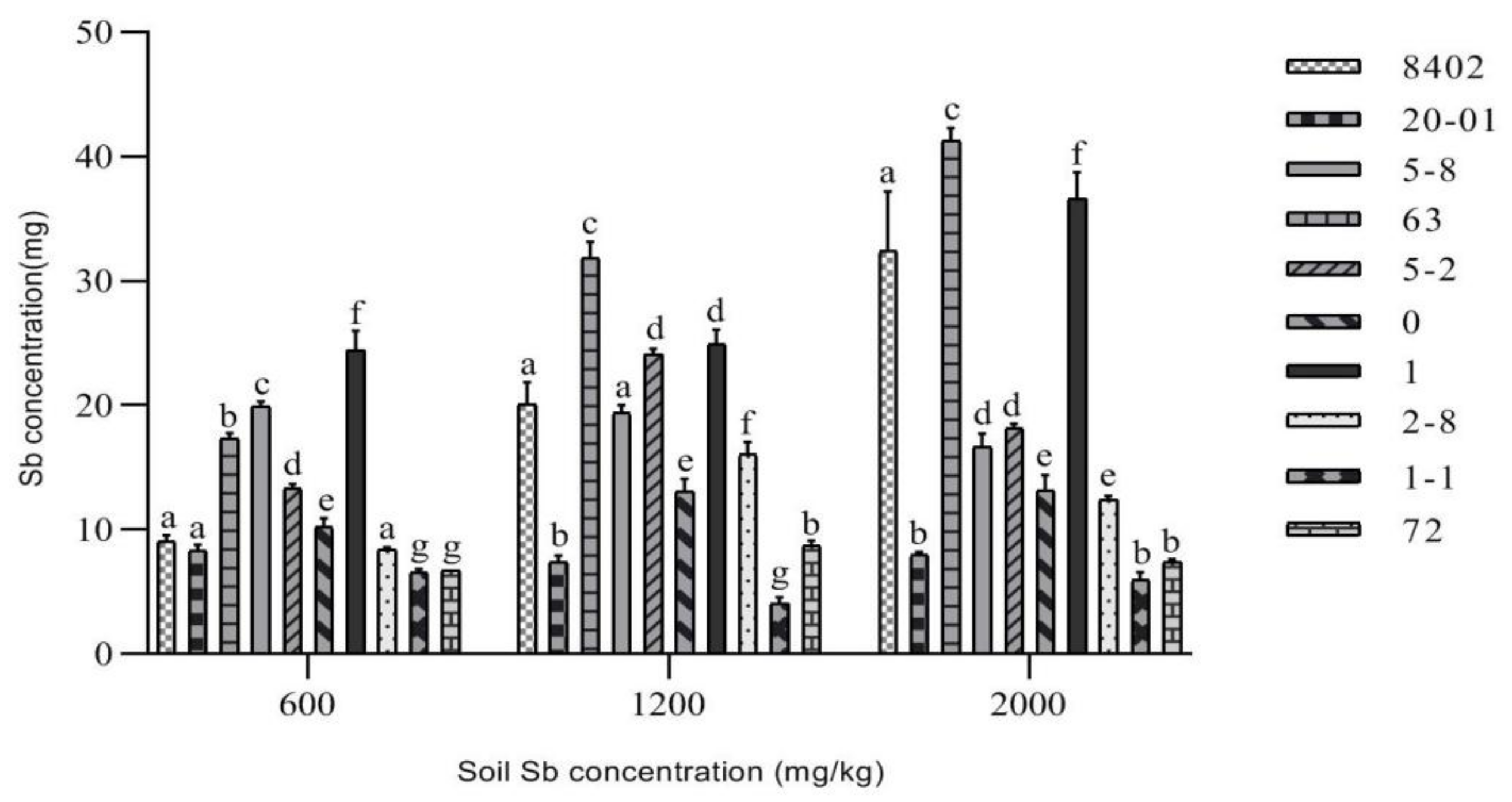
3.4. Root Sb Accumulation in 10 C. bungei Genotypes by Sb Concentration
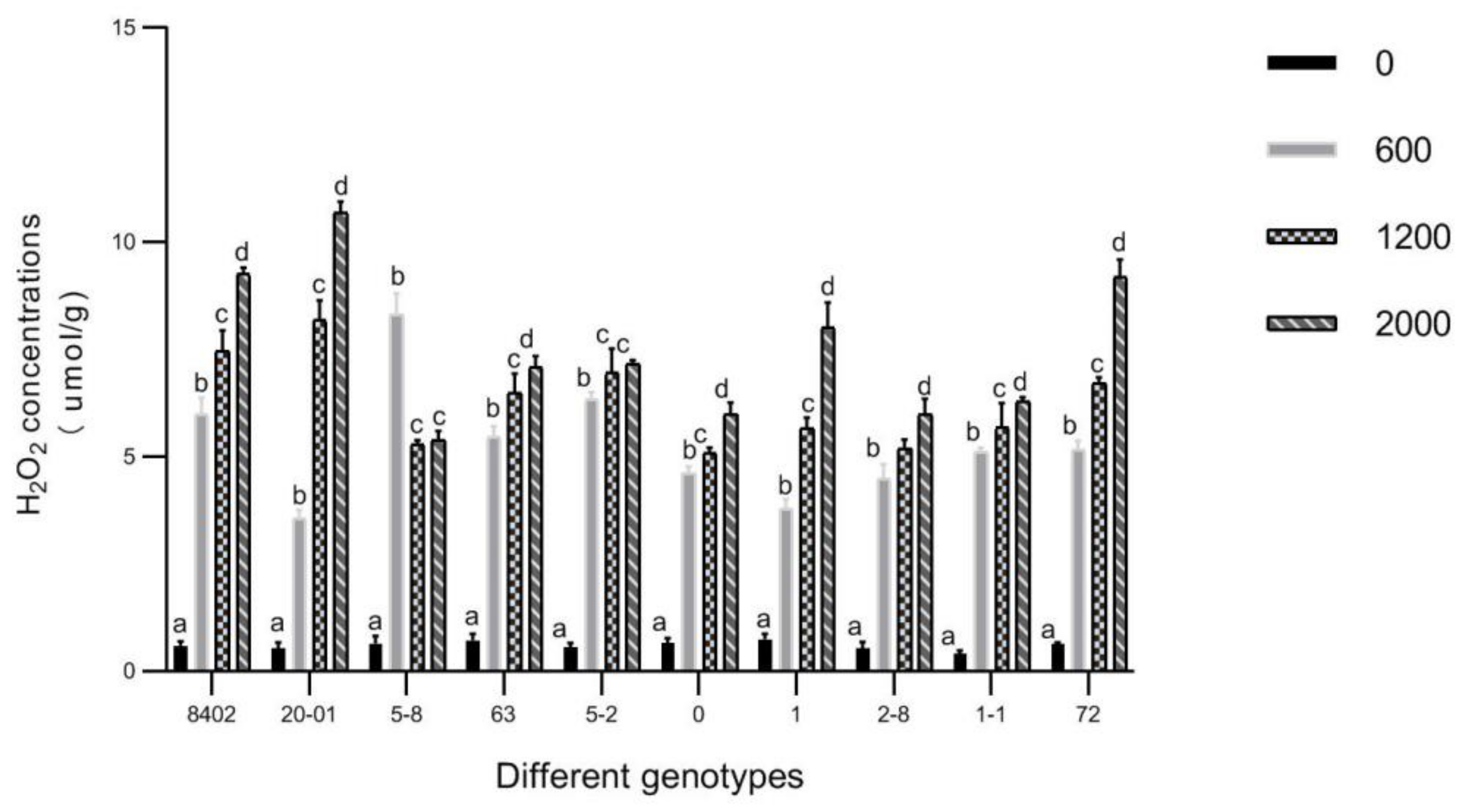
3.5. O2▪− and H2O2 in Leaves of C. bungei by Sb Concentration
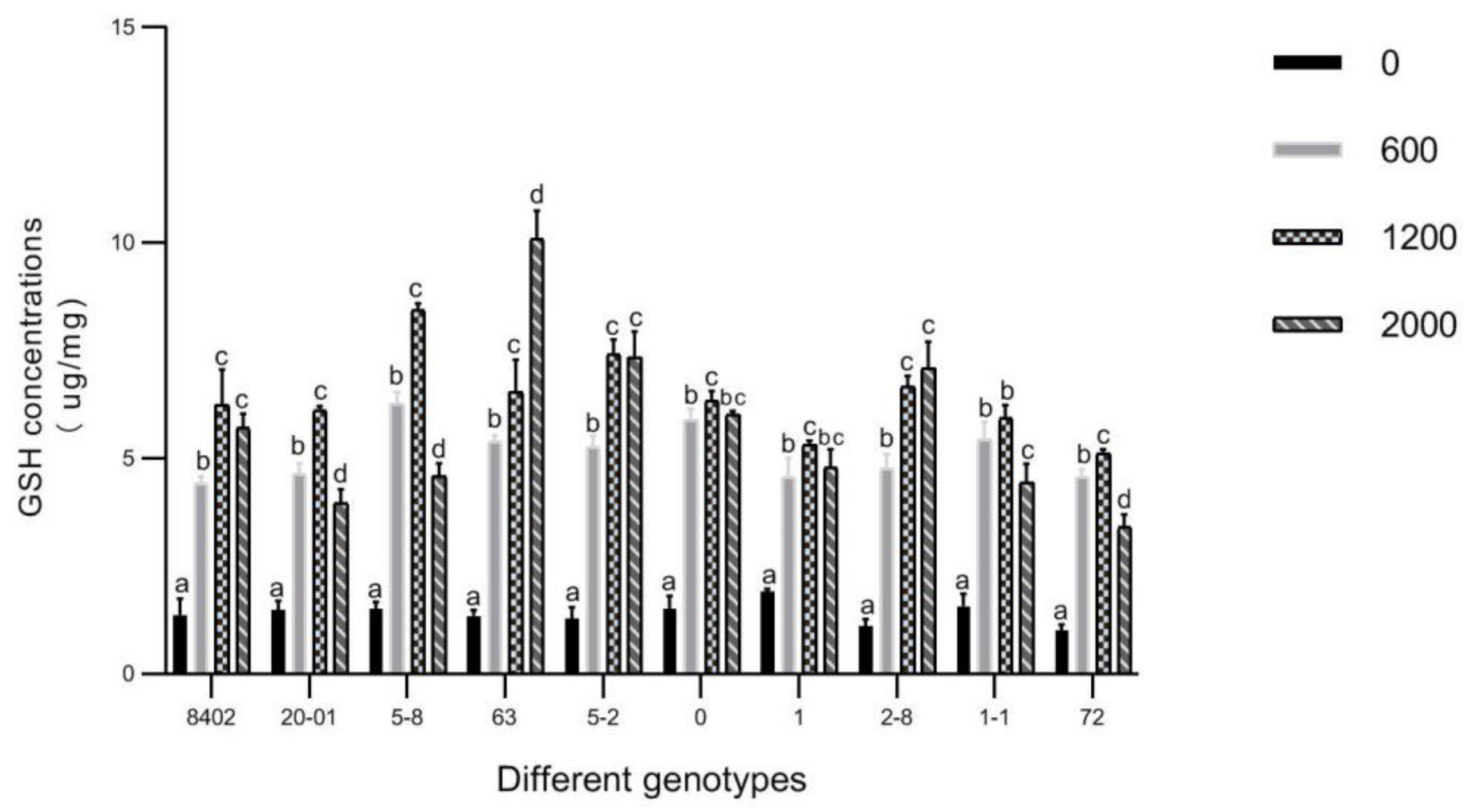
3.6. GSH in Leaves of C. bungei by Sb Concentration
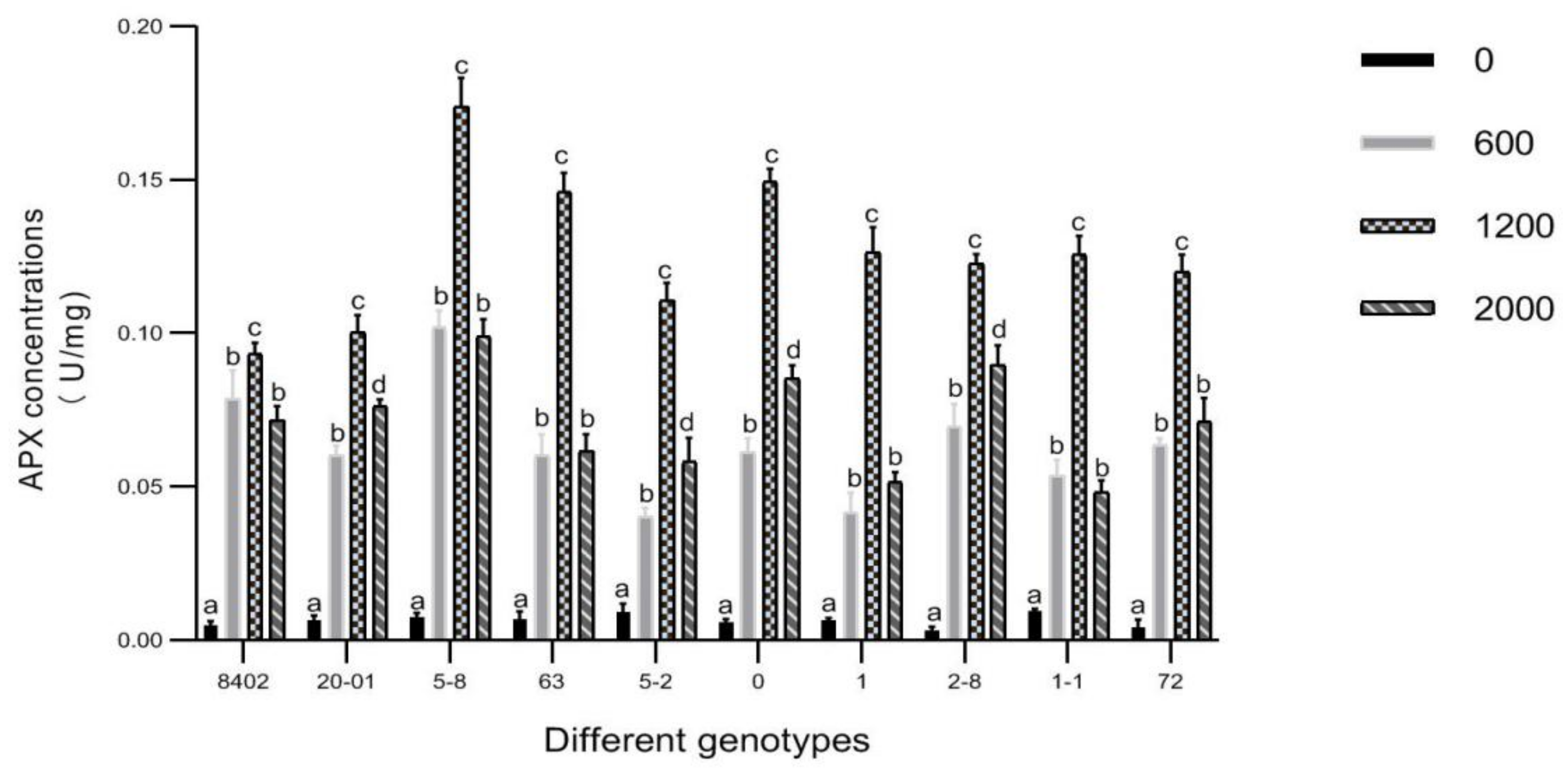
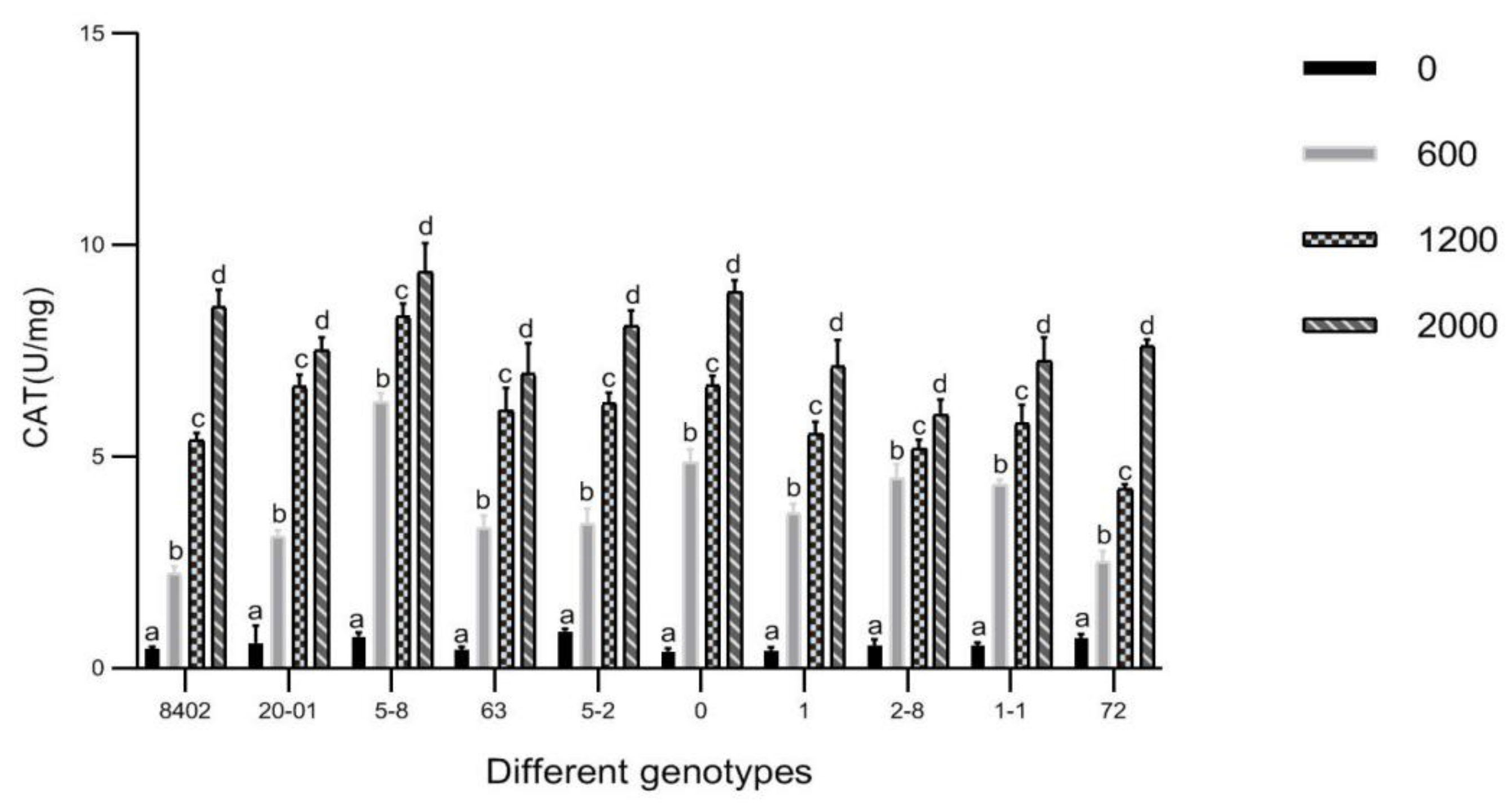
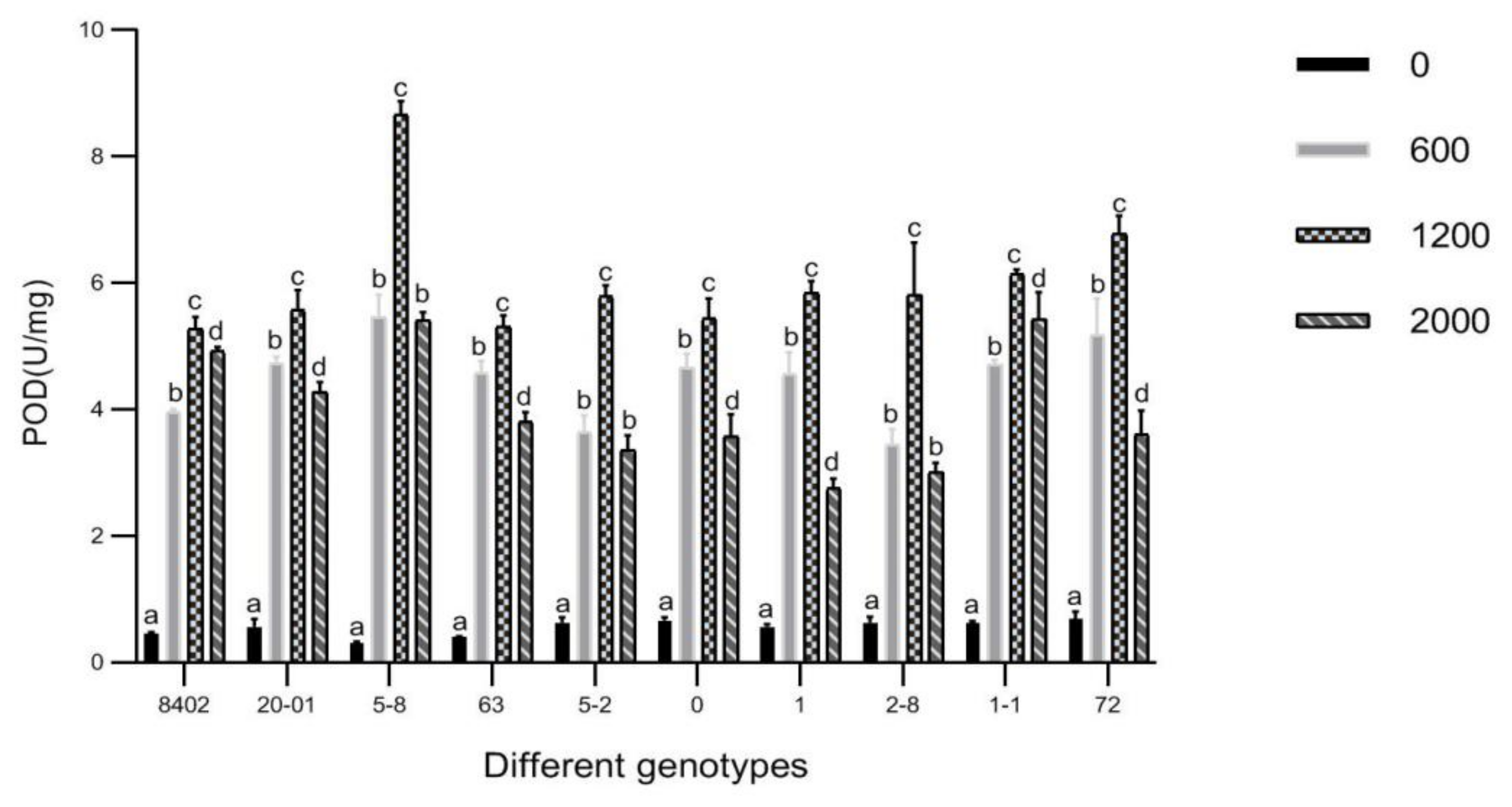
3.7. Effects of Sb Concentration on Antioxidant Enzyme Activities in Leaves of C. bungei
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xing, W.; Liu, H.; Banet, T.; Wang, H.; Ippolito, J.A.; Li, L. Cadmium, copper, lead and zinc accumulation in wild plant species near a lead smelter. Ecotoxicol. Environ. Saf. 2020, 198, 110683. [Google Scholar] [CrossRef]
- Ma, C.; He, M.; Zhong, Q.; Ouyang, W.; Lin, C.; Liu, X. Uptake, translocation and phytotoxicity of antimonite in wheat (Triticum aestivum). Sci. Total Environ. 2019, 669, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Smichowski, P. Antimony in the environment as a global pollutant: A review on analytical methodologies for its determination in atmospheric aerosols. Talanta 2008, 75, 2–14. [Google Scholar] [CrossRef]
- Chai, L.Y.; Mubarak, H.; Yang, Z.H.; Yong, W.; Tang, C.-J.; Mirza, N. Growth, photosynthesis, and defense mechanism of antimony (Sb)-contaminated Boehmeria nivea L. Environ. Sci. Pollut. Res. 2015, 23, 7470–7481. [Google Scholar] [CrossRef]
- Wilson, N.J.; Craw, D.; Hunter, K. Antimony distribution and environmental mobility at an historic antimony smelter site, New Zealand. Environ. Pollut. 2004, 129, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, D.; Iavicoli, I.; Setini, A.; Marinaccio, A.; Perniconi, B.; Carelli, G.; Iavicoli, S. Genotoxic risk and oxidative DNA damage in workers exposed to antimony trioxide. Environ. Mol. Mutagenesis 2002, 40, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Herath, I.; Vithanage, M.; Bundschuh, J. Antimony as a global dilemma: Geochemistry, mobility, fate and transport. Environ. Pollut. 2017, 223, 545–559. [Google Scholar] [CrossRef]
- Jamali Hajiani, N.; Ghaderian, S.M.; Karimi, N.; Schat, H. A comparison of antimony accumulation and tolerance among Achillea wilhelmsii, Silene vulgaris and Thlaspi arvense. Plant Soil 2016, 412, 267–281. [Google Scholar] [CrossRef]
- Feng, R.; Lei, L.; Su, J.; Zhang, R.; Zhu, Y.; Chen, W.; Wang, L.; Wang, R.; Dai, J.; Lin, Z.; et al. Toxicity of different forms of antimony to rice plant: Effects on root exudates, cell wall components, endogenous hormones and antioxidant system. Sci. Total Environ. 2020, 711, 134589. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Yuan, C.; Li, B.; Liu, T.; Liu, C.; Liu, C. The translocation of antimony in soil-rice system with comparisons to arsenic: Alleviation of their accumulation in rice by simultaneous use of Fe(II) and NO3−. Sci. Total Environ. 2019, 650, 633–641. [Google Scholar] [CrossRef]
- Baroni, F.; Boscagli, A.; Protano, G.; Riccobono, F. Antimony accumulation in Achillea ageratum, Plantago lanceolata and Silene vulgaris growing in an old Sb-mining area. Environ. Pollut. 2000, 109, 347–352. [Google Scholar] [CrossRef]
- Tschan, M.; Robinson, B.H.; Schulin, R. Antimony in the soil-plant system—A review. Environ. Chem. 2009, 6, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Cidu, R.; Biddau, R.; Dore, E.; Vacca, A.; Marini, L. Antimony in the soil–water–plant system at the Su Suergiu abandoned mine (Sardinia, Italy): Strategies to mitigate contamination. Sci. Total Environ. 2014, 497–498, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Han, F.; Pan, B.; Niu, Y.; Nie, G.; Lv, L. Antimony(V) removal from water by hydrated ferric oxides supported by calcite sand and polymeric anion exchanger. J. Environ. Sci. 2014, 26, 307–314. [Google Scholar] [CrossRef]
- He, M. Distribution and phytoavailability of antimony at an antimony mining and smelting area, Hunan, China. Environ. Geochem. Health 2007, 29, 209–219. [Google Scholar] [CrossRef]
- Corrales, I.; Barceló, J.; Bech, J.; Poschenrieder, C. Antimony accumulation and toxicity tolerance mechanisms in Trifolium species. J. Geochem. Explor. 2014, 147, 167–172. [Google Scholar] [CrossRef]
- Remans, T.; Opdenakker, K.; Guisez, Y.; Carleer, R.; Schat, H.; Vangronsveld, J.; Cuypers, A. Exposure of Arabidopsis thaliana to excess Zn reveals a Zn-specific oxidative stress signature. Environ. Exp. Bot. 2012, 84, 61–71. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, X.; Ma, W.; Song, J.; Rahman, S.U.; Wang, J.; Zhang, Y. Morphological and physiological responses to cyclic drought in two contrasting genotypes of Catalpa bungei. Environ. Exp. Bot. 2017, 138, 77–87. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Yang, Y.; Duan, B. Soil cadmium toxicity and nitrogen deposition differently affect growth and physiology in Toxicodendron vernicifluum seedlings. Acta Physiol. Plant. 2012, 35, 529–540. [Google Scholar] [CrossRef]
- Xue, L.; Ren, H.; Li, S.; Gao, M.; Shi, S.; Chang, E.; Wei, Y.; Yao, X.; Jiang, Z.; Liu, J. Comparative proteomic analysis in Miscanthus sinensis exposed to antimony stress. Environ. Pollut. 2015, 201, 150–160. [Google Scholar] [CrossRef]
- Dahmani-Muller, H.; van Oort, F.; Gélie, B.; Balabane, M. Strategies of heavy metal uptake by three plant species growing near a metal smelter. Environ. Pollut. 2000, 109, 231–238. [Google Scholar] [CrossRef]
- Shtangeeva, I.; Steinnes, E.; Lierhagen, S. Uptake of different forms of antimony by wheat and rye seedlings. Environ. Sci. Pollut. Res. Int. 2012, 19, 502–509. [Google Scholar] [CrossRef]
- Wang, P.; Ma, L.; Li, Y.; Li, L.; Yang, R.; Ma, Y.; Wang, Q. Transcriptome profiling of indole-3-butyric acid-induced adventitious root formation in softwood cuttings of the Catalpa bungei variety ‘YU-1’ at different developmental stages. Genes Genom. 2015, 38, 145–162. [Google Scholar] [CrossRef]
- Cai, F.; Ren, J.; Tao, S.; Wang, X. Uptake, translocation and transformation of antimony in rice (Oryza sativa L.) seedlings. Environ. Pollut. 2016, 209, 169–176. [Google Scholar] [CrossRef]
- Ghosh, M.; Singh, S.P. A comparative study of cadmium phytoextraction by accumulator and weed species. Environ. Pollut. 2005, 133, 365–371. [Google Scholar] [CrossRef]
- Xue, L.; Liu, J.; Shi, S.; Wei, Y.; Chang, E.; Gao, M.; Chen, L.; Jiang, Z. Uptake of Heavy Metals by Native Herbaceous Plants in an Antimony Mine (Hunan, China). CLEAN – Soil, Air, Water 2014, 42, 81–87. [Google Scholar] [CrossRef]
- Zhang, W.; Cai, Y.; Tu, C.; Ma, L.Q. Arsenic speciation and distribution in an arsenic hyperaccumulating plant. Sci. Total Environ. 2002, 300, 167–177. [Google Scholar] [CrossRef]
- Fitz, W.J.; Wenzel, W.W. Arsenic transformations in the soil-rhizosphere-plant system: Fundamentals and potential application to phytoremediation. J. Biotechnol. 2002, 99, 259–278. [Google Scholar] [CrossRef]
- Hongjiang, Z.; Xizhou, Z.; Tingxuan, L.; Fu, H. Variation of cadmium uptake, translocation among rice lines and detecting for potential cadmium-safe cultivars. Environ. Earth Sci. 2013, 71, 277–286. [Google Scholar] [CrossRef]
- Tang, L.; Luo, W.; Tian, S.; He, Z.; Stoffella, P.J.; Yang, X. Genotypic differences in cadmium and nitrate co-accumulation among the Chinese cabbage genotypes under field conditions. Sci. Hortic. 2016, 201, 92–100. [Google Scholar] [CrossRef]
- Liu, J.; Mei, C.; Cai, H.; Wang, M. Relationships Between Subcellular Distribution and Translocation and Grain Accumulation of Pb in Different Rice Cultivars. Water Air Soil Pollut. 2015, 226. [Google Scholar] [CrossRef]
- Zhuang, P.; Shu, W.; Li, Z.; Liao, B.; Li, J.; Shao, J. Removal of metals by sorghum plants from contaminated land. J. Environ. Sci. 2009, 21, 1432–1437. [Google Scholar] [CrossRef]
- Ren, J.H.; Ma, L.Q.; Sun, H.J.; Cai, F.; Luo, J. Antimony uptake, translocation and speciation in rice plants exposed to antimonite and antimonate. Sci. Total Environ. 2014, 475, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Wei, C.; Tu, S.; Ding, Y.; Wang, R.; Guo, J. The uptake and detoxification of antimony by plants: A review. Environ. Exp. Botany 2013, 96, 28–34. [Google Scholar] [CrossRef]




| pH | Cd | Pb | Hg | As | Sb | Total N | Total P | Total K | Organic Matter | Available P | Available K | Hydrolytic N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4.80 | 0.06 | 16.80 | 0.07 | 9.08 | 1.94 | 0.74 | 0.15 | 11.30 | 10.50 | 0.51 | 39.20 | 63.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Ma, W.; Tong, F.; Wang, J. Effects of Antimony Stress on Growth and Physiology of 10 Genotypes of Catalpa bungei. Forests 2021, 12, 1036. https://doi.org/10.3390/f12081036
Liu Z, Ma W, Tong F, Wang J. Effects of Antimony Stress on Growth and Physiology of 10 Genotypes of Catalpa bungei. Forests. 2021; 12(8):1036. https://doi.org/10.3390/f12081036
Chicago/Turabian StyleLiu, Zhenhua, Wenjun Ma, Fangping Tong, and Junhui Wang. 2021. "Effects of Antimony Stress on Growth and Physiology of 10 Genotypes of Catalpa bungei" Forests 12, no. 8: 1036. https://doi.org/10.3390/f12081036
APA StyleLiu, Z., Ma, W., Tong, F., & Wang, J. (2021). Effects of Antimony Stress on Growth and Physiology of 10 Genotypes of Catalpa bungei. Forests, 12(8), 1036. https://doi.org/10.3390/f12081036





