Abstract
Silver and downy birch (Betula pendula Roth and B. pubescens Ehrhs) are pioneer species which play an important role in forest regeneration in disturbed areas. Knowledge of birch seed production and dispersal is key to making good predictions of the persistence and colonization of birch. Both processes can be affected by the density of trees in the neighbourhood. In this study, we studied the seed production and dispersal of birch trees in two plots in Wytham Woods, UK, in 2015, and investigated the potential effect of neighbourhood tree density. We applied inverse modelling to seed trap data, incorporating tree density around the source tree and on the seed path to estimate birch fecundity and the dispersal kernel of the seeds. We show that the pattern of dispersed seeds was best explained by a model that included an effect of tree density on seed dispersal. There was no strong evidence that conspecific or heterospecific tree density had an effect on birch fecundity in Wytham Woods. A birch with diameter at breast height (DBH) of 20 cm is estimated to have produced ~137,000 seeds in 2015. Mean dispersal distance in an open area is estimated to be 65 m but would be reduced to 38 m in a closed stand. Both the mean dispersal distance and the probability of long-distance dispersal of birch decreases in dense environments. Areas with higher tree density also would intercept more seeds. These results highlight the importance of considering tree density in the neighbourhood and in the overall landscape when predicting the colonization and recruitment of birch.
1. Introduction
Birch (Betula pendula Roth and B. pubescens Ehrhs.) are common tree species of high ecological value in Europe. Birch is an important pioneer tree species that produces a large seed crop, has long dispersal distances by wind and is characterized by a fast juvenile growth rate [1]. It is adapted to the colonisation of open areas created by disturbance and habitat unsuitable for other tree species [1]. Birch is known to be a soil improver, increasing soil fertility and soil nutrient cycle rate, and facilitating the establishment of other plants [2,3,4]. Birch is also important for maintaining biodiversity by supporting a high number of invertebrate species and provides habitats for various bird and mammal species [4,5]. The ecological characteristics of birch make it important for forest regeneration after disturbance. In Western Europe, birch is an undervalued indigenous species for forestry but has high potential [6]. With global change, frequencies and intensities of disturbance like extreme weather events, pathogens and pests are likely to increase [7]. Disturbances can cause heavy tree mortality and create unforested areas. The presence of birch in a forest can contribute to the alleviation of the negative consequences of disturbances and increased resilience of both natural forest and plantations, making birch of high ecological and silvicultural importance [6,8].
Given its important ecological and silvicultural value, knowledge of birch seed production and dispersal is necessary to predict the probability of birch regeneration in disturbed areas [9]. It enables us to decide if forest restoration by birch in disturbed areas is limited by the number of seed sources or by limited dispersal distance. In mixed broadleaved forest, birch is often outcompeted by more shade-tolerant and competitive tree species. Understanding the seed production and dispersal of birch in mixed woodland can help predict the persistence of a birch population and decide to what extent management intervention might be needed to help birch recruitment.
Measuring seed production and tracking dispersed seeds from a tree directly is a very difficult task and requires a large amount of work. Thus, most researchers study seed production and dispersal by fitting statistical models to observed seed shadow data and the spatial distribution of source trees, an approach known as inverse modelling [10,11]. Several studies have been conducted to estimate the fecundity and dispersal distance of Betula species using the inverse modelling method [9,12,13]. But most models did not include the environmental heterogeneity experienced by birch trees. It is known that neighbouring environment has a significant effect on the seed production and dispersal of plants [14,15,16]. Trees in dense environments often have smaller seed crops than those in the open due to competition for resources and reduced crown size and volume. Thus, neighbourhood tree density can reflect competition intensity for resources needed for seed production, such as light, water and space available for the growth of the crown. Therefore, incorporation of neighbourhood tree density may help better predict tree fecundity. Furthermore, intra- and interspecific competition may have different impacts on tree fecundity. Hajek et al. [17] showed that in mixed forests, the strength of canopy interactions like crown shyness differs between different species combinations. Thus, the shape and size of the crown of a tree is affected by the species composition of its neighbours, which may be reflected in seed production. As a result, not only the number and size of neighbours but also the species composition may need to be considered when one is predicting seed production. For a wind-dispersed tree like birch, neighbourhood tree density can have important and complex effects on seed dispersal. Tree density near the seed source and on the seed dispersal path can affect both wind velocity and turbulence conditions, and other trees can physically intercept released seeds. Wind speed below canopy is usually lower in denser environments. However, Schurr et al. [15] found trees in dense environments have longer mean dispersal distance due to the greater tree height in dense environments. Nathan & Katul [18] showed seeds released above sparse canopy have longer dispersal distances, which is the result of two conflicting mechanisms, a lower wind velocity above a sparse canopy and a higher chance of seeds being uplifted and experiencing a strong wind. Therefore, it is worth considering the effect of the neighbourhood when estimating both the fecundity and seed dispersal of trees.
In this study, we investigated the seed production and seed dispersal of birch, and explored the effects of tree density on these two processes in Wytham Woods, UK. We used seed traps to collect dispersed seeds in 2015 and fitted inverse models to observed data using an approach developed by Schurr et al. [15], which allowed us to explicitly incorporate neighbourhood tree density as a factor. The aim of this piece of work is to determine (1) whether including a neighbourhood effect enables improved predictions of birch seed production and/or dispersal, (2) whether there is interspecific variation in the strength of the neighbourhood effect on birch fecundity and (3) whether tree density near the source tree and on the seed dispersal path significantly affects birch seed dispersal.
2. Materials and Method
2.1. Study Site
The study site was in Wytham Woods, Oxfordshire, UK (51°46′ N, 1°20′ W). Wytham Woods covers over 400 ha, with an altitude range of 60–164 m above sea level, a mean annual temperature of 10 °C and a mean annual rainfall of 726 mm [19,20]. The woodland compromises disturbed ancient woodland, naturally generated secondary woodland and plantations [21]. The two dominant canopy trees species are ash (Fraxinus excelsior L.) and sycamore (Acer pseudoplatanus L.). Other common canopy tree species include birch (Betula pendula Roth and B. pubescens Ehrhs), oak (Quercus robur L.), beech (Fagus sylvatica L.) and field maple (Acer campestre L.).
2.2. Data Collection
Birch is a species in decline in Wytham Woods [22]. It is widely distributed across the woods but in low numbers. To collect birch seed dispersal data, we set up two plots with locally abundant birch trees as study sites (hereafter called plots A and B). Both plots were located in the disturbed ancient woodland area of Wytham Woods, was managed as a coppice with standards since the early twentieth century, but has been converted to high forest more recently [23]. The most frequent coppice species is hazel (Corylus avellane L.). There were 15 and 27 birch trees in plots A and B, respectively. Ash and sycamore were the most common species in both plots. There were no living birch trees within 70 m of the boundary of the plots, so the impact of trees outside the plots is unlikely to be significant. The two plots were 430 m away from each other. We set up seed traps to collect dispersed seed with nine traps placed in each plot. The traps in a plot were placed in two crossing lines, with traps spaced at ~10 m intervals. The central trap was located at the centre of the plot. Each seed trap was made of a mesh screen with area of 0.25 m2 supported by a PVC frame with a height of 1 m.
Each plot was 70 m × 70 m in size. We collected and counted seeds in traps every one or two weeks from September to February in the years 2015 and 2016. Coordinates of all trees with diameter at breast height (DBH) >15 cm were mapped with a laser rangefinder (Hilti PD 40, Schaan, Liechtenstein) and a compass, and DBH was also measured with a tape measure to the nearest 0.1 cm. The living birch trees measured were considered as possible source trees. A windstorm blew down traps in plot A in 2016 during the seed dispersal season, so we excluded 2016 data.
2.3. Modelling Seed Dispersal
We used the extended inverse modelling (IM) approach developed by Schurr et al. [15] to model birch fecundity and dispersal. The general principle of the IM approach is to estimate plant fecundity and dispersal distance distribution via observed seed trap data and spatial distribution of potential seed sources, finding a set of parameters that are most likely to produce the observed data. Seed count data is assumed to follow a Poisson or negative binominal distribution and parameters are estimated via maximizing the likelihood of data [10,12,24].
The seed shadow of a single tree consists of two components: a fecundity function Q which predicts the number of seeds produced according to tree size and a dispersal kernel f that describes the probability distribution of seed dispersal distances.
For the fecundity function, we used basal area as a measure of tree size following Clark et al. [12]. Basal area/DBH also has a good allometry relationship with the crown area of a tree, which is closely related to seed production [25,26]. The number of seeds produced by a tree of basal area G is:
where G is basal area and b is the fecundity parameter.
Q(G) = bG
For the choice of dispersal kernel, we used the lognormal kernel [15,27]. The lognormal kernel is derived from the micrometeorological mechanism and performs well in many wind-dispersed studies [28,29].
The equation of the lognormal kernel is as follows:
where the parameter u is the scale parameter and mean dispersal distance. Parameter p is the shape parameter. r is the distance from seed source.
The number of seeds collected by a seed trap i of area A is calculated as the sum of seeds dispersed to it from all T possible parent trees in the plot the trap is located in.
2.4. Incorporation of Tree Density Effects
We incorporated the effects of tree density on the fecundity function and dispersal kernel into Equation (3). The approach we used, proposed by Schurr et al. [15], classifies environmental effects into source effects and path effects, which mean the effect of the environment around the source tree and on the seed dispersal path, respectively. For fecundity, we used two variables, conspecific and heterospecific neighbourhood tree density, to test if there was a difference in effect strength between intraspecific and interspecific neighbourhood effects. For the source effect and path effect on seed dispersal, we used a single variable, density of all trees, as tree density mainly affects seed dispersal via its impact on wind velocity and turbulence conditions. This effect is unlikely to be species-specific. To calculate neighbourhood tree density, a grid with 10 m × 10 m cells was superimposed on the study plots and the number of trees was counted in each cell.
To model the source effect, fecundity and dispersal parameters were included as functions of the tree density around the source tree in a standard inverse model like Equation (3).
For example, the fecundity parameter b of tree i was modelled as:
where Tc and Th are conspecific and heterospecific tree density in the cell of tree i. b0, bc and bh can be viewed as the ‘intercept’ and ‘slopes’ of the model respectively. When values of bc and bh are zero, the model is equivalent to a standard inverse model without tree density effects. Dispersal parameters u and p were also modelled with this approach to reflect the source tree density effect on the dispersal kernel:
where T is the total tree density in the grid in which tree i is located. While ut and pt are coefficients of tree density like Equation (4).
bi = exp (b0 + bcTc + bhTh)
ui = exp (u0 + utT)
pi = exp (p0 + ptT)
The path effect indicates the resistance that tree density imposes on the dispersal of seeds. To model the path effect, the real distance between a seed trap and source tree was transformed into an effective distance dependent on the environmental conditions (here this means tree density) seeds encountered on the dispersal path. For simplicity, the dispersal path was considered a straight line between seed trap and source tree. With this approach, regions of high resistance could enlarge the effective distance between a seed trap and a source tree by decreasing the probabilities of seeds moving through the landscape [16]. The effective distance was calculated as:
where:
r’ is the effective distance between the seed trap and a source tree.
c means all cells intersected by a straight line between the source tree and the cell the seed trap is located in.
dc is the length of the straight-line segment in each cell.
wc is the environmental resistance in cell c.
The environmental resistance wc was further modelled as a function of tree density:
where is the coefficient of the path effect. Tc is the number of trees in each cell as in the incorporation of source effect above.
With the effective distance changed, the effective area of the seed traps also needs to be changed so the dispersal kernel can still integrate to 1. Schurr et al. [15] used an approximate way to express the transformed area:
where:
A’ is the effective area and A is the actual area of the trap.
r is the actual distance from source to cell and r’ is the effective distance calculated by Equation (7).
TA is the number of trees in the cell where the seed trap was located.
As a result, grids with higher environmental resistance will capture more seeds because they have larger effective area A’.
Finally, parameters were fitted using observed seed trap data and tree distribution data from both plots with the maximum likelihood method using R [30].
2.5. Model Selection
To test which combination of source and path effect best explain the data, 16 alternative models with different combinations of source effect and path effect and variables (conspecific and heterospecific tree density) were fitted. A standard model with no environment effects was also included. The models were fitted with data from both plots.
To correct for over-dispersion in seed count data, we used the QAIC value instead of the AIC value to compare the performance of the models [31]. A variance inflation factor was estimated according to Symonds & Moussalli [32] and this factor was used to calculate the QAIC value [33]. A lower QAIC value indicates better model performance. The model with the lowest QAIC value is considered the best model, but models with a difference in QAIC between the best model (ΔQAIC) <2 are considered equally good in performance. A ΔQAIC within 4–7 means considerably less support for the models and a difference greater than 10 means essentially no support compared to the best model [31]. All analyses were conducted in R [30].
3. Results
3.1. Data Collection
In total we collected 2814 birch seeds (599 in plot A and 2215 in plot B) in 2015. A total of 292 trees were mapped in two plots; 42 of them were birches (15 in plot A and 27 in plot B) (Figure 1). The remaining trees consisted of ash, sycamore, oak and beech. Average tree density was 0.03 trees m−2. Average conspecific tree density for birches was 0.014 trees m−2 and average heterospecific tree density was 0.02 trees m−2. Conspecific and heterospecific tree density in cells were not correlated in either plot.
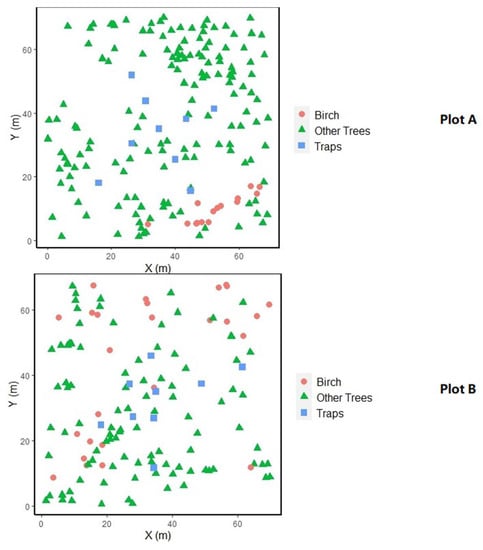
Figure 1.
Tree distribution maps of study plot A (top) and B (bottom).
3.2. Tree Density Effects on Source and Path
Almost all models that include a tree density effect have better performance than the standard model (Table 1). The best fitting model has an effect of tree density around the source tree on dispersal parameters plus a tree density effect on dispersal path resistance, with a QAIC value of 47.6 (Table 1). It has well-supported superior performance to other models, with a ΔQAIC >2 compared to any other model. The addition of a tree density effect on fecundity to the best model made performance worse. The 2nd and 3rd ranked model included an effect of tree density on fecundity, where tree density has a negative effect on fecundity in both models (Table 1). A full table of model parameters with standard error and 95% CI can be found in the supplement material (Table S1).

Table 1.
Parameter estimations and QAIC values of all 16 models ranked according to increasing QAIC. Parameters with 95% CI that did not include zero were marked in bold.
In the best model, the neighbourhood tree density around the source tree had a negative effect on both parameters u and p. Thus, a tree in a dense grid cell had a lower mean dispersal distance, lighter distribution tail and lower probability of long-distance dispersal (Figure 2). The prediction of seeds caught by the traps by the best model is well correlated (Pearson’s r = 0.97) to the observed seed number caught (Figure 3). A tree density effect on the dispersal path also appeared in the best model. Grid cells with higher tree density had a higher chance of intercepting seeds, and a location behind grid cells with a high tree density would then receive fewer seeds. A birch in an open location is predicted to have a mean dispersal distance of 65 m (exp (4.18) ≈ 65). The best model predicted that a birch tree will produce ~4 million seeds per m−2 basal area (exp (15.23) ≈ 4,100,000). A predicted seed number distribution by the best model is shown in Figure 4. It can be seen that in the lower part of plot B, where birches and other trees were aggerated, a very high concentration of seeds (~1500–3500 /m2) was predicted, where other parts have a predicted seed number of ~80–1000 /m2 (Figure 4).
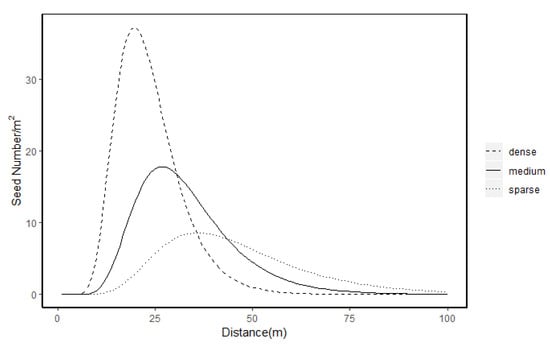
Figure 2.
Dispersal kernels of a birch tree producing 10,000 seeds based on parameters of the best model in different environments. The different curves denote dense (0.05 trees/m2), medium (0.03 trees/m2) and sparse (0.01 trees/m2) neighbouring environment where the source tree is located.
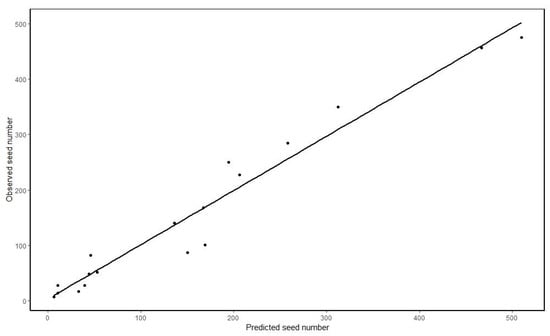
Figure 3.
Number of seeds captured by all seed traps vs predicted number of seeds by the best model.
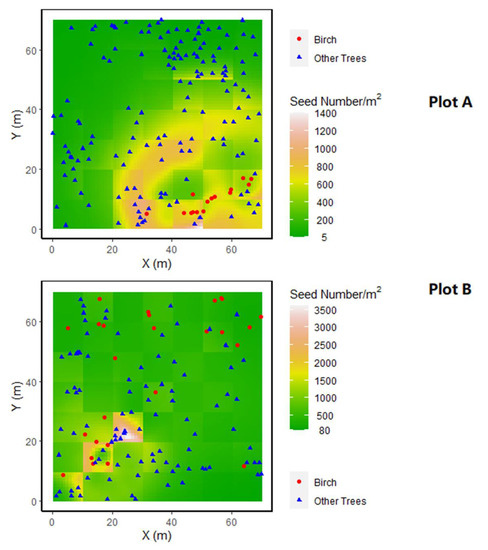
Figure 4.
Predicted distribution of seed number per m2 of plot A (top) and plot B (bottom) by the best model with locations of trees. Please note the minimum predicted seed number is not zero.
4. Discussion
Our study shows that neighbourhood tree density is a good predictor of the seed dispersal of birch. The best model was one with an effect of tree density on both the dispersal parameters and the path effect; it performed better than other models, and the improvement compared to the standard model was substantial. However, the effect of tree density on fecundity was not included in the best model. This result suggests neighbourhood tree density has a more profound effect on seed dispersal than seed production for birch, at least in this year in our study site. Neighbourhood density is a predictor widely used in models for tree growth and mortality. Given its importance in seed dispersal, it should also be considered when predicting tree dispersal. As the best model is well-supported by QAIC values, we will discuss the effects of tree density mainly based on the parameters of the best model.
Tree density can affect seed dispersal in a complex manner via wind velocity, turbulence characteristic and other factors [18,34,35]. According to the best model, a denser environment has a negative effect on both the mean dispersal distance u and shape parameter p. The result is contrary to Schurr et al. [15], who found Aleppo pines (Pinus halepensis, Miller) in dense environments have increased mean dispersal distances, which they attributed to trees in dense environment being taller and their cones being concentrated towards the canopy top. We estimated birch height from DBH using the allometry equation in Evans et al. [25] and found no significant relationship between birch height and neighbourhood tree density (Pearson’s r, p = 0.8, N.S.). This observation can explain our difference with Schurr et al. [15]. Our best model shows that a birch tree has a mean dispersal distance of 65 m in an open environment. For a tree in a grid cell of average tree density (0.03 trees m−2), mean dispersal distance is predicted to be 38 m. This figure is comparable to other studies on birch. Studies on birch in closed stands have estimated mean dispersal distance for B. alleghaniensis, B. lenta and B. pendula to be in the range 37–46 m [24,36], which is close to our result. On the other hand, Tiebel et al. [9] found mean dispersal distance for silver birch (B. pendula) in open space can range from 86–97 m (uphill) and 360–389 m (downhill). The difference between our result and theirs is probably due to very different site conditions, as our study was done in a closed stand on levelled ground while theirs was on mountain slopes. The difference underlines the fact that the stand environment has a significant effect on birch dispersal distance. An increase in tree density near the source tree also decreases the shape parameter p, which leads to a lighter tail of the dispersal distance distribution. Thus, trees in a dense environment have lower chance of dispersing seeds a long distance. However long-distance dispersal is known to be very difficult to characterize accurately using simple statistical models like those used here [37]. The plot area is also not large enough to capture real long-distance dispersal events, so prediction by dispersal kernel should be used only for short or medium distances.
Like Schurr et al. [15], we found higher tree densities increased dispersal resistance, reflected by the fact that a path effect was included in the best model. Compared to a dispersal path in open space, a dispersal path in a dense environment has a longer effective distance due to the path effect. The estimated value of parameter w has a value of 0.22, meaning a grid with tree density of 0.03 tree m−2 (three trees per grid cell) has twice the resistance of an area without trees (exp (0.22 × 3) ≈ 1.9), which is very close to the estimation of path effect by Schurr et al. [15] for a pure stand of Aleppo pines. In both studies, only the density of trees was used to estimate path effect yet the effect size was very close. A further study considering not just tree density but total crown area in a gridded environment as a proxy of path effect could be conducted to investigate whether it produces better performance than the use of tree density alone. In this approach, location in a dense environment is expected to capture more seeds as the area in ‘movement’ space is inflated (Equation (9)). An open space suitable for birch recruitment can be ‘shaded’ if surrounded by dense forest and does not receive enough seeds. In another study, Zhao et al. [38] found seed traps under an open canopy receive more birch seeds. However, it is difficult to compare our result with theirs due to the very different research methods employed. Zhao et al. [38] did not use inverse modelling, and the exact locations of mature trees and the environment between source trees and traps were not available. Our results about the source and path effect of tree density on dispersal is in agreement with Nathan and Katul [18], who found that seeds released over a sparse canopy have an overall higher probability of long-distance dispersal. However, the frequency of seeds uplifted above the canopy is considered to be very low [15]. Thus, the most important mechanism underlying the negative effect of tree density on mean dispersal distance and resistance on seed dispersal is probably lower wind velocity in dense environments. A potential important factor affecting seed dispersal we did not investigate is the dominant wind direction. However, the wind direction in our study site was highly variable during the study period, with a mean standard deviation of 90° of daily wind direction according to the meteorology data collected by the automatic weather station in Wytham Woods [39]. In the study by Tiebel et al. [9], no obvious directionality caused by wind in birch seed dispersal was found, probably due to a long sample period and the high variability of wind direction during the study period (standard deviation of monthly mean wind direction from 25 to 102°). Given the highly variable wind direction, we do not expect that dominant wind direction had a large impact on our results.
Contrary to the hypothesis that birch seed production would suffer from neighbourhood competition, the best model did not include an effect of conspecific or heterospecific tree density on birch fecundity. The second-best and third-best model both included effects of conspecific and heterospecific tree density on fecundity in addition to source effects on the dispersal and path effect. There was a negative effect of both density factors on fecundity, but the effect was very weak and the 95% CIs included zero (Table 1). Overall, the incorporation of a density effect on fecundity did not improve upon the best model, which included only dispersal and path effects. Thus, our results did not show support for birch fecundity being affected by intra- or inter-specific competition at Wytham Woods. According to the best model, a birch tree of 20 cm DBH produced ~137,000 seeds in 2015. The number is low compared to Tiebel et al. [9], where they found a birch of the same size produces ~350,000 seeds in a non-mast year and 1,500,000 in a medium year. The difference could be that they began to collect seeds two months earlier than us. Birch is known to have very variable interannual seed production, which is affected by the climatic conditions of the previous year [40,41]. The year 2015 was probably a non-mast year for birch in Wytham Woods. Perhaps a longer study would have found a more profound effect of neighbourhood interaction on fecundity in mast years, when birch allocate more resources into seed production.
Understanding seed dispersal and production can help predict the dynamics of a birch population in a closed forest or open environment. Previous studies have shown that birch is a declining species in Wytham Woods, as the number of adult trees is decreasing and numbers of birch seedlings and saplings are very low [21,42]. Birch is particularly susceptible to windthrow, which probably contributes to its decline in Wytham Woods, in addition to the low shade tolerance [42]. In Wytham Woods, most birches are in the disturbed semi-natural woodlands and others are scattered over the whole woodland in low numbers [20,42]. Apart from being a shade-intolerant species and getting out-competed in closed canopy, our results show birch dispersal is also very sensitive to stand tree density. Ash dieback disease reached Wytham Woods in 2017 and it has the potential to create large deforested areas in this ash-dominated woodland [43]. Birch could play an important role in forest restoration in this scenario. However, the low number of birch trees and the dispersal limitation posed by a dense environment could hamper the recruitment of birch. Successful establishment of birch in deforested area would rely on the number and spatial distribution of seed source trees. A dispersal model such as that presented here can help predict if existing birch can colonize, once open source is created by disturbance, and if management is needed to ensure persistence of the current birch population.
5. Conclusions
We applied inverse modelling to investigate the effect of neighbourhood tree density on the seed production and dispersal of birch, which is important for predicting the persistence and expansion of birch. Our studies highlighted the importance of both tree density near the seed source and the dispersal path on seed dispersal. The incorporation of tree density can greatly improve the prediction of birch seed disposition. However, the effects of tree density on fecundity and dispersal were not equal. There was a lack of strong evidence on the effect of tree density on birch fecundity. A long-term study may help us better understand the role of neighbouring trees in birch seed production. Factors other than tree density, like the crown size of neighbouring trees, could be introduced in a future study for further improvement. In management practice, the spatial distribution of tree density is an important factor to be considered if the successful natural recruitment of birch is of interest, due to its strong effect on birch dispersal.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/f12070929/s1, Table S1: A full table of model parameters with standard error and 95% CI.
Author Contributions
Conceptualization, Z.L. and M.E.; Methodology, Z.L.; Formal Analysis, Z.L.; Investigation, Z.L. and M.E.; Resources, M.E.; Data Curation, Z.L.; Writing–Original Draft Preparation, Z.L.; Writing–Review & Editing, M.E.; Visualization, Z.L.; Supervision, M.E.; All authors have read and agreed to the published version of the manuscript.
Funding
Z.L. was funded by the China Scholarship Council.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank the University of Oxford for access to Wytham Woods, Aristides Moustakas for help with field work and Andrew Leitch for guidance on Z.L.’s project, of which this study is a part. We are grateful to two anonymous reviewers for their helpful comments and suggestions.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Atkinson, M. Betula Pendula Roth (B. Verrucosa Ehrh.) and B. Pubescens Ehrh. J. Ecol. 1992, 80, 837. [Google Scholar] [CrossRef]
- Brandtberg, P.-O.; Lundkvist, H.; Bengtsson, J. Changes in forest-floor chemistry caused by a birch admixture in Norway spruce stands. For. Ecol. Manag. 2000, 130, 253–264. [Google Scholar] [CrossRef]
- Schua, K.; Wende, S.; Wagner, S.; Feger, K.-H. Soil Chemical and Microbial Properties in a Mixed Stand of Spruce and Birch in the Ore Mountains (Germany)—A Case Study. Forests 2015, 6, 1949–1965. [Google Scholar] [CrossRef]
- Patterson, G.S. The Value of Birch in Upland Forests for Wildlife Conservation; Forestry Commission Bulletin 109; HMSO: London, UK, 1993. [Google Scholar]
- Woodcock, B.A.; Leather, S.R.; Watt, A. Changing management in Scottish birch woodlands: A potential threat to local invertebrate biodiversity. Bull. Entomol. Res. 2003, 93, 159–167. [Google Scholar] [CrossRef]
- Dubois, H.; Verkasalo, E.; Claessens, H. Potential of Birch (Betula Pendula Roth and B. pubescens Ehrh.) for Forestry and Forest-Based Industry Sector within the Changing Climatic and Socio-Economic Context of Western Europe. Forests 2020, 11, 336. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef]
- Tiebel, K.; Huth, F.; Wagner, S. Soil seed banks of pioneer tree species in European temperate forests: A review. Iforest Biogeosciences For. 2018, 11, 48–57. [Google Scholar] [CrossRef]
- Tiebel, K.; Huth, F.; Frischbier, N.; Wagner, S. Restrictions on natural regeneration of storm-felled spruce sites by silver birch (Betula Pendula Roth) through limitations in fructification and seed dispersal. Eur. J. For. Res. 2020, 139, 731–745. [Google Scholar] [CrossRef]
- Ribbens, E.; Silander, J.A.; Pacala, S.W. Seedling Recruitment in Forests: Calibrating Models to Predict Patterns of Tree Seedling Dispersion. Ecology 1994, 75, 1794–1806. [Google Scholar] [CrossRef]
- Canham, C.D.; Uriarte, M. Analysis of Neighborhood Dynamics of Forest Ecosystems Using Likelihood Methods and Modeling. Ecol. Appl. 2006, 16, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.; Silman, M.; Kern, R. Seed Dispersal near and Far: Patterns across Temperate and Tropical Forests. Ecology 1999, 80, 1475–1494. [Google Scholar] [CrossRef]
- Greene, D.F.; Canham, C.D.; Coates, K.D.; Lepage, P.T. An evaluation of alternative dispersal functions for trees. J. Ecol. 2004, 92, 758–766. [Google Scholar] [CrossRef]
- Mack, R.N.; Harper, J.L. Interference in Dune Annuals: Spatial Pattern and Neighbourhood Effects. J. Ecol. 1977, 65, 345. [Google Scholar] [CrossRef]
- Schurr, F.M.; Steinitz, O.; Nathan, R. Plant fecundity and seed dispersal in spatially heterogeneous environments: Models, mechanisms and estimation. J. Ecol. 2008, 96, 628–641. [Google Scholar] [CrossRef]
- Herrera, J.M.; Morales, J.M.; García, D. Differential effects of fruit availability and habitat cover for frugivore-mediated seed dispersal in a heterogeneous landscape. J. Ecol. 2011, 99, 1100–1107. [Google Scholar] [CrossRef]
- Hajek, P.; Seidel, D.; Leuschner, C. Mechanical abrasion, and not competition for light, is the dominant canopy interaction in a temperate mixed forest. For. Ecol. Manag. 2015, 348, 108–116. [Google Scholar] [CrossRef]
- Nathan, R.; Katul, G. Foliage shedding in deciduous forests lifts up long-distance seed dispersal by wind. Proc. Natl. Acad. Sci. USA 2005, 102, 8251–8256. [Google Scholar] [CrossRef]
- Savill, P.S.; Perrins, C.M.; Jones, D.M.; Smith, A.R.; Gibson, C.W.D.W.D.C.; Kirby, K.J.J.; More-croft, M.D.D.; Buesching, C.D. Wytham Woods: Oxford’s Ecological Laboratory; Oxford University Press: Oxford, UK, 2010; ISBN 9781483289120. [Google Scholar]
- Butt, N.; Campbell, G.; Malhi, Y. Initial Results from Establishment of a Long-Term Broadleaf Monitoring Plot at Wytham Woods, Oxford, UK; University of Oxford: Oxford, UK, 2009. [Google Scholar]
- Mihók, B.; Kenderes, K.; Kirby, K.J.; Paviour-Smith, K.; Elbourn, C.A. Forty-year changes in the canopy and the understorey in Wytham Woods. Forestry 2009, 82, 515–527. [Google Scholar] [CrossRef]
- Kirby, K.J.; Bazely, D.R.; Goldberg, E.A.; Hall, J.E.; Isted, R.; Perry, S.C.; Thomas, R.C. Changes in the tree and shrub layer of Wytham Woods (Southern England) 1974–2012: Local and national trends compared. Forestry 2014, 87, 663–673. [Google Scholar] [CrossRef]
- Morecroft, M.D.; Stokes, V.; Taylor, M.E.; Morison, J.I. Effects of climate and management history on the distribution and growth of sycamore (Acer pseudoplatanus L.) in a southern British woodland in comparison to native competitors. Forestry 2008, 81, 59–74. [Google Scholar] [CrossRef]
- Clark, J.S.; Macklin, E.; Wood, L. Stages and Spatial Scales of Recruitment Limitation in Southern Appalachian Forests. Ecol. Monogr. 1998, 68, 213. [Google Scholar] [CrossRef]
- Evans, M.R.; Moustakas, A.; Carey, G.; Malhi, Y.; Butt, N.; Benham, S.; Pallett, D.; Schäfer, S. Allometry and growth of eight tree taxa in United Kingdom woodlands. Sci. Data 2015, 2, 150006. [Google Scholar] [CrossRef]
- Blanchard, E.; Birnbaum, P.; Ibanez, T.; Boutreux, T.; Antin, C.; Ploton, P.; Vincent, G.; Pouteau, R.; Vandrot, H.; Hequet, V.; et al. Contrasted allometries between stem diameter, crown area, and tree height in five tropical biogeographic areas. Trees 2016, 30, 1953–1968. [Google Scholar] [CrossRef]
- Stoyan, D.; Wagner, S. Estimating the fruit dispersion of anemochorous forest trees. Ecol. Model. 2001, 145, 35–47. [Google Scholar] [CrossRef]
- Greene, D.F.; Johnson, E.A. A Model of Wind Dispersal of Winged or Plumed Seeds. Ecology 1989, 70, 339–347. [Google Scholar] [CrossRef]
- Clobert, J.; Baguette, M.; Benton, T.; Bullock, J. Dispersal Ecology and Evolution; Oxford University Press: Oxford, UK, 2012; ISBN 9780199608898. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.r-project.org/ (accessed on 5 September 2016).
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 1988; ISBN 0387953647. [Google Scholar]
- Symonds, M.R.E.; Moussalli, A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav. Ecol. Sociobiol. 2010, 65, 13–21. [Google Scholar] [CrossRef]
- Richards, S.A. Dealing with overdispersed count data in applied ecology. J. Appl. Ecol. 2007, 45, 218–227. [Google Scholar] [CrossRef]
- Pounden, E.; Greene, D.F.; Quesada, M.; Sánchez, J.M.C. The effect of collisions with vegetation elements on the dispersal of winged and plumed seeds. J. Ecol. 2008, 96, 591–598. [Google Scholar] [CrossRef]
- Bohrer, G.; Katul, G.; Nathan, R.; Walko, R.L.; Avissar, R. Effects of canopy heterogeneity, seed abscission and inertia on wind-driven dispersal kernels of tree seeds. J. Ecol. 2008, 96, 569–580. [Google Scholar] [CrossRef]
- Wagner, S.; Wälder, K.; Ribbens, E.; Zeibig, A. Directionality in fruit dispersal models for anemochorous forest trees. Ecol. Model. 2004, 179, 487–498. [Google Scholar] [CrossRef]
- Jordano, P. What is long-distance dispersal? And a taxonomy of dispersal events. J. Ecol. 2017, 105, 75–84. [Google Scholar] [CrossRef]
- Zhao, F.; Qi, L.; Fang, L.; Yang, J. Influencing factors of seed long-distance dispersal on a fragmented forest landscape on Changbai Mountains, China. Chin. Geogr. Sci. 2015, 26, 68–77. [Google Scholar] [CrossRef]
- Rennie, S.; Adamson, J.; Anderson, R.; Andrews, C.; Bater, J.; Bayfield, N.; Beaton, K.; Beaumont, D.; Benham, S.; Bowmaker, V. Environmental Change Network (ECN) Meteorology Data: 1991–2015; NERC Environmental Information Data Centre: Gardine, UK, 2017. [Google Scholar]
- Kullman, L. Tree limit dynamics of Betula pubescens ssp. Tortuosain relation to climate variability: Evidence from central Sweden. J. Veg. Sci. 1993, 4, 765–772. [Google Scholar] [CrossRef]
- Holmström, E.; Karlsson, M.; Nilsson, U. Modeling birch seed supply and seedling establishment during forest regeneration. Ecol. Model. 2017, 352, 31–39. [Google Scholar] [CrossRef]
- Kirby, K.J.; Thomas, R.C.; Dawkins, H.C. Monitoring of changes in tree and shrub layers in Wytham Woods (Oxfordshire), 1974–1991. Forestry 1996, 69, 319–334. [Google Scholar] [CrossRef][Green Version]
- Kirby, K.J. The Ash Population in Wytham Woods. 2020. Available online: https://anhso.org.uk/wp-content/uploads/Fritillary/frit8-ashdieback.pdf (accessed on 12 June 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).