Characterization of Riparian Tree Communities along a River Basin in the Pacific Slope of Guatemala
Abstract
:1. Introduction
2. Materials and Methods
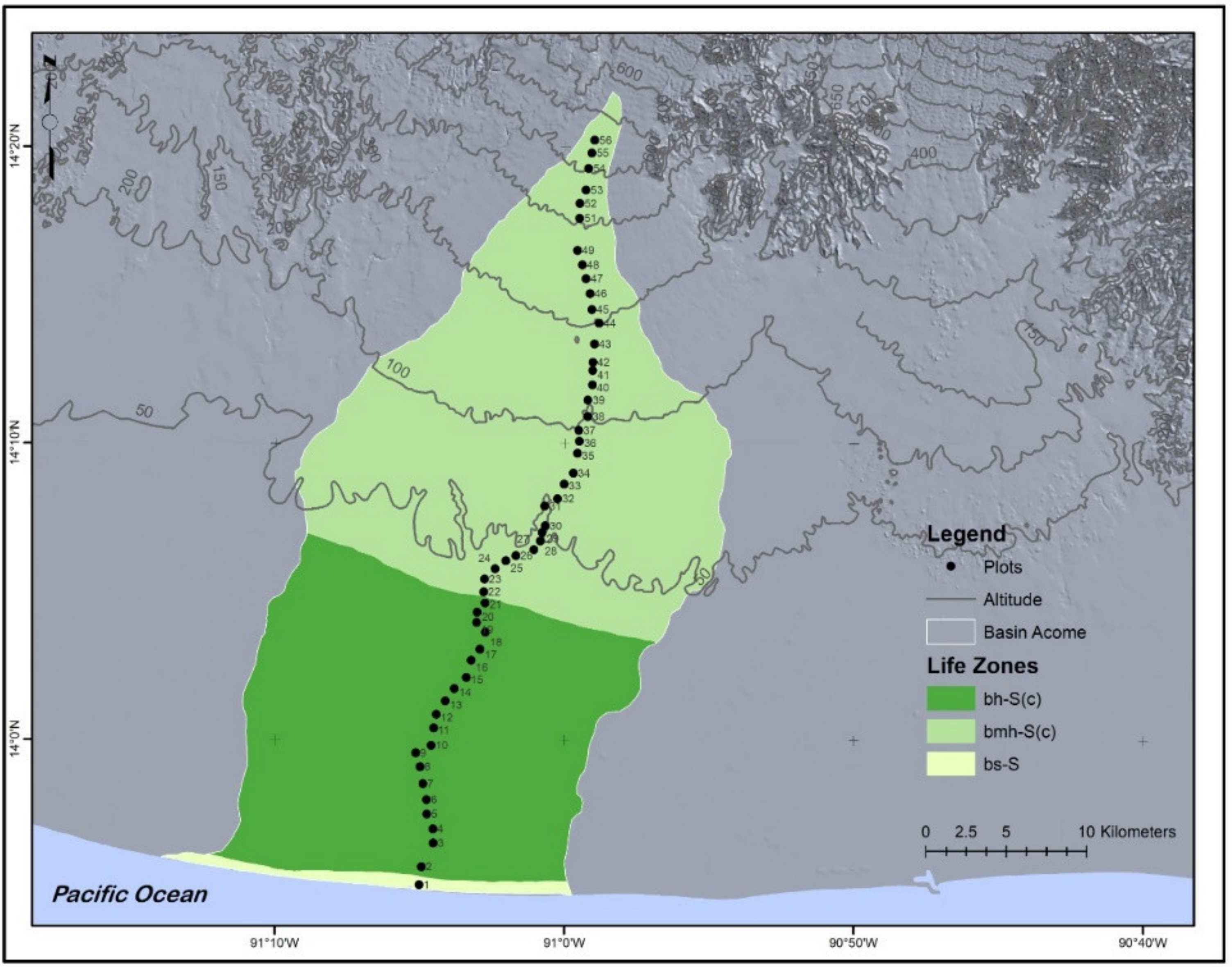
2.1. Study Site
2.2. Plant Sampling
2.3. Plant Identification
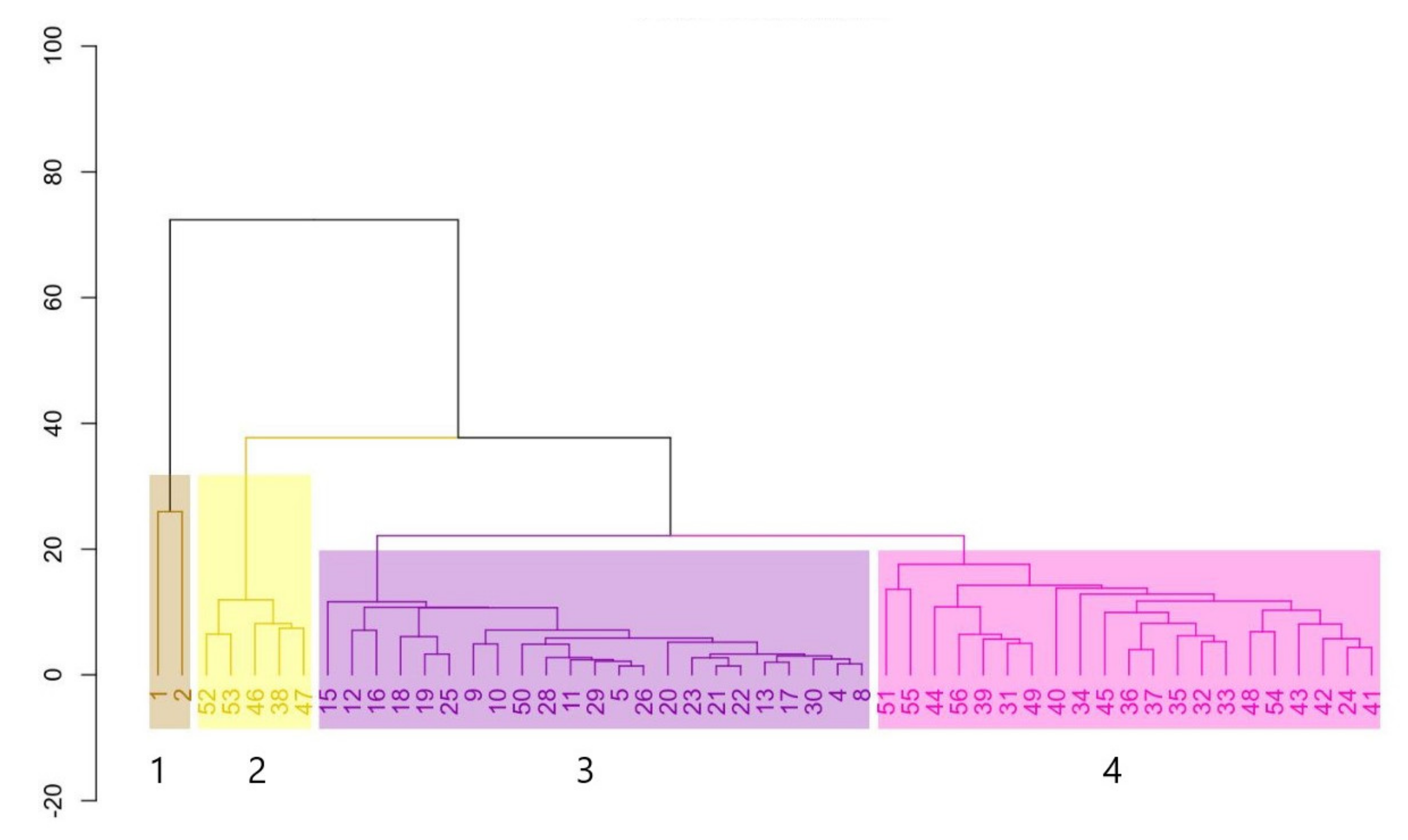
2.4. Vegetation Classification
2.5. Floristic Composition and Species Diversity
3. Results
3.1. Tree Species Identification
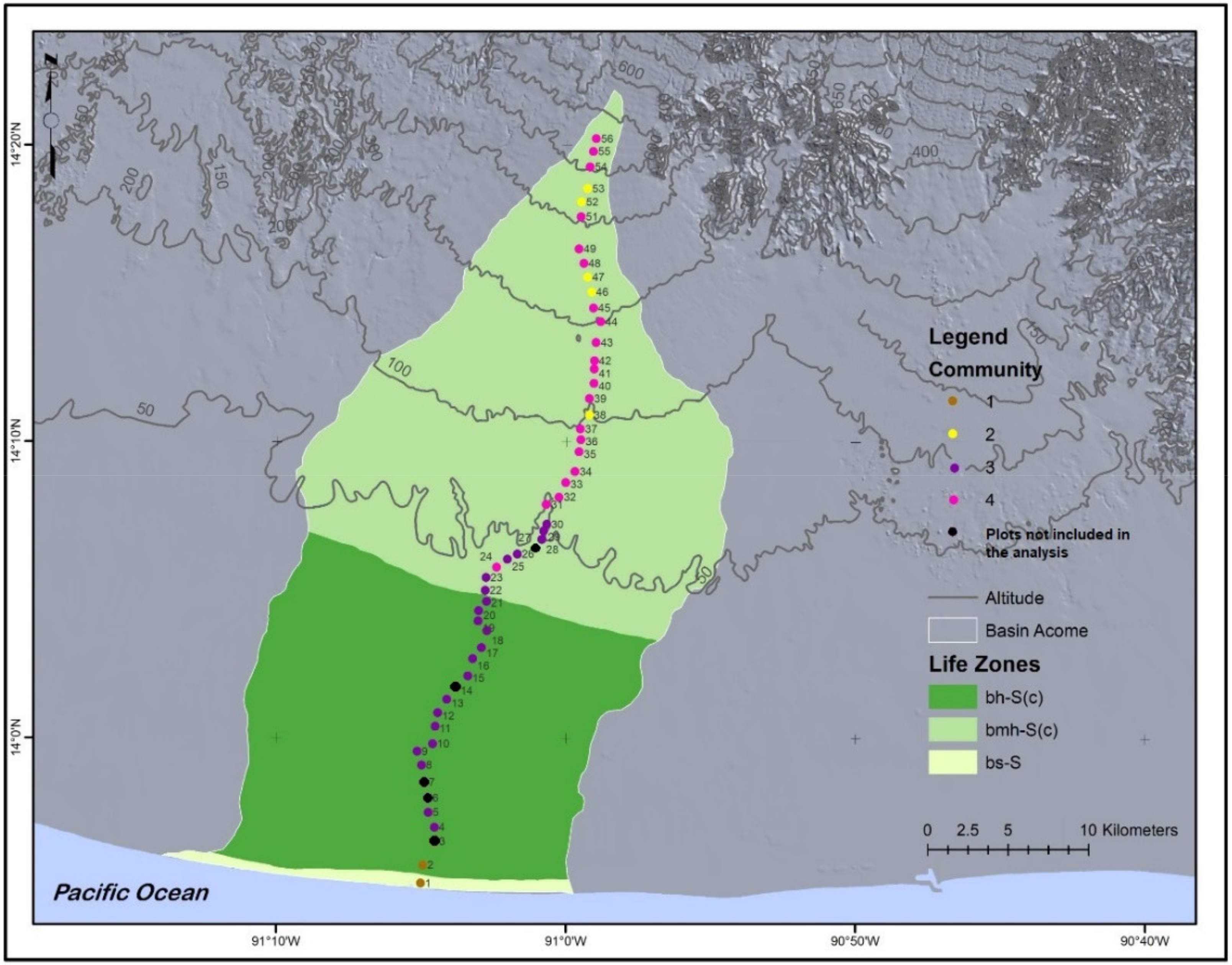
3.2. Vegetation Classification
3.3. Floristic Composition in the Riparian Tree Communities
3.4. Species Diversity of the Riparian Tree Communities
4. Discussion
5. Conclusions and Future Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laurance, W.F.; Sayer, J.; Cassman, K.G. Agricultural expansion and its impacts on tropical nature. Trends Ecol. Evol. 2014, 29, 107–116. [Google Scholar] [CrossRef]
- Phalan, B.; Bertzky, M.; Butchart, S.H.; Donald, P.F.; Scharlemann, J.P.; Stattersfield, A.J.; Balmford, A. Crop expansion and conservation priorities in tropical countries. PLoS ONE 2013, 8, e51759. [Google Scholar]
- Sempris, E.; Carrillo, R.; Portillo, R.H.O.; Carranza, A.C.; Fuller, C.; Peters, R.E.; Stokes, C.; Castañon, C.C.; Irwin, D.; Barraza, E. Potential Impacts of Climate Change on Biodiversity in Central America, Mexico and the Dominican Republic; Water Center for the Humid Tropics of Latin America and the Caribbean: Panama City, Panama, 2008. [Google Scholar]
- DeClerck, F.A.; Chazdon, R.; Holl, K.D.; Milder, J.C.; Finegan, B.; Martinez-Salinas, A.; Imbach, P.; Canet, L.; Ramos, Z. Biodiversity conservation in human-modified landscapes of Mesoamerica: Past, present and future. Biol. Conserv. 2010, 143, 2301–2313. [Google Scholar] [CrossRef]
- Dos Santos, F.B.; Ferreira, F.C.; Esteves, K.E. Assessing the importance of the riparian zone for stream fish communities in a sugarcane dominated landscape (Piracicaba River Basin, Southeast Brazil). Environ. Biol. Fishes 2015, 98, 1895–1912. [Google Scholar] [CrossRef]
- Collier, K.; Cooper, A.; Davies-Colley, R.; Rutherford, J.; Smith, C.; Williamson, R.B. Managing Riparian Zones: A Contribution to Protecting New Zealand’s Rivers and Streams; Department of Conservation: Wellington, New Zealand, 1995; Volume 2. [Google Scholar]
- Stanley, V.; Swanson, F.; Mckee, W.; Cummins, K. An Ecosystem Perspective of Riparian Zones, Focus on links between land and water. Bioscience 1991, 41, 540–551. [Google Scholar]
- Van Der Maarel, E.; Franklin, J. Vegetation ecology: Historical notes and outline. Veg. Ecol. 2013, 2, 1–27. [Google Scholar]
- Burton, M.L.; Samuelson, L.J.; Pan, S. Riparian woody plant diversity and forest structure along an urban-rural gradient. Urban Ecosyst. 2005, 8, 93–106. [Google Scholar] [CrossRef]
- Gregory, S.V.; Swanson, F.J.; McKee, W.A.; Cummins, K.W. An ecosystem perspective of riparian zones. BioScience 1991, 41, 540–551. [Google Scholar] [CrossRef]
- Naiman, R.J.; Decamps, H.; McClain, M.E. Riparia: Ecology, Conservation, and Management of Streamside Communities; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Naiman, R.J.; Decamps, H.; Pollock, M. The role of riparian corridors in maintaining regional biodiversity. Ecol. Appl. 1993, 3, 209–212. [Google Scholar] [CrossRef]
- Pysek, P.; Prach, K. How important are rivers for supporting plant invasions. In Ecology and Management of Invasive Riverside Plants; John Wiley & Sons Ltd.: Chichester, UK, 1994; pp. 19–26. [Google Scholar]
- Sunil, C.; Somashekar, R.; Nagaraja, B. Impact of anthropogenic disturbances on riparian forest ecology and ecosystem services in Southern India. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2011, 7, 273–282. [Google Scholar] [CrossRef] [Green Version]
- Naiman, R.J. New perspectives for watershed management: Balancing long-term sustainability with cumulative environmental change. In Watershed Management; Springer: New York, NY, USA, 1992. [Google Scholar]
- NSW Government. Guidelines for Riparian Corridors on Waterfront Land; Department of Trade and Investment, Regional Infrastructure and Services: Parramatta, Australia, 2018.
- Platts, W.S. Methods for Evaluating Riparian Habitats with Applications to Management; US Department of Agriculture, Forest Service, Intermountain Research Service: Ogden, UT, USA, 1987; Volume 221.
- Regalado, O.; Villagrán, X.; Pérez, G.; Castellanos, E.; Martínez, G.; Incer, D. Mapa de Cobertura Forestal de Guatemala 2010 y Dinámica de la Cobertura Forestal 2006–2010; National Forestry Institute (INAB), National Council for Protected Areas (CONAP), Universidad del Valle de Guatemala, Universidad Rafael Landívar: Guatemala City, Guatemala, 2012.
- MAGA. Capa Digital a Escala 1:50,000 de la República de Guatemala; Ministerio de Agricultura, Ganadería y Alimentación de Guatemala (MAGA): Guatemala City, Guatemala, 2009.
- Herrera Ibáñez, I.R. Manual de hidrología. In Facultad de Agronomía; Universidad de San Carlos de Guatemala: Guatemala City, Guatemala, 1995. [Google Scholar]
- CONRED. Mapa de Elevación Dígital 12.5; Coordinadora Nacional para la Reducción de Desastres: Guatemala City, Guatemala, 2016. [Google Scholar]
- ICC. Resumen Meteorológico: Resultados del Sistema Meteorológico ICC; Instituto Privado de Investigación sobre Cambio Climático: Santa Lucía Cotzumalguapa, Guatemala, 2015; 31p. [Google Scholar]
- De la Cruz, R. Clasificación de Zonas de vida de Guatemala Basada en el Sistema Holdridge; Instituto Nacional Forestal: Guatemala, 1976. [Google Scholar]
- IARNA-URL (Instituto de Investigación y Proyección sobre Ambiente Natural y Sociedad de la Universidad Rafael Landívar). Ecosistemas de Guatemala Basado en el Sistema de Clasificación de Zonas de Vida; Instituto de Investigación y Proyección sobre Ambiente Natural y Sociedad de la Universidad Rafael Landívar: Guatemala City, Guatemala, 2018. [Google Scholar]
- Blanco, M.A.; Whitten, W.M.; Penneys, D.S.; Williams, N.H.; Neubig, K.M.; Endara, L. A simple and safe method for rapid drying of plant specimens using forced-air space heaters. Selbyana 2006, 27, 83–87. [Google Scholar]
- Standley, P.C.; Steyermark, J.A. Flora of Guatemala. V. Fieldiana Bot. 1946, 24, 502. [Google Scholar]
- Stevens, W.D.; Ulloa, C.; Pool, A.; Montiel, O.M. Flora de Nicaragua; Missouri Botanical Garden Press: St. Louis, MO, USA, 2001; Volume 85. [Google Scholar]
- Flora Mesoamericana; British Museum of Natural History: London, UK, 1995.
- The Plant List, Version 1.1. 2013: Published on the Internet. Available online: http://www.theplantlist.org/ (accessed on 15 January 2021).
- WFO. World Flora Online. 2021: Published on the Internet. Available online: http://www.worldfloraonline.org/ (accessed on 15 January 2021).
- Gauch, H.G.; Gauch, H.G., Jr. Multivariate Analysis in Community Ecology; Cambridge University Press: Cambridge, UK, 1982. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Galili, T. Dendextend: An R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics 2015, 31, 3718–3720. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Oksanen, J.; Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P. Vegan: Community Ecology Package. R Package Version 2.5-6. 2019; Boston, MA, USA, 2019. [Google Scholar]
- Kindt, R. BiodiversityR: Package for Community Ecology and Suitability Analysis, Version 2.7-1; 2016. [Google Scholar]
- Pither, R.; Kellman, M. Tree species diversity in small, tropical riparian forest fragments in Belize, Central America. Biodivers. Conserv. 2002, 11, 1623–1636. [Google Scholar] [CrossRef]
- Vivero, J.L.; Szejner, M.; Gordon, J.; Magin, G. The Red List of Trees of Guatemala; UNEP: Ginebra, Switzerland; IUCN: Gland, Switzerland; FFI: Cambridge, UK; BGCI: Surrey, UK, 2006. [Google Scholar]
- Hoffman, J.D.; Narumalani, S.; Mishra, D.R.; Merani, P.; Wilson, R.G. Predicting potential occurrence and spread of invasive plant species along the North Platte River, Nebraska. Invasive Plant Sci. Manag. 2008, 1, 359–367. [Google Scholar] [CrossRef]
- Ríos, B.; Raga, G.B. Spatio-temporal distribution of burned areas by ecoregions in Mexico and Central America. Int. J. Remote Sens. 2018, 39, 949–970. [Google Scholar] [CrossRef]
- Lugo, A.E.; Snedaker, S.C. The ecology of mangroves. Annu. Rev. Ecol. Syst. 1974, 5, 39–64. [Google Scholar] [CrossRef]
- Henao Bravo, E.I.; Ordóñez, Y.; Camino Velozo, R.D.; Soto, R.V.; Gambeta, F.C. El Bosque Secundario en Centroamérica: Un Recurso Potencial de uso Limitado por Procedimientos y Normativas Inadecuadas; CATIE: Cartago, Costa Rica, 2015. [Google Scholar]
- Fahrig, L. Habitat fragmentation: A long and tangled tale. Glob. Ecol. Biogeogr. 2019, 28, 33–41. [Google Scholar] [CrossRef]
- Wilson, E.O. Threats to biodiversity. Sci. Am. 1989, 261, 108–117. [Google Scholar] [CrossRef]
- Chazdon, R.L.J.S. Tropical forests—Log’em or leave’em? Science 1998, 281, 1295–1296. [Google Scholar] [CrossRef]
- Noss, R.F. Assessing and monitoring forest biodiversity: A suggested framework and indicators. For. Ecol. Manag. 1999, 115, 135–146. [Google Scholar] [CrossRef]
| Family | Species | Common Name |
|---|---|---|
| Acanthaceae | Avicennia germinans (L.) L. | Mangle negro |
| Anacardiaceae | Spondias mombin L. | Jocote jobo |
| Annonaceae | Annona purpurea Moc. & Sessé ex Dunal | Chincuya |
| Rollinia mucosa (Jacq.) Baill. | Anona | |
| Apocynaceae | Aspidosperma megalocarpon Müll.Arg. | Chichique |
| Tabernaemontana donnell-smithii Rose ex J.D.Sm. | Cojón | |
| Araliaceae | Dendropanax arboreus (L.) Decne. & Planch. | Mano de león |
| Bignoniaceae | Crescentia cujete L. | Morro |
| Spathodea campanulata P.Beauv. | Llama del bosque | |
| Tabebuia rosea (Bertol.) Bertero ex A.DC. | Matilisguate | |
| Boraginaceae | Cordia alba (Jacq.) Roem. & Schult. | Tigüilote, Upay |
| Cordia alliodora (Ruiz & Pav.) Oken | Laurel | |
| Burseraceae | Bursera simaruba (L.) Sarg. | Palo de jiote |
| Cactaceae | Cynometra retusa Britton & Rose | |
| Cannabaceae | Celtis iguanaea (Jacq.) Sarg. | |
| Trema micrantha (L.) Blume | Capulín | |
| Chrysobalanaceae | Chrysobalanus icaco L. | Icaco |
| Clethraceae | Clethra mexicana DC. | Zapotillo |
| Clusiaceae | Calophyllum brasiliense var. rekoi (Standl.) Standl. | Palo mario |
| Clusia guatemalensis Hemsl. | ||
| Combretaceae | Conocarpus erectus L. | Botoncillo |
| Laguncularia racemosa (L.) C.F.Gaertn. | Mangle blanco | |
| Terminalia arborea Koord. | Volador | |
| Dichapetalaceae | Dichapetalum donnell-smithii Engl. | |
| Ebenaceae | Diospyros nigra (J.F.Gmel.) Perrier | |
| Fabaceae | Acacia cornigera (L.) Willd. | Ixcanal blanco |
| Acacia hindsii Benth. | Ixcanal negro | |
| Acacia polyphylla DC. | Alacrán | |
| Albizia adinocephala (Donn.Sm.) Record | Conacaste blanco | |
| Albizia saman (Jacq.) Merr. | Cenicero | |
| Andira inermis (Wright) DC. | Almendro cimarrón | |
| Calliandra magdalenae var. colombiana (Britton & Killip) Barneby | Chalí | |
| Dalbergia cuscatlanica (Standl.) Standl. | Granadillo | |
| Delonix regia (Hook.) Raf. | Flambollano | |
| Diphysa americana (Mill.) M.Sousa | Guachipilín | |
| Enterolobium cyclocarpum (Jacq.) Griseb. | Conacaste | |
| Erythrina mexicana Krukoff | Palo de pito | |
| Gliricidia sepium (Jacq.) Walp. | Madrecacao | |
| Hymenaea courbaril L. | Guapinol | |
| Inga edulis Mart. | Cuje | |
| Inga laurina (Sw.) Willd. | Caspirol | |
| Inga paterno Harms | Paterna | |
| Inga sapindoides Willd. | Cushin | |
| Lonchocarpus macrocarpus Benth. | Quebracho | |
| Lonchocarpus salvadorensis Pittier | Chaperno | |
| Lonchocarpus sericeus (Poir.) DC. | Matabuey | |
| Pithecellobium dulce (Roxb.) Benth. | Guachimol | |
| Platymiscium dimorphandrum Donn.Sm. | Hormigo | |
| Poeppigia procera C.Presl | Tepemisque | |
| Schizolobium parahyba (Vell.) S.F.Blake | Plumillo | |
| Senna reticulata (Willd.) H.S.Irwin & Barneby | Varajito, Arípin | |
| Senna spectabilis (DC.) H.S.Irwin & Barneby | ||
| Vatairea lundellii (Standl.) Record | Palo negro | |
| Lamiaceae | Gmelina arborea Roxb. | Melina |
| Lauraceae | Nectandra membranacea (Sw.) Griseb. | |
| Ocotea sinuata (Mez) Rohwer | Canoj | |
| Lythraceae | Lagerstroemia indica L. | |
| Malpighiaceae | Bunchosia guatemalensis Nied. | |
| Bunchosia odorata (Jacq.) Juss. | ||
| Byrsonima crassifolia (L.) Kunth | Nance | |
| Malvaceae | Guazuma ulmifolia Lam. | Caulote |
| Hampea rovirosae Standl. | ||
| Heliocarpus donnellsmithii Rose | ||
| Pachira aquatica Aubl. | Pumpujush, zapotón, pumpo | |
| Quararibea funebris (La Llave) Vischer | Molinillo | |
| Sterculia apetala (Jacq.) H.Karst. | Castaño | |
| Trichospermum mexicanum (DC.) Baill. | Cajete | |
| Melastomataceae | Conostegia xalapensis (Bonpl.) D. Don ex DC. | |
| Miconia laevigata (L.) D. Don | Cacho de venado | |
| Meliaceae | Guarea glabra Vahl | Anicillo |
| Guarea megantha A.Juss. | Trompillo | |
| Swietenia macrophylla King | Caoba | |
| Trichilia havanensis Jacq. | ||
| Trichilia martiana C.DC. | Chile amate | |
| Moraceae | Brosimum costaricanum Liebm. | Ujushte, Ramón |
| Castilla elastica Cerv. | Palo de hule | |
| Ficus aurea Nutt. | Amate | |
| Ficus benjamina L. | Amate | |
| Ficus costaricana (Liebm.) Miq. | Amate | |
| Ficus crocata (Miq.) Mart. ex Miq. | Amate | |
| Ficus hemsleyana King | Amate | |
| Ficus insipida Willd. | Amate | |
| Ficus maxima Mill. | Amate | |
| Ficus obtusifolia Kunth | Amate | |
| Ficus pertusa L.f. | Amate | |
| Ficus sp. | Amate | |
| Maclura tinctoria (L.) D.Don ex Steud. | Mora | |
| Trophis racemosa (L.) Urb. | ||
| Muntingiaceae | Muntingia calabura L. | Capulín blanco |
| Nyctaginaceae | Neea psychotrioides Donn.Sm. | Siete camisas |
| Olacaceae | Ximenia americana L. | |
| Phyllanthaceae | Astrocasia austinii (Standl.) G.L.Webster | Pimiento |
| Polygonaceae | Coccoloba barbadensis Jacq. | Papaturro |
| Coccoloba escuintlensis Lundell | Papaturro | |
| Triplaris melaenodendron (Bertol.) Standl. & Steyerm. | Mulato | |
| Primulaceae | Bonellia macrocarpa (Cav.) B.Ståhl & Källersjö | Naranjillo |
| Rhamnaceae | Colubrina arborescens (Mill.) Sarg. | Coshte |
| Rhizophoraceae | Rhizophora mangle L. | Mangle Rojo |
| Rubiaceae | Hamelia patens Jacq. | Chichipín |
| Psychotria limonensis K.Krause | ||
| Simira salvadorensis (Standl.) Steyerm. | Puntero | |
| Rutaceae | Zanthoxylum riedelianum subsp. kellermanii (P.Wilson) Reynel ex C.Nelson | Lagarto |
| Salicaceae | Casearia arguta Kunth | Raspa lengua |
| Salix humboldtiana Willd. | Sauce | |
| Sapindaceae | Sapindus saponaria L. | Jaboncillo |
| Sapotaceae | Pouteria sapota (Jacq.) H.E.Moore & Stearn | Zapote |
| Sideroxylon capiri subsp. tempisque (Pittier) T.D.Penn. | Tempisque | |
| Sideroxylon celastrinum (Kunth) T.D.Penn. | ||
| Sideroxylon capiri (A.DC.) Pittier | Tempisque | |
| Simaroubaceae | Simarouba amara Aubl. | Aceituno |
| Solanaceae | Cestrum racemosum Ruiz & Pav. | Huele de noche |
| Solanum diphyllum L. | ||
| Staphyleaceae | Turpinia occidentalis (Sw.) G.Don | |
| Urticaceae | Cecropia obtusifolia Bertol. | Guarumo |
| Verbenaceae | Citharexylum affine D.Don | Cola de iguana |
| Community | Species | IV (%) |
|---|---|---|
| 1 | Rhizophora mangle L. | 41.6 |
| Avicennia germinans (L.) L. | 22.7 | |
| Pithecellobium dulce (Roxb.) Benth. | 6.8 | |
| Laguncularia racemosa (L.) C.F.Gaertn. | 5.6 | |
| 2 | Cecropia obtusifolia Bertol. | 19.1 |
| Guazuma ulmifolia Lam. | 10.9 | |
| Andira inermis (Wright) DC. | 8.8 | |
| Ficus aurea Nutt. | 7.7 | |
| 3 | Guazuma ulmifolia Lam. | 18.4 |
| Ceiba pentandra (L.) Gaertn. | 17.3 | |
| Enterolobium cyclocarpum (Jacq.) Griseb. | 11.3 | |
| Salix humboldtiana Willd. | 7.2 | |
| 4 | Brosimum costaricanum Liebm. | 9.2 |
| Acacia polyphylla DC. | 7.7 | |
| Cecropia obtusifolia Bertol. | 7.0 | |
| Ceiba pentandra (L.) Gaertn. | 5.9 |
| Diversity Index | Riparian Tree Community | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Number of plots | 2 | 5 | 23 | 21 |
| Total number of species | 12 | 28 | 31 | 54 |
| Richness per plot (s) * | 8 | 10.8 | 4.2 | 11.3 |
| Evenness (J’) * | 0.64 | 0.78 | 0.92 | 0.91 |
| Simpson diversity (Ds) * | 0.63 | 0.77 | 0.7 | 0.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfaro Pinto, A.; Castillo Mont, J.J.; Mendieta Jiménez, D.E.; Guerra Noriega, A.; Jiménez Barrios, J.; Clavijo McCormick, A. Characterization of Riparian Tree Communities along a River Basin in the Pacific Slope of Guatemala. Forests 2021, 12, 898. https://doi.org/10.3390/f12070898
Alfaro Pinto A, Castillo Mont JJ, Mendieta Jiménez DE, Guerra Noriega A, Jiménez Barrios J, Clavijo McCormick A. Characterization of Riparian Tree Communities along a River Basin in the Pacific Slope of Guatemala. Forests. 2021; 12(7):898. https://doi.org/10.3390/f12070898
Chicago/Turabian StyleAlfaro Pinto, Alejandra, Juan J. Castillo Mont, David E. Mendieta Jiménez, Alex Guerra Noriega, Jorge Jiménez Barrios, and Andrea Clavijo McCormick. 2021. "Characterization of Riparian Tree Communities along a River Basin in the Pacific Slope of Guatemala" Forests 12, no. 7: 898. https://doi.org/10.3390/f12070898
APA StyleAlfaro Pinto, A., Castillo Mont, J. J., Mendieta Jiménez, D. E., Guerra Noriega, A., Jiménez Barrios, J., & Clavijo McCormick, A. (2021). Characterization of Riparian Tree Communities along a River Basin in the Pacific Slope of Guatemala. Forests, 12(7), 898. https://doi.org/10.3390/f12070898








