Species-Specific Nitrogen Resorption Efficiency in Quercus mongolica and Acer mono in Response to Elevated CO2 and Soil N Deficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Elevated CO2 and Soil N Supply Treatments
2.3. Growth and Biomass Allocation
2.4. Gas Exchange Measurements
2.5. Leaf Characters
2.6. N Resorption Efficiency before Senescence
2.7. Statistical Analysis
3. Results
3.1. Growth Responses of Q. mongolica and A. mono Seedlings to CO2 and N Treatments
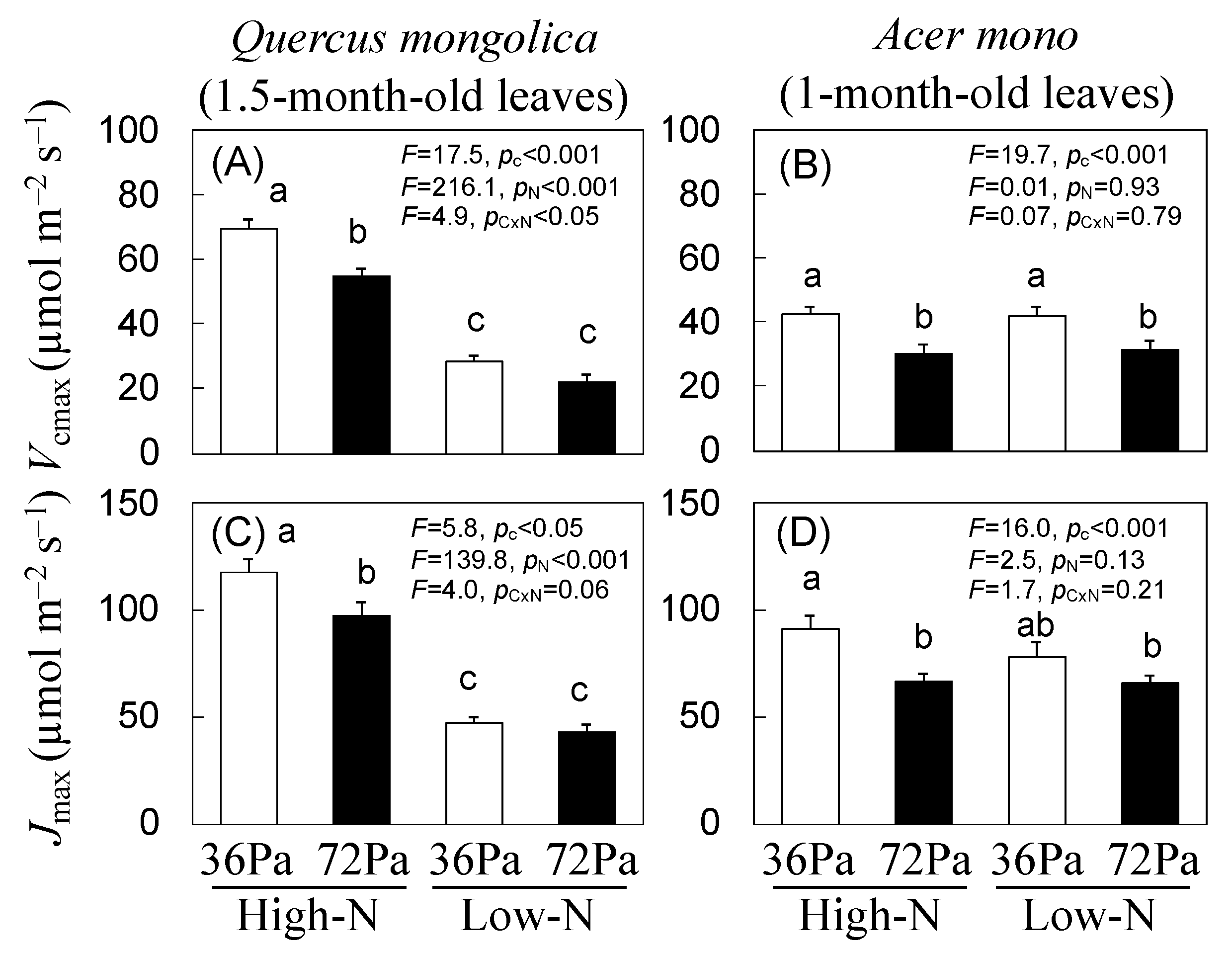
3.2. Maximum Carbon Fixation Rate (Vcmax) and Maximum Electron Transfer Rate (Jmax) of Q. mongolica and A. mono Seedlings under CO2 and N Treatments
3.3. Characteristics of Green Mature Leaves of Q. mongolica and A. mono Seedlings under CO2 and N Treatments
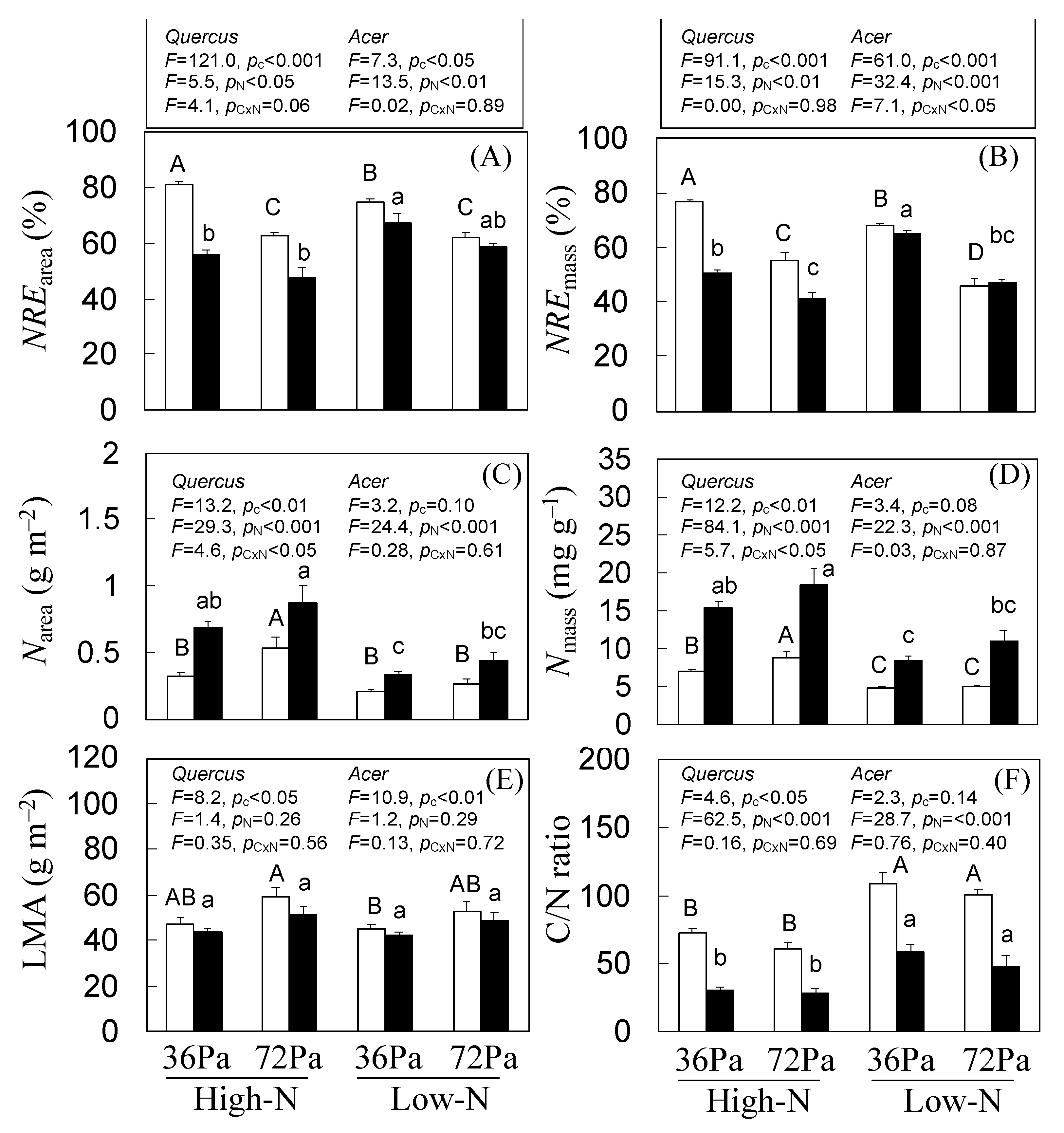
3.4. Indirect Estimation of N Partitioning within Leaves of Q. mongolica and A. mono Seedlings under CO2 and N Treatments
3.5. N Resorption Efficiency of Q. mongolica and A. mono Seedlings under CO2 and N Treatments
4. Discussion
4.1. N Content in Senesced Leaves of Q. mongolica and A. mono Seedlings Showed No Decline under Elevated CO2 Regardless of N Conditions
4.2. N Resorption Efficiency of Q. mongolica and A. mono Seedlings under CO2 and N Treatments
4.3. Relationship between NRE and N Partitioning within Leaves of Q. mongolica and A. mono Seedlings under CO2 and N Treatments
4.4. Response of NRE with N2 Fixer and Non-N2 Fixer (Q. mongolica and A. mono) to CO2 and N Treatments
4.5. Phtosynthetic Responses of Q. mongolica and A. mono Seedlings under CO2 and N Treatments
4.6. Evaluation Method of N Resorption Efficiency
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate change 2007: Impacts, adaptation and vulnerability. In Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- IPCC. Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- van Vuuren, D.P.; Edmonds, J.; Kainuma, M.; Riahi, K.; Thomson, A.; Hibbard, K.; Hurtt, G.C.; Kram, T.; Krey, V.; Lamarque, J.F.; et al. The representative concentration pathways: An overview. Clim. Chang. 2011, 109, 5–31. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Lemonnier, P.; Wedow, J.M. The influence of rising tropospheric carbon dioxide and ozone on plant productivity. Plant Biol. 2020, 22, 5–11. [Google Scholar] [CrossRef]
- Norby, R.J.; Warren, J.M.; Iversen, C.M.; Medlyn, B.E.; McMurtrie, R.E. CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proc. Natl. Acad. Sci. USA 2010, 107, 19368–19373. [Google Scholar] [CrossRef]
- Zak, D.R.; Pregitzer, K.S.; Kubiske, M.E.; Burton, A.J. Forest productivity under elevated CO2 and O3: Positive feedbacks to soil N cycling sustain decade-long net primary productivity enhancement by CO2. Ecol. Lett. 2011, 14, 1220–1226. [Google Scholar] [CrossRef][Green Version]
- Sigurdsson, B.D.; Medhurst, J.L.; Wallin, G.; Eggertsson, O.; Linder, S. Growth of mature boreal Norway spruce was not affected by elevated [CO2] and/or air temperature unless nutrient availability was improved. Tree Physiol. 2013, 33, 1192–1205. [Google Scholar] [CrossRef]
- Walker, A.P.; De Kauwe, M.G.; Medlyn, B.E.; Zaehle, S.; Iversen, C.M.; Asao, S.; Guenet, B.; Harper, A.; Hickler, T.; Hungate, B.A.; et al. Decadal biomass increment in early secondary succession woody ecosystems is increased by CO2 enrichment. Nat. Commun. 2019, 10, 454. [Google Scholar] [CrossRef] [PubMed]
- Terrer, C.; Jackson, R.B.; Prentice, I.C.; Keenan, T.F.; Kaiser, C.; Vicca, S.; Fisher, J.B.; Reich, P.B.; Stocker, B.D.; Hungate, B.A.; et al. Nitrogen and phosphorus constrain the CO2 fertilization of global plant biomass. Nat. Clim. Chang. 2019, 9, 684–689. [Google Scholar] [CrossRef]
- Klein, T.; Bader, M.K.-F.; Leuzinger, S.; Mildner, M.; Schleppi, P.; Slegwolf, R.T.W.; Körner, C. Growth and carbon relations of mature Picea abies trees under 5 years of free-air CO2 enrichment. J. Ecol. 2016, 104, 1720–1733. [Google Scholar] [CrossRef]
- Terrer, C.; Vicca, S.; Stocker, D.; Hungate, B.A.; Phillips, R.P.; Reich, P.B.; Finzi, A.C.; Prentice, I.C. Ecosystem responses to elevated CO2 governed by plant–soil interactions and the cost of nitrogen acquisition. New Phytol. 2018, 217, 507–522. [Google Scholar] [CrossRef]
- Yoon, T.K.; Noh, N.J.; Lee, S.H.J.; Son, Y. Soil moisture effects on leaf litter decomposition and soil carbon dioxide efflux in wetland and upland forests. Soil Sci. Soc. Am. J. 2014, 78, 1804–1816. [Google Scholar] [CrossRef]
- Parsons, W.F.J.; Bockheim, J.G.; Lindroth, R.L. Independent, interactive, and species-specific responses of leaf litter decomposition to elevated CO2 and O3 in a northern hardwood forest. Ecosystems 2008, 11, 505–519. [Google Scholar] [CrossRef]
- Liu, L.L.; King, J.S.; Booker, F.L.; Giardiana, C.P. Enhanced litter input rather than changes in litter chemistry drive soil carbon and nitrogen cycles under elevated CO2: A microcosm study. Glob. Chang. Biol. 2009, 15, 441. [Google Scholar] [CrossRef]
- Cha, S.; Chae, H.M.; Lee, S.H.; Shim, J.K. Effect of elevated atmospheric CO2 concentration on growth and leaf litter decomposition of Quercus actissima and Fraxinus rhynchophylla. PLoS ONE 2017, 12, e01171197. [Google Scholar] [CrossRef]
- Norby, R.J.; Cotrufo, M.F.; Ineson, P.; O’Neill, E.G.; Canadell, J.G. Elevated CO2, litter chemistry, and decomposition: A synthesis. Oecologia 2001, 127, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, M.F.; Ineson, P.; Scott, A. Elevated CO2 reduces the nitrogen concentration of plant tissues. Glob. Chang. Biol. 2010, 4, 43–54. [Google Scholar] [CrossRef]
- Strain, B.R.; Bazzaz, F.A. Terrestrial plant communities. In CO2 and Plants; Lemon, E.R., Ed.; Westview Press: Boulder, CO, USA, 1983; pp. 177–222. [Google Scholar]
- Curtis, P.S.; Wang, X. A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 1998, 113, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Kasurinen, A.; Peltonen, P.; Julkunen-Tiitto, R.; Apaavuori, E.; Nuutinen, V.; Holopainen, T.; Holopainen, J. Effects of elevated CO2 and O3 on leaf litter phenolics and subsequent performance of litter-feeding soil macrofauna. Plant Soil 2007, 292, 25–43. [Google Scholar] [CrossRef]
- Vizoso, S.; Gerant, D.; Guehl, J.M.; Joffre, R.; Chalot, M.; Gross, P.; Maillard, P. Do elevated CO2 concentration and nitrogen fertilization alter storage and remobilization of carbon and nitrogen in pedunculate oak saplings? Tree Physiol. 2008, 28, 1729–1739. [Google Scholar] [CrossRef]
- Norby, R.J.; Long, T.M.; Hartz-Rubin, J.S.; O’Neill, E.G. Nitrogen resorption in senescing tree leaves in a warmer, CO2-enriched atmosphere. Plant Soil 2000, 224, 15–29. [Google Scholar] [CrossRef]
- Li, L.; Manning, W.; Wang, X. Elevated CO2 increased root mass and leaf nitrogen resorption in red maple (Acer rubrum L.). Forests 2019, 10, 420. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Briones, M.A.J.I.; Ineson, P. Elevated CO2 affects field decomposition rate and palatability of tree leaf litter: Importance of changes in substrate quality. Soil Biol. Biochem. 1998, 30, 1565–1571. [Google Scholar] [CrossRef]
- Parsons, W.F.J.; Lindroth, R.I.; Bockheim, J.G. Decomposition of Betula papyrifera leaf litter under the independent and interactive effects of elevated CO2 and O3. Glob. Chang. Biol. 2004, 10, 1666–1677. [Google Scholar] [CrossRef]
- Liu, L.L.; King, J.S.; Giardiana, C.P. Effects of elevated atmospheric CO2 and tropospheric O3 on leaf litter production and chemistry in trembling aspen and paper birch ecosystems. Tree Physiol. 2005, 15, 1511–1522. [Google Scholar] [CrossRef]
- Vitousek, P.M. Nutrient cycling and nutrient use efficiency. Am. Nat. 1982, 4, 553–572. [Google Scholar] [CrossRef]
- Aerts, R.; Chapin, F.S., III. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv. Ecol. Res. 2000, 30, 1–67. [Google Scholar]
- Killingbeck, K. Nutrients in senesced leaves: Keys to the search for potential resorption and resorption proficiency. Ecology 1996, 77, 1716–1727. [Google Scholar] [CrossRef]
- Van Heerwaarden, L.M.; Toet, S.; Aerts, R. Nitrogen and phosphorus resorption efficiency and proficiency in six-arctic bog species after 4 years of nitrogen fertilization. J. Ecol. 2003, 91, 1060–1070. [Google Scholar] [CrossRef]
- Maeda, Y.; Tashiro, N.; Enoki, T.; Urakawa, R.; Hishi, T. Effects of species replacement on the relationship between net primary production and soil nitrogen availability along a topographical gradient: Comparison of belowground allocation and nitrogen use efficiency between natural forests and plantations. For. Ecol. Manag. 2018, 422, 214–222. [Google Scholar] [CrossRef]
- Li, L.-H.; Dijkstra, P.; Hymus, G.J.; Wheeler, R.M.; Piastuch, W.C.; Hinkle, C.R.; Drake, B.G. Leaf senescence of Quercus myrtifolia as affected by long-term CO2 enrichment in its native environment. Glob. Chang. Biol. 2000, 6, 727–733. [Google Scholar] [CrossRef]
- Mediavilla, S.; García-Iglesias, J.; González-Zurdo, P.; Escudero, A. Nitrogen resorption efficiency in mature trees and seedlings of four tree species co-occurring in a Mediterranean environment. Plant Soil 2014, 385, 205–215. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, J.; Yang, H.; Zhang, X.; Luo, C.; Zhao, Y. Resorption of nitrogen, phosphorus and potassium from leaves of lucerne stands of different ages. Plant Soil 2014, 383, 301–312. [Google Scholar] [CrossRef]
- Deng, J.; Wang, S.; Ren, C.; Zhang, W.; Zhao, F.; Li, X.; Zhang, D.; Han, X.; Yang, G. Nitrogen and phosphorus resorption in relation to nutrition limitation along the chronosequence of black locust (Robinia pseudoacacia L.) plantation. Forests 2019, 10, 261. [Google Scholar] [CrossRef]
- Vergutz, L.; Manzonl, S.; Porporato, A.; Novals, R.F.; Jackson, R.B. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol. Monogr. 2012, 82, 205–220. [Google Scholar] [CrossRef]
- Jiang, D.; Gzeng, Q.; Li, Q.; Luo, Y.; Vogel, J.; Shi, Z.; Ruan, H.; Xu, X. Nitrogen and phosphorus resorption in planted forests worldwide. Forests 2019, 10, 201. [Google Scholar] [CrossRef]
- Jiang, D.; Yang, B.; Cheng, X.; Chen, H.Y.H.; Ruan, H.; Xu, X. The stoichiometry of leaf nitrogen and phosphorus resorption in plantation forests. For. Ecol. Manag. 2021, 483, 2021. [Google Scholar] [CrossRef]
- Arp, W.J. Effects of source–sink relations on photosynthetic acclimation to elevated CO2. Plant Cell Environ. 1991, 14, 869–875. [Google Scholar] [CrossRef]
- Pugnaire, F.I.; Chapin, F.S., III. Controls over nutrient resorption from leaves of evergreen Mediterranean species. Ecology 1993, 74, 124–129. [Google Scholar] [CrossRef]
- Norby, R.J.; Wullschleger, S.D.; Gjunderson, C.A.; Johnson, D.W.; Ceulemans, R. Tree responses to rising CO2 in field experiments: Implications for the future forest. Plant Cell Environ. 1999, 22, 683–714. [Google Scholar] [CrossRef]
- Norby, R.J.; Iversen, C.M. Nitrogen uptake, distribution, turnover, and efficiency of use in a CO2-enriched sweetgum forest. Ecology 2006, 87, 5–14. [Google Scholar] [CrossRef]
- Finzi, A.C.; Allen, A.S.; DeLucia, E.H.; Ellsworth, D.S.; Schlesinger, W.H. Forest litter production, chemistry, and decomposition following two years of free-air CO2 enrichment. Ecology 2001, 82, 470–484. [Google Scholar]
- Billings, S.A.; Zitzer, S.F.; Weatherly, H.; Schaeffer, S.M.; Charlet, T.; Arnon, J.A., III; Evans, R.D. Effects of elevated carbon dioxide on green leaf tissue and leaf litter quality in an intact Mojave Desert ecosystem. Glob. Chang. Biol. 2003, 9, 729–735. [Google Scholar] [CrossRef]
- Housman, D.C.; Killingbeck, K.T.; Evans, R.D.; Charlet, T.N.; Smith, S.D. Foliar nutrient resorption in two Mojave Desert shrubs exposed to free-air CO2 enrichment (FACE). J. Arid Environ. 2012, 78, 26–32. [Google Scholar] [CrossRef]
- Calfapietra, C.; Angelis, P.D.; Gielen, B.; Lukac, M.; Moscatelli, M.C.; Avino, G.; Lagomarsino, A.; Polle, A.; Ceulemans, R.; Mugnozza, G.S.; et al. Increased nitrogen-use efficiency of a short-rotation poplar plantation in elevated CO2 concentration. Tree Physiol. 2007, 27, 1153–1163. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, L.; Wang, X.; Manning, W.J. Effects of elevated CO2 on leaf senescence, leaf nitrogen resorption, and late-season photosynthesis in Tilia americana L. Front Plant Sci. 2019, 10, 1217. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Field, C. Allocation leaf nitrogen for the maximization of carbon gain: Leaf age as a control on the allocation program. Oecologia 1983, 56, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Mae, T. Leaf senescence and nitrogen metabolism. In Plant Cell Death Process; Noodén, L.D., Ed.; Elsevier Academic Press: San Diego, CA, USA, 2004; pp. 157–168. [Google Scholar]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Riding atmospheric carbon dioxide: Plants FACE the future. Ann. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.; Ainsworth, E.A. The response of foliar carbohydrates to elevated carbon dioxide concentration. In Managed Ecosystems and CO2. Case Studies, Processes and Perspectives; Nörsberger, J., Long, S.P., Norby, R.J., Stitt, M., Hendrey, G.R., Blum, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 293–308. [Google Scholar]
- Koike, T.; Watanabe, M.; Watanabe, Y.; Agathokleous, E.; Eguchi, N.; Takagi, K.; Satoh, F.; Kitaoka, S.; Funada, R. Ecophysiology of deciduous trees native to Northeast Asia grown under FACE (Free Air CO2 Enrichment). J. Agric. Meteorol. 2015, 71, 174–184. [Google Scholar] [CrossRef]
- Yasumura, Y.; Hikosaka, K.; Hirose, T. Resource allocation to vegetative and reproductive growth in relation to mast seedlings in Fagus crenata. For. Ecol. Manag. 2006, 229, 228–233. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A.; Nelson, R.; Long, S.P. Testing the “source–sink” hypothesis of down-regulation of photosynthesis in elevated [CO2] in the field with single gene substitutions in glycine max. Agric. For. Meteorol. 2004, 122, 85–94. [Google Scholar] [CrossRef]
- Temperton, V.M.; Grayston, S.J.; Jackson, G.; Barton, C.V.M.; Millard, P.; Jarvis, P.G. Effects of elevated carbon dioxide concentration on growth and nitrogen fixation in Alnus glutinosa in a long-term field experiment. Tree Physiol. 2003, 23, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.S.; Ellsworth, D.S.; Smith, S.D. Functional responses of plants to elevated atmospheric CO2—Do photosynthetic and productivity data from FACE experiments support early predictions? New Phytol. 2004, 162, 253–280. [Google Scholar] [CrossRef]
- Tobita, H.; Kitao, M.; Koike, T.; Maruyama, Y. Effects of elevated CO2 and nitrogen availability on nodulation of Alnus hirsuta Turcz. Phyton 2005, 45, 125–131. [Google Scholar]
- Tobita, H.; Uemura, A.; Kitao, M.; Kitaoka, S.; Maruyama, Y.; Utsugi, H. Effects of elevated atmospheric carbon dioxide, soil nutrients and water conditions on photosynthetic and growth responses of Alnus hirsuta. Funct. Plant Biol. 2011, 38, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Koike, T. Leaf structure and photosynthetic performance as related to the forest succession of deciduous broad-leaved trees. Plant Species Biol. 1988, 3, 77–87. [Google Scholar] [CrossRef]
- Ishizuka, M.; Sugawara, S.; Kanazawa, Y. The relationship between diffused light intensity under canopy and leader growth of some hardwoods saplings. Trans. Mtg. Hokkaido Br. Jpn. For. Soc. 1988, 36, 48–50. [Google Scholar]
- Kitao, M.; Lei, T.T.; Koike, T.; Tobita, H.; Maruyama, Y. Tradeoff between shade adaptation and mitigation of photoinhibition in leaves of Quercus mongolica and Acer mono acclimated to deep shade. Tree Physiol. 2006, 26, 441–448. [Google Scholar] [CrossRef]
- Terasawa, K.; Usui, G.; Gosyu, K. Relationship between site and stand structure of mizunara oak (Quercus mongolica var. grosseserrata) secondary forests in central Hokkaido. Bull. Hokkaido For. Exp. Stn. 1989, 26, 97–106. [Google Scholar]
- Tomioka, E.; Amano, Y. Studies on the base-status of soils in northern-east Hokkaido. Res. Ball. Hokkaido Natul. Agric. Exp. Stn. 1978, 123, 79–87. [Google Scholar]
- Usui, G. An environmental study on the coastal natural stands to establish the disaster prevention forests in north west Hokkaido. Bull. Hokkaido For. Res. Inst. 1990, 28, 1–52. (In Japanese) [Google Scholar]
- Watanabe, Y.; Tobita, H.; Kitao, M.; Maruyama, Y.; Choi, D.; Sasa, K.; Funada, R.; Koike, T. Effects of elevated CO2 and nitrogen on wood structure related to water transport in seedlings of two deciduous broad-leaved tree species. Trees 2008, 22, 403–411. [Google Scholar] [CrossRef]
- Asher, C.J.; Edwards, D.G. Modern solution culture techniques. In Inorganic Plant Nutrition; Encyclopedia of Plant Physiology (New Series); Läuchli, A., Bieleski, R.L., Eds.; Springer: Berlin/Heidelberg, Germany, 1983; Volume 15, pp. 94–119. [Google Scholar]
- R Core Team. 2020 R: A Language and Environment for Statistical Computing, 4.0.3 ed.; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Duursma, R.A. Plantecophys—An R Package for analysing and modelling Leaf Gas Exchange Data. PLoS ONE 2015, 10, e0143346. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic acclimation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef]
- Dreyer, E.; Roux, X.L.; Montpied, P.; Daudet, F.; Masson, F. Temperature response of leaf photosynthetic capacity in seedlings from seven temperate tree species. Tree Physiol. 2001, 214, 223–232. [Google Scholar] [CrossRef]
- Kabeya, D.; Sakai, A.; Matsui, K.; Sakai, S. Resprouting ability of Quercus crispula seedlings depends on the vegetation cover of their microhabitats. J. Plant Res. 2003, 116, 207–216. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Tenhunen, J.D. A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant Cell Environ. 1997, 20, 845–866. [Google Scholar] [CrossRef]
- Jordan, D.B.; Ogren, W.L. The CO2/O2 specificity of ribulose 1,5-bisphosphate carboxylase/oxygenase. Planta 1984, 161, 308–313. [Google Scholar] [CrossRef]
- Evans, J.R.; Seeman, J.R. The allocation of protein nitrogen in the photosynthetic apparatus: Costs, consequences, and control. In Photosynthesis; Brigs, W.R., Alan, R., Eds.; Liss Press: New York, NY, USA, 1989; pp. 183–205. [Google Scholar]
- Frak, E.; LeRoux, X.; Millard, P.; Dreyer, E.; Jaouen, G.; Saint-Joanis, B.; Wendler, R. Changes in total leaf nitrogen and partitioning of leaf nitrogen drive photosynthetic acclimation to light in fully developed walnut leaves. Plant Cell Environ. 2001, 24, 1279–1288. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Tenhunen, J.D. Within-canopy variation in the rate of development of photosynthetic capacity is proportional to integrated quantum flux density in temperate deciduous trees. Plant Cell Environ. 2004, 27, 293–313. [Google Scholar] [CrossRef]
- Tateno, R.; Kawaguchi, H. Differences in nitrogen use efficiency between leaves from canopy and subcanopy trees. Ecol. Res. 2002, 17, 695–704. [Google Scholar] [CrossRef]
- Wang, W.Q.; Wang, M.; Lin, P. Seasonal changes in element contents in mangrove element retranslocation during leaf senescence. Plant Soil 2003, 252, 187–193. [Google Scholar] [CrossRef]
- Aerts, R.; Cornelissen, J.H.C.; van Loghestijn, R.S.P.; Callaghan, T.V. Climate change has only a minor impact on nutrient resorption parameters in a high-latitude peatland. Oecologia 2007, 151, 132–139. [Google Scholar] [CrossRef]
- Parker, G.G. Throughfall and stemflow in the forest nutrient cycle. Adv. Ecol. Res. 1983, 13, 57–133. [Google Scholar]
- Dray, M.W.; Crowther, T.W.; Thomas, S.M.; A’Bear, A.D.; Godbold, D.L. Effects of elevated CO2 on litter chemistry and subsequent invertebrate detritivore feeding responses. PLoS ONE 2014, 9, e86246. [Google Scholar] [CrossRef]
- Chapman, J.A.; King, J.S.; Pregitzer, K.S.; Zak, D.R. Effects of elevated concentrations of atmospheric CO2 and tropospheric O3 on decomposition of tree fine roots. Tree Physiol. 2005, 25, 1501–1510. [Google Scholar] [CrossRef]
- Gill, R.A.; Anderson, L.J.; Polley, H.W.; Johnson, H.B.; Jackson, R.B. Potential nitrogen constraints on soil carbon sequestration under low and elevated atmospheric CO2. Ecology 2006, 87, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.C.; Stiling, P.; Moon, D.C.; Drake, B.G.; Hunter, M.D. Effects of elevated CO2 and herbivore damage on litter quality in a scrub oak ecosystem. J. Chem. Ecol. 2005, 31, 2343–2356. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S., III; Kedrowski, R.A. Seasonal changes in nitrogen and phosphorus fractions and autumn retranslocation in evergreen and deciduous taiga trees. Ecology 1983, 64, 376–391. [Google Scholar] [CrossRef]
- Crous, K.Y.; Wujeska-Klause, A.; Jiang, M.; Medlyn, B.E.; Ellsworth, D.S. Nitrogen and phosphorus retranslocation of leaves and stemwood in a mature Eucalyptus forest exposed to 5 years of elevated CO2. Front. Plant Sci. 2019, 10, 664. [Google Scholar] [CrossRef]
- Tateno, R.; Hishi, T.; Takeda, H. Above- and belowground biomass and net primary production in a cool-temperate deciduous forest in relation to topographical changes in soil nitrogen. For. Ecol. Manag. 2004, 193, 297–306. [Google Scholar] [CrossRef]
- Aerts, R. Nutrient Resorption from Senescing Leaves of Perennials: Are there General Patterns? J. Ecol. 1996, 84, 597–608. [Google Scholar] [CrossRef]
- Charles-Edwards, D.A.; Stutzel, H.; Ferraris, R.; Beech, D.F. An analysis of spatial variation in the nitrogen content of leaves from different horizons within a canopy. Ann. Bot. 1987, 60, 421–426. [Google Scholar] [CrossRef]
- O’Neill, E.G.; Norby, R.J. Litter quality and decomposition rates of foliar litter produced under CO2 enrichment. In Carbon Dioxide and Terrestrial Ecosystems; Koch, G.W., Mooney, H.A., Eds.; Academic Press: San Diego, CA, USA, 1996; pp. 87–103. [Google Scholar]
- Hikosaka, K. A model of dynamics of leaves and nitrogen in a plant canopy: An integration of canopy photosynthesis, leaf life span, and nitrogen use efficiency. Am. Nat. 2003, 162, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü. Distribution patterns of foliar carbon and nitrogen as affected by tree dimensions and relative light conditions in the canopy of Picea abies. Trees 1997, 11, 144–154. [Google Scholar]
- Onoda, Y.; Hikosaka, K.; Hirose, T. Allocation of nitrogen to cell walls decreases photosynthetic nitrogen use efficiency. Funct. Ecol. 2004, 18, 419–425. [Google Scholar] [CrossRef]
- Takashima, T.; Hikosaka, K.; Hirose, T. Photosynthesis or persistence: Nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ. 2004, 27, 1047–1054. [Google Scholar] [CrossRef]
- Hikosaka, K. Interspecific difference in the photosynthesis-nitrogen relationship: Patterns, physiological causes, and ecological importance. J. Plant Res. 2004, 117, 481–494. [Google Scholar] [CrossRef]

| Characteristics | Species | High N | Low N | Source of Variance | ||||
|---|---|---|---|---|---|---|---|---|
| Ambient | Elevated | Ambient | Elevated | CO2 | N | CO2 × N | ||
| LMA (g m−2) | Quercus | 64.3 (1.7) | 71.3 (2.1) | 63.4 (1.7) | 78.9 (2.8) | 25.3 *** | 2.9 | 4.2 |
| Acer | 42.7 (1.2) | 51.2 (2.6) | 41.2 (1.5) | 48.3 (2.8) | 11.8 ** | 1.0 | 0.09 | |
| Nmass (mg g−1) | Quercus | 29.8 (1.3) | 23.6 (1.4) | 10.8 (0.4) | 8.4 (0.3) | 12.8 ** | 286.0 *** | 3.6 |
| Acer | 26.1 (2.0) | 21.7 (1.1) | 20.6 (1.5) | 17.0 (1.2) | 7.9 * | 13.3 ** | 0.09 | |
| Narea (g m−2) | Quercus | 1.9 (0.10) | 1.7 (0.10) | 0.68 (0.03) | 0.66 (0.04) | 1.2 | 192.8 *** | 2.1 |
| Acer | 1.1 (0.09) | 1.1 (0.03) | 0.85 (0.08) | 0.81 (0.05) | 0.17 | 18.4 *** | 0.04 | |
| SPAD value | Quercus | 42.0 (1.3) | 41.9 (1.4) | 21.1 (1.6) | 20.8 (1.0) | 0.73 | 247.8 *** | 0.003 |
| Acer | 33.1 (0.6) | 29.7 (1.1) | 30.4 (1.7) | 27.8 (1.4) | 5.4 * | 3.3 | 0.13 | |
| TNC (%) | Quercus | 7.6 (1.3) | 12.6 (2.7) | 15.4 (1.5) | 20.1 (1.6) | 4.9 * | 19.4 *** | 0.004 |
| Acer | 7.8 (1.4) | 13.1 (2.6) | 7.5 (1.4) | 14.3 (2.8) | 10.6 ** | 0.06 | 0.15 | |
| Characteristics | Species | High N | Low N | Source of Variance | ||||
|---|---|---|---|---|---|---|---|---|
| Ambient | Elevated | Ambient | Elevated | CO2 | N | CO2 × N | ||
| Fr | Quercus | 0.29 (0.01) | 0.26 (0.01) | 0.33 (0.01) | 0.26 (0.02) | 17.2 *** | 1.3 | 1.1 |
| Acer | 0.30 (0.01) | 0.22 (0.02) | 0.40 (0.02) | 0.31 (0.03) | 17.6 *** | 18.8 *** | 0.005 | |
| Fb | Quercus | 0.050 (0.002) | 0.047 (0.002) | 0.054 (0.002) | 0.051 (0.003) | 4.4 * | 2.6 | 0.12 |
| Acer | 0.066 (0.004) | 0.048 (0.003) | 0.074 (0.003) | 0.065 (0.005) | 12.0 ** | 10.2 ** | 1.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tobita, H.; Kitao, M.; Uemura, A.; Utsugi, H. Species-Specific Nitrogen Resorption Efficiency in Quercus mongolica and Acer mono in Response to Elevated CO2 and Soil N Deficiency. Forests 2021, 12, 1034. https://doi.org/10.3390/f12081034
Tobita H, Kitao M, Uemura A, Utsugi H. Species-Specific Nitrogen Resorption Efficiency in Quercus mongolica and Acer mono in Response to Elevated CO2 and Soil N Deficiency. Forests. 2021; 12(8):1034. https://doi.org/10.3390/f12081034
Chicago/Turabian StyleTobita, Hiroyuki, Mitsutoshi Kitao, Akira Uemura, and Hajime Utsugi. 2021. "Species-Specific Nitrogen Resorption Efficiency in Quercus mongolica and Acer mono in Response to Elevated CO2 and Soil N Deficiency" Forests 12, no. 8: 1034. https://doi.org/10.3390/f12081034
APA StyleTobita, H., Kitao, M., Uemura, A., & Utsugi, H. (2021). Species-Specific Nitrogen Resorption Efficiency in Quercus mongolica and Acer mono in Response to Elevated CO2 and Soil N Deficiency. Forests, 12(8), 1034. https://doi.org/10.3390/f12081034






