Allometry and Post-Drought Growth Resilience of Pedunculate Oak (Quercus robur L.) Varieties
Abstract
1. Introduction
Aim
2. Materials and Methods
2.1. Site Description
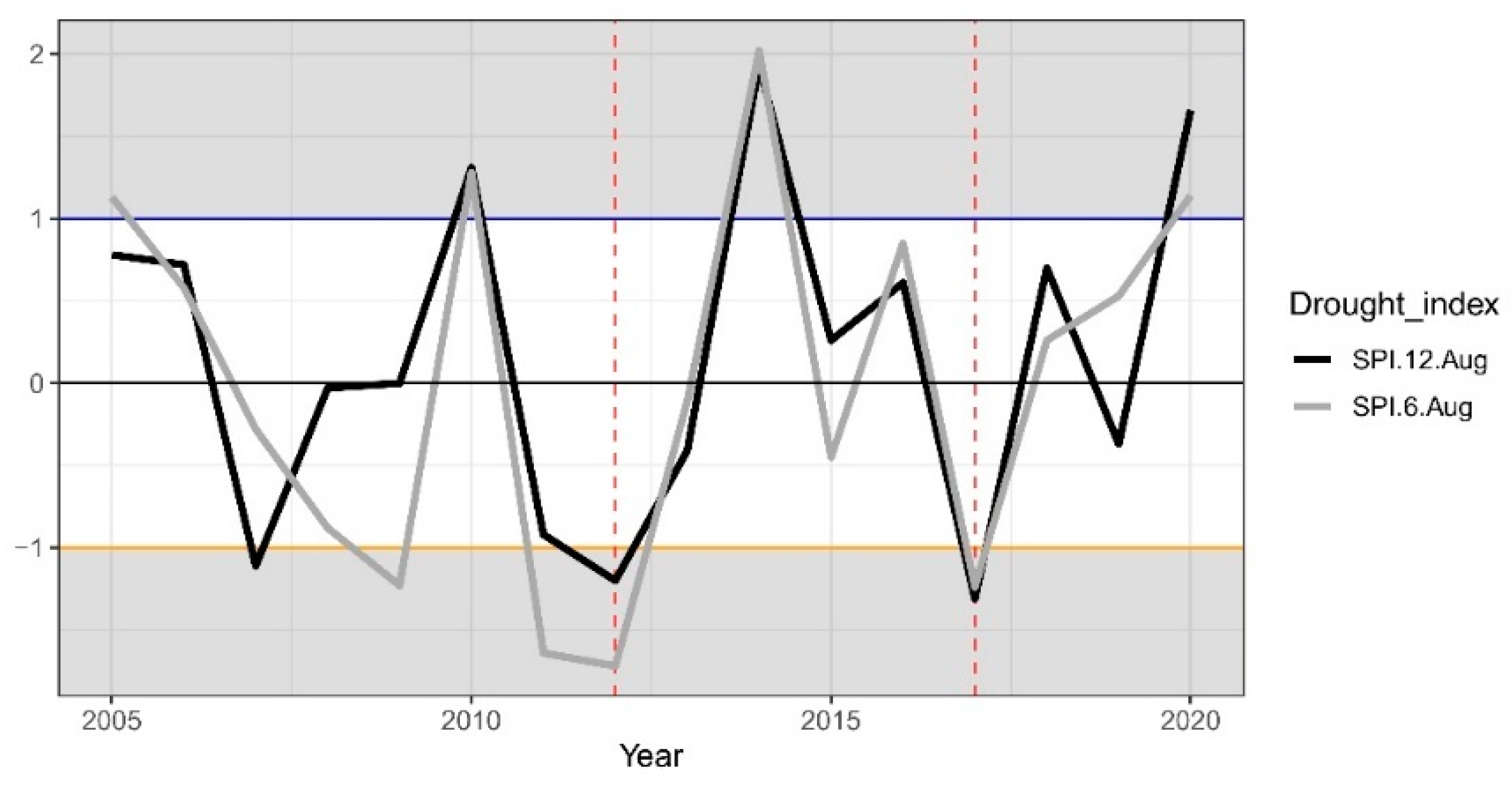
2.1.1. Climate Conditions and Drought Events
2.1.2. Trial Setup
2.2. Dendrochronological and Biometric Analyses
2.3. Statistical Analyses
3. Results
3.1. Climate Conditions, Stand Characteristics, and Drought Events
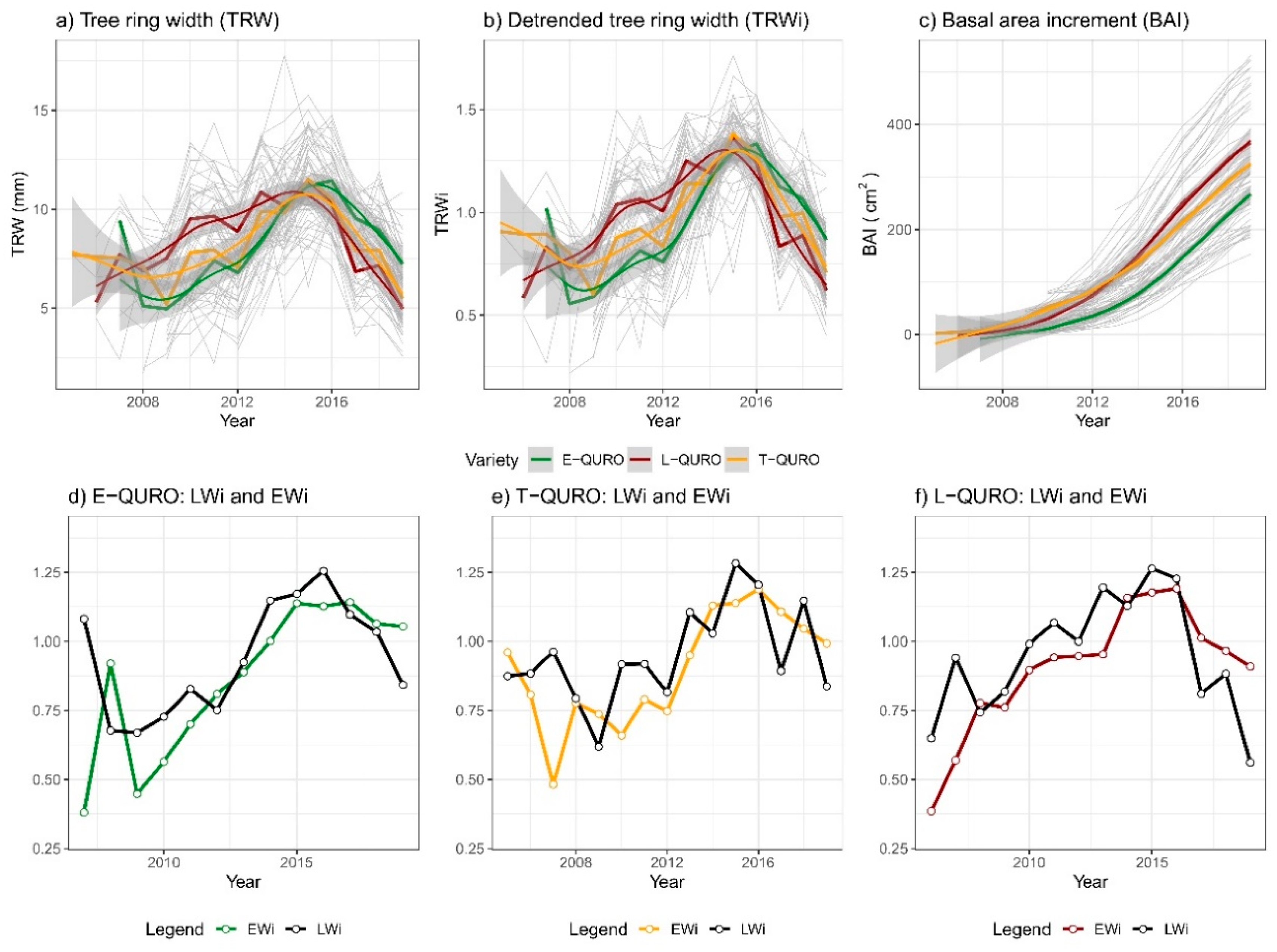
3.2. Radial Growth Performance
3.3. Early and Late Wood Characteristics
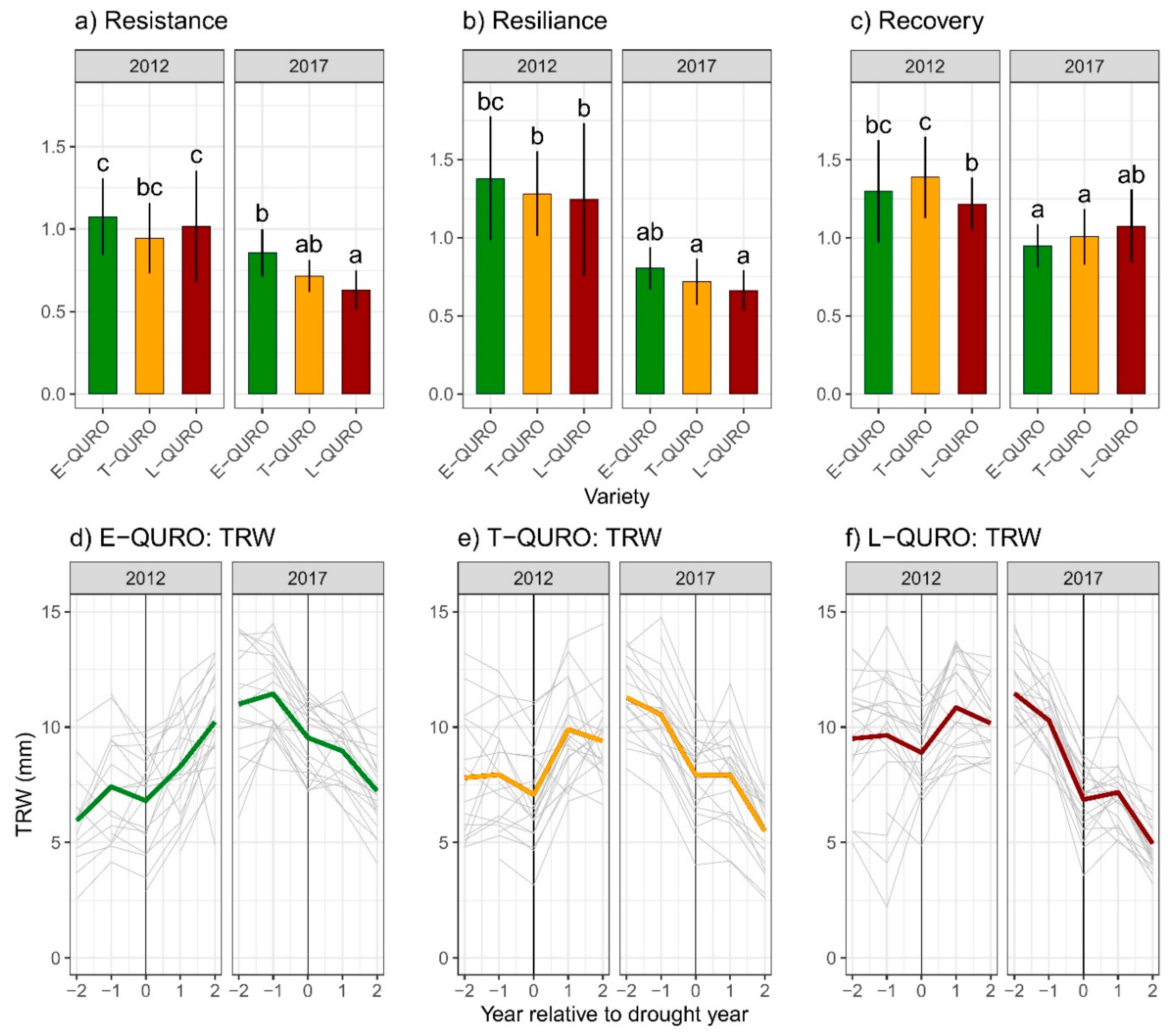
3.4. Drought Resistance, Resilience, and Recovery Potential
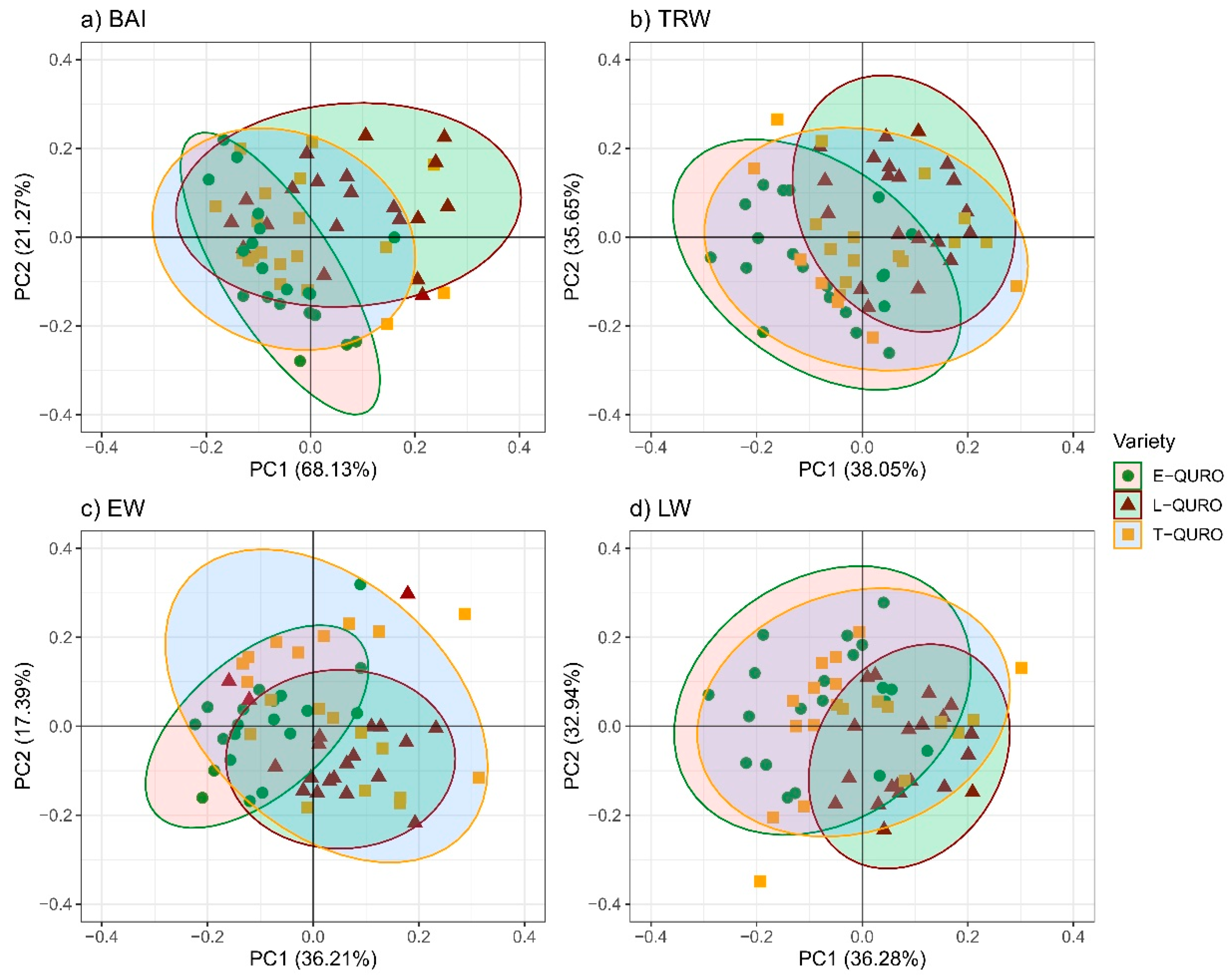
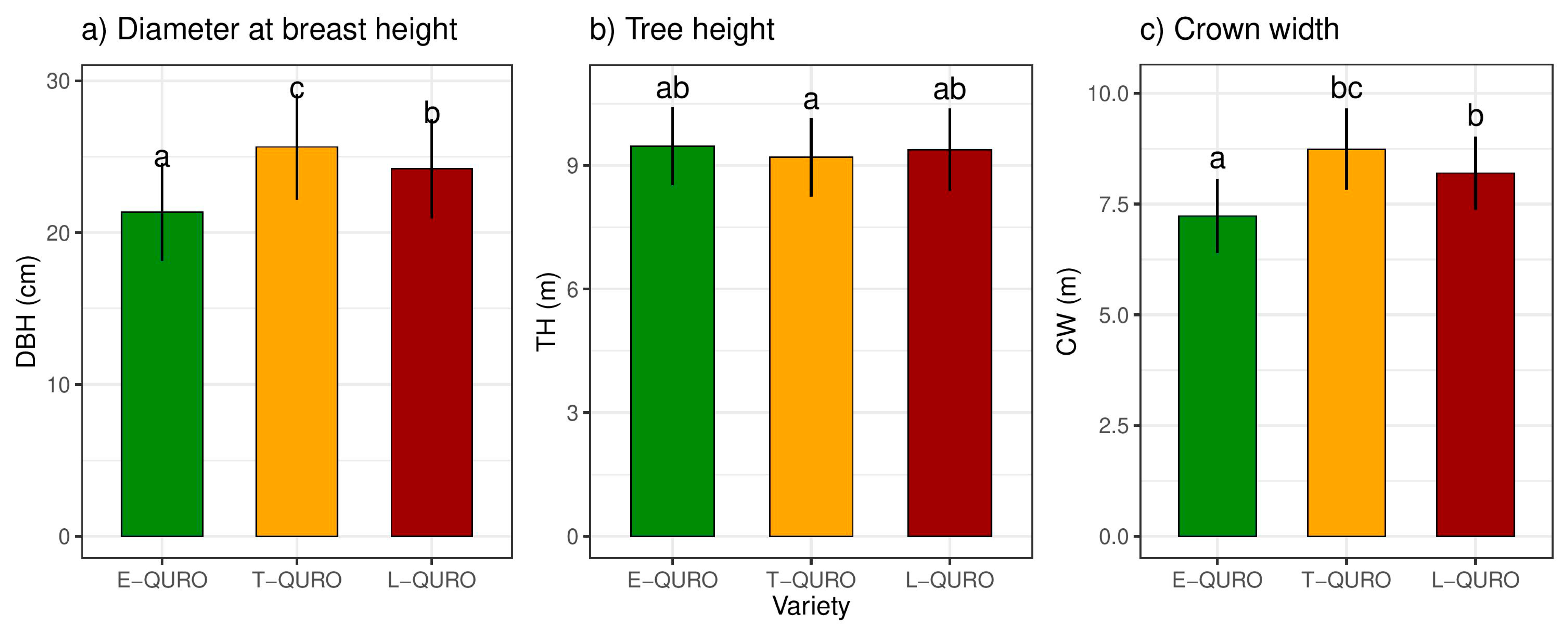
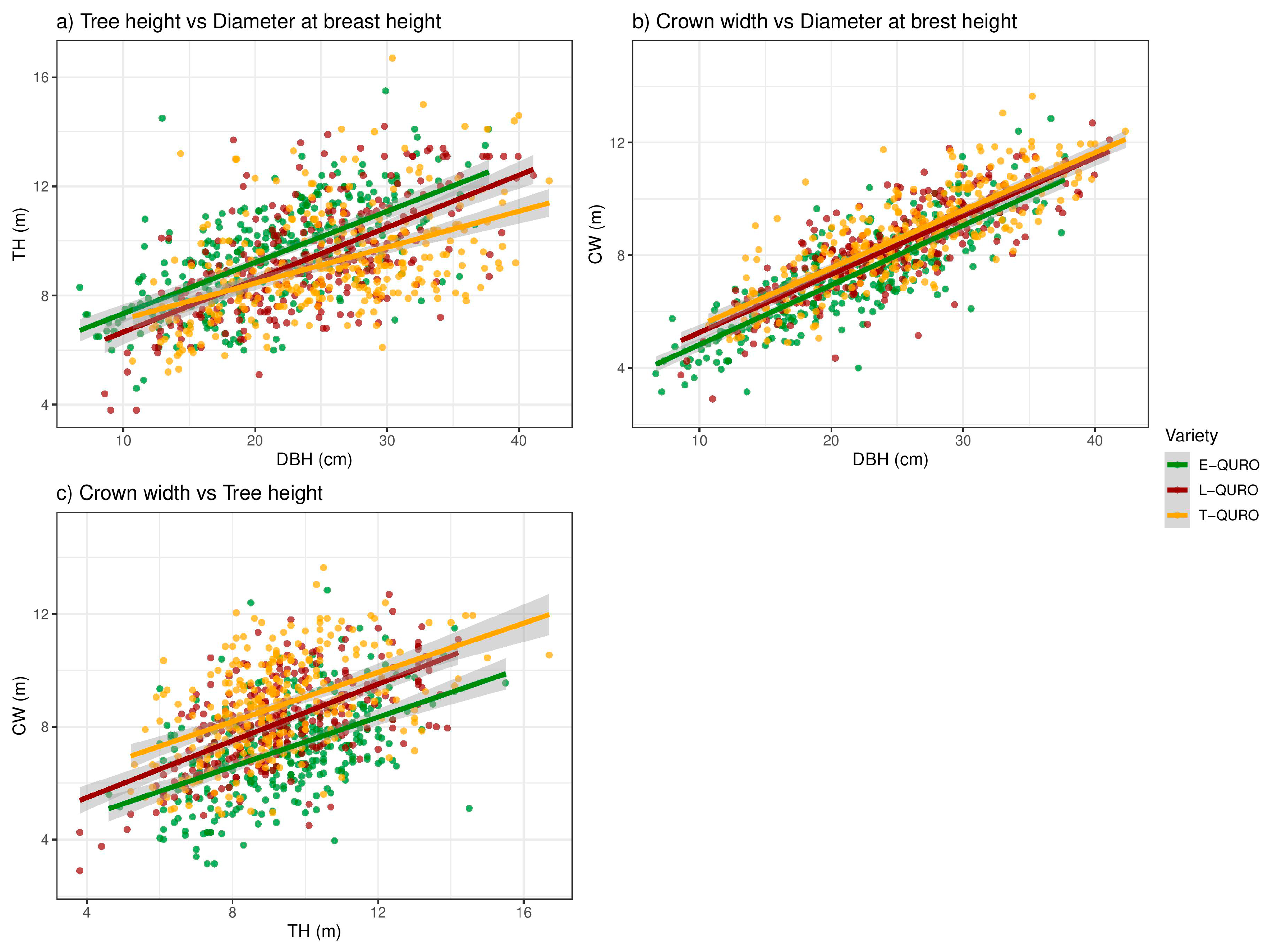
3.5. Tree Dimensions and Allometry
4. Discussion
4.1. Intra-Variety Deviations in Radial Growth Dynamics
4.2. Drought Sensitivity of Different QURO Varieties during Juvenile and Mature Tree Growth Phases
4.3. QURO Inter-Variety Deviations in Tree Dimensions and Allometry
4.4. Perspective of Implications Phenological QURO Varieties for Climate-Resistant Forestry
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santopuoli, G.; Temperli, C.; Alberdi, I.; Barbeito, I.; Bosela, M.; Bottero, A.; Klopčič, M.; Lesinski, J.; Panzacchi, P.; Tognetti, R. Pan-European sustainable forest management indicators for assessing Climate-Smart Forestry in Europe. Can. J. For. Res. 2021, 51, 999. [Google Scholar] [CrossRef]
- Jandl, R.; Ledermann, T.; Kindermann, G.; Freudenschuss, A.; Gschwantner, T.; Weiss, P. Strategies for climate-smart forest management in Austria. Forests 2018, 9, 592. [Google Scholar] [CrossRef]
- Williams, M.I.; Dumroese, R.K. Preparing for climate change: Forestry and assisted migration. J. For. 2013, 111, 287–297. [Google Scholar] [CrossRef]
- Bowditch, E.; Santopuoli, G.; Binder, F.; del Río, M.; La Porta, N.; Kluvankova, T.; Lesinski, J.; Motta, R.; Pach, M.; Panzacchi, P.; et al. What is Climate-Smart Forestry? A definition from a multinational collaborative process focused on mountain regions of Europe. Ecosyst. Serv. 2020, 43, 101113. [Google Scholar] [CrossRef]
- Buras, A.; Sass-Klaassen, U.; Verbeek, I.; Copini, P. Provenance selection and site conditions determine growth performance of pedunculate oak. Dendrochronologia 2020, 61, 125705. [Google Scholar] [CrossRef]
- Orlović, S.; Šimunovački, D.; Djorđević, Z.; Pilipović, A.; Radosavljević, N. Očuvanje Genofonda I Proizvodnja Semena Hrasta Lužnjaka (Quercus robur L.). In 250 Godina Ravnog Srema; Vojvodinašume: Petrovaradin, Serbia, 2008; ISBN 978-86-906665-1-5. [Google Scholar]
- Ljubojević, M.; Sebolt, A.; Ognjanov, V.; Iezzoni, A. heritability of anatomical characteristics in cherry interspecific hybrids. J. Plant Growth Regul. 2021, 1–18. [Google Scholar] [CrossRef]
- Pellizzari, E.; Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Carrer, M. Wood anatomy and carbon-isotope discrimination support long-term hydraulic deterioration as a major cause of drought-induced dieback. Glob. Chang. Biol. 2016, 22, 2125–2137. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, D.B.; Levanič, T.; Matović, B.; Orlović, S. Growth decrease and mortality of oak floodplain forests as a response to change of water regime and climate. Eur. J. For. Res. 2015, 134, 555–567. [Google Scholar] [CrossRef]
- Zheng, W.; Gou, X.; Su, J.; Fan, H.; Yu, A.; Liu, W.; Deng, Y.; Manzanedo, R.D.; Fonti, P. Physiological and growth responses to increasing drought of an endangered tree species in Southwest China. Forests 2019, 10, 514. [Google Scholar] [CrossRef]
- Lévesque, M.; Walthert, L.; Weber, P. Soil nutrients influence growth response of temperate tree species to drought. J. Ecol. 2016, 104, 377–387. [Google Scholar] [CrossRef]
- Kostić, S.; Kesić, L.; Matović, B.; Orlović, S.; Srđan, S.; Stojanović, D.B. Soil properties are significant modifiers of pedunculate oak (Quercus robur L.) radial increment variations and their sensitivity to drought. Dendrochronologia 2021, 67, 125838. [Google Scholar] [CrossRef]
- Losseau, J.; Jonard, M.; Vincke, C. Pedunculate oak decline in southern Belgium: A long-term process highlighting the complex interplay among drought, winter frost, biotic attacks, and masting. Can. J. For. Res. 2020, 50, 380–389. [Google Scholar] [CrossRef]
- Camarero, J.J.; Colangelo, M.; Gazol, A.; Azorín-Molina, C. Drought and cold spells trigger dieback of temperate oak and beech forests in northern Spain. Dendrochronologia 2021, 66, 125812. [Google Scholar] [CrossRef]
- Mölder, A.; Sennhenn-Reulen, H.; Fischer, C.; Rumpf, H.; Schönfelder, E.; Stockmann, J.; Nagel, R.V. Success factors for high-quality oak forest (Quercus robur, Q. petraea) regeneration. For. Ecosyst. 2019, 6. [Google Scholar] [CrossRef]
- Banković, S.; Medarević, M.; Pantić, D.; Petrović, N. National Forest Inventory of Republic of Serbia; Orlović, S., Ed.; Ministry of Agriculture, Forestry and Water Management: Belgrade, Serbia, 2008.
- Stojanović, D.; Levanič, T.; Matović, B.; Bravo-Oviedo, A. Climate change impact on a mixed lowland oak stand in Serbia. Ann. Silvic. Res. 2015, 39, 94–99. [Google Scholar] [CrossRef]
- Euforgen Distribution Map of Pedunculate Oak (Quercus robur). Available online: http://www.euforgen.org (accessed on 15 May 2021).
- Josifović, M. Flora SR Serbia; Serbian Academy of Sciences and Arts: Belgrade, Serbia, 1977. [Google Scholar]
- Wenden, B.; Mariadassou, M.; Chmielewski, F.M.; Vitasse, Y. Shifts in the temperature-sensitive periods for spring phenology in European beech and pedunculate oak clones across latitudes and over recent decades. Glob. Chang. Biol. 2020, 26, 1808–1819. [Google Scholar] [CrossRef]
- Batos, B. Diversity of Pedunculate Oak (Quercus robur L.); Foundation Andrejević: Belgrade, Serbia, 2012; ISBN 1450-801X. [Google Scholar]
- Bobinac, M.; Batos, B.; Miljković, D.; Radulović, S. Polycyclism and phenological variability in the common oak (Quercus robur L.). Arch. Biol. Sci. 2012, 64, 97–105. [Google Scholar] [CrossRef]
- Batos, B. Population and Individual Variability of Chemical Markers-Flavonoid and Morpho-Anatomical Characteristics of the Pedunculate Oak (Quercus robur L.). Ph.D. Thesis, Unoversity of Novi Sad, Novi Sad, Serbia, 2012. [Google Scholar]
- Karazac, J. Kasni (pozni) hrast (Quercus pedunculata var. tardissima Simonkai). Šumarski List. 1898, 22, 41–53. [Google Scholar]
- Puchałka, R.; Koprowski, M.; Gričar, J.; Przybylak, R. Does tree-ring formation follow leaf phenology in pedunculate oak (Quercus robur L.)? Eur. J. For. Res. 2017, 136, 259–268. [Google Scholar] [CrossRef]
- Wesołowski, T.; Rowiǹski, P. Late leaf development in pedunculate oak (Quercus robur): An antiherbivore defence? Scand. J. For. Res. 2008, 23, 386–394. [Google Scholar] [CrossRef]
- Rubtsov, V.V.; Utkina, I.A. Adaptatsionnyye Reaktsii Duba na Defoliatsiyu; GRIF: Angarsk, Russia, 2008. [Google Scholar]
- Izdebski, K. Wstępne badania nad ekologią i rozmieszczeniem dębu szypułkowego (Quercus robur L.) w Polsce. Sylwan 1956, 11, 415–506. [Google Scholar]
- Koval, I.M.; Kostyashkin, D.C. The influence of climate and recreation on formation of layers of annual wood of early and late forms Quercus robur L. in Kharkiv Greenbelt. Sci. Bull. UNFU 2015, 25, 52–58. [Google Scholar]
- Utkina, I.A.; Rubtsov, V.V. Studies of phenological forms of pedunculate oak. Contemp. Probl. Ecol. 2017, 10, 804–811. [Google Scholar] [CrossRef]
- Takamatsu, S.; Braun, U.; Limkaisang, S.; Kom-un, S.; Sato, Y.; Cunnington, J.H. Phylogeny and taxonomy of the oak powdery mildew Erysiphe alphitoides sensu lato. Mycol. Res. 2007, 111, 809–826. [Google Scholar] [CrossRef] [PubMed]
- Solla, A.; Moreno, G.; Malewski, T.; Jung, T.; Klisz, M.; Tkaczyk, M.; Siebyla, M.; Pérez, A.; Cubera, E.; Hrynyk, H.; et al. Phosphite spray for the control of oak decline induced by Phytophthora in Europe. For. Ecol. Manag. 2021, 2021, 118938. [Google Scholar] [CrossRef]
- Oszako, T. Oak declines in Europe’s forest-history, causes and hypothesis. In Recent Advances on Oak Health in Europe; Instytut Badawczy Leśnictwa (Forest Research Institute): Warsaw, Poland, 2000; pp. 11–41. [Google Scholar]
- Dobor, L.; Barcza, Z.; Hlásny, T.; Havasi, A.; Horváth, F.; Ittzés, P.; Bartholy, J. Bridging the gap between climate models and impact studies: The Foresee Database. Geosci. Data J. 2015, 2, 1–11. [Google Scholar] [CrossRef]
- McKee, T.B.; Doesken, N.J.; Kleist, J. The relationship of drought frequency and duration to time scales. In Proceedings of the 8th Conference on Applied Climatology, Anaheim, CA, USA, 17–22 January 1993. [Google Scholar]
- Fritts, H. Tree Rings and Climate; Blackburn Press: Caldwell, ID, USA, 2001. [Google Scholar]
- Wang, Y.; Zhang, R.; Zhou, Z. Radial variation of wood anatomical properties determines the demarcation of juvenile-mature wood in Schima superba. Forests 2021, 12, 512. [Google Scholar] [CrossRef]
- Schwarz, J.; Skiadaresis, G.; Kohler, M.; Kunz, J.; Schnabel, F.; Vitali, V.; Bauhus, J. Quantifying growth responses of trees to drought—A critique of commonly used resilience indices and recommendations for future studies. Curr. For. Rep. 2020, 6, 185–200. [Google Scholar] [CrossRef]
- Vannoppen, A.; Treydte, K.; Boeckx, P.; Kint, V.; Ponette, Q.; Verheyen, K.; Muys, B. Tree species diversity improves beech growth and alters its physiological response to drought. Trees Struct. Funct. 2020, 34, 1059–1073. [Google Scholar] [CrossRef]
- Lloret, F.; Keeling, E.G.; Sala, A. Components of tree resilience: Effects of successive low-growth episodes in old ponderosa pine forests. Oikos 2011, 120, 1909–1920. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Bunn, A.; Korpela, M.; Biondi, F.; Campelo, F.; Merian, P.; Qeadan, F.; Zang, C. Time series analysis in dplR. Process. dplR 2016.
- Wickham, H. Ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Bjerknes, J. Atmospheric teleconnections from the equatorial Pacific. Mon. Weather Rev. 1969, 97, 526–535. [Google Scholar] [CrossRef]
- Bert, D.; Lebourgeois, F.; Ponton, S.; Musch, B.; Ducousso, A. Which oak provenances for the 22nd century in Western Europe? Dendroclimatology in common gardens. PLoS ONE 2020, 15, e0234583. [Google Scholar] [CrossRef] [PubMed]
- Netsvetov, M.; Sergeyev, M.; Nikulina, V.; Korniyenko, V.; Prokopuk, Y. The climate to growth relationships of pedunculate oak in steppe. Dendrochronologia 2017, 44, 31–38. [Google Scholar] [CrossRef]
- Hartl, C.; Düthorn, E.; Tejedor, E.; Kirchhefer, A.J.; Timonen, M.; Holzkämper, S.; Büntgen, U.; Esper, J. Micro-site conditions affect Fennoscandian forest growth. Dendrochronologia 2021, 65, 125787. [Google Scholar] [CrossRef]
- Mátyás, C. Adaptive pattern of phenotypic plasticity and inherent growth reveal the potential for assisted transfer in sessile oak (Quercus petraea L.). For. Ecol. Manag. 2021, 482. [Google Scholar] [CrossRef]
- McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.; Aukema, B.H.; Bond-Lamberty, B.; Chini, L.; Clark, J.S.; Dietze, M.; Grossiord, C.; Hanbury-Brown, A.; et al. Pervasive shifts in forest dynamics in a changing world. Science 2020, 368. [Google Scholar] [CrossRef]
- Zhu, J.; Nakano, T.; Hirakawa, Y. Effects of radial growth rate on selected indices for juvenile and mature wood of the Japanese larch. J. Wood Sci. 2000, 46, 417–422. [Google Scholar] [CrossRef]
- McGowran, E.; Douglas, G.C.; Parkinson, M. Morphological and physiological markers of juvenility and maturity in shoot cultures of oak (Quercus robur and Q. petraea). Tree Physiol. 1998, 18, 251–257. [Google Scholar] [CrossRef][Green Version]
- Vitasse, Y.; Bottero, A.; Cailleret, M.; Bigler, C.; Fonti, P.; Gessler, A.; Lévesque, M.; Rohner, B.; Weber, P.; Rigling, A.; et al. Contrasting resistance and resilience to extreme drought and late spring frost in five major European tree species. Glob. Chang. Biol. 2019, 25, 3781–3792. [Google Scholar] [CrossRef]
- Hilker, M.; Schmulling, T. Stress priming, memory, and signalling in plants. Plant Cell Environ. 2019, 753–761. [Google Scholar] [CrossRef]
- Wang, X.; Vignjevic, M.; Liu, F.; Jacobsen, S.; Jiang, D.; Wollenweber, B. Drought priming at vegetative growth stages improves tolerance to drought and heat stresses occurring during grain filling in spring wheat. Plant Growth Regul. 2015, 75, 677–687. [Google Scholar] [CrossRef]
- Nardini, A.; Lo Gullo, M.A.; Trifilò, P.; Salleo, S. The challenge of the Mediterranean climate to plant hydraulics: Responses and adaptations. Environ. Exp. Bot. 2014, 103, 68–79. [Google Scholar] [CrossRef]
- Venturas, M.D.; Sperry, J.S.; Hacke, U.G. Plant xylem hydraulics: What we understand, current research, and future challenges. J. Integr. Plant Biol. 2017, 59, 356–389. [Google Scholar] [CrossRef]
- Kostić, S.; Čukanović, J.; Orlović, S.; Ljubojević, M.; Mladenović, E. Allometric relations of sycamore maple (Acer pseudoplatanus) and its red leaf cultivar (A. pseudoplatanus “Atropurpureum”) in street and park habitats of Novi Sad (Serbia, Europe). J. For. 2019, 117, 114–127. [Google Scholar] [CrossRef]
- Pretzsch, H. The effect of tree crown allometry on community dynamics in mixed-species stands versus monocultures. A review and perspectives for modeling and silvicultural regulation. Forests 2019, 10, 810. [Google Scholar] [CrossRef]
- Dai, J.; Liu, H.; Wang, Y.; Guo, Q.; Hu, T.; Quine, T.; Green, S.; Hartmann, H.; Xu, C.; Liu, X.; et al. Drought-modulated allometric patterns of trees in semi-arid forests. Commun. Biol. 2020, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hallgrímsson, B.; Katz, D.C.; Aponte, J.D.; Larson, J.R.; Devine, J.; Gonzalez, P.N.; Young, N.M.; Roseman, C.C.; Marcucio, R.S. Integration and the Developmental genetics of allometry. Integr. Comp. Biol. 2019, 59, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Antin, C.; Pélissier, R.; Vincent, G.; Couteron, P. Crown allometries are less responsive than stem allometry to tree size and habitat variations in an Indian monsoon forest. Trees Struct. Funct. 2013, 27, 1485–1495. [Google Scholar] [CrossRef]
- Krejza, J.; Světlík, J.; Bednář, P. Allometric relationship and biomass expansion factors (BEFs) for above- and below-ground biomass prediction and stem volume estimation for ash (Fraxinus excelsior L.) and oak (Quercus robur L.). Trees Struct. Funct. 2017, 31, 1303–1316. [Google Scholar] [CrossRef]
- Biondi, F. From dendrochronology to allometry. Forests 2020, 11, 146. [Google Scholar] [CrossRef]
- Knight, J.; Harrison, S. The impacts of climate change on terrestrial Earth surface systems. Nat. Clim. Chang. 2013, 3, 24–29. [Google Scholar] [CrossRef]
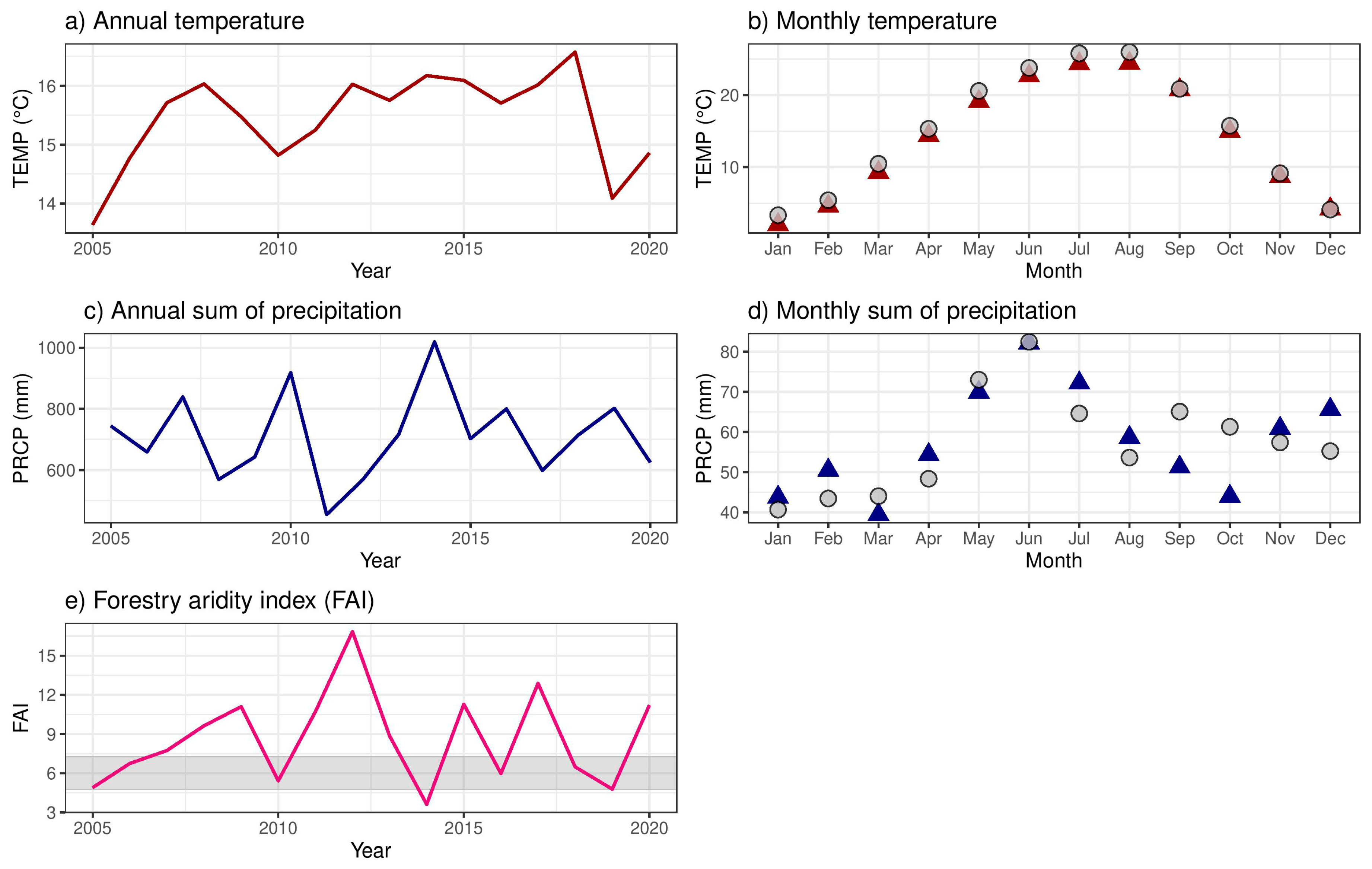
| Timespan | TEMP (°C) | TEMPMAM (°C) | TEMPJJA (°C) | PRCP (mm m−2) | PRCPMAM (mm m−2) | PRCPJJA (mm m−2) |
|---|---|---|---|---|---|---|
| 1950–2020 | 14.47 ± 0.98 | 14.75 ± 1.51 | 24.47 ± 1.39 | 684.0 ± 128.5 | 165.1 ± 57.7 | 207.8 ± 73.5 |
| 2005–2020 | 15.35 ± 0.88 | 15.57 ± 1.26 | 25.42 ± 1.6 | 716.1 ± 145.7 | 197.9 ± 73.3 | 207.9 ± 88.5 |
| 2012 | 15.92 | 16.01 | 27.82 | 568.0 | 180.4 | 57.3 |
| 2017 | 15.97 | 16.33 | 27.44 | 598.7 | 178.5 | 107.7 |
| DBH | TH | CW | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor | SS | F | p | SS | F | p | SS | F | p |
| Variety | 2179 | 261.34 | 9.53 × 10−12 ** | 18.05 | 2.48 | 0.09 NS | 147.10 | 25.89 | 1.19 × 10−11 ** |
| Half-sib | 54 | 26.14 | 0.26 NS | 10.57 | 2.91 | 0.09 NS | 64.58 | 1.98 | 0.34 NS |
| Variety x half-sib | 1315 | 1.30 | 1.88 × 10−7 ** | 48.47 | 6.67 | 0.00 ** | 51.60 | 9.09 | 4.60 × 10−4 ** |
| Residuals | 36,063 | 3142 | 2456 | ||||||
| Relationship | Variety | n | a | b | Adj. r2 | F (p) |
|---|---|---|---|---|---|---|
| TH/DBH | E-QURO | 310 | 5.47 | 0.19 | 0.41 | 217.8 ** |
| T-QURO | 291 | 5.82 | 0.13 | 0.23 | 87.0 ** | |
| L-QURO | 270 | 4.74 | 0.19 | 0.39 | 174.0 ** | |
| CW/DBH | E-QURO | 310 | 2.71 | 0.22 | 0.67 | 629.1 ** |
| T-QURO | 291 | 3.45 | 0.19 | 0.61 | 451.5 ** | |
| L-QURO | 270 | 3.19 | 0.21 | 0.67 | 560.6 ** | |
| CW/TH | E-QURO | 310 | 3.07 | 0.43 | 0.24 | 99.7 ** |
| T-QURO | 291 | 4.61 | 0.45 | 0.22 | 80.4 ** | |
| L-QURO | 270 | 3.47 | 0.50 | 0.38 | 159.9 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostić, S.; Orlović, S.; Karaklić, V.; Kesić, L.; Zorić, M.; Stojanović, D.B. Allometry and Post-Drought Growth Resilience of Pedunculate Oak (Quercus robur L.) Varieties. Forests 2021, 12, 930. https://doi.org/10.3390/f12070930
Kostić S, Orlović S, Karaklić V, Kesić L, Zorić M, Stojanović DB. Allometry and Post-Drought Growth Resilience of Pedunculate Oak (Quercus robur L.) Varieties. Forests. 2021; 12(7):930. https://doi.org/10.3390/f12070930
Chicago/Turabian StyleKostić, Saša, Saša Orlović, Velisav Karaklić, Lazar Kesić, Martina Zorić, and Dejan B. Stojanović. 2021. "Allometry and Post-Drought Growth Resilience of Pedunculate Oak (Quercus robur L.) Varieties" Forests 12, no. 7: 930. https://doi.org/10.3390/f12070930
APA StyleKostić, S., Orlović, S., Karaklić, V., Kesić, L., Zorić, M., & Stojanović, D. B. (2021). Allometry and Post-Drought Growth Resilience of Pedunculate Oak (Quercus robur L.) Varieties. Forests, 12(7), 930. https://doi.org/10.3390/f12070930









